Significance
We investigated the mechanistic target of rapamycin (mTOR) signaling cascade, which is commonly implicated in neurodevelopmental autism spectrum disorders but is almost never studied in juveniles. We demonstrated that experience-dependent activation of mTOR has functional consequences for complex learned behavior using a songbird model that has strong developmental, social, behavioral, neural, and genomic parallels with human vocal communication. Our results highlight the value of investigations that integrate age and experience to understand molecular mechanisms and behavioral outcomes of neurodevelopmental disorders and typical neural development.
Keywords: zebra finch, development, critical period, rapamycin, learning
Abstract
Early life experiences can have long-lasting behavioral consequences because they are encoded when the brain is most malleable. The mechanistic target of rapamycin (mTOR) signaling cascade modulates experience-dependent synaptic plasticity, among other processes. mTOR has been almost exclusively examined in adult rodent learning models, but may be especially important in organizing neural circuits required for developmental acquisition of meaningful complex behaviors. It is among the most commonly implicated factors in neurodevelopmental autism spectrum disorders (ASD), characterized, in part, by distinct social and communication phenotypes. Here, we investigated mTOR in juvenile zebra finch songbirds. Much as children learn language, young male zebra finches need to interact socially with an adult tutor to learn a meaningful song. The memory of the tutor’s song structure guides the juvenile’s own song, which it uses to communicate for the rest of its life. We hypothesized that mTOR is required for juveniles to learn song. To this end, we first discovered that hearing song activates mTOR signaling in a brain area required for tutor song memorization in males old enough to copy song but not in younger males or females, who cannot sing. We then showed that both inhibition and constitutive activation of mTOR during tutor experiences significantly diminished tutor song copying. Finally, we found that constitutive mTOR activation lowered a behavioral measure of the juvenile’s social engagement during tutor experiences, mirroring the relationship in humans. These studies therefore advance understanding about the effects of experience in the context of neurodevelopmental disorders and typical neural development.
Learned behavior depends on mechanisms that reconfigure synaptic connections in response to experience. Neural plasticity is greater during development than in adulthood. Experience-dependent processes that alter synaptic function during development may therefore have particularly robust influences on lasting patterns of learned behavior, and are best studied in models in which effects of age and experience can be parsed.
The mechanistic target of rapamycin (mTOR) signaling cascade is well positioned to direct experience-dependent synaptic plasticity. As part of two multiprotein complexes, mTOR integrates environmental signals provided by multiple upstream receptor systems. The mTOR complex 1, in particular, affects synaptic function by regulating protein synthesis via its downstream effector proteins eukaryotic initiation factor 4E-binding protein (4EBP1) and S6 kinase (S6K) (1–4) (Fig. 1A). Protein synthesis is a key feature of long-term memory formation, and mTOR signaling contributes to learned behavior in a variety of paradigms in adult rodents (1, 5, 6). Some of the strongest evidence that mTOR may also be required developmentally comes from genetic mutations associated with neurodevelopmental disorders.
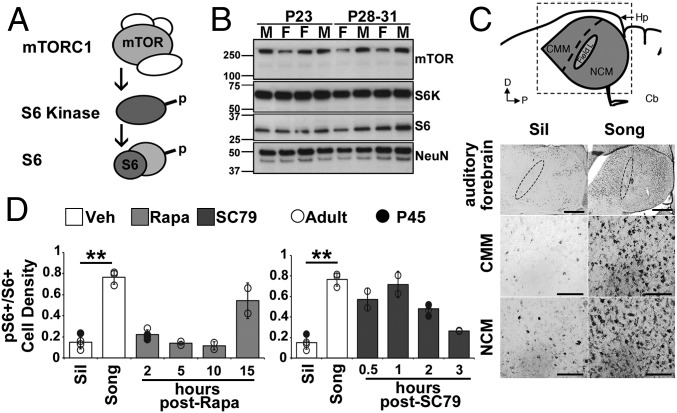
Fig. 1.
mTOR-S6K-S6 signaling in auditory forebrain is song-responsive. (A) Simplified schematic of the mTOR cascade showing the relationship between mTOR complex 1 (mTORC1), S6K, and S6. p, phosphorylation. (B) Key components of the mTOR signaling cascade are present in the juvenile auditory forebrain. Immunoblot of mTOR, S6K, S6, and NeuN (marker for mature neurons) in male (M) and female (F) birds at P23 or P28–31. There is precedence for a double-band pattern for NeuN (www.emdmillipore.com). Protein standards are marked in kilodaltons on the left (n = 2 males and n = 2 females per age). (C) Schematic depicts the portion of the telencephalon that contains the auditory forebrain (highlighted in gray), cerebellum (Cb), and hippocampus (Hp). The axis denotes that dorsal (D) is up and posterior (P) is to the right. The area imaged (dashed box) includes Field L and two higher order auditory processing regions: NCM and CMM. Images show pS6+ cells throughout the entire auditory forebrain. (Scale bar: 500 μm.) The position of Field L is indicated with a dashed oval. Images also show specific cellular staining in CMM and NCM. Images demonstrate the greater abundance of pS6+ cells after novel song playbacks (Song) compared with baseline [Silence (Sil)], as quantified in the white bars in D for the adult validation experiment and time course. Brightness and contrast were adjusted for figure clarity, but not for analysis. (Scale bars: 100 μm.) (D) Time course of Rapa-mediated attenuation of pS6+/S6+ cell density after novel song playbacks (Left) and song playback-independent increases in pS6+/S6+ cell density by SC79 (Right). Circles denote the individual birds (○, adult females; ●, P45 males). White bars denote DMSO Veh, light gray bars denote Rapa, and dark gray bars denote SC79. **P < 0.01 between indicated groups [n = 3 Veh and n = 2 per drug treatment group (adult); n = 1 Veh and n = 2 per drug treatment group (P45)].
