Significance
Bacteria cope with changing external environments and must therefore sense and respond to their local conditions. In addition to sensing numerous chemical cues, in this paper we show that Escherichia coli can sense the local mechanical environment through voltage-induced calcium flux. This mechanism is similar to sensory neurons in vertebrates, suggesting an ancient process of voltage-regulated mechanotransduction. Mechanical contact drives pathogenicity in several species of bacteria and we now propose a potential mechanism for this sensation.
Keywords: electrophysiology, voltage, calcium, Escherichia, mechanosensation
Abstract
Electrically excitable cells harness voltage-coupled calcium influx to transmit intracellular signals, typically studied in neurons and cardiomyocytes. Despite intense study in higher organisms, investigations of voltage and calcium signaling in bacteria have lagged due to their small size and a lack of sensitive tools. Only recently were bacteria shown to modulate their membrane potential on the timescale of seconds, and little is known about the downstream effects from this modulation. In this paper, we report on the effects of electrophysiology in individual bacteria. A genetically encoded calcium sensor expressed in Escherichia coli revealed calcium transients in single cells. A fusion sensor that simultaneously reports voltage and calcium indicated that calcium influx is induced by voltage depolarizations, similar to metazoan action potentials. Cytoplasmic calcium levels and transients increased upon mechanical stimulation with a hydrogel, and single cells altered protein concentrations dependent on the mechanical environment. Blocking voltage and calcium flux altered mechanically induced changes in protein concentration, while inducing calcium flux reproduced these changes. Thus, voltage and calcium relay a bacterial sense of touch and alter cellular lifestyle. Although the calcium effectors remain unknown, these data open a host of new questions about E. coli, including the identity of the underlying molecular players, as well as other signals conveyed by voltage and calcium. These data also provide evidence that dynamic voltage and calcium exists as a signaling modality in the oldest domain of life, and therefore studying electrophysiology beyond canonical electrically excitable cells could yield exciting new findings.
Calcium is a universal and indispensable signaling ion used in all known eukaryotes (1). Calcium concentration gradients across the plasma membrane and intracellular organelles enable highly dynamic fluxes via orchestrated channel openings to generate tightly controlled spatial and temporal patterns. The dynamics in both space and time are encoded and decoded into varying and sometimes opposing cellular signals (2, 3). In electrically excitable cells, including neurons and muscle cells, voltage-gated calcium channels (VGCCs) couple membrane depolarization to calcium influx, which can then alter cellular physiology. The myriad uses of calcium by cells highlight its crucial role in cellular maintenance.
Despite calcium’s ubiquity and utility across biological domains, little is known about how calcium is regulated in bacteria, especially at the single cell level (4). Fluorescent calcium dyes used in eukaryotic cells are not able to pass through the cell wall without pretreatment with the chelating agent EDTA, prohibiting studies of calcium with high temporal and spatial resolution in bacteria. Isotope labeling and luminescent probes have contributed population-level measurements of calcium, but were unable to resolve any potential cellular heterogeneity. In population measurements, cytoplasmic calcium concentration was correlated with processes, including bacterial differentiation (5), chemotaxis (6), pathogenicity (7), and sporulation (8). Furthermore, significant transcriptomic changes occur in bacterial species upon addition of external calcium (9, 10), but it remains unknown if there is any heterogeneity in a population.
Bacteria must cope with a variety of environments and changing conditions. In addition to chemical cues, bacteria navigate a range of mechanical substrates, from freely swimming to adhered to a surface (11), and these substrates can alter physiology and growth (12). How bacteria sense and respond to mechanical cues is critical to understanding how bacteria interact with their environment and adjust their behavior, including biofilm formation and infection. Virulent bacteria, such as Escherichia coli, Salmonella, Pseudomonas, and Yersinia, induce pathogenic factor expression upon contact with host cells or when exposed to shear stress (13–16). However, the mechanisms that relay bacterial mechanosensation remain unknown.
E. coli express a complement of mechanosensitive channels (17, 18), which enhance survival during osmotic transitions by altering ion conductance. These mechanosensitive channels were initially characterized using standard electrophysiology techniques from in vitro preparations and were shown to be voltage and tension gated. There is also evidence of a nonproteinaceous polymer acting as a VGCC in E. coli (19), highlighting a potential role for calcium signaling via electrophysiology. Despite extensive electrophysiological characterization of bacterial channels, it has not been possible to explore how the mechanical environment influences electrophysiology in vivo due to a lack of capable tools.
