Significance
Chronic cocaine use leads to both increased motivation for the drug and long-lasting morphological changes in the form of increased dendritic spine density in medium spiny neurons of the nucleus accumbens. Here we show that the cocaine-induced morphological changes are mediated by local brain-derived neurotrophic factor (BDNF)-tyrosine kinase B (TrkB) signaling, similar to activity-dependent dendritic spine increases in other brain regions. However, the same BDNF-TrkB signaling pathway can reverse these spine increases after their formation, suggesting BDNF-TrkB mediates a form of “cataplasticity” that resets accumbens neurons to their preaddiction morphological state. Importantly, however, the changes in spine density are dissociated from BDNF-TrkB effects on motivation for cocaine, demonstrating that the increased dendritic spine density is likely not a causal factor in maintaining cocaine addiction.
Keywords: accumbens shell, cocaine addiction, BDNF-TrkB, PLC, dendritic spines
Abstract
Chronic cocaine use is associated with prominent morphological changes in nucleus accumbens shell (NACsh) neurons, including increases in dendritic spine density along with enhanced motivation for cocaine, but a functional relationship between these morphological and behavioral phenomena has not been shown. Here we show that brain-derived neurotrophic factor (BDNF) signaling through tyrosine kinase B (TrkB) receptors in NACsh neurons is necessary for cocaine-induced dendritic spine formation by using either localized TrkB knockout or viral-mediated expression of a dominant negative, kinase-dead TrkB mutant. Interestingly, augmenting wild-type TrkB expression after chronic cocaine self-administration reverses the sustained increase in dendritic spine density, an effect mediated by TrkB signaling pathways that converge on extracellular regulated kinase. Loss of TrkB function after cocaine self-administration, however, leaves spine density intact but markedly enhances the motivation for cocaine, an effect mediated by specific loss of TrkB signaling through phospholipase Cgamma1 (PLCγ1). Conversely, overexpression of PLCγ1 both reduces the motivation for cocaine and reverses dendritic spine density, suggesting a potential target for the treatment of addiction in chronic users. Together, these findings indicate that BDNF-TrkB signaling both mediates and reverses cocaine-induced increases in dendritic spine density in NACsh neurons, and these morphological changes are entirely dissociable from changes in addictive behavior.
Cocaine addiction is a chronic, debilitating disorder with strong negative effects on society and a lack of effective treatments (1). One potential target for treatment is brain-derived neurotrophic factor (BDNF) and its tyrosine receptor kinase B (TrkB) (2–5). BDNF is critical for normal brain development in early life stages, but also plays a role in neuronal maintenance and neuroplasticity in the mature brain (6). We previously found that limited daily access to cocaine self-administration (CSA) induces transient increases in BDNF and TrkB levels in the nucleus accumbens shell (NACsh) (2, 3), and that inducible and localized deletion of either BDNF or TrkB in NACsh neurons reduces CSA behavior (2, 3). Others have shown that overexpression of BDNF in the NAC increases cocaine sensitization and conditioned place preference (4). Since deleting BDNF-TrkB signaling in NACsh neurons can reduce addictive behavior, drugs that block BDNF-TrkB signaling could be an effective treatment for cocaine addiction (7).
In addition to altering BDNF-TrkB signaling, cocaine also induces dendritic spine formation in NAC neurons (8). Dendritic spine formation is functionally linked to activity-dependent BDNF-TrkB signaling in hippocampal pyramidal neurons (9), but whether local BDNF-TrkB signaling is involved in cocaine-induced spine formation in medium spiny NACsh neurons is unknown. Thus, we tested whether BDNF-TrkB signaling is necessary for cocaine-induced dendritic spine formation in NACsh neurons. Using transient viral-mediated expression of a dominant negative TrkB mutant, we also tested whether BDNF-TrkB signaling is important for maintaining the stability of cocaine-induced spines once formed and, surprisingly, found that BDNF-TrkB signaling plays opposing roles in the induction and maintenance of cocaine-induced dendritic spines.
BDNF binds to cell surface TrkB receptors to activate neurons by via numerous intracellular pathways (6). BDNF activation of TrkB autophosphorylates several tyrosines, including TYR515 and TYR816, that produce differential effects on neurite growth, survival, and neuroplasticity (6). Autophosphorylation of TYR816 recruits and phosphorylates PLCγ; this leads to downstream regulation of protein kinase C and calcium signaling (10). Our prior work found that CSA increases PLCγ phosphorylation (pPLC) in NACsh in a BDNF-dependent manner (3). Autophosphorylation of another TrkB site, TYR515, recruits a docking complex containing Src homology 2 domain-containing protein (SHC), culminating in Ras-dependent extracellular regulated kinase (ERK) phosphorylation (pERK) and activation. ERK has a prominent role in mediating BDNF-induced dendritic spine formation in hippocampal neurons (9) and could therefore play a role in spine formation in NAcSh neurons after chronic CSA. Thus, we compared the necessary and sufficient roles of these distinct TrkB signaling pathways on modulation of cocaine-induced dendritic spines, along with the modulation of CSA behavior. Ultimately, our findings indicate that postcocaine activation of PLCγ signaling reduces the motivation for cocaine completely independent of effects on morphological changes in NACsh neurons.