Mutations in the mTOR cascade are associated with multiple neurodevelopmental disorders and are among the most commonly implicated single-gene contributions to the neurodevelopmental autism spectrum disorders (ASDs); mTOR has been proposed as a shared mechanism across ASD symptoms (7–12). ASDs arise early in life and are characterized by perseverative interests and behaviors, as well as deficits in social interactions and communication (refs. 13–15; https://www.nimh.nih.gov/index.shtml). Behavioral interventions can be effective in ameliorating social and language symptoms, especially when treatment starts early in childhood (16–20). mTOR signaling may therefore encode early life experiences into organizing neural circuits required for meaningful complex behaviors. Surprisingly, little investigation has been done on the behavioral effects of mTOR function in young animals (6, 21–23).
The zebra finch songbird presents a unique opportunity to investigate mTOR signaling in a model for persistent behavioral effects of developmental experience. Juvenile males learn to sing from a “tutor” bird during one developmentally sensitive period [females cannot sing (24–26)]. Social interactions with the tutor during this period promote memorization of the tutor song, which the juvenile uses to guide the patterning of its own song. Tutor song memorization largely determines the structure of the stereotyped song the bird produces for the entirety of its adult life. We hypothesized that mTOR signaling in the auditory forebrain, a region required for tutor song memorization, was regulated during development to influence song learning (27, 28). We first used a song playback paradigm in young males and females to assay how age and experience contribute to mTOR activation upon song exposure, and to examine if mTOR signaling was sex-dependent, because there is a 4:1 bias toward boys in ASD diagnoses. We then bidirectionally manipulated mTOR cascade activation in vivo to test the contribution of experience-dependent mTOR signaling to tutor song copying. We also assessed the juvenile’s social behavior during tutor sessions. Our results are among the first to demonstrate a functional requirement for mTOR signaling during developmental learning of complex behavior, and establish a system for meaningful investigation into mechanisms affecting behavioral outcomes of neurodevelopmental disorders and typical neural organization.
Results
mTOR Machinery Is Present in the Juvenile Zebra Finch Auditory Forebrain.
We were not aware of previous reports of mTOR cascade proteins in songbirds. We therefore first established that key components of the complex 1 mTOR signaling cascade—mTOR, ribosomal protein S6K, and the 40S subunit ribosomal protein S6 (S6)—were present in the auditory forebrain of juvenile males and females reared normally (Fig. 1A). Western blots revealed bands of the expected size for each protein (Fig. 1B).
Hearing Song Playbacks Activates mTOR in the Auditory Forebrain of Posthatch Day 30 Males.
We then tested if hearing song activated mTOR signaling in juvenile auditory forebrain. We were particularly interested in examining conditions that would inform about the ability to learn from tutor experience. In males, tutor song experience exclusively before posthatch day 30 (P30) does not support tutor song copying, whereas experience with a tutor starting at P30 does (26). Hence, we examined two developmental points, one a week before the onset of demonstrable song copying (P23) and the other at the beginning of demonstrable song copying (P30). Further, because tutor song experience contributes to the end of the sensitive period for tutor song memorization, we were interested in examining if prior song exposure affected cascade responsivity at P30 (29, 30). Additionally, we included males and females because both learn to discriminate songs; sex differences indicate possible mechanisms that support tutor song memorization separately from discrimination. We therefore compared males and females raised normally in the aviaries (P23 and P30) or housed individually with an adult female (i.e., isolated from hearing song) in a sound-attenuating chamber for 1 wk before P30 (P30i).
We assessed mTOR cascade activation after a short (75 s) playback of novel conspecific song (Song). We used phosphorylation of S6 (pS6) as a functional readout of mTOR activation because its kinase (S6K) is itself directly phosphorylated by mTOR complex 1 kinase activity (3, 31, 32) (Fig. 1A). We first verified that this paradigm, initially used for immediate early gene induction in adults, also induces pS6 above Silence in adult auditory forebrain but not in an adjacent region used to control for technical variation in staining (33) (P > 0.52; Fig. 1 C and D and SI Appendix, SI Materials and Methods). We then used this paradigm for the juvenile playback experiment. We calculated a normalized measure of pS6/total S6 cell density (pS6+/S6+) for each bird, and compared Song pS6+/S6+ densities with pS6+/S6+ densities of birds left in Silence. Because pS6 staining was essentially absent from the primary auditory cortex, Field L (Figs. 1C and 2C and SI Appendix, Fig. S1), we quantified normalized pS6 levels in the two secondary auditory processing areas, caudomedial nidopallium (NCM) and caudomedial mesopallium (CMM).
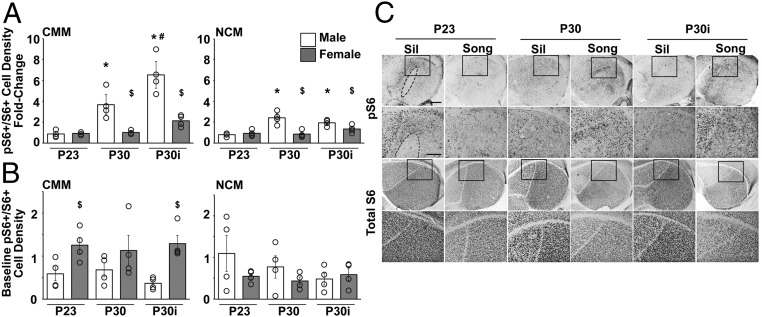
Fig. 2.