Recent advances in genetically encoded fluorescent biosensors are opening a new era in single cell physiology. Improvements in both sensor brightness and sensitivity have enabled measurements in individual cells in crowded environments. The GCaMP6 series of cpGFP-based calcium sensors have brightness, sensitivity, and kinetics that compare favorably to organic calcium sensitive dyes (20). GCaMP sensors have been heavily used in eukaryotic biology across a variety of cell types and model organisms. Fluorescent voltage indicators based on rhodopsins have been used in numerous cell types, including neurons, cardiomyocytes, and bacteria (21–23). These improved sensors enable measurements in very small cells and avoid potential exclusion by the outer membrane and cell wall.
In this paper, we use fluorescent biosensors in E. coli to show that voltage transients induce calcium influx in bacteria, highlighting their activity as electrically excitable cells capable of using calcium as a second messenger. We found that calcium influx can be both induced and blocked chemically. Furthermore, we showed that calcium transients are strongly increased by mechanical stimulation, and these calcium changes are necessary and sufficient to induce changes in protein concentration. Thus, bacteria use voltage and calcium as a mechanosensitive relay, similar to sensory neurons in higher organisms, and we hope to uncover the channels and effectors that mediate this relay in future work.
Results
Individual Bacteria Exhibit Calcium Transients.
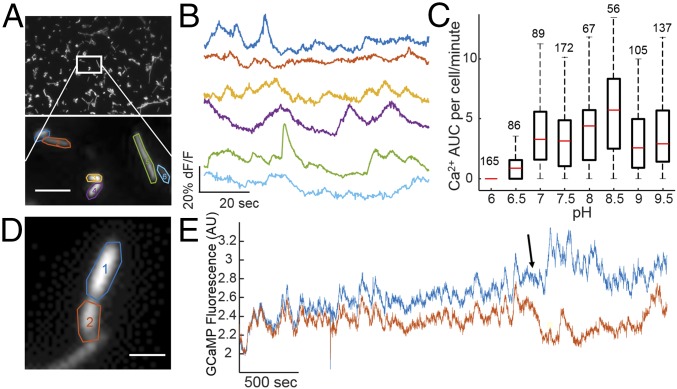
We hypothesized that E. coli, which were shown to have spontaneous voltage transients (23), would exhibit cytoplasmic calcium flux similar to eukaryotic electrically excitable cells. E. coli maintain tight control of cytoplasmic calcium and adjust calcium concentration in response to changing external conditions (24, 25). To test if E. coli displayed calcium dynamics, we imaged single cells expressing a genetically encoded calcium sensor, GCaMP6f (20) tethered to the calcium-insensitive fluorophore mRuby3 (26) to ensure proper expression in cases with low cytoplasmic calcium concentration. Constitutive expression of GCaMP6f resulted in slower growth compared with nonexpressing cells (SI Appendix, Fig. S1), and single cells fluoresced with a 488-nm excitation (Fig. 1A). Under typical imaging conditions of cells sandwiched between a glass coverslip and a 1% agarose pad made from M9 salts with glucose at pH 7.5 with no external calcium, individual cells showed transient bursts of fluorescence on the timescale of seconds (Fig. 1B and Movie S1). Cells also showed calcium transients under agarose pads made with supplemented minimal medium, phosphate-free medium without calcium, and phosphate-free medium with calcium (SI Appendix, Fig. S1), all at pH 7.5. The increase in free cytoplasmic calcium could come from the periplasm as E. coli maintain a larger periplasmic store of calcium relative to the cytoplasm (25).
Fig. 1.
Individual bacteria exhibit calcium transients. (A) Image of E. coli constitutively expressing GCaMP6f imaged under an agarose pad (Top). Zoomed view of individually segmented bacterial cells (Bottom). (Scale bar, 3 µm.) (B) Fluorescence time traces of spontaneous calcium transients in highlighted cells from A. (C) Quantification of calcium transients as a function of external pH. Red line indicates median, black box indicates 75/25%, and black dashed lines indicate 90/10% of the maxima. Outliers were analyzed but are not shown. The numbers above each boxplot are the number of cells analyzed. (D) Image of E. coli constitutively expressing GCaMP6f dividing during long-term image acquisition. (Scale bar, 2 µm.) (E) Fluorescence time traces of highlighted regions in D with a cell division marked with the arrow.