Results
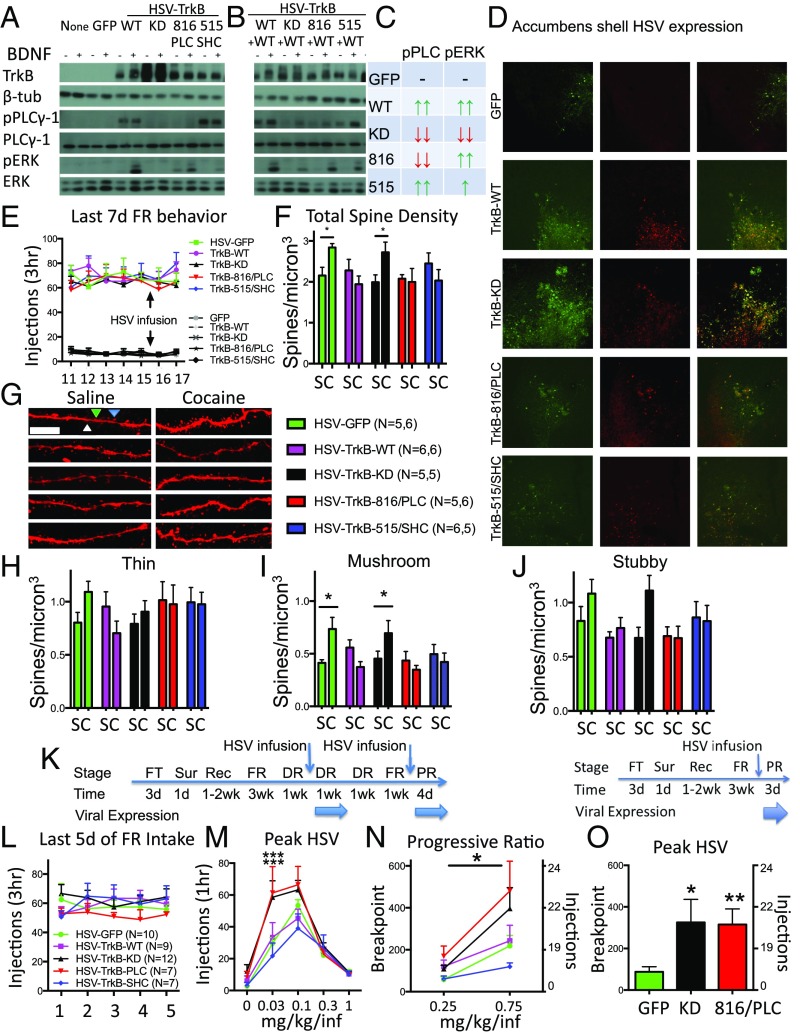
Given the role of BDNF-TrkB activity in dendritic spine formation in other brain regions (e.g., hippocampus) (9), we hypothesized that TrkB would have a critical role in the induction of cocaine-induce dendritic spines in medium spiny neurons of the NACsh. We used two methods to attenuate TrkB function in NACsh neurons. First, a herpes simplex viral vector (HSV)-encoding Cre recombinase with bicistronic GFP expression was infused into the NAC of floxed TrkB mice to delete the TrkB gene selectively from NAC neurons. Second, we infused an HSV vector expressing a kinase-dead (KD) dominant negative TrkB mutant (HSV-TrkB-KD) in the NAC. Fig. 1A shows that ectopic expression of either Cre or TrkB-KD aligned with GFP labeling in NACsh neurons. HSV-Cre expression in the NAC produced no evidence for retrograde infection in areas projecting to the NACsh (Fig. S1). Neither HSV-Cre nor HSV-TrkB-KD expression altered baseline dendritic spine density in distal dendrites of medium spiny neurons of the NACsh in saline-treated animals compared with control infection with HSV-GFP alone (Fig. 1 B and C). After a binge-like cocaine dosing regimen previously shown to induce an increase in spine density during HSV peak expression times (5 × 20 mg/kg i.p. injections over the course of 3 d) (11), total spine density appeared to increase in HSV-GFP–infected controls, but not in neurons expressing Cre or TrkB-KD (effect of vector, F2,18 = 5.286; P = 0.0156), although a lack of significant interaction precludes pairwise comparisons (Fig. 1 B and C). However, further analysis of spine morphology indicates that binge cocaine exposure induced primarily thin (Fig. 1D), but not mushroom-shaped (Fig. 1E) or stubby, spines (Fig. 1F), consistent with the profile for immature early spine induction immediately after similar cocaine treatment regimens (11). The thin spines were prevented from forming by either localized TrkB knockout or dominant negative TrkB mutant expression in NACsh neurons [Fig. 1D; effect of vector, F2,18 = 6.616 (P = 0.007); effect of drug, F1,18 = 8.051 (P = 0.0109); interaction, F2,18 = 4.964 (P = 0.0192)]. These results demonstrate that local BDNF-TrkB signaling in NACsh neurons is necessary for the increase in spine density induced by cocaine. Together with our previous work showing that CSA induces BDNF expression, release, and TrkB activation in NACsh, and that BDNF or TrkB knockout in NAC neurons decreases CSA behavior (2, 3), these findings indicate BDNF-TrkB signaling during initial cocaine exposure is necessary for both the induction of morphological plasticity and the maintenance of cocaine reinforcement.
Fig. 1.
Cocaine induction of spines is blocked by TrkB knockdown. (A) Localized expression of GFP (green) and TrkB (blue) after intra-NACsh infusions of HSV-GFP, HSV-Cre, or HSV-TrkB-KD (with bicistronic GFP) in floxed TrkB mice. (Magnification, 10×.) (B) Total spine density of saline- and cocaine-treated (5 × 20 mg/kg, i.p.) mice during HSV expression. (C) Representative images of saline- and cocaine-treated dendrites expressing GFP only, Cre recombinase, or TrkB-KD. Representative spines are labeled white (thin), blue (mushroom), and green (stubby) to illustrate their categorization. (Scale bar, 10 µm.) (D) Thin, (E) mushroom, and (F) stubby spine density after binge cocaine-dosing paradigm. Data are expressed as mean ± SEM. **P < 0.01 compared with saline.
Fig. S1.
Lack of evidence for retrograde infection with NACsh infusions of HSV-Cre. GFP from the HSV-Cre virus was detected via IHC in the NAC, but not in areas projecting to the NAC including ventral hippocampus (vHIPP), prelimbic prefrontal cortex (PL), and ventral tegmental area (VTA). Representative images are shown. (Magnification, 4×.)
Cocaine-induced spines can remain significantly increased for a month after CSA (12, 13), and we hypothesized that knocking down TrkB function after chronic CSA would reverse these spine increases similar to blocking their induction. We compared the effects of knocking down TrkB signaling with HSV-TrkB-KD to the effects of increasing TrkB function by overexpressing wild-type TrkB (HSV-TrkB-WT). We also investigated the role of specific TrkB signaling pathways TrkB-SHC or TrkB-PLCγ. This was accomplished by constructing HSV vectors expressing two additional TrkB mutants: HSV-TrkB-515/SHC contains a phosphorylation-resistant tyrosine to phenylalanine mutation at TYR515 that prevents TrkB coupling with SHC complexes to activate ERK, but preserves TrkB-PLCγ signaling (10). Conversely, HSV-TrkB-816/PLC has a phosphorylation-resistant mutation of TYR816 that prevents TrkB-PLCγ signaling while leaving TrkB-SHC activation of pERK intact.