Song playbacks induce pS6 in the P30 male auditory forebrain. (A) Fold change in Song/Sil pS6+/S6+ cell density in the CMM (Left) and NCM (Right). Bars (white, males; gray, females) represent the experimental group mean ± SEM. Open circles (○) denote individual birds. *P < 0.005, compared with the sex-matched P23 group; #P < 0.005, compared with the sex-matched P30 group; $P < 0.05 between age-matched sexes (n = 4 birds per Sex and Age/Rearing condition). (B) Baseline (Silence) pS6+/S6+ cell densities in the CMM (Left) and NCM (Right). (C) Representative bright-field images of pS6+ and total S6+ cells in P23, P30, and P30i male auditory forebrain; below each is a higher magnification image of the boxed Inset. The dashed oval indicates the position of Field L. Brightness and contrast were adjusted for figure clarity, but not for analysis. (Scale bars: auditory forebrain, 500 μm; Insets, 250 μm.)
Novel conspecific song playbacks significantly induced mTOR signaling in P30 males, but not in females or P23 males (Fig. 2A). In both NCM and CMM, we found a significant main effect of Sex [NCM: F(1,18) = 20.82, P = 0.002; CMM: F(1,18) = 35.16, P = 0.0001], and Age/Rearing Condition [P23, P30, and P30i; NCM: F(2,18) = 12.21, P = 0.005; CMM: F(2,18) = 25.53, P = 0.0001], as well as a Sex * Age/Rearing Condition interaction [NCM: F(2,23) = 10.98, P = 0.0008; CMM: F(2,23) = 10.82, P = 0.0008]. Post hoc analysis showed that compared with P23, song playbacks significantly increased the normalized density of pS6+ cells in P30 and P30i males, but not in females (NCM P30: P = 0.0001; NCM P30i: P = 0.03; CMM P30: P = 0.001; CMM P30i: P = 0.001). In CMM, P30i males also had greater song-induced pS6+/S6+ cell densities than P30 males (P = 0.002).
We also considered the fact that increased Song/Silence ratios can arise from increased Song values or decreased Silence values (e.g., ref. 34). We therefore compared the baseline Silence measures across groups. For the most part, Silence levels did not systematically vary with Song measures, although there was a sex difference in CMM [F(1.18) = 16.29, P = 0.0008], with females having overall higher Silence pS6+/S6+ cell densities (Fig. 2B; nonsignificant main effects are provided in SI Appendix, SI Materials and Methods). We did note that Silence measures were different between P30 (0.68) and P30i (0.37) males, but in the opposite direction of the Song/Silence comparison. The Song pS6+/S6+ cell densities showed little difference (2.48 for P30 and 2.34 for P30i, a 0.94-fold difference) before normalizing to Silence levels. It may be that the magnitude of the fold change between P30 and P30i in the Song/Silence cell density measures (a 1.78-fold difference) derives not from song induction, but from a difference in baseline Silence measures (P30/P30i Silence = 1.85).
In Vivo, Bidirectional Manipulation of mTOR Diminishes Tutor Song Copying.
Given that hearing song playbacks selectively activates the mTOR cascade in males at an age when tutor experience affects later song structure, we hypothesized that experience-dependent mTOR signaling in the auditory forebrain contributes to tutor song copying. To test this hypothesis, we combined in vivo manipulation of mTOR activation with controlled tutor experiences. We used an established paradigm that supports normal levels of tutor song copying when molecular mechanisms are not disrupted (27) (SI Appendix, SI Materials and Methods). Briefly, juvenile males were removed from the aviary at P21, and were thereafter socially housed with an adult female. The only exposure to song the juveniles experienced was eight daily, 1.5-h tutor sessions starting on P42. Here, we used two tutor males (Tutor A and Tutor B); each juvenile experienced only one (Fig. 3A).
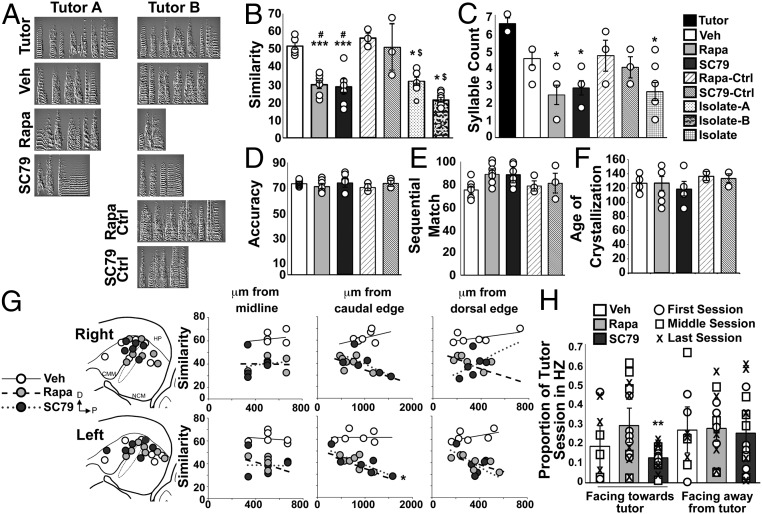
Fig. 3.
Both inhibition and constitutive activation of the mTOR cascade during tutoring experiences significantly reduces tutor song copying. (A) Representative sonograms of the Tutor songs and songs from each experimental group. (B–F) Bars [black, Tutors; white, DMSO Veh; light gray, Rapa; dark gray, SC79; wide-striped, Rapa-Ctrl; narrow-striped, SC79-Ctrl; dotted, Isolate A comparisons; mottled, Isolate B comparisons; checkers, Isolate] represent the mean ± SEM for global Song Similarity score (B), Syllable Count (C), Accuracy (D), Sequential Match (E), and age of crystallization in days posthatch (F). Circles denote individual birds. *P < 0.05 and ***P < 0.001 for the indicated experimental group compared with Veh; #P < 0.01 for Rapa or SC79 compared with drug-matched control groups; $P < 0.05 for the Isolate comparisons vs. the Rapa-Ctrl and SC79-Ctrl groups [n = 6 (Veh and SC79), n = 7 (Rapa), n = 3 (Rapa-Ctrl and SC79-Ctrl), and n = 8 (Isolates)]. (G) Correlation between infusion sites and Song Similarity scores. Schematics represent the rostral-caudal (R-C) and dorsal-ventral (D-V) cannula tip positions (infusion sites) in the right and left hemispheres. For simplicity, all medial-lateral (M-L) cannula tip positions were collapsed for depiction on one M-L plane (∼500 μm from midline). White circles, Veh; light gray circles, Rapa; dark gray circles, SC79. Graphs show regression analysis of Song Similarity scores and M-L (Left; range = 330–660 μm from midline), R-C (Center; range = 286–1,300 μm from the caudal edge), and D-V (Right; range = 154–722 μm from the dorsal edge) cannula tip positions in each hemisphere. Trend lines: solid, Veh; dashed, Rapa; dotted, SC79. *P < 0.05 for the SC79 group and P = 0.096 for the Rapa group. (H) Proportion of session time spent in proximity to the tutor (HZ), either facing toward or facing away from the tutor’s cage. Bars represent the mean ± SEM. Each data point marks an individual during the first (○), middle (□), and last (x) tutoring sessions. **P < 0.01 between the Rapa- and SC79-treated groups in the facing toward tutor measurement [n = 3 (Veh) and n = 5 (Rapa and SC79)].
Molecular and cellular data indicate that balanced mTOR signaling is required for function (4). Although mTOR signaling has been activated and inhibited in various adult learning paradigms, we found no studies that directly compared the effect of bidirectional mTOR manipulation within the same design. We therefore infused either SC79, which constitutively activates mTOR signaling, or rapamycin (Rapa), which selectively inhibits mTOR signaling, via a bilateral cannula targeted to the auditory forebrain (Fig. 1D and SI Appendix, SI Materials and Methods). Before combining in vivo mTOR manipulations with the controlled tutor experience paradigm, we needed to (i) verify that drug infused 30 min before each of the 1.5-h tutor sessions would be effective throughout the experience and (ii) consider the effective half-lives of the drugs to schedule the temporally offset infusions for tutor song memorization control (discussed below) appropriately. We found no previous reports of in vivo effective half-lives for either Rapa or SC79, although there is one record of an in vitro Rapa half-life (35). We therefore performed time-course experiments infusing drugs into the auditory forebrain before initiating the tutoring experiment (SI Appendix, SI Materials and Methods). Results indicated that 200 ng/μL SC79 and 1 μg/μL Rapa constitutively activated or inhibited, respectively, mTOR signaling appropriate for our two objectives (Fig. 1D).
We performed quantitative song analysis comparing crystallized songs from experimental birds with their tutor’s song (36) (SI Appendix, SI Materials and Methods). Our three main groups tested the effect of Rapa, SC79, or vehicle (Veh) infusions 30 min before each tutor session. A fourth group included songs from juveniles raised without hearing any tutor song (Isolate), which permits assessment of song similarity between two zebra finch songs that have no relation to each other.
Rapa and SC79 infusions just before tutor sessions significantly lowered the fidelity of tutor song copying [F(6,34) = 19.68; P = 9.27E−10; Fig. 3B]. Post hoc tests revealed that the Rapa, SC79, and Isolate Song Similarity scores were not statistically different from each other, but that the Rapa and SC79 scores were different from Veh (Rapa: P = 0.00013, SC79: P = 0.00011). Song Similarity scores did not differ between birds tutored by Tutor A or Tutor B in the Veh [F(1,4) = 0.18, P = 0.7], Rapa [F(1,5) = 1.9, P = 0.22], or SC79 [F(1,4) = 0.22, P = 0.66] groups, and Isolate scores did not differ when compared with Tutor A or Tutor B (P = 0.065).
The Rapa, SC79, and Isolate birds sang significantly fewer syllables than the Tutors [F(6,28) = 5.18, P = 0.0011; Rapa: P = 0.004; SC79: P = 0.014; Isolate: P = 0.006; Fig. 3C]. The number of syllables is related to the duration of a bout [R2 = 0.59; F(1,25) = 37.16; P = 2.26E−6], and there was also a significant difference in song bout duration [F(7,33) = 3.42, P = 0.007]; post hoc tests show Rapa songs are shorter than Tutor songs (A: P = 0.02, B: P = 0.02). We did not detect a significant effect of Drug treatment on Accuracy, a fine-grained (10 ms) measure of how faithfully each song element that met similarity criteria was reproduced [F(4,20) = 0.58; P = 0.68; Fig. 3D], or Sequential Match, a measure of the ordering of copied elements [F(4,20) = 2.49, P = 0.08; Fig. 3E] across tutored groups. We also did not find a significant difference between the songs of our experimental birds and the Tutors on measures of Pitch [F(5,21) = 1.43, P = 0.25], Frequency Modulation [FM; F(5,21) = 1.96, P = 0.13], or Goodness of pitch [F(5,21) = 0.61, P = 0.69; SI Appendix, Table S1]. There was a significant main effect on Wiener entropy [F(5,21) = 3.07, P = 0.03], but no significant pairwise differences (SI Appendix, Table S1), and a significant main effect of Amplitude Modulation [AM; F(5,21) = 3.74, P = 0.01]; Rapa and SC79 birds’ songs had significantly lower AM than the Tutors’ (Rapa: P = 0.049; SC79: P = 0.013; SI Appendix, Table S1).
Cannula Analysis.
After completion of behavioral analysis, we verified that all cannula tips were located within the auditory forebrain (SI Appendix, SI Materials and Methods). There were no significant differences in dorsal-ventral [left hemisphere: F(4,20) = 0.84, P = 0.52; right hemisphere: F(4,20) = 0.45, P = 0.77], rostral-caudal [left hemisphere: F(4,20) = 1.14, P = 0.37; right hemisphere: F(4,20) = 2.83, P = 0.052], and medial-lateral [left hemisphere: F(4,20) = 0.35, P = 0.84; right hemisphere: F(4,20) = 0.68, P = 0.62] cannula tip position coordinates across groups. The only statistically significant correlation between cannula tip position and Song Similarity score was in the left hemisphere rostral-caudal dimension in the SC79 group [R2 = 0.71, F(1,4) = 9.59, P = 0.036; Fig. 3G], although both Rapa and SC79 groups tended to show lower Song Similarity scores with more rostral cannula positions (Fig. 3G).
Controls for Sensory Song Learning.
We assessed four possible alternative explanations for effects of drug during tutor sessions. One is that Rapa or SC79 prevents auditory perception. To check that the birds could still hear, we performed a sharp noise outside of the bird’s visual field after infusion and before tutor sessions to ensure that all birds responded to this sound. A second possibility is that the 0.5-μL infusions spread to other functional areas (27). To assess this possibility, we measured the area of drug-altered pS6 staining on serial immunohistochemistry sections and then calculated the volume of brain affected. Infusions affected 67% (Rapa) and 34% (SC79) of the auditory forebrain. We found no spread of either drug beyond the anatomical boundaries of the auditory forebrain. Third, it was possible that drugs had extended effects on how much the birds practiced singing or how quickly their song became stereotyped. We recorded the birds every 10 d starting at P90. From these data, we quantified singing bouts and compared the age of song crystallization. We found no difference in the number of rehearsal bouts per day across tutored groups [F(4,20) = 1.65, P = 0.20] and no difference in the age of song crystallization [F(4,20) = 0.60, P = 0.67; Fig. 3F]. Fourth, it was possible that the drugs had adverse nonspecific effects on auditory forebrain function or disrupted sensorimotor processes invoked during singing practice. To address this possibility, we first watched videotapes of tutor sessions 1, 4, and 8 (first, middle, and last) for each of the experimental juveniles to confirm that they did not sing during sessions, consistent with previous reports (27). Further, we performed an additional tutoring experiment on separate sets of birds to test for effects of Rapa and SC79 in the auditory forebrain at times other than the tutor sessions, when the birds could be rehearsing and therefore performing sensorimotor processing. We infused this independent set of birds with either Rapa or SC79 temporally offset from the tutor sessions so that drugs were not effective during tutor experiences (SI Appendix, SI Materials and Methods). We measured the extent of tutor song copying as for the other conditions; both control groups were included in the tutor song copying statistics above. Song Similarity score post hoc analysis revealed significant differences between the Rapa and SC79 groups and their respective control groups (Rapa-Ctrl: P = 0.00016; SC79-Ctrl: P = 0.0016), but no significant differences in the level of tutor song copying between the Veh group and the offset temporal control groups (Rapa-Ctrl: P = 0.97; SC79-Ctrl: P = 0.99; Fig. 3B).
Increasing mTOR Activation Decreases a Behavioral Measure of Social Engagement.
Increased neural mTOR activity correlates with lower levels of social interactions (6, 11, 37, 38). We therefore predicted that SC79 birds would display diminished social interactions during tutor sessions. Because the juvenile and the Tutor were in separate cages, we quantified the proportion of time that the juveniles spent in the vicinity of the tutor bird’s cage, which we termed the Hot Zone (HZ), and facing the tutor, as a proxy for intent to interact (SI Appendix, SI Materials and Methods). We found a significant main effect of Drug [F(2,30) = 3.91, P = 0.03; Fig. 3H] on the proportion of time juveniles spent in the HZ facing the tutor’s cage, but not in how long the juveniles were in the HZ but facing away from the tutor’s cage [F(2,30) = 0.06, P = 0.94; Fig. 3H]. Post hoc analysis revealed a significant difference in HZ time facing toward the tutor between Rapa- and SC79-treated birds (P = 0.009); there were no differences between the Veh group and either the Rapa (P = 0.13) or SC79 (P = 0.39) group. Both behaviors were unaffected by Session [facing toward: F(2,30) = 0.12, P = 0.88; facing away: F(2,30) = 0.28, P = 0.76] or Session * Drug interaction [facing toward: F(4,38) = 0.65, P = 0.63; facing away: F(4,38) = 0.40, P = 0.81]. Examination of linear correlations between the proportion of time each juvenile spent in the HZ suggested no relationship with the Song Similarity score for Rapa or SC79 birds. In addition, cannula tip positions largely do not affect HZ behaviors; of the 54 possible relationships (3D coordinates, two hemispheres, three drug treatment conditions, and three sessions), only three reached significance.
Discussion
Zebra finches are a powerful system in which to examine the long-term neural and behavioral legacies of developmental experiences. The mTOR cascade is implicated in neurodevelopmental disorders that affect complex social and communication behaviors but is little studied in juveniles. We causally established the functional relevance of experience-dependent mTOR cascade activation in the socially mediated learning required for juveniles to acquire song. Our use of temporally restricted, bidirectional, and localized drug administration provided high specificity that emphasizes the importance of precise experience-dependent signaling for behavioral learning.
Inhibition and constitutive activation of mTOR signaling in the auditory forebrain during tutor experiences significantly lowered the fidelity of tutor song copying. Nonlinear relationships between levels of signaling factors and learning outcomes do occur, and balanced mTOR signaling is a feature of learning and memory in other models (e.g., refs. 2, 4, 39, 40). For example, diminished learning is observed in rodents after manipulations that either increase or decrease mTOR function, perhaps because the proper complement of proteins required for long-term memory formation cannot be synthesized (e.g., refs. 41, 42). Additionally, Rapa and SC79 affect the number of pS6+ cells in the auditory forebrain. It is possible that high-fidelity tutor song copying requires synaptic remodeling across a select set of cells; modifying the scale of the cellular network activated during tutor experiences may thus disrupt tutor song memorization (43, 44).
Indeed, the drugs do not need to affect the entire auditory forebrain directly to reduce tutor song copying. Rapa and SC79 songs were no more similar to Tutor song than to Isolate song, and like Isolate songs, included fewer syllables than the Tutor songs. Accuracy and Sequential Match scores indicated that the tutor song elements that Rapa and SC79 birds did copy were as faithfully perceived, processed, and produced as in tutored control birds. This finding may reflect distinct neural control or preserved functionality in unaffected portions of the auditory forebrain. Just as for humans, deficits in learned vocal communication are meaningful for zebra finches. Simpler songs evoke distinct molecular and behavioral responses in females, and males who produce them are less preferred in mate choice paradigms (45–50).
Social interactions enhance vocal learning in juvenile zebra finches and children (24, 25, 51–53). Although our tutor sessions do not allow physical contact between juveniles and Tutors, they do permit social interaction. During tutor sessions, SC79 birds spent less time in close proximity facing the Tutor than Rapa birds, consistent with correlations between increased mTOR signaling and ASD social and communication difficulties (10, 11, 54). This observation has mechanistic implications, because we do not yet understand how social interactions improve vocal learning. Notably, mTOR influences on social interactions are partially dissociable from the mTOR influences that regulate tutor song copying: Rapa diminishes tutor song copying without altering our measure of social behavior, but SC79 disrupts both behaviors. A combination of molecular and circuit properties likely explains this pattern. For example, Rapa and SC79 may create different cellular perturbations and invoke distinct compensatory or feedback processes that affect distinct neural networks for each behavior (e.g., refs. 55, 56). Additionally, it may be that the functional relationship between the two behaviors is not serial; perhaps an attentional or motivational component of social engagement is separable from tutor song copying.
Baseline and experience-dependent patterns of gene expression change as the role of the auditory forebrain shifts from juvenile tutor song memorization to adult song recognition learning, indicating that molecular mechanisms can provide insight into function (27, 34, 57, 58). Indeed, song-induced mTOR activation and tutor song memorization appear to emerge at the same age in males, and song playback-induced pS6 levels are twice as high in the CMM compared with NCM, mirroring the trend between rostral cannula position and lower fidelity of tutor song copying (26). Further, we failed to find song induction in juvenile females, which can form discriminatory auditory memories but cannot sing (59, 60). mTOR activation in the auditory forebrain may therefore be a useful marker for broader cellular processes that contribute specifically to tutor song memorization.
Additionally, the mTOR cascade may be among the first to come “online” for encoding tutor experience. ERK is the other signaling cascade known to be required for tutor song memorization (27). ERK and mTOR cascades can intersect, with ERK typically positioned upstream of mTOR (61). It is intriguing to consider that the song-copying effects of ERK were a consequence of disrupted mTOR signaling. However, in P30 males, novel song playbacks induce mTOR activation but not ZENK (zif268, egr-1, ngfi-a, krox24) transcription, a readout of ERK activation (34, 62). Further investigation into age- and experience-dependent regulation of these two molecular cascades will inform about acquisition of complex behaviors.
This study tests the role of mTOR signaling in developmental learning. Our results have immediate relevance to developmental disruptions in sensory processing of social interactions that have persistent consequences for behaviors, such as vocal communication. They also open the possibility that mTOR could regulate other components of developmentally acquired behaviors. For example, production of meaningful song depends on dynamic integration of sensory and motor functions across a distributed neural circuit; mTOR could mediate plasticity in several of these other brain areas. More broadly, knowledge gained here provides a platform for multiple lines of inquiry into the fundamental question of how early life experiences are encoded to affect brain function and behavior.
Materials and Methods
Detailed procedures are provided in SI Appendix, SI Materials and Methods. All procedures were conducted in accordance with the NIH guidelines for the care and use of animals for experimentation, and were approved by the University of Chicago Institutional Animal Care and Use Committee (ACUP no. 72220).
Song Playback-Induced mTOR Activation in Juveniles.
After ∼16 h alone in acoustic chambers, juveniles were either exposed to a 75-s playback of triple song (Song) or left in silence [Silence; n = 4 for all Sex, Age/Rearing (P23, P30, P30i), and playback combinations]. Immediately after playbacks, or within 10 min in the case of the Silence birds, the right hemisphere was fixed in 4% paraformaldehyde in 0.025 M PBS) in preparation for pS6 and S6 immunohistochemistry. The left hemisphere was used for another experiment.
Tutor Song Memorization in Juvenile Males.
We followed a previously established paradigm (27). On P40, we surgically implanted a bilateral guide cannula into the auditory forebrain of males as previously described (27, 57, 62). Thirty minutes before each 1.5-h tutor session, experimental groups received 0.5-μL bilateral infusions of either Rapa (1 μg/μL; n = 7) or SC79 (200 ng/μL; n = 6) in DMSO. The Veh control group (n = 6) received 0.5-μL infusions of undiluted DMSO as in the study by London and Clayton (27). Drug infusions and tutor sessions were conducted once daily for eight consecutive days, from P42 to P49. All juveniles experienced four tutor sessions in the first 7 h of lights-on (except for the first hour after the lights are on: AM), and four in the second 7 h of lights-on (except for the last hour of the day: PM). Males continued to live with their companion female within a sound-attenuating chamber until their songs were crystallized. We performed several controls (SI Appendix, SI Materials and Methods), including groups that received temporally offset drug [Rapa-Ctrl, SC79-Ctrl (n = 3 per group), final group numbers were determined by statistical power analysis using data acquired from preliminary studies] infusions. Based on results from the time-course experiment, SC79-Ctrl birds were infused 2 h after the completion of an AM tutor session and 2 h before the start of a PM tutor session, and Rapa-Ctrl birds received drug infusions 4 h after the conclusion of each tutoring session. All aspects of housing, tutoring, data acquisition, and analysis were consistent across all experimental groups, except Isolates, which were not exposed to tutor sessions.
Song Similarity Analysis.
Experimental birds were recorded every 10 d, beginning at P90, until their songs were crystallized. Acoustic analysis and similarity scoring were conducted using Sound Analysis Pro2011 (SAP2011), excluding songs recorded during the first 3 h after lights-on (36).
Tutor Session Behavioral Scoring.
A perch placed ∼3 inches from the end of the juvenile’s cage adjacent to the tutor’ cage delineated an HZ. Reviewers blind to condition used JWatcher (63) to quantify how long the juvenile spent in the HZ either not facing the tutor’s cage or facing the tutor’s cage and not engaged in any other behaviors. We scored the entire 90 min for tutor sessions 1, 4, and 8 (first, middle, and last). Due to technical difficulties, this dataset contains a subset of birds included for the song similarity scoring (n = 3 for Veh and n = 5 each for the Rapa and SC79 groups).
Cannula Placement Analysis, Immunoblots, Immunohistochemistry, Imaging, and Quantification.
Details are provided in SI Appendix, SI Materials and Methods.
Statistics.
StatPlus software (AnalystSoft) was used to run all statistical tests (α = 0.05), including the Student’s t test, one- and two-way ANOVAs, and linear regressions. In the instance of significant main effects or interactions from ANOVAs, either a post hoc Tukey’s honest significant difference test (equal sample sizes) or Tukey–Kramer (unequal sample sizes) test was used to identify significant pairwise differences.
Supplementary Material
Acknowledgments
We thank research assistants Charissa Newkirk and Sylvia Lobo for behavioral scoring, Megan Garvey and Elisa Gores for careful reading of the manuscript, Elisa Gores for brain sectioning, and Latisha Haskell for bird care. This study was supported by NIH Grant T32MH020065 (to S.A.) and University of Chicago funds (S.E.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 9240.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701829114/-/DCSupplemental.
References
- 1.Garza-Lombó C, Gonsebatt ME. Mammalian target of rapamycin: Its role in early neural development and in adult and aged brain function. Front Cell Neurosci. 2016;10:157. doi: 10.3389/fncel.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn Mem. 2013;20:518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- 3.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 4.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannini MG, Lana D. mTOR involvement in the mechanisms of memory: An overview of animal studies. In: Maiese K, editor. Molecules to Medicine with mTOR. Academic; Boston: 2016. pp. 169–184. [Google Scholar]
- 6.Kazdoba TM, Leach PT, Crawley JN. Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 2016;15:7–26. doi: 10.1111/gbb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Alberts I, Li X. Dysregulation of the IGF-I/PI3K/AKT/mTOR signaling pathway in autism spectrum disorders. Int J Dev Neurosci. 2014;35:35–41. doi: 10.1016/j.ijdevneu.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Crino PB. The mTOR signalling cascade: Paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–392. doi: 10.1038/nrneurol.2016.81. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimi-Fakhari D, Sahin M. Autism and the synapse: Emerging mechanisms and mechanism-based therapies. Curr Opin Neurol. 2015;28:91–102. doi: 10.1097/WCO.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 10.Sato A. mTOR, a potential target to treat autism spectrum disorder. CNS Neurol Disord Drug Targets. 2016;15:533–543. doi: 10.2174/1871527315666160413120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawicka K, Zukin RS. Dysregulation of mTOR signaling in neuropsychiatric disorders: Therapeutic implications. Neuropsychopharmacology. 2012;37:305–306. doi: 10.1038/npp.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian M, Timmerman CK, Schwartz JL, Pham DL, Meffert MK. Characterizing autism spectrum disorders by key biochemical pathways. Front Neurosci. 2015;9:313. doi: 10.3389/fnins.2015.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiCicco-Bloom E, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanner L, Eisenberg L. Early infantile autism, 1943-1955. Psychiatr Res Rep Am Psychiatr Assoc. 1957;7:55–65. doi: 10.4159/harvard.9780674367012.c2. [DOI] [PubMed] [Google Scholar]
- 15.Ziats MN, Rennert OM. The evolving diagnostic and genetic landscapes of autism spectrum disorder. Front Genet. 2016;7:65. doi: 10.3389/fgene.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris SL, Handleman JS. Age and IQ at intake as predictors of placement for young children with autism: A four- to six-year follow-up. J Autism Dev Disord. 2000;30:137–142. doi: 10.1023/a:1005459606120. [DOI] [PubMed] [Google Scholar]
- 17.Cohen H, Amerine-Dickens M, Smith T. Early intensive behavioral treatment: Replication of the UCLA model in a community setting. J Dev Behav Pediatr. 2006;27(2 Suppl):S145–S155. doi: 10.1097/00004703-200604002-00013. [DOI] [PubMed] [Google Scholar]
- 18.Remington B, et al. Early intensive behavioral intervention: Outcomes for children with autism and their parents after two years. Am J Ment Retard. 2007;112:418–438. doi: 10.1352/0895-8017(2007)112[418:EIBIOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Dawson G, et al. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren Z, et al. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303–e1311. doi: 10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- 21.Halloran J, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113, and erratum (2015) 306:151. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber KM, Klann E, Costa-Mattioli M, Zukin RS. Dysregulation of mammalian target of rapamycin signaling in mouse models of autism. J Neurosci. 2015;35:13836–13842. doi: 10.1523/JNEUROSCI.2656-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tallot L, et al. Updating of aversive memories after temporal error detection is differentially modulated by mTOR across development. Learn Mem. 2017;24:115–122. doi: 10.1101/lm.043083.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Matheson LE, Sakata JT. Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc Natl Acad Sci USA. 2016;113:6641–6646. doi: 10.1073/pnas.1522306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derégnaucourt S, Poirier C, Kant AV, Linden AV, Gahr M. Comparisons of different methods to train a young zebra finch (Taeniopygia guttata) to learn a song. J Physiol Paris. 2013;107:210–218. doi: 10.1016/j.jphysparis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology. 2006;112:458–470. [Google Scholar]
- 27.London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagihara S, Yazaki-Sugiyama Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun. 2016;7:11946. doi: 10.1038/ncomms11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eales LA. Song learning in zebra finches: Some effects of song model availability on what is learnt and when. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 30.Eales LA. Song learning in female-raised zebra finches: Another look at the sensitive phase. Anim Behav. 1987;35:1356–1365. [Google Scholar]
- 31.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 32.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 33.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 35.Hosoi H, et al. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59:886–894. [PubMed] [Google Scholar]
- 36.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 37.Kwon CH, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burket JA, Benson AD, Tang AH, Deutsch SI. NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:60–65. doi: 10.1016/j.pnpbp.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber TA, Kimbrough TN. Memantine improves observational learning in day-old chicks. Behav Pharmacol. 2015;26:407–410. doi: 10.1097/FBP.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 40.Salehi B, Cordero MI, Sandi C. Learning under stress: The inverted-U-shape function revisited. Learn Mem. 2010;17:522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- 41.Banko JL, et al. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogerson T, et al. Synaptic tagging during memory allocation. Nat Rev Neurosci. 2014;15:157–169. doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 45.Holveck M-J, Riebel K. Female zebra finches learn to prefer more than one song and from more than one tutor. Anim Behav. 2014;88:125–135. [Google Scholar]
- 46.Lin LC, Vanier DR, London SE. Social information embedded in vocalizations induces neurogenomic and behavioral responses. PLoS One. 2014;9:e112905. doi: 10.1371/journal.pone.0112905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller DB. Long-term recognition of father’s song by female zebra finches. Nature. 1979;280:389–391. [Google Scholar]
- 48.Tchernichovski O, Schwabl H, Nottebohm F. Context determines the sex appeal of male zebra finch song. Anim Behav. 1998;55:1003–1010. doi: 10.1006/anbe.1997.0673. [DOI] [PubMed] [Google Scholar]
- 49.Vignal C, Andru J, Mathevon N. Social context modulates behavioural and brain immediate early gene responses to sound in male songbird. Eur J Neurosci. 2005;22:949–955. doi: 10.1111/j.1460-9568.2005.04254.x. [DOI] [PubMed] [Google Scholar]
- 50.Clayton NS. Song discrimination learning in zebra finches. Anim Behav. 1988;36:1016–1024. [Google Scholar]
- 51.Adret P. Vocal imitation in blindfolded zebra finches (Taeniopygia guttata) is facilitated in the presence of a non-singing conspecific female. J Ethol. 2004;22:29–35. [Google Scholar]
- 52.Yu C, Ballard DH. A unified model of early word learning: Integrating statistical and social cues. Neurocomputing. 2007;70:2149–2165. [Google Scholar]
- 53.Clayton NS. Song tutor choice in zebra finches. Anim Behav. 1987;35:714–721. doi: 10.1006/anbe.1998.0924. [DOI] [PubMed] [Google Scholar]
- 54.Baribeau DA, Anagnostou E. Social communication is an emerging target for pharmacotherapy in autism spectrum disorder - A review of the literature on potential agents. J Can Acad Child Adolesc Psychiatry. 2014;23:20–30. [PMC free article] [PubMed] [Google Scholar]
- 55.Antion MD, et al. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn Mem. 2008;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhattacharya A, et al. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron. 2012;76:325–337. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong S, et al. Discrete molecular states in the brain accompany changing responses to a vocal signal. Proc Natl Acad Sci USA. 2009;106:11364–11369. doi: 10.1073/pnas.0812998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mello CV, Clayton DF. The opportunities and challenges of large-scale molecular approaches to songbird neurobiology. Neurosci Biobehav Rev. 2015;50:70–76. doi: 10.1016/j.neubiorev.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braaten RF. Song recognition in zebra finches: Are there sensitive periods for song memorization? Learn Motiv. 2010;41:202–212. [Google Scholar]
- 60.Riebel K. Advances in the Study of Behavior. Vol 40. Academic Press; London: 2009. Song and female mate choice in zebra finches: A review; pp. 197–238. [Google Scholar]
- 61.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng HY, Clayton DF. Activation and habituation of extracellular signal-regulated kinase phosphorylation in zebra finch auditory forebrain during song presentation. J Neurosci. 2004;24:7503–7513. doi: 10.1523/JNEUROSCI.1405-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumstein DT, Daniel JC. Quantifying Behavior the JWatcher Way. Sinauer Associates; Sunderland, MA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.