We sought to determine if intracellular calcium concentration was sensitive to external conditions. Addition of medium containing 5 mM of calcium caused an increase in the cytoplasmic concentration of free calcium similar to previous results (24), while addition of medium containing extracellular EGTA reduced the calcium levels (SI Appendix, Fig. S2). The number and amplitude of calcium transients was sensitive to external pH, with very few transients at acidic pH, and an increasing number with increasing pH (Fig. 1C). After identifying calcium transient peaks, the area under the curve (AUC) was calculated for each peak and summed for individual cells. Each cell AUC was then plotted as a member of a box plot. Highest activity was measured at pH 8–8.5, which is consistent with previously measured voltage transients in E. coli (23).
The high brightness and sensitivity of GCaMP6f enabled imaging at low light intensities for long periods of time without measurable phototoxicity. Hour-long continuous movies at room temperature showed examples of single cells growing and dividing, changing from correlated to uncorrelated calcium transients indicative of the formation of two independent cells (Fig. 1D). These data showed that our imaging conditions did not affect their ability to divide; bacteria exhibited calcium transients both before and after cell division (Fig. 1E). We concluded bacteria have rapid and heterogeneous changes in intracellular calcium concentration, on a timescale similar to voltage depolarizations.
Voltage Depolarization Induces Calcium Influx.
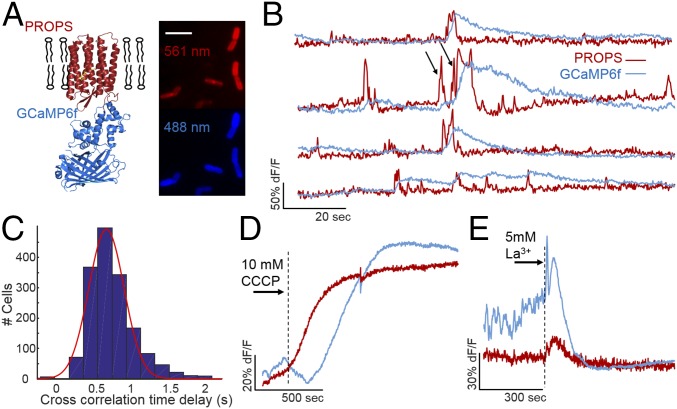
We hypothesized E. coli may be electrically excitable cells, using voltage depolarization to trigger calcium influx. To test the relationship between voltage and calcium in bacteria, we created a fusion of the PROPS voltage sensor with GCaMP6f (Fig. 2A), similar to the eukaryotic construct CaViar (27). We named the bacterial fusion protein CaPR. Fluorescence excited by both 488-nm (GCaMP6f) and 561-nm (PROPS) light confirmed proper folding of both proteins as well as minimal spectral overlap. CaPR was localized to the plasma membrane as both 488 and 561 nm of excited fluorescence showed enhanced intensity on the cell periphery (SI Appendix, Fig. S3). Constitutive expression of the CaPR construct resulted in a slowed growth compared with nonexpressing cells or cytoplasmic GCaMP6, and both the PROPS and GCaMP signals were lower than when expressing either protein alone. The lower CaPR expression compared with PROPS alone, combined with the required dual excitation, reduced the signal-to-noise ratio (SI Appendix, Fig. S3) and the amount of time before observed phototoxicity, as measured by a loss in membrane polarization (SI Appendix, Fig. S4). We therefore restricted continuous CaPR measurements with both 561-nm and 488-nm light to less than 2 min to avoid potential artifacts.
Fig. 2.
Voltage depolarizations induce cytoplasmic calcium influx in E. coli. (A) Design of the CaPR sensor. PROPS was fused to GCaMP6f. These two fluorophores were spectrally distinct (Left). Image of E. coli constitutively expressing CaPR imaged under an agarose pad (Right). (Scale bar, 5 µm.) (B) Time traces of single cells during simultaneous voltage (red) and calcium (blue) imaging. (C) Cross-correlation of voltage and calcium across entire traces calculated for each cell peaks at a 690-ms lag of calcium from voltage. (D and E) The average voltage (red) and calcium (blue) response to 10 µM CCCP (D) or 5 mM La3+ (E).