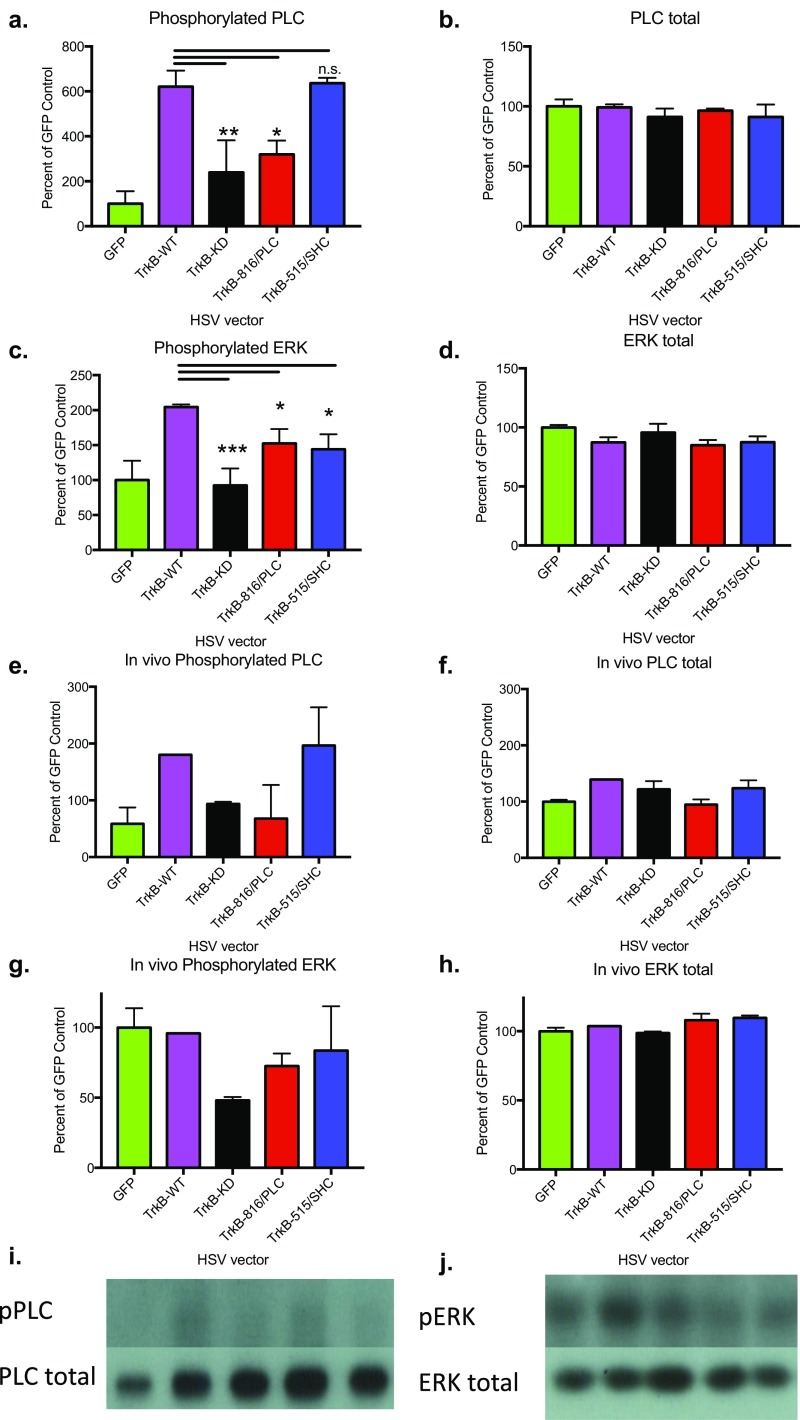
In heterologous cells in culture (HEK293), all four vectors increased TrkB protein expression, but only TrkB-WT enhanced BDNF-TrkB-mediated phosphorylation of both PLCγ and ERK compared with no vector or HSV-GFP–infected controls. In contrast, HSV-TrkB-KD had no detectable increases in BDNF-TrkB-WT-induced pPLCγ and pERK. Cells treated with HSV-TrkB-816/PLC lacked BDNF-stimulated PLCγ phosphorylation, but showed marked BDNF-induced pERK, whereas cells treated with HSV-TrkB-515/SHC had very low BDNF-induced pERK, but showed increased pPLCγ similar to HSV-TrkB-WT (Fig. 2A and Fig. S2 A–D).
Fig. 2.
BDNF-TrkB signaling reverses dendritic spines induced by chronic CSA and is dissociated from increased CSA behaviors caused by the selective loss of BDNF-TrkB-PLC activity in NACsh neurons. (A) Protein levels of TrkB, β-tubulin, pPLCγ, PLCγ, pERK, and ERK either with or without the addition of BDNF. Results from HEK293 cells infected with no vector, HSV-GFP, HSV-TrkB-WT, HSV-TrkB-KD, HSV-TrkB-816/PLC, or HSV-TrkB-515/SHC alone. (B) Coinfection with HSV-TrkB-WT reveals pathway-specific dominant negative profiles of TrkB signaling mutants. (C) Summary of TrkB-WT and dominant negative TrkBs HSV on pPLC and pERK. (D) Localized expression of GFP (green) and TrkB (red) 3 d after intra-NACsh infusions of HSV-GFP and HSV-TrkB vectors. (Magnification, 10×.) (E) Cocaine and saline SA rates before and after infusions of HSV vectors. (F) Quantification of total spine density in saline and CSA expressing TrkB viral vectors. (G) Representative images of dendritic segments expressing HSV vectors from saline and CSA animals. Representative spines are labeled white (thin), blue (mushroom), and green (stubby) to illustrate their categorization. (Scale bar, 10 µm.) (H) Quantification of thin, (I) mushroom, and (J) stubby spine density after SA. (K, Left) Experimental time course depicting operant training with food pellets (FT), surgery (Sur), recovery (Rec), and fixed ratio (FR) CSA training, followed by dose–response (DR) before, during, and after HSV infusion. A second HSV infusion is given before CSA testing on a PR reinforcement schedule. (K, Right) A modified experimental time course depicting 1 HSV infusion before PR. (L) Average CSA (FR5) in study groups before (M) dose–response testing during peak HSV-mediated expression. (N) CSA on a PR schedule during HSV expression after a second HSV infusion; the asterisk above a line represents a significant main effect of vector after a mixed factorial ANOVA. (O) CSA (0.75 mg/kg/injection) on a PR schedule during HSV expression after a single HSV infusion. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 compared with HSV-GFP. C, cocaine; S, saline.
Fig. S2.
Mutant TrkB receptors selectively alter BDNF-induced PLC and ERK phosphorylation events. HEK293 cells were infected with HSV-GFP (n = 3), HSV-TrkB-WT (n = 6), HSV-TrkB-KD (n = 2), HSV-TrkB-816/PLC (n = 3), or HSV-TrkB-515/SHC (n = 3), and then 100 ng/mL BDNF or PBS was added 1 d later and cells were harvested after 30 min. Results from Western blots are shown here, and BDNF-treated samples were analyzed with a one-way ANOVA. (A) TrkB vectors had significant effects on phosphorylated PLC (F4,12 = 9.81; P = 0.0009), but not (B) PLC total protein levels. (C) TrkB vectors also had significant effects on phosphorylated ERK (F4,12 = 8.342; P = 0.0019), but not (D) total ERK protein levels. Similar effects were also observed in vivo. Rats received cannulation surgery and, after recovery, were infused with HSV-GFP (n = 5), HSV-TrkB-WT (n = 1), HSV-TrkB-KD (n = 3), HSV-TrkB-816/PLC (n = 2), or HSV-TrkB-515/SHC (n = 2). After 3 d, during peak expression HSV times, rats were infused with 1 μL BDNF (0.5 mg/μL) and sacked 30 min later. Compared with in vitro cell culture data, similar profiles were observed for (E) phosphorylated PLC, (F) PLC total protein, (G) phosphorylated ERK, and (H) ERK total, suggesting these TrkB mutants work the same in vivo. Qualitative representative images are shown for (I) pPLC/PLC total and (J) pERK/ERK total. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 compared with WT control. n.s., not significant.
In HEK293 cells that lack endogenous TrkB expression, the TrkB signaling mutants form homodimers that generally act to enhance TrkB function through their remaining intact signaling pathway (as shown in Fig. 2A). However, when expressed in combination with WT TrkB, they can mediate pathway-selective, dominant negative effects on their mutated signaling pathway via formation of heterodimers with WT TrkB (14), similar to TrkB-KD. To demonstrate the specificity of these signaling phenomena, Fig. 2B shows that HSV-TrkB-816/PLC attenuated WT TrkB-mediated pPLCγ while maintaining pERK similar to TrkB-WT alone. The pathway-specific effects of HSV-TrkB-515/SHC are less prominent, as BDNF-induced pERK is reduced but not completely blocked, while pPLCγ is retained (Fig. 2B). One possibility is that intact PLCγ signaling can indirectly lead to pERK through downstream Ca2+ signaling cascades (10) (and see following). The pPLCγ and pERK response to intra-NACsh BDNF infusions in vivo showed similar dominant negative profiles in animals expressing these HSV-TrkB vectors (Fig. S2 E–J). These complementary gain and loss of signaling interactions with WT TrkB are summarized in Fig. 2C for clarity. Importantly, the role of gain versus loss of pathway-specific TrkB signaling in mediating morphological and behavioral effects can only be revealed when comparable effects are produced by WT (gain) or KD (loss) TrkB.
After 3 wk of CSA in daily 3-h sessions, rats were given intra-NACsh infusions of HSV vector and allowed access to CSA for 2 additional days (Fig. 2E). Brain tissue was collected 1 d after the final SA session for subsequent analysis of spine density. No viral vector significantly altered CSA baselines during the final 2 d of SA training when rats had relatively unrestricted access to cocaine injections (1 lever press for each injection). Moreover, blockade of endogenous TrkB signaling with HSV-TrkB-KD failed to affect the increase in total spine density induced by chronic CSA compared with GFP-only–expressing neurons (Fig. 2 F and G), in marked contrast to its ability to prevent the initial induction of spine formation (Fig. 1). Instead, surprisingly, overexpression of WT TrkB completely reversed the increase in total spine density produced by chronic CSA (drug × vector interaction, F4,46 = 2.726; P = 0.041), and spine density levels were similar to those seen in GFP-expressing neurons from saline SA animals. In contrast to early spine induction shown in Fig. 1, increases in spine density after chronic CSA were represented by increases in mushroom-shaped spines, but not thin or stubby spines (Fig. 2 H–J), consistent with maturation and stabilization of the newly formed thin dendritic spines with prolonged cocaine use (15). However, the increase in mushroom-shaped spines at the end of CSA was reversed by TrkB-WT, TrkB-816/PLC, and TrkB-515/SHC overexpression (Fig. 2I; drug × vector interaction, F4,46 = 4.078; P = 0.0065). Moreover, a similar TrkB-WT reversal of cocaine-induced mushroom spines was seen after 15 d of experimenter-delivered i.p. cocaine injections in mice (Fig. S3). Thus, BDNF-TrkB activity has opposing roles on the induction versus stability of cocaine-induced dendritic spines in NACsh neurons, being both necessary for spine formation and sufficient for their later reversal, regardless of the mode of cocaine administration.
Fig. S3.
Cocaine-induced spines are reversed by TrkB-WT, but not TrkB-KD, overexpression after 17 d of daily 20 mg/kg i.p. cocaine injections in mice. Mice were infused with HSV-GFP, HSV-TrkB-WT, or HSV-TrkB-KD before the 2 additional i.p. injections and perfused 1 d later to mimic the 17-d CSA treatment conditions shown in Fig. 2 E–J. (A) Cocaine i.p. injections increased total spine density in HSV-GFP mice compared with saline-injected controls. This increase was reversed in mice infused with HSV-TrkB-WT, but not HSV-TrkB-KD (effect of drug, F1,17 = 6.799 [P = 0.0184]; interaction, F2,17 = 5.496 [P = 0.0144\). (B) The increases in total spine density is reflected by an increase in mushroom spine density (effect of drug, F1,17 = 17.09 [P = 0.0007]; interaction, F2,17 = 4.105 [P = 0.0351]), but not (C) thin nor (D) stubby spines similar to findings in CSA rats. Data are expressed as mean ± SEM. *P < 0.05 compared with saline control. Coc, cocaine; Sal, saline.
Since WT, but not KD, TrkB produces a similar reversal in spine increases, these results indicate that enhanced BDNF-TrkB signaling through either PLCγ or ERK is sufficient for this reversal. However, given that some degree of pERK is shared by TrkB-WT, TrkB-816/PLC, and TrkB-515/SHC (Fig. 2 A–C), it is possible that downstream ERK activation ultimately is sufficient to reverse spine formation induced by CSA. In any event, these findings indicate that BDNF-TrkB signaling can mediate bidirectional effects on the induction of new spine formation and their maintenance during and after chronic CSA.
Since increased BDNF-TrkB signaling in NACsh neurons reverses cocaine-induced morphological changes in spine density, we hypothesized that this effect may be associated with a reduction in addictive behavior. To test this hypothesis, animals were subjected to a similar CSA regimen, and the effects of HSV vectors on subsequent CSA behavior were measured in sequential SA tests on fixed and progressive ratio (PR) reinforcement schedules (Fig. 2K, Left). Before HSV infusions, stabilized CSA was counterbalanced across the 5 study groups (Fig. 2L), and no group differences were apparent in baseline SA dose–response curves during within-session testing (Fig. S4A). Three days after intra-NACsh HSV infusions and during peak HSV-mediated expression, animals receiving the dominant negative TrkB-KD or TrkB-816/PLC vector showed a transient leftward shift in dose-sensitivity for maintaining CSA compared with GFP-expressing controls (Fig. 2M; effect of vector, F4,39 = 3.389, P = 0.018; effect of dose, F4,156 = 67.0, P < 0.001; interaction, F16,156 = 2.133, P = 0.009), an effect that recovered during the following week of testing (Fig. S4B), when HSV-expression is no longer evident (16). Rats were given an additional week of CSA to restabilize on the training dose, and no statistical differences in cocaine intake were evident (Fig. S4C). Rats subsequently received a second intra-NACsh infusion with the same HSV vector, and the degree of effort they would exert to maintain SA behavior was assessed on the PR reinforcement schedule. HSV-TrkB-KD or HSV-TrkB-816/PLC treatment enhanced the motivation for cocaine, as the number of cocaine injections earned increased (effect of vector, F4,35 = 2.696, P = 0.047; effect of dose, F1,34 = 33.06, P < 0.001), along with the highest ratio of lever-presses/cocaine injection achieved (Fig. 2N). Similar statistically significant increases in breakpoints at the 0.75 mg/kg injection dose were observed in a separate cohort after a single infusion (Fig. 2K, Right) of HSV-TrkB-KD or HSV-TrkB-816/PLC (Fig. S4D and Fig. 2O; F2,11 = 6.203; P = 0.0157), indicating a direct effect on CSA despite a lack of spine changes at a similar time of testing. Thus, transient loss of TrkB function via TrkB-KD after CSA enhanced cocaine reinforcement while not affecting cocaine-induced spine density in NACsh neurons (Fig. 2 F–J). Conversely, enhanced TrkB function produced by HSV-TrkB-WT reversed spine density increases without altering CSA behavior. Therefore, we conclude that these morphological and behavioral phenomena are not functionally related with respect to motivation for cocaine. These findings also indicate that selective loss of TrkB-PLCγ, but not TrkB-SHC-ERK, signaling conveys the motivational effects of global loss of TrkB function in NACsh neurons.
Fig. S4.
Similar baseline SA for fixed ratio dose–response testing is seen in all HSV groups, and no HSV vector caused long-lasting changes in postexpression CSA behavior. (A) Dose–response testing was not significantly different between groups before GFP, TrkB-WT, TrkB-KD, TrkB-816/PLC, or TrkB-515/SHC expression (effect of dose, F4,152 = 159.2; P < 0.001). (B) Dose–response testing was not significantly different after the second week of HSV expression (effect of dose, F4,152 = 102.7 [P < 0.001]). (C) No significant change in FR5 intake was evident the third week after HSV expression. (D) No significant differences in FR intake was evident for rats before expression of GFP, TrkB-KD, or TrkB-816/PLC before PR testing after a single infusion of HSV vector. (E) Dose–response testing was not significantly different before GFP or PLCγ1 expression (effect of dose, F4,52 = 52.38 [P < 0.001]). (F) Dose–response testing was not significantly different after the second week of GFP or PLCγ1 expression (effect of dose, F4,52 = 46.73 [P < 0.001]). (G) No significant change in FR5 intake was evident the third week after GFP or PLCγ1 expression.
Given that gain of TrkB signaling (through PLCγ or ERK) reverses initial increases in spine density after chronic CSA, and loss of TrkB-PLCγ signaling enhances motivation for cocaine, we tested the effects of generalized PLCγ overexpression in NACsh on these morphological and behavioral measures. These experiments used a previously validated HSV-PLCγ vector that increases levels of pPLCγ to a greater extent than HSV-TrkB-WT in HEK293 cells (Fig. 3A) and also increases pERK (17) potentially due to downstream Ca2+ signaling cascades, as discussed earlier. Indeed, direct stimulation of PLCγ in HEK293 cells with the PLC activator m-3M3FBS increased pERK in a time-dependent manner (Fig. 3B) consistent with the notion that PLCγ can indirectly enhance pERK. This HSV-PLCγ vector showed prominent expression in NACsh neurons (Fig. 3C) and reversed increases in mushroom spines induced by chronic CSA (Fig. 3D) after 1 d of withdrawal to levels seen in saline SA controls (Fig. 3E; interaction: F1,23 = 4.481; P = 0.0453; Fig. S5). Taken together, these findings suggest reversal of cocaine-induced mushroom-shaped spines with gain of TrkB-PLCγ function ultimately may involve enhanced pERK signaling.
Fig. 3.
PLCγ overexpression both reverses dendritic spines induced by chronic CSA and reduces CSA behaviors. (A) Western blot depicting increased phosphorylated and total PLCγ in HEK293 cells infected with HSV-PLCγ compared with HSV-TrkB-WT. (B) Time course of pERK after addition of the PLC activator m-3M3FBS (100 µM) over the course of 30 min. (C) Localized expression of GFP (green) and PLCγ (blue) 3 d after intra-NACsh infusions of HSV-GFP or HSV-PLCγ vectors. (D) Cocaine and saline SA rates before and after HSV-PLCγ or HSV-GFP infusions. (E) Quantification of mushroom spine density in saline and CSA overexpressing PLCγ or GFP. (F) Representative images of dendritic segments expressing HSV- PLCγ from saline and CSA animals. Representative spines are labeled white (thin), blue (mushroom), and green (stubby) to illustrate their categorization. (Scale bar, 10 µm.) (G) Average CSA (FR5) in study groups before (H) dose–response testing during peak HSV-mediated expression of PLCγ. (I) CSA on a PR schedule during GFP or PLCγ expression after a second HSV infusion (Fig. 2K, Left for time line). The asterisk above a line represents a significant main effect of vector after a mixed factorial ANOVA. Data are expressed as mean ± SEM. *P < 0.05 compared with HSV-GFP controls. C, cocaine; n.s., not significant; S, saline.
Fig. S5.
The effect of HSV-PLC versus HSV-GFP on total, thin, or stubby spines in the NACsh. Saline- and cocaine-treated rats infused with HSV-GFP or HSV-PLC were analyzed for (A) total (effect of drug, F1,23 = 6.71 [P = 0.0164]), (B) thin, and (C) stubby spines. Data are expressed as mean ± SEM. Coc, cocaine; Sal, saline.
We next hypothesized that directly increasing PLCγ signaling would have an opposite effect on CSA behavior compared with the loss of TrkB-PLCγ signaling (Figs. 2 M–O). Rats were trained to SA cocaine as described earlier (Fig. 2K, Left), and subsequently tested in SA dose–response before (Fig. S4E), during (Fig. 3H; effect of dose, F4,52 = 38.89; P < 0.001), and after (Fig. S4F) HSV-mediated PLCγ overexpression in NACsh neurons. In contrast to loss of TrkB-PLC signaling, but similar to TrkB-WT, overexpression of PLC had no significant effect on CSA compared with GFP controls in dose–response tests on the fixed ratio schedule. After restabilization of CSA (Fig. S4G) and a second HSV injection, overexpression of PLCγ reduced the number of infusions earned on the PR reinforcement schedule at both doses [Fig. 3I; effect of vector, F1,11 = 6.88 (P = 0.024); effect of dose, F1,13 = 14.48 [P = 0.002]), and strongly depressed the final response/injection ratio achieved before cessation of SA behavior. The inability of HSV-PLCγ to reduce peak SA rates in fixed ratio testing indicates reduced PR responding was not related to a generalized suppression of operant behavior. The effect of enhanced PLCγ function on PR is opposite to the effect of eliminating TrkB-PLCγ signaling (Fig. 2 N and O). In addition, the ability of PLCγ to reduce CSA is discordant with the failure of TrkB-WT and TrkB-515/SHC to alter CSA, although all three vectors produce a similar reversal of cocaine-induced spine density in NACsh neurons.
Discussion
Chronic cocaine exposure increases spine density in NAC, and these morphological changes can persist for a month or more after withdrawal from repeated cocaine exposure (8). Dendritic spine formation is a hallmark of activity-dependent morphological changes in other brain regions, and BDNF-TrkB signaling is known to be a key player in these events (9). Here we found that local BDNF-TrkB signaling in NACsh neurons is necessary for the initial induction of spine density increases with repeated cocaine exposure. Thus, both inducible localized deletion of the TrkB gene, or limited transient expression of a dominant negative TrkB mutant, before the onset of repeated cocaine exposure prevented the formation of cocaine-induced thin spines without affecting basal density of any spine subtype. This loss of spine formation is associated with a reduction in mouse CSA behavior using similar constitutive deletion of either BDNF or TrkB in NAC neurons (2, 3). As CSA induces BDNF synthesis, release, and TrkB activation concomitant with each daily exposure (2, 3), it is likely that such dynamic BDNF-TrkB activity mediates the formation of new spines in the NACsh. In contrast, loss of TrkB function failed to alter spine increases after chronic CSA, suggesting ongoing TrkB activity is not necessary for maintaining newly formed spines, and probably is not necessary for spine maintenance after withdrawal from CSA. These findings suggest BDNF-TrkB signaling is necessary to maintain sensitivity to cocaine reinforcement, but argue against a functional relationship between spine growth and cocaine reinforcement.
However, we found that increasing TrkB function actually reversed cocaine-induced spines in NACsh neurons after their formation had occurred. This suggests BDNF-TrkB signaling is capable of mediating bidirectional effects on the induction and maintenance of morphological plasticity and potentially signifies an avenue for reversing relatively enduring structural changes in neurons due to prolonged chronic use of abused drugs. The reversal of cocaine-induced spines is consistent with BDNF’s neurotrophic role that may unlock structural rigidity in NACsh neurons, a process we term cataplasticity (from Greek cata; reversal), possibly allowing for subsequent de novo synaptic alterations to occur. This process can be mediated by either TrkB-SHC signaling through the small GTPase Ras or TrkB-PLCγ signaling, but since both pathways converge downstream to increase pERK (10), ERK activation ultimately may mediate the reversal in cocaine-induced spines. CSA increases pERK in many brain areas, including the NACsh (18), and while ERK has a prominent role in mediating BDNF-induced spine formation in hippocampal neurons (9), there are reports of spine density reductions with similar sustained TrkB and ERK activity (19, 20). An alternative interpretation could be that TrkB reduces a different spine population from those induced by cocaine. This is unlikely, since no TrkB vector decreased basal spine density in saline animals, and only the subset of spines increased by cocaine (mushroom) was altered.
It is interesting that TrkB-mediated reversal of cocaine-induced dendritic spines is not accompanied by changes in sensitivity to low-dose cocaine reinforcement, or the effort rats exert to self-administer higher cocaine doses on the PR schedule. Instead, both dose sensitivity and the motivation for cocaine are enhanced by transient loss of TrkB function in NACsh neurons after chronic CSA. The effect on SA behavior clearly differs from constitutive and local knockout of TrkB in the NAC of cocaine self-administering mice discussed earlier (3). A similar enhancement in cocaine-seeking after early withdrawal is reported with viral expression of truncated TrkB or TrkB RNAi in the NAC core, but not shell, in more posterior NAc target regions in rats (5). These discrepancies in behavioral outcomes between TrkB knockout in mice, or knockdown of TrkB function in shell versus core in rats, may reflect species or subregional differences within the NACsh, but also could relate to gene deletion before cocaine versus functional knockdown after cocaine approaches. It also should be noted that our findings do not rule out potential effects of spine density on drug-seeking behavior in a drug-free state, as often measured in extinction/cue reinstatement tests.
Loss of TrkB-PLCγ, and not TrkB-SHC, signaling in NACsh increases CSA behaviors, suggesting the TrkB-PLCγ signaling pathway opposes addictive behavior. Moreover, the increase in motivation for cocaine with loss of TrkB-PLCγ is concurrent with reversal of cocaine-induced dendritic spines. However, since increased CSA is recapitulated with the dominant negative TrkB-KD that fails to alter spine density, this behavior probably reflects loss of other TrkB-PLCγ signaling events, rather than reversal of spine increases. In contrast, since a similar reversal of spine increases was found with TrkB-WT, the residual gain of TrkB-SHC-ERK function due to overexpression of the TrkB-PLCγ mutant likely explains the morphological effects. Contrary to the loss of TrkB-PLCγ signaling, overexpression of PLCγ itself is sufficient to reduce CSA behavior in PR reinforcement schedules. Thus, PLCγ signaling in NACsh neurons plays a pivotal role in regulating the motivation for cocaine reinforcement, and could represent a target for therapeutic intervention in the treatment of cocaine addiction. The specific mechanism of the anti-addictive effects of PLC is currently unknown, but could involve a direct interaction with the small GTPase Rac1 (21) that has a well-known role in spine morphology (22), or via Ca2+ signaling through protein kinase C that has been shown to alter drug conditioned place preference (23). Although overexpression of PLCγ also reverses cocaine-induced spines, other TrkB vectors (TrkB-WT and TrkB-SHC) all produce a similar reversal of spine changes without altering CSA, and so it is difficult to attribute decreases in CSA with PLCγ overexpression to reversal of spine increases. In any event, the ability of PLCγ overexpression to increase pERK supports the notion that convergence on sustained downstream ERK activity ultimately mediates reversal of spine density increases, since all three TrkB vectors that decrease spine density also activate ERK. PLC can activate MEK-ERK signaling via Gab-1 or Ras-Raf mechanisms (10), but either TrkB-PLC or TrkB-SHC signaling also converges on other pathways, including PI3K, PKB, and Rac1, that may ultimately mediate these morphological effects (10).
Cocaine-induced increases in spine density can persist for several weeks after cessation of cocaine exposure (13, 24, 25), although chronic opiate exposure decreases spine density in the NAC (25), further questioning the relevance of these changes to maintenance of drug SA and vulnerability to relapse in withdrawal. Indeed, some investigators have suggested that increased spine density may represent a homeostatic counteradaptive response that diminishes drug sensitivity, or plays no role in locomotor sensitization to cocaine (26). One could speculate that spine formation reflects de novo generation of silent synapses in NAC neurons that ultimately recruit AMPA receptors after a prolonged period of withdrawal along with incubation of cocaine craving (27). In this sense, a BDNF-TrkB–mediated reversal of cocaine-induced spines could convey prominent anti-addictive properties, but only after prolonged abstinence. However, our prior work suggests daily CSA elevates transient BDNF-TrkB signaling in the NACsh, and not core (2, 3), where maturation of silent synapses and recruitment of synaptic AMPA receptors attenuates cocaine-seeking behavior (16, 28). Moreover, most studies show that dendritic spine increases revert to normal after about 1 mo from the last cocaine exposure (13). Thus, the role of spine density increases in drug addiction would be limited to perpetuating ongoing drug use, rather than promoting craving and relapse after long-term abstinence.
However, the present data refute a direct positive or negative relationship between such morphological changes and the motivation for cocaine, as TrkB-mediated reversal of sustained spine increases is not associated with changes in motivation for cocaine, loss of overall TrkB function enhances motivation for cocaine while leaving spine increases intact, and gain of PLCγ function reduces both behavioral and morphological measures. Given the multiple dissociations of morphological and behavioral effects in our study, we conclude that neither increases nor decreases in CSA behavior are mediated by alterations in total or mushroom-shaped spine density in the NACsh. Nevertheless, morphological cataplasticity is an attractive therapeutic target because treatments are administered after chronic cocaine use and reversion of spines to premorbid states could allow for new morphological responses to beneficial behavioral repertoires.
Methods
Complete methodology is available in the supplemental material. In brief, HSV vectors were constructed as previously described (29). In vitro HSV characterization was completed in HEK293 cells, and in vivo characterization was completed in NACsh tissue with Western blots using standard protocols (30) and IHC. CSA assays were performed similarly to previous methods (30). Dendritic spine analysis was performed with Volocity and Neuronstudio software similar to previously published methods (24). These studies were approved by the UTSW institutional Animal Care and Use Committee, and facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care. All procedures were conducted in accordance with the guidelines established by the National Institutes of Health and the National Research Council.
SI Methods
Animal Use and Care.
Adult male Sprague-Dawley rats (Charles-River) initially weighing 250–300 g, or floxed TrkB mice [male and female, ≤5 per cage, bred at UT Southwestern Medical Center (UTSW)], were housed in a climate-controlled environment (21 °C) on a 12-h light-dark cycle (lights on at 6:00 AM). All animals were habituated to the housing environment for at least 7 d before use in experiments and had ad libitum access to food and water, except when rats received operant training with sucrose pellets. All experiments except for sucrose training were performed during the light cycle. These studies were approved by the UTSW Institutional Animal Care and Use Committee, and facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care. All procedures were conducted in accordance with the guidelines established by the National Institutes of Health and the National Research Council.
Vector Construction.
A bicistronic herpes simplex viral vector (HSV) was used to construct the HSV-GFP, HSV-Cre recombinase, HSV-PLCγ, and all HSV-TrkB WT and mutant vectors, as previously described (29). GFP, Cre recombinase, TrkB-WT, and mutant TrkB cDNAs were inserted into an HSV-PrpUC amplicon and packaged using 5dl1.2 helper virus. Viruses were purified on a 10% sucrose gradient. Transgene expression was driven by an IE 4/5 promoter with GFP that was coexpressed by a CMV promoter. Mutant TrkB vectors are described as follows: HSV-TrkB-KD: kinase dead dominant negative due to mutation of the ATP-presenting site (lysine 540) to asparagine that eliminates tyrosine kinase activity; HSV-TrkB-816/PLC: dominant negative PLCγ docking mutant due to tyrosine 816 to phenylalanine mutation; HSV-TrkB-515/SHC: dominant negative docking SHC mutant due to tyrosine 515 to phenylalanine mutation.
Mouse HSV Infusions and Cocaine Treatments.
Two separate protocols for examining the effects of HSVs on cocaine-induced spines were performed.
First, induction of cocaine-induced spines with i.p. cocaine. Floxed TrkB mice received acute stereotaxic HSV infusions under full anesthesia (ketamine/xylazine 100/10 mg/kg). HSV-GFP, HSV-TrkB-KD, or HSV-Cre was infused bilaterally (0.5–1.0 µL/side over the course of 5–10 min; anterior–posterior (AP) + 1.5, dorsal–ventral (DV), −4.4 medial–lateral (ML), ±1.5, 10° angle), and mice were given penicillin and Ketofen during the postoperative period. The day after HSV infusion, mice were randomly assigned to receive i.p. injections of cocaine hydrochloride (20 mg/kg, i.p.; Research Triangle International) or saline 5 times over the course of 3 d to ensure cocaine exposure occurred during peak HSV expression. Two injections were given on the first and second day, spaced 8 h apart. Four to 6 h after the fifth injection on day 3, mice were anesthetized with chloral hydrate (0.5–1.0 mg/kg, s.c.) and perfused with 1% paraformaldehyde in PBS for 1–2 min, followed by 4% paraformaldehyde for an additional 10 min. The extracted brains were stored for at least 24 h in paraformaldehyde before sectioning on a Vibratome (75–100-µm slices) and slices stored in PBS with sodium azide.
Second, reversal of the maintenance of cocaine-induced spines. In separate randomly assigned mice, cocaine or saline i.p. injections were given for 15 d. One to 4 d later, mice were stereotaxically injected with HSV, given 2 more days of saline or cocaine, and then perfused on peak HSV expression day 3.
Immunohistochemical Labeling of TrkB/GFP, FLAG/GFP, PLC/GFP.
Sections were blocked in buffer (3% Normal donkey serum and 0.1% Triton-X in PBS) for 1 h, followed by incubation with primary antibodies against GFP (Aves, chicken, 1:4,000, cat:GFP-1020) colabeled with either anti-TrkB (Millipore, rabbit, 1:500, cat:07–225) or anti-PLCγ1 (Millipore, mouse, 1:4,000, cat:05–163) overnight at 4 °C. After three 10-min washes, buffer with secondary antibodies was added as follows: Cy3 donkey anti-rabbit (cat:711–165-152) or Cy5 donkey anti-rabbit (cat:111–175-144) for TrkB, or Cy5 donkey anti-mouse (cat:715–175-151) for PLCγ (Jackson ImmunoResearch, 1:1,000), all with fluorescein anti-chicken (Aves, 1:1,000, cat:F-1005). After washing 3 times with buffer, the sections were mounted on slides in PBS, coverslipped, and sealed with a nitrocellulose lacquer. A similar protocol for dendritic spine labeling was used, but with anti-GFP primary from Abcam (rabbit, 1:200, cat:ab290) overnight at room temperature (RT), and incubation with Cy3 donkey anti-rabbit for a second overnight at RT. TrkB vectors were tested at least twice for colabeling.
Dendritic Spine Imaging and Counting.
All images were obtained with a Zeiss LSM 510 confocal microscope, and spines were measured only in dendritic segments of at least 20 µm with a branch order of 3 or more to limit analysis to distal dendritic segments where psychostimulant-induced changes in spine density have been previously observed (31). Measurements of dendritic segments from at least 5 different neurons per animal were averaged to represent a single value for each animal. Z-stacks were taken at 0.21-µm intervals and analyzed with Volocity software (Improvision). Spine counts were limited to spines possessing a protruding head visibly connected to the dendrite, and were conducted by investigators blinded to experimental conditions. Spine density was defined as the number of spines per dendritic volume. Three measures of dendritic diameter (beginning, middle, and end) were averaged and then combined with segment length, using the cylindrical volume formula (V = πr2h). Values were therefore expressed as spines per cubic micrometer. A secondary analysis was conducted with Neuronstudio software (Computational Neurobiology and Imaging Center, Mount Sinai School of Medicine). This program was used to automatically count and characterize spines into 3 categories on the basis of the following criteria: thin spines have a visible neck with a similar head size (head-to-neck ratio less than 1.1 and a length-to-spine head diameter greater than 2.5); mushroom-shaped spines also have a visible neck but with a larger head size (head-to-neck ratio of 1.1 or above and a spine head diameter of at least 0.35 µm); and stubby spines lack a discernable neck and do not fit the criteria of thin or mushroom spines.
TrkB and PLC Signaling in Cell Culture.
HEK293 cells were grown in 12-well plates in DMEM supplemented with 10% FBS and penicillin/streptavidin to 80–90% confluence. Media was replaced with 0% FBS DMEM with or without HSV vectors added. Each well was infected with 0.1 µL HSV virus in 0.5 mL DMEM. A second experiment examined the ability of the mutated TrkB vectors (0.5 µL) to knockdown TrkB-WT (0.5 µL) signaling. One day after HSV infection, 100 ng/mL BDNF protein was added for 30 min. Cells were harvested after rinsing with 4 °C PBS by the addition of 100–200 µL homogenization buffer, scraping and pipetting into a tube for immediate sonication before freezing on dry ice. For the PLC activation, m-3M3FBS (100 µM) was added to control cells and harvested after 0, 2, 5, 15, or 30 min. Cell culture experiments were run multiple times, but one representative image was selected for the figures.
Western Blotting.
Blots were run according to the methods in Larson et al. (30), except where noted. Briefly, samples were quantified with a Lowry assay and 40 µg protein was electrophoresed on 7.5% polyacrylamide gels, transferred to PVDF membranes, blocked in 5% BSA or 5% dry milk in Tween/Tris-buffered saline (TTBS) for 1–2 h before addition of primary antibodies for overnight incubation at 4 °C. The next day, three 10-min washes in buffer were performed and secondary antibodies were added for at least 1 h in TTBS. After three more 10-min washes, blots were coated with ECL 2 (Thermo Pierce) and exposed to film (Kodak). Primaries: anti-TrkB (cat:07–225), anti–phospho-PLCγ1 (tyr 783, cat:44696G), anti-PLCγ1 (Millipore, cat:05–163), anti–phospho-ERK (thr 202/tyr 204, cat:9106L), anti-ERK (Cell Signaling, cat:9102), anti-beta-tubulin III (cat:TUJ), and anti-GFP (Aves, cat:GFP-1020). Secondaries: anti-rabbit or anti-mouse HRP (BioRad, cat:170–6515&170–6516) and anti-chicken HRP (Aves, cat:H-1004). Blots were first probed for anti–phospho-ERK or anti–phospho-PLCγ1, stripped with Restore buffer (Thermo), reblocked, and reprobed with anti-ERK and anti-PLCγ1, respectively. Phospho and total protein levels were analyzed with ImageJ software.
Rat Stereotaxic Cannulation, Intravenous Catheterization, and CSA.
For immunohistochemistry colocalization experiments, rats were anesthetized (ketamine/xylazine, 100/10 mg/kg, i.p.), and bilateral guide cannulae were implanted above the NACsh (AP, +1.7 mm; ML, −0.75 mm; DV, −5.7 mm ventral to dura) fixed in place with skull screws and dental cement. After recovery for 5–7 d, 2.0 µL HSV vector was infused bilaterally over the course of 10 min, using infusion needles that projected 1.0 mm below the guide cannula (−6.7 mm ventral to the dura). Rats were perfused after 3 d as described earlier for immunohistochemical analysis of HSV-mediated expression.
For CSA experiments, food-restricted rats initially trained to lever press for vanilla sucrose pellets (45 mg) in operant chambers (Med Associates) on a fixed ratio 1 (FR1) reinforcement schedule until they achieved 100 pellets in a 1-h session (1–3 d). After lever training, rats were fed ad libitum at least 48 h before surgical implantation of both bilateral guide cannulae and chronic indwelling intrajugular catheter, as described (30). Catheters were flushed daily with 0.2 mL heparinized bacteriostatic saline containing gentamycin sulfate (0.33 mg/mL) to maintain patency. After at least 5 d recovery, rats were placed in operant chambers with their catheters attached to syringe pumps, where a single lever press response delivered 0.5 mg/kg cocaine hydrochloride in 50 µL sterile saline over 2.5 s, concurrent with a cue light above the active lever. House lights were off during the injection and for an additional 12.5-s timeout period. SA training sessions lasted 3 h for 5 d/week for 3–4 wk until criteria for stable responding criteria was obtained (<15% variance over the final 3 d of SA training). Cocaine intake during the final 5 d of SA training was counterbalanced across study groups before intra-NACsh HSV infusions, as described earlier. After 2 additional days of FR1 CSA and a 24-h withdrawal period (day 3 after HSV), rats were perfused and brain tissue processed for analysis of dendritic spine density.
Cocaine Self-Administration on FR and PR Reinforcement Schedules.
Rats received 2 consecutive HSV infusions during FR and PR SA tests, as described (30) and outlined in Fig. 2K, Left. An additional cohort of rats received only 1 HSV infusion during PR SA tests, as outlined in Fig. 2K, Right. CSA training was conducted as earlier, with the exception that the FR requirement was incrementally increased first to FR3 and then to FR5 by the end of the 3–4-wk training period. After CSA training and stabilization, rats were subjected to within-session CSA DR testing before, during, and after transient (1–5 d) HSV expression. Each within-session DR test began with a 30-min loading phase with access to the training dose (0.5 mg/kg/injection), followed by 5 consecutive 30 min components when descending injections doses were available (1.0, 0.3, 0.1, 0.03, and 0 mg/kg, i.v.). CSA data were averaged across 2 SA days before HSV (days 4–5) for baseline measures, and rats were assigned to counterbalanced groups based on baseline FR and DR responding, the third day after HSV infusion was used for HSV-TrkB peak expression, and data were averaged again on days 4–5 during the following week after HSV expression is absent for the postinfusion data (11–12 d after initial HSV infusion). CSA was restabilized for an additional week of FR5 SA at the training dose, and rats received a second HSV infusion with the same vector for analysis of CSA on a PR reinforcement schedule. Here the ratio of lever presses/cocaine injection progressively increased for each successive injection in an exponential fashion (1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901, 1,102, 1,347, 1,646, etc.), and breakpoints were defined as the last completed ratio before a 1-h period when no further injections were earned. CSA on the PR schedule was conducted with 2 injection doses (0.25 and 0.75 mg/kg/injection) in counterbalanced order, each tested in 2 consecutive sessions. The second test day at each dose was used for analysis. At the end of both DR and PR testing phases, catheter patency was confirmed by brief induction of anesthesia with i.v. methohexital injection. After CSA testing, rats were injected with chloral hydrate (400 mg/kg, i.p.), and cresyl violet (0.5 µL) was infused bilaterally into the guide cannula. Brains were removed, and 1-mm-thick slices were collected to identify the infusion sites. Animals with infusions outside the NACsh were excluded from the study. If an animal made it through the FR and all 3 wk of DR, they were included in the study. However, if issues such as illness or operant box issues affected later stages such as FR restabilization or PR, these rats were excluded from the last 2 wk of testing only.
Statistics.
Spine density measures were analyzed with two-way ANOVA; differences between saline and cocaine groups were compared with least significance difference post hoc tests. Behavioral data were analyzed with mixed factorial ANOVA with one independent and one repeated factor; differences between vector groups were compared with least significance difference post hoc tests. Only main or interactive effects with significant P values from mixed factorial ANOVAs are reported. A one-way ANOVA was used to analyze in vitro HSV characterization, Western blot data, and high-dose PR breakpoints after a single infusion of HSV. Also, only significant one-way ANOVAs results are reported in the results section. All statistics were performed with GraphPad Prism, and P < 0.05 was considered significant. All relevant data are available from the authors.
Acknowledgments
We thank Luis Parada (Memorial Sloan Kettering Cancer Center) for the floxed TrkB mice. This work was funded by NIH Grants T32 DA 007290 and P01 DA 08227.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702441114/-/DCSupplemental.
References
- 1.Dennis ML, Foss MA, Scott CK. An eight-year perspective on the relationship between the duration of abstinence and other aspects of recovery. Eval Rev. 2007;31:585–612. doi: 10.1177/0193841X07307771. [DOI] [PubMed] [Google Scholar]
- 2.Graham DL, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: Region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 4.Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- 5.Li X, et al. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibel M, Barde YA. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 7.Verheij MM, et al. Systemic delivery of a brain-penetrant TrkB antagonist reduces cocaine self-administration and normalizes TrkB signaling in the nucleus accumbens and prefrontal cortex. J Neurosci. 2016;36:8149–8159. doi: 10.1523/JNEUROSCI.2711-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci. 2003;17:2529–2539. doi: 10.1046/j.1460-9568.2003.02694.x. [DOI] [PubMed] [Google Scholar]
- 10.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 11.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Anderson EM, Self DW. It’s only a matter of time: Longevity of cocaine-induced changes in dendritic spine density in the nucleus accumbens. Curr Opin Behav Sci. 2017;13:117–123. doi: 10.1016/j.cobeha.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luikart BW, Nef S, Shipman T, Parada LF. In vivo role of truncated trkb receptors during sensory ganglion neurogenesis. Neuroscience. 2003;117:847–858. doi: 10.1016/s0306-4522(02)00719-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton MA, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 17.Bolaños CA, et al. Phospholipase Cgamma in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J Neurosci. 2003;23:7569–7576. doi: 10.1523/JNEUROSCI.23-20-07569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW. Emergence of context-associated GluR(1) and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol. 2011;16:450–457. doi: 10.1111/j.1369-1600.2010.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapleau CA, Pozzo-Miller L. Divergent roles of p75NTR and Trk receptors in BDNF’s effects on dendritic spine density and morphology. Neural Plast. 2012;2012:578057. doi: 10.1155/2012/578057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, et al. Up-regulation of Ras/Raf/ERK1/2 signaling impairs cultured neuronal cell migration, neurogenesis, synapse formation, and dendritic spine development. Brain Struct Funct. 2013;218:669–682. doi: 10.1007/s00429-012-0420-7. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Wang Q, Wang Y, Chen X, Wang Z. PLC-gamma1 and Rac1 coregulate EGF-induced cytoskeleton remodeling and cell migration. Mol Endocrinol. 2009;23:901–913. doi: 10.1210/me.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo L, et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 23.Li YQ, et al. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Pulipparacharuvil S, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39:381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- 30.Larson EB, et al. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]