Abstract
BACKGROUND
Routine resection of cavity shave margins (additional tissue circumferentially around the cavity left by partial mastectomy) may reduce the rates of positive margins (margins positive for tumor) and reexcision among patients undergoing partial mastectomy for breast cancer.
METHODS
In this randomized, controlled trial, we assigned, in a 1:1 ratio, 235 patients with breast cancer of stage 0 to III who were undergoing partial mastectomy, with or without resection of selective margins, to have further cavity shave margins resected (shave group) or not to have further cavity shave margins resected (no-shave group). Randomization occurred intraoperatively after surgeons had completed standard partial mastectomy. Positive margins were defined as tumor touching the edge of the specimen that was removed in the case of invasive cancer and tumor that was within 1 mm of the edge of the specimen removed in the case of ductal carcinoma in situ. The rate of positive margins was the primary outcome measure; secondary outcome measures included cosmesis and the volume of tissue resected.
RESULTS
The median age of the patients was 61 years (range, 33 to 94). On final pathological testing, 54 patients (23%) had invasive cancer, 45 (19%) had ductal carcinoma in situ, and 125 (53%) had both; 11 patients had no further disease. The median size of the tumor in the greatest diameter was 1.1 cm (range, 0 to 6.5) in patients with invasive carcinoma and 1.0 cm (range, 0 to 9.3) in patients with ductal carcinoma in situ. Groups were well matched at baseline with respect to demographic and clinicopathological characteristics. The rate of positive margins after partial mastectomy (before randomization) was similar in the shave group and the no-shave group (36% and 34%, respectively; P= 0.69). After randomization, patients in the shave group had a significantly lower rate of positive margins than did those in the no-shave group (19% vs. 34%, P= 0.01), as well as a lower rate of second surgery for margin clearance (10% vs. 21%, P= 0.02). There was no significant difference in complications between the two groups.
CONCLUSIONS
Cavity shaving halved the rates of positive margins and reexcision among patients with partial mastectomy. (Funded by the Yale Cancer Center; ClinicalTrials.gov number, NCT01452399.)
Many women who receive a diagnosis of early-stage breast cancer opt for breast-conserving surgery with partial mastectomy.1 Although the survival rate with such surgery is equivalent to that with total mastectomy, margin status is a critical determinant of local recurrence.2
Approximately 20 to 40% of patients have positive margins (margins positive for tumor) after partial mastectomy and require a second operation for margin clearance.3,4 Retrospective studies have shown that taking additional tissue circumferentially around the cavity left by partial mastectomy (also known as cavity shave margins) may reduce the rate of positive margins. However, others have argued that it may be sufficient to excise selective margins where the tumor appears to be close to the edge of the specimen on the basis of intraoperative imaging and gross assessment. We sought to determine, in a prospective randomized, controlled trial, the effect of routine excision of circumferential cavity shave margins versus standard partial mastectomy, including excision of selective margins, on outcomes after breast-conserving surgery.
Methods
Study Design
We conducted a randomized, controlled trial involving 235 patients 18 years of age or older who had breast cancer of stage 0 to III that had been diagnosed by means of core-needle biopsy and who were undergoing breast-conserving surgery. Patients who had undergone an excisional biopsy or attempted partial mastectomy previously were excluded. Patients who had undergone neoadjuvant chemotherapy and were candidates for partial mastectomy were eligible. Preoperative imaging and localization of nonpalpable tumors with the use of a needle or wire were performed at the discretion of the surgeon.
After written informed consent was obtained, patients were enrolled in the study, with stratification into one of two groups: patients with stage 0, I, or II cancer and those with stage III cancer. In each stratum, patients were randomly assigned in a 1:1 ratio to having either additional circumferential cavity shave margins resected (shave group) or no further tissue removed (no-shave group). Sealed randomization envelopes were assigned on the basis of a randomization list generated a priori at the Yale Center for Analytical Sciences. Study personnel were unaware of the study-group assignments until the point of randomization intraoperatively.
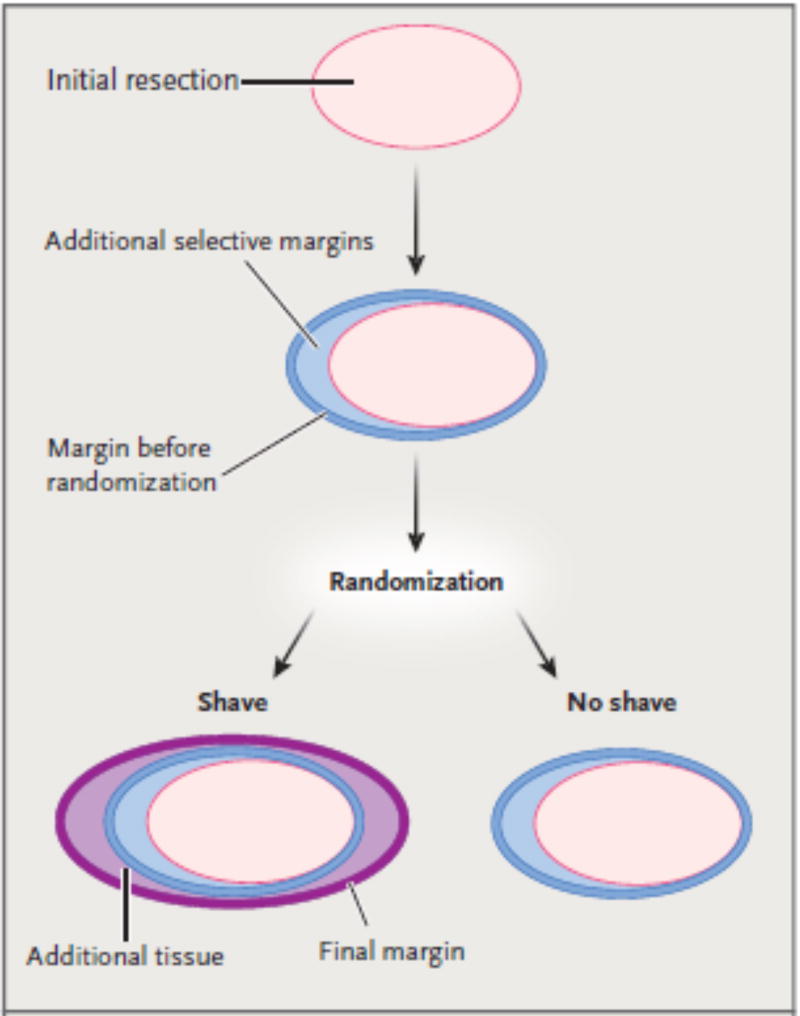
Four surgeons participated in the study. Surgeons were instructed to perform standard partial mastectomy according to their usual practice, including resection of margins where the tumor was believed to be close to the edge of the specimen on the basis of standard intraoperative imaging or their own gross evaluation (or both). Neither the specimen obtained during partial mastectomy nor any additional margins were sent for intraoperative pathological evaluation by means of frozen-section examination. The sealed randomization envelopes were opened intraoperatively after the surgeon completed the partial mastectomy. Surgeons were instructed either to resect additional circumferential margins (shave group) or to close with no further excision (no-shave group) (Fig. 1). For patients in the shave group, surgeons were instructed to resect additional tissue such that cavity shave margins encompassing the entire cavity were removed. Superior, inferior, medial, and lateral shave margins were mandated, along with anterior and posterior margins if the resection had not extended to the dermis and pectoralis fascia, respectively. The volume of the cavity shave margins could not be standardized given the varied tumor size and body habitus of the patients; however, participating surgeons were instructed that cavity shave margins should encompass the entire cavity. The specimen obtained during partial mastectomy was oriented with sutures to designate a minimum of two orthogonal faces (e.g., superior and lateral). All the additional tissue that was removed was marked with regard to its location and oriented to designate the true margin.
Figure 1. Study Design and Margin Designation.
After the initial resection, surgeons were permitted to excise additional selective margins, according to their usual practice, on the basis of intraoperative gross and radiographic findings. The margin before randomization represents, by definition, the final margin after randomization for patients randomly assigned to the no-shave group. For patients randomly assigned to the shave group, additional tissue was excised to encompass the entire cavity.
Postoperatively, specimens obtained during partial mastectomy were sectioned into 0.40-cm slices for gross evaluation and sliced-specimen radiography. Representative sections were submitted for histologic evaluation with a map of the specimen for the correlation of gross, imaging, and microscopic findings. Specimens obtained during partial mastectomy that were smaller than 5 cm in the greatest dimension were submitted for histologic evaluation in their entirety. A minimum of two sections perpendicular to each margin of the specimen obtained during partial mastectomy were evaluated. Additional margins were serially sectioned perpendicular to the true margin and were evaluated grossly and by means of specimen radiography in the same way as the other specimens obtained during partial mastectomy. Quantitative margin distances were recorded to the nearest millimeter. Pathologists were unaware of which patients were participating in the trial so that their interpretation of margins would not be biased.
The study was designed by the first author, who also conducted the analysis and wrote the initial draft of the manuscript. Two authors gathered the data, and two other authors created the randomization lists and verified the statistical analysis. All the authors contributed to the final draft of the manuscript, vouch for the data and analyses reported and for the adherence of the study to the protocol, and made the decision to submit the manuscript for publication. The protocol is available with the full text of this article at NEJM.org.
Study Oversight
This study was approved by the Yale University Human Investigations Committee. The study was monitored by the data and safety monitoring committee of the Yale Cancer Center, with internal audits conducted by the Yale Center for Clinical Investigation Office of Quality Assurance and Training.
Study End Points
The primary end point was the rate of positive margins on final pathological testing. Positive margins were defined as tumor touching the edge of the specimen that was removed5 in patients with invasive cancer and tumor that was within 1 mm of the edge of the specimen removed in those with ductal carcinoma in situ. Reexcision rates, defined as the proportion of patients who were returned to the operating room for further surgery for margin clearance, were also recorded. Although it was expected that surgeons would perform reexcision on patients whose final margin was positive, this decision was left to the surgeons’ discretion.4 Secondary end points included the volume of tissue excised, defined as cubic measurements (length×width×height) of all pieces of tissue removed, and patient-reported cosmesis on a 4-point Likert scale (with 1 indicating poor, 2 fair, 3 good, and 4 excellent). Here, we report results regarding patient-reported cosmesis at their postoperative visit before the patients became aware of their randomization group. Multiple measures of cosmesis (including the 4-point Likert scale) have been described in the literature.6 Photographs of the patients were taken before and after surgery to provide a visual record of the cosmetic outcomes.
Statistical Analysis
Sample-size calculation was performed with the use of the Inequality Tests for Two Proportions module in PASS 2008 software (NCSS Statistical Software) on the basis of the normal approximation. We estimated that a sample of 250 patients would provide the study with 80% power to detect a difference between the anticipated rate of positive margins of 30% in the no-shave group and a rate of positive margins of 15% in the shave group, at a one-sided significance level of 1.25. Group comparisons were performed with the use of Fisher’s exact test or chi-square tests for categorical variables and Mann–Whitney U tests for continuous variables, as appropriate. Multivariate logistic regression was used to assess the effect of excision of cavity shave margins after adjustment for potential confounding effects. SPSS software, version 21.0 (SPSS), was used for statistical analysis.
Results
Study Participants
Between October 21, 2011, and November 25, 2013, a total of 235 patients were enrolled in the trial. The median age of the patients was 61 years (range, 33 to 94). At the time of this analysis, the median follow-up was 22 months (range, 0 to 39). On final pathological testing, 54 patients (23%) had invasive cancer, 45 (19%) had ductal carcinoma in situ, and 125 (53%) had both. A total of 11 patients (5%) had no further disease at the time of surgery, including 2 who had a pathological complete response after neoadjuvant chemotherapy and 9 who had the focus of disease excised completely at the preoperative core biopsy. On final pathological testing, the median size of invasive tumor in the greatest diameter was 1.1 cm (range, 0 to 6.5) and the median size of ductal carcinoma in situ in the greatest diameter was 1.0 cm (range, 0 to 9.3). The median largest tumor deposit (regardless of patient status with respect to in situ or invasive disease) was 1.6 cm (range, 0 to 9.3).
A total of 119 patients were randomly assigned to the shave group, and 116 to the no-shave group. The groups were well matched with respect to demographic and clinicopathological characteristics at baseline (P>0.05 for all comparisons) (Table 1). The distribution of cases to individual surgeons was similar in the two groups (P=0.87). Before randomization, the rate of positive margins (Fig. 1) did not differ significantly between the shave group and the no-shave group (36% and 34%, respectively; P= 0.69).
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Shave (N = 119) |
No Shave (N = 116) |
|---|---|---|
| Age — yr | ||
| Median | 62 | 60 |
| Range | 35–88 | 33–94 |
| Race — no. (%)† | ||
| White | 93 (78) | 90 (78) |
| Black | 15 (13) | 15 (13) |
| Asian | 2 (2) | 2 (2) |
| Other | 9 (8) | 9 (8) |
| Hispanic ethnic group — no./total no. (%)† | 3/96 (3) | 3/96 (3) |
| Palpable tumor — no. (%) | 26 (22) | 26 (22) |
| Pathological stage — no. (%) | ||
| 0 | 24 (20) | 32 (28) |
| 1 | 69 (58) | 53 (46) |
| II | 25 (21) | 29 (25) |
| III | 1 (1) | 2 (2) |
| Invasive tumor size in greatest diameter — cm | ||
| Median | 1.0 | 1.1 |
| Range | 0–6.0 | 0–6.5 |
| Invasive histologic subtype — no./total no. (%) | ||
| Ductal | 80/95 (84) | 73/84 (87) |
| Lobular | 10/95 (11) | 6/84 (7) |
| Other | 5/95 (5) | 5/84 (6) |
| Node-positive disease — no./total no. (%) | 11/98 (11) | 13/89 (15) |
| DCIS component — no. (%) | 83 (70) | 87 (75) |
| DCIS size in greatest diameter — cm | ||
| Median | 1.0 | 1.0 |
| Range | 0–9.3 | 0–8.1 |
| Neoadjuvant chemotherapy — no. (%) | 4 (3) | 3 (3) |
| No residual disease — no. (%) | 4 (3) | 7 (6) |
| Initial volume of tissue resected, including selective margins, before randomization — cm3 | ||
| Median | 74.3 | 74.2 |
| Range | 12.5–427.5 | 2.5–480.0 |
| Positive margins before randomization — no. (%) | 43 (36) | 39 (34) |
Patients with breast cancer of stage 0 to III who undergoing partial mastectomy, with or without resection of selective margins, were assigned to have further cavity shave margins resected (shave group) or not to have further cavity shave margins resected (no-shave group). There were no significant differences between the two study groups. DCIS denotes ductal carcinoma in situ.
Race and ethnic group were self-reported.
End Points
Rates of Positive Margins and Reexcision
After randomization, patients who had been assigned to the shave group had a significantly lower rate of positive margins than did those randomly assigned to the no-shave group (19% vs. 34%, P= 0.01). Of the 119 patients in the shave group, 43 (36%) had positive margins before randomization, 23 of whom (53%) had the tumor cleared with the additional cavity shaving. Of the 76 patients in the shave group who were classified as having negative margins before randomization, 9 (12%) were found to have further cancer in the cavity shave margins; in 3 patients (4%), the new true margin was positive.
Margin positivity did not vary according to surgeon either before or after randomization (P = 0.16 and P= 0.26, respectively). Factors correlating with final margin positivity are shown in Table 2. The age of the patients and the size of invasive tumor were not correlated with margin status (P= 0.85 and P= 0.68, respectively). Patients with a greater extent of ductal carcinoma in situ, however, were more likely than those with a lesser extent to have positive margins (median size of tumor, 2.0 cm among those with positive margins vs. 0.6 cm among those with negative margins; P<0.001). In a multivariate analysis with adjustment for factors that were found to be significant in bivariate analysis, the effect of excising cavity shave margins trended toward significance in reducing the odds of positive margins (P= 0.06) (Table 3).
Table 2.
Factors Associated with Margin Positivity.*
| Variable | Patients with Positive Margins (N = 62) |
P value |
|---|---|---|
| Study group — no./total no. (%) | 0.01 | |
| Shave | 23/119 (19) | |
| No shave | 39/116 (34) | |
| Race — no./total no. (%)† | 0.33 | |
| White | 44/183 (24) | |
| Black | 12/30 (40) | |
| Asian | 1/4 (25) | |
| Other | 5/18 (28) | |
| Hispanic ethnic group — no./total no. (%)† | 0.05 | |
| Yes | 4/6 (67) | |
| No | 49/186 (26) | |
| Palpable tumor — no./total no. (%) | 0.65 | |
| Yes | 15/52 (29) | |
| No | 47/183 (26) | |
| Invasive histologic subtype — no./total no. (%) | 0.92 | |
| Ductal | 37/153 (24) | |
| Lobular | 4/16 (25) | |
| Other | 3/10 (30) | |
| DCIS component — no./total no. (%) | 0.002 | |
| Yes | 54/170 (32) | |
| No | 8/65 (12) | |
| Median DCIS size — cm‡ | 2.0 | <0.001 |
| Neoadjuvant chemotherapy — no./total no. (%) | 1.00 | |
| Yes | 2/7 (29) | |
| No | 60/228 (26) |
Total numbers in this table are the total numbers of patients with the particular demographic or baseline clinical characteristic.
Race and ethnic group were self-reported.
The comparison was with the group of patients with negative margins, who had a median DCIS size of 0.6 cm.
Table 3.
Multivariate Analysis of Margin Positivity.*
| Factor | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Hispanic ethnic group | 4.76 (0.77–29.43) | 0.09 |
| Presence of DCIS | 1.11 (0.36–3.39) | 0.86 |
| Size of DCIS | 1.87 (1.40–2.49) | <0.001 |
| Standard partial mastectomy | 2.06 (0.98–4.32) | 0.06 |
The comparator groups for the listed factors are as follows: for Hispanic ethnic group, the comparator group was non-Hispanic ethnic group; for presence of DCIS, absence of DCIS; and for standard partial mastectomy, cavity shave margin. The size of the tumor in patients with DCIS was analyzed as a continuous variable, such that the odds ratio reflects the odds of having positive margins per incremental centimeter. CI denotes confidence interval.
There was a clear association between the rate of positive margins and the rate of reexcision; 86% of the patients who had a reexcision had it because of positive margins (P<0.001 for the comparison with the group of patients with negative margins). Patients who had been randomly assigned to the shave group had a significantly lower rate of reexcision than those assigned to the no-shave group (10% vs. 21%, P= 0.02). Not all the patients who had final positive margins had a reexcision; some patients had a positive anterior or posterior margin in which no further tissue could be taken. The rate at which surgeons opted not to perform reexcision on patients with positive margins did not differ significantly between the shave group and the no-shave group (57% and 46%, respectively; P=0.43). Patients in the no-shave group were also more likely than those in the shave group to have a second or third reexcision; of the 6 patients who required more than one reexcision, 5 (83%) were in the no-shave group, but this finding did not reach statistical significance (P= 0.09).
Volume of Tissue Excised
The volume of tissue resected before randomization did not differ significantly between the shave group and the no-shave group (median, 74.3 cm3 and 74.2 cm3, respectively; P = 0.92). Among patients randomly assigned to the shave group, the median volume of each margin was 7.8 cm3 (range, 0.4 to 88.0). The median number of shave margins resected was 4 (range, 3 to 6), and the median total volume of shaved margins after randomization was 36.1 cm3 (range, 2.1 to 440.2). The volume of the shaved margins was directly correlated with the volume of tissue resected before randomization (Spearman’s correlation coefficient, 0.547; P<0.001), indicating that the variation in volume of the cavity shave margin was due to differences in the cavity itself. The total volume of tissue excised was significantly larger in the shave group than in the no-shave group (median, 115.1 cm3 vs. 74.2 cm3; P<0.001).
Postoperative Findings
There was no significant difference between the two groups in the patients’ perception of their cosmetic outcomes (P= 0.69) (Table 4). Hematomas developed postoperatively in three patients, all of whom were in the no-shave group; this finding did not reach statistical significance (P = 0.08). Surgeons varied in their technique for closure of the partial-mastectomy cavity, with some opting for complex closures routinely and others leaving the cavity to fill with a seroma. There was no significant difference between the two groups in the rate of complex rearrangements for cavity closure (P= 0.22) (Table 4), and no patient had a seroma of the partial-mastectomy cavity that required drainage.
Table 4.
Postoperative Results.
| Variable | Shave (N = 119) |
No Shave (N = 116) |
|---|---|---|
| Cosmetic outcome — no./total no. (%)* | ||
| Poor | 3/116 (3) | 1/113 (1) |
| Fair | 12/116 (10) | 9/113 (8) |
| Good | 58/116 (50) | 61/113 (54) |
| Excellent | 43/116 (37) | 42/113 (37) |
| Hematoma — no. (%) | 0 | 3 (3) |
| Complex wound closure — no. (%) | 20 (17) | 27 (23) |
Cosmesis was graded by the patients on a 4-point Likert scale (with 1 indicating poor, 2 fair, 3 good, and 4 excellent).
Discussion
We conducted a prospective, randomized, controlled trial to evaluate routine excision of cavity shave margins as a technique for reducing the rates of positive margins and reexcision. We found that excision of a cavity shave margin reduced the rate of positive margins by nearly 50% and more than halved the rate of reexcision, as compared with standard partial mastectomy, performed with or without the excision of selective margins.
Several retrospective studies have shown similar findings. In a study involving 138 patients, Kobbermann et al. found that routine cavity shaving was associated with a lower rate of reoperation for margin clearance than was standard partial mastectomy (22% vs. 42%, P= 0.01) and was a significant predictor of negative margins on multivariate analysis.7 Unzeitig et al. found that routine cavity shaving resulted in nearly half the reexcision rate associated with standard partial mastectomy (24% vs. 47%, P<0.001).8 Similarly, Marudanayagam et al. found that before the introduction of cavity shaving, 49 of 392 patients (12%) underwent reoperation for margin clearance, whereas afterward, only 22 of 394 patients (6%) who underwent cavity shaving required further surgery.9 Cao et al. found that 59% of 103 patients who had positive margins on their initial specimen had negative margins after cavity shaving.10 Tengher-Barna et al. similarly found that 42% of 47 patients who had positive margins on their initial specimen had negative margins with cavity shaving.11 Jacobson et al. found that routine cavity shaving eliminated the need for a second surgery for margin clearance in 49% of 125 patients.12 All these studies were retrospective and did not evaluate the volume of resection or cosmesis. Furthermore, none of these studies evaluated the role of excision of selective margins.
Mook et al., in a retrospective study, found that cavity shaving was associated with a smaller volume of excised tissue than was standard partial mastectomy (80.7 cm3 vs. 165.1 cm3), which raises the possibility that surgeons who perform cavity shaving routinely excise less tissue initially.13 In a retrospective study involving 171 patients, Huston et al. found that cavity shaving was associated with larger total specimen volumes than was partial mastectomy, with or without intraoperative selective margin resection (129.2 cm3 vs. 46.0 cm3 and 37.4 cm3, respectively).14 The median volume resected in the shave group in our study was in the range of these studies, suggesting that resections performed in this study were within the norm. Feron et al. found that cavity shaving reduced the need for reexcision in 24% of patients and that this was independent of the volume of tissue resected.15
Few studies have evaluated the effect of excising cavity shave margins on cosmesis. In a subgroup of 24 patients, Mook et al. found that cavity shaving was associated with improved cosmesis.13 However, this finding was based on the assessments of a multidisciplinary expert panel rather than on patients’ perception and may be correlated with the finding that patients who had cavity shaving also had less tissue removed. We found that the perception of the cosmetic outcome was equivalent in the two groups among patients who were unaware of their study-group assignment, despite the fact that the shave group had more tissue excised.
Although some have argued that routine cavity shaving may not be needed if surgeons excise margins where the tumor is deemed to be close to the edge of the specimen on the basis of intraoperative imaging or gross evaluation, we found that selective intraoperative resection of margins was insufficient to reduce the rates of positive margins. Although 27% of our patients underwent resection of selective margins before randomization, the rate of positive margins was more than 30%. Patients who had selective margins resected before randomization were no less likely to have positive margins before randomization than were those who did not (38% and 34%, respectively; P= 0.53); with routine cavity shaving, the rate of positive margins was 19%. These data echo the findings of Huston et al., who found that patients undergoing partial mastectomy with no further margins resected or with selective margins resected had reoperation rates of 39% and 32%, respectively; those with cavity shaving had a reoperation rate of 18%.14
Our finding that routine cavity shaving resulted in cancer being found in 12% of patients who were previously deemed to have negative margins calls into question the accuracy of margin status in predicting residual disease. These patients had multifocal disease that was detected only after cavity shaving. Tang et al. found that 19% of patients who had negative margins after lumpectomy had cancer found in additional shave margins16 — a finding similar to that in our study. Cao et al. found that 9% of patients who had negative margins initially had cancer found in shave margins, which rendered their final margin positive.10 Similarly, Hequet et al. found that cavity shaving resulted in the finding of previously unexpected multifocal disease in 8% of patients.17 Huston et al. found that 2% of patients with negative margins had further cancer that yielded positive margins after cavity shaving,14 which is similar to our finding of 4%. Although one could argue that finding additional occult disease may not affect outcome,18 excising additional disease in more than 10% of patients may have a significant long-term effect on the rate of local recurrence.
In conclusion, we found that cavity shaving resulted in a halving of the rates of positive margins and reoperation among patients undergoing breast-conserving surgery for breast cancer of stage 0 to III, with no decrement in patient-perceived cosmesis.
Acknowledgments
Supported by the Yale Cancer Center.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004–2010. JAMA Surg. 2014;149:1296–305. doi: 10.1001/jamasurg.2014.926. [DOI] [PubMed] [Google Scholar]
- 4.McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307:467–75. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 5.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21:704–16. doi: 10.1245/s10434-014-3481-4. [DOI] [PubMed] [Google Scholar]
- 6.Racz JM, Hong NL, Latosinsky S. In search of a gold standard scoring system for the subjective evaluation of cosmetic outcomes following breast-conserving therapy. Breast J. 2015 doi: 10.1111/tbj.12423. [DOI] [PubMed] [Google Scholar]
- 7.Kobbermann A, Unzeitig A, Xie XJ, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann Surg Oncol. 2011;18:1349–55. doi: 10.1245/s10434-010-1420-6. [DOI] [PubMed] [Google Scholar]
- 8.Unzeitig A, Kobbermann A, Xie XJ, et al. Influence of surgical technique on mastectomy and reexcision rates in breast-conserving therapy for cancer. Int J Surg Oncol. 2012:725121. doi: 10.1155/2012/725121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marudanayagam R, Singhal R, Tanchel B, O’Connor B, Balasubramanian B, Paterson I. Effect of cavity shaving on reoperation rate following breast-conserving surgery. Breast J. 2008;14:570–3. doi: 10.1111/j.1524-4741.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 10.Cao D, Lin C, Woo SH, Vang R, Tsangaris TN, Argani P. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29:1625–32. doi: 10.1097/01.pas.0000180448.08203.70. [DOI] [PubMed] [Google Scholar]
- 11.Tengher-Barna I, Hequet D, Reboul-Marty J, et al. Prevalence and predictive factors for the detection of carcinoma in cavity margin performed at the time of breast lumpectomy. Mod Pathol. 2009;22:299–305. doi: 10.1038/modpathol.2008.186. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson AF, Asad J, Boolbol SK, Osborne MP, Boachie-Adjei K, Feldman SM. Do additional shaved margins at the time of lumpectomy eliminate the need for reexcision? Am J Surg. 2008;196:556–8. doi: 10.1016/j.amjsurg.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Mook J, Klein R, Kobbermann A, et al. Volume of excision and cosmesis with routine cavity shave margins technique. Ann Surg Oncol. 2012;19:886–91. doi: 10.1245/s10434-011-1982-y. [DOI] [PubMed] [Google Scholar]
- 14.Huston TL, Pigalarga R, Osborne MP, Tousimis E. The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg. 2006;192:509–12. doi: 10.1016/j.amjsurg.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Feron JG, Nguyen A, Bézu C, et al. Interest in cavity shaving in breast conservative treatment does not depend on lumpectomy technique. Breast. 2011;20:358–64. doi: 10.1016/j.breast.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Tang R, Coopey SB, Specht MC, et al. Lumpectomy specimen margins are not reliable in predicting residual disease in breast conserving surgery. Am J Surg. 2014 Dec 13; doi: 10.1016/j.amjsurg.2014.09.029. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 17.Hequet D, Bricou A, Koual M, et al. Systematic cavity shaving: modifications of breast cancer management and long-term local recurrence, a multicentre study. Eur J Surg Oncol. 2013;39:899–905. doi: 10.1016/j.ejso.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Vos EL, Jager A, Verhoef C, Voogd AC, Koppert LB. Overall survival in patients with a re-excision following breast conserving surgery compared to those without in a large population-based cohort. Eur J Cancer. 2015;51:282–91. doi: 10.1016/j.ejca.2014.12.003. [DOI] [PubMed] [Google Scholar]