Under simultaneous illumination with both 561-nm and 488-nm light, we observed that voltage depolarizations were followed by calcium influx into the cell (Fig. 2B). Cross-correlation between voltage and calcium time traces showed the maximum correlation with the calcium signal lagging the voltage signal by 690 ms (Fig. 2C). PROPS has a reported response time constant of 4 ms, and GCaMP6f has a reported response time constant of 60 ms, so the observed delays are unlikely a result of sensor kinetics. A Granger causality test also indicated that voltage depolarizations predicted calcium transients, and that calcium transients did not predict voltage depolarizations (SI Appendix, Fig. S5). We confirmed that the presence of yellow light does not affect the number of measured calcium transients within a 2-min measurement (SI Appendix, Fig. S6). A second plasmid was generated using an inducible dual ORF expression system (petDUET) with both proteins present but not fused (i.e., cytoplasmic GCaMP6). Cells imaged using this system also had voltage-gated calcium influx (SI Appendix, Fig. S7).
Both PROPS and GCaMP6 are sensitive to pH, as well as voltage and calcium, respectively. To confirm that the transients we observed are due to calcium, we tried several red indicators of pH or calcium in E. coli to image both modalities simultaneously. However, no tested combination of pH and calcium sensors was functional in E. coli (SI Appendix, Table S1). We then used a combination of PROPS and super ecliptic pHluorin (28) to show that voltage transients are accompanied at most by a very small acidification (<0.1 pH units) of the cytoplasm (SI Appendix, Fig. S8). These acidifications would cause a small decrease in the GCaMP6 signal. In contrast, when imaging CaPR-expressing cells, voltage depolarizations were followed by large increases in GCaMP6 fluorescence (Fig. 2B), which indicated that the signal change is not due to changes in pH, but to increases in cytoplasmic calcium. Other intracellular ionic concentrations could also be altered during a voltage depolarization, which may have important functional cellular consequences, but we lacked suitable tools to measure additional ionic species at the single cell level.
Unlike traditional eukaryotic action potentials, E. coli have a wide range of amplitudes and durations of voltage depolarizations. This variability could be due to the small numbers of channels, large surface-to-volume ratio, or multiple distinct channels with different permeabilities. Transient heterogeneity, along with a changing baseline, made automatic peak identification challenging. To identify peaks, we adapted an algorithm based on clustering points in delay space (29) to call peaks in the PROPS channel (SI Appendix, Fig. S9 and SI Methods). Compared with a human-truthed sample dataset, the algorithm correctly identified 75% of voltage peaks with a false positive rate of 8%.
Cells expressing CaPR showed that unlike neuronal or cardiac action potentials, a single amplitude threshold in voltage did not consistently induce calcium influx. Two depolarizations of the same amplitude, but different widths, showed different calcium responses (Fig. 2B, black arrows). To identify the required voltage input to generate a calcium response, we identified 1,780 voltage peaks from a population of 300 cells expressing CaPR. For each voltage transient, we calculated the voltage depolarization time, voltage AUC, and calcium AUC (SI Appendix, Fig. S10). Voltage peaks of the same duration were binned, and the mean calcium AUC was calculated. Voltage transients less than 3 s in duration had very little calcium influx (31% of transients). Longer voltage transients between 3 and 6 s in duration showed a linearly increasing calcium response (61%). The 8% of voltage transients that were longer than 6 s in duration showed large GCaMP6 responses. The differences in calcium influx in response to voltage depolarization could be due to different channels with differential kinetics; a fast channel (1–3 s) depolarizes, but does not induce calcium, and a slow channel that does mediate calcium influx. It could also be two modes of the same channel, but our data cannot distinguish between these possibilities.
E. coli voltage transients require respiration and a proton motive force (PMF) (23). To test the energetic requirements of voltage-induced calcium flux, we imaged CaPR-expressing cells during application of small molecule inhibitors. These drug addition experiments were performed at pH 8.2 to eliminate changes in internal pH (23) upon drug application, as drug-induced changes in pH will affect both PROPS and GCaMP6 fluorescence. PMF was completely dissipated by 10 μM of the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) indicated by a rise in the PROPS signal (Fig. 2D). The GCaMP6 signal was reduced at the same time, and the loss of PMF eliminated both voltage and calcium transients. On average, 2.6 min after depolarization, the GCaMP6 signal slowly increased, indicating a rise in cytoplasmic calcium, consistent with previous findings that calcium efflux is ATP dependent (24). Lanthanum chloride was previously shown to block eukaryotic calcium channels as well as depress E. coli calcium concentration (30). Application of 5 mM lanthanum chloride blocked all voltage and calcium transients, but maintained cellular polarization (Fig. 2E). Unlike CCCP, cells did not show influx of calcium after lanthanum treatment, which suggested cells were still generating ATP. Exposure to 100 μM of the L-type calcium channel blockers verapamil and nifedipine did not block the calcium transients (SI Appendix, Fig. S11); however, this could be due to a unique VGCC that is not antagonized by these compounds or the bacteria were actively extruding the drugs from the periplasm. These data showed that actively respiring cells with a PMF are critical to voltage and calcium transients. Furthermore, bacteria required voltage transients to have calcium influx into the cytoplasm.
Calcium Transients Are Induced by Mechanical Stimulation.
Little is known about how bacterial cytoplasmic calcium concentration affects cellular physiology, although it has been implicated in numerous processes. To identify potential situations in which calcium transients affect cellular physiology, we varied environmental conditions to find differential calcium dynamics. We noticed that calcium transients in bacteria immobilized on glass in minimal medium were reduced compared with cells immobilized on glass under an agarose pad of the same medium composition. We hypothesized that calcium transients could be a mechanical signal relay for bacteria to modulate their lifestyle in response to changing environmental conditions.
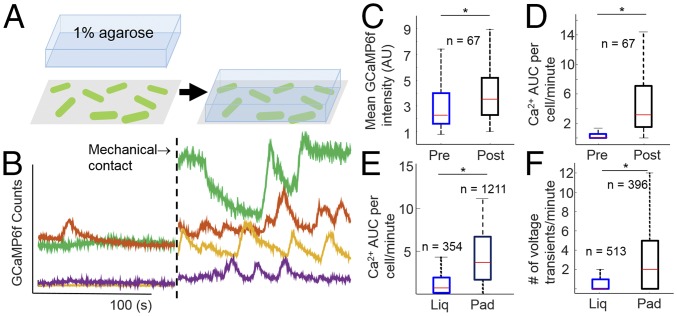
To explore the mechanical response of bacteria, we first tested if electrophysiology was influenced by mechanical substrate. Cells were immobilized on glass using poly-l-lysine (PLL), and calcium was imaged using GCaMP6f fluorescence in M9 minimal medium at pH 7.5 (Fig. 3A). The PLL-immobilized cells showed low calcium activity (Fig. 3B). Placement of a 1% agarose pad composed of the same medium on top of the bacteria induced a rise in the mean calcium levels as well as an increase in calcium transients (Fig. 3B and Movie S2). Comparing the same cells before and after the addition of the agarose pad showed an increase in the median GCaMP fluorescence intensity by 54% (Fig. 3C) and the median calcium AUC per cell from 0 to 3.2 (Fig. 3D). We controlled for optical artifacts by performing the same experiment while monitoring the tethered mRuby3, a calcium-insensitive red fluorescent protein (SI Appendix, Fig. S12). Adhered cells expressing the CaPR fusion or the dual ORF CaPR imaged with 488-nm light showed similar calcium increases upon placement of an agarose pad on adhered cells (SI Appendix, Fig. S13).
Fig. 3.
Calcium transients are induced by mechanical stimulation. (A) Schematic of the mechanical stimulation experiment. Cells expressing GCaMP6f (green) are adhered directly onto a glass coverslip with PLL, then immersed in liquid. These cells are continuously imaged before and after being sandwiched under an agarose pad. (B) Single cell calcium recordings before and after mechanical stimulation (dotted line) with a 1% low-melt agarose pad. Colors correspond to the same cell before and after stimulation. (C) Mean GCaMP6f intensity of the same cells before and after mechanical stimulation with an agarose pad. (D) Single cell calcium AUC per minute calculated for cells in C. (C and D) *P < 0.001 in a paired Student t test with unequal variance. (E) Calcium AUC measurements for populations of cells expressing GCaMP6f either adhered in liquid or under an agarose pad. (F) Number of voltage transients measured from populations of cells expressing PROPS adhered in liquid or adhered under an agarose pad. (E and F) *P < 0.001 in an unpaired Student t test with unequal variance. (C–F) Red line indicates median, blue/black box indicates 75/25%, and black dashed lines indicate 90/10% of the maxima. Outliers were analyzed but are not shown. n, number of cells analyzed.
The increased calcium influx suggested a concomitant increase in voltage transients in response to mechanical stimulation. However, the high light intensity required to image PROPS induced phototoxicity before completion of the experiment. Therefore, populations of cells were immobilized with PLL and immersed in minimal medium liquid or a minimal medium agarose pad, which we refer to as adhered-liquid and adhered-pad conditions, respectively. We confirmed that GCaMP6-expressing cells recapitulated the increased transients in adhered-pad compared with adhered-liquid conditions (Fig. 3E), and that the calcium response was triggered by mechanical contact, rather than chemical changes by varying the hydrogel composition (gelatin vs. agarose) and chemical adherent (PLL vs. polyethylenimine vs. concanavilin) (SI Appendix, Fig. S14). We then compared cells expressing PROPS alone in the adhered-liquid vs. adhered-pad conditions and found the mean number of voltage peaks per cell increased by a factor of 3.8, similar to the changes in calcium flux (Fig. 3F). The median duration of a voltage transient did not change, but the median AUC per transient increased 38% under the pad and indicated larger amplitude and more frequent depolarizations (SI Appendix, Fig. S15). These data confirmed that bacteria respond to changing mechanical environments with increased voltage-induced calcium flux.
No Individual Mechanosensitive Channel Controls Mechanically Induced Calcium Transients.
To identify channels that mediate mechanosensation, we used the Keio collection of nonessential E. coli knockouts (31). Knockouts were transformed with the constitutively expressing CaPR plasmid and imaged under an agarose pad. We first tested the seven known mechanosensitive channel proteins in E. coli (32) from two independently generated knockout strains. No single mechanosensitive knockout was sufficient to eliminate the calcium transients under an agarose pad (SI Appendix, Fig. S16). A triple knockout of the three most highly expressed mechanosensitive channels (33) also exhibited calcium transients. Increased osmotic pressure through addition of sorbitol did not induce calcium transients (SI Appendix, Fig. S17), which suggested the msc family of genes may not mediate the pad-induced calcium response.
We expanded our search for knockouts that eliminated calcium flux under an agarose pad. A total of 175 knockout strains enriched in membrane proteins, pili, and flagellar genes were measured for calcium transients, but no individual knockout eliminated calcium flux (SI Appendix, Figs. S18 and S19, Table S2, and SI Text). We then sought to determine if the knockouts we tested had any difference in the observed calcium transients relative to WT. All 175 knockouts were then compared with WT for the average transient duration, amplitude, and AUC. A single knockout, visC, was larger than 3σ away from the WT distributions with lower amplitude and higher duration transients (SI Appendix, Fig. S20). VisC is involved in energy production for the cell and a knockout reduced membrane potential in uropathogenic E. coli (34). The visC results suggested our measurements were sensitive enough to find variants that alter calcium flux, but the 175 genes tested are unlikely to relay mechanosensation or the voltage-gated calcium current.
Mechanically Induced Protein Concentration Changes Require Calcium Transients.
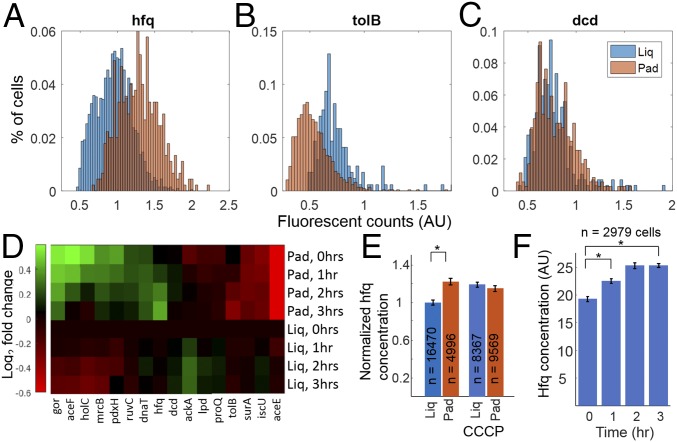
To test potential physiological roles of dynamic changes in cytoplasmic calcium, we compared protein concentration in cells in the adhered-liquid vs. adhered-pad conditions. Previous microarray data showed that 943 genes change expression levels upon addition of external calcium (24), so we hypothesized increased cytoplasmic calcium could affect protein concentrations. We used a previously generated library of endogenous proteins tagged with Venus, a YFP (35) to measure protein concentration of individual gene products. To optimize the likelihood of identifying mechanically sensitive proteins, we obtained 16 tagged strains of genes that were identified in a genome-wide screen to modulate growth in agarose (12). YFP-expressing strains were compared in minimal medium in the adhered-liquid vs. adhered-pad conditions. Images of protein concentration were compared from cells under each condition by extracting single cell YFP intensity (SI Appendix, Fig. S21). Measurements were taken hourly for 3 h. After 3 h, several genes had different protein concentrations dependent on the mechanical environment (Fig. 4 A–C). Of the 16 strains tested, 6 had increased and 4 had decreased protein concentration in the adhered-pad condition compared with the initial adhered-liquid condition cells (Fig. 4D). These data confirmed that E. coli sense and respond to the mechanical environment by changing cellular protein concentrations.
Fig. 4.
Mechanically induced protein concentration changes require calcium transients. (A–C) Single cell protein concentration was determined via YFP fluorescence under liquid (blue) or agarose pad (orange) immobilization after 4 h. The YFP-tagged gene products shown are hfq (RNA binding protein), tolB (periplasmic export protein), and dcd (deoxycytidine deaminase). (D) Heat map showing the log2 fold change in concentration at 1, 2, and 3 h after immobilization for the 16 genes measured. (E) Mechanosensation is lost in the presence of CCCP, which inhibits voltage and calcium transients as indicated by the hfq concentration measured at 2 h. (F) Hfq concentration when cells were adhered to glass and then exposed to oscillating levels of external calcium (30-s intervals of 5 mM and 0 mM) for the duration of the experiment. The cells were measured once per hour for 3 h. (E and F) *P value < 0.001 in an unpaired t test with unequal variance.
We sought to determine if mechanically induced changes in protein concentration were influenced by electrophysiology. Of the 16 YFP-tagged proteins tested, we initially focused on hfq, an RNA binding protein involved in regulating translation, which is essential for virulence in Salmonella enterica (36) and E. coli (37). We observed mean hfq concentration increased in the adhered-pad condition compared with adhered-liquid condition by 1.32 times (Fig. 4A) and hypothesized that blocking voltage and calcium flux would inhibit this increase in concentration. CCCP (10 μM) inhibited voltage and calcium flux (Fig. 2), and thus we measured hfq concentration in adhered-pad and -liquid conditions, with and without the drug. CCCP eliminated mechanically induced hfq concentration difference (Fig. 4E and SI Appendix, Fig. S22), but did not kill the cells as measured by their ability to divide after treatment (SI Appendix, Fig. S22). Compared with the untreated adhered-pad vs. adhered-liquid condition, the addition of CCCP left hfq concentration levels identical to the adhered-liquid condition without drug. Additional proteins were also shown to have altered concentration levels in the presence of CCCP, including aceF and dcd (SI Appendix, Fig. S23). The changes in protein concentration with CCCP may be due to the differences in voltage-induced calcium, although there are many other potential physiological effects. However, these data support the hypothesis that dynamic changes in cytoplasmic calcium levels can influence protein concentration in E. coli.
Inhibiting calcium transients altered mechanically induced protein concentration differences, so we next tested if externally induced cytoplasmic calcium transients could reproduce the differences in concentration in the absence of the agarose pad. We attempted several optogenetic and chemogenetic systems used in eukaryotic systems, without success (SI Appendix, Table S3). Unable to find an orthogonal actuator, we instead measured hfq concentration in cells adhered on glass while oscillating the level of external calcium. Using a flow cell, a protocol was developed to change the medium every 30 s from 0 to 5 mM calcium. This protocol was sufficient to induce increased cytoplasmic calcium as measured by GCaMP6 fluorescence (SI Appendix, Fig. S24). Hfq concentration was increased by 35% under periodic external calcium flow, similar to the concentration under a pad (Fig. 4F). A control flow experiment with constant external calcium showed no difference in hfq expression (SI Appendix, Fig. S25). AceF (SI Appendix, Fig. S26) and tolB (SI Appendix, Fig. S27) also replicated the pad vs. adhered changes in protein concentration under external calcium flow. These data showed that dynamic changes in cytoplasmic calcium levels are sufficient to drive changes in protein concentration to levels similar to mechanically activated cells.
Discussion
Electrically excitable cells are most often considered in the context of neurons, muscle, and β-cells. In these cells voltage depolarization is coupled with calcium influx, which then affects cellular physiology. Our findings indicate that E. coli also exhibit cytoplasmic calcium influx during voltage depolarization. Both voltage and calcium are known to regulate bacterial function. ATP generation (38), cell division (39), and persister formation (40) have all been tied to membrane potential. Calcium regulates Pseudomonas aeruginosa surface motility and integrin binding through PilY (41), a gene that encodes a calcium-sensitive pilus biogenesis factor (42). Additionally, a calcium-sensitive kinase, LadS, was shown to act as a virulence switch in P. aeruginosa, providing direct mechanistic evidence that calcium is used as a signal in bacteria (43). In agreement with these studies our findings that correlate calcium fluctuations and protein concentrations highlight calcium as an important signal for causing lifestyle changes in bacteria. These results, in conjunction with recent studies that show Bacillus subtilis in biofilms communicate electrically (44, 45), contribute to a growing body of evidence that bacterial electrophysiology can signal at both the intracellular single cell and intercellular community levels.
Our results indicate that E. coli have heterogeneous electrophysiological responses to the mechanical environment, and it will be important to characterize the heterogeneity of phenotypic responses. To conduct these measurements on an individual bacterium new and improved tools are required to probe dynamic cellular states. Sensitive new fluorescent probes enabled the study of calcium at the single bacterium level with high temporal resolution, in vivo. We expect that improved voltage sensors, ratiometric calcium sensors, and optogenetic actuators designed for bacteria will encourage broad adoption by the community. The fact that bacteria contain voltage-gated calcium fluctuations suggests that electrically excitable cells are more widely distributed across nature than previously realized; we expect better tools will uncover many novel uses for voltage and calcium signaling, in E. coli, as well as other bacterial species.
Bacteria change their lifestyle upon exposure to external forces or surface attachment, consistent with the broad range of potential mechanical substrates in the natural environment. Our data show E. coli alter both cytoplasmic calcium levels and protein concentrations dependent on the presence or absence of a hydrogel, suggesting they too mediate their lifestyle dependent on external forces. Both pathogenic E. coli and S. enterica increase pathogenicity during shear forces and surface adhesion (13, 46). We hypothesize that the mechanically induced calcium signal could up-regulate cellular concentration of pathogenic factors, including hfq, and thus bacteria use both mechanical and chemical cues to initiate infection of host cells. Future work will elucidate the specific forces that initiate mechanically induced changes in dynamic E. coli electrophysiology, as well as if this mode of mechanical signaling is more broadly distributed in the bacterial domain.
Vertebrate sensory neurons relay mechanosensory information by using voltage-induced calcium flux, mediated by force-activated TRP and Piezo channels (47, 48). We show that E. coli also use voltage-induced calcium flux to relay information about their mechanical environment, suggesting an ancient evolutionary origin for electrically excitable mechanosensation. Our data further imply the known mechanosensitive channels in E. coli do not mediate this response on their own, and a critical issue remains to identify the molecular players responsible for mechanosensation, calcium influx, and calcium-dependent transcription. Additionally, we have not ruled out other ionic species that could change during voltage transients, which may also have important physiological consequences. Although numerous questions remain, we hope GCaMP6 will become broadly used to help define the role of electrophysiology in numerous bacterial species. Overall this work indicates that voltage-coupled calcium dynamics could be used extensively across the biological domains.
Materials and Methods
Genes.
Sequences for voltage (PROPS), calcium (GCaMP6f), pH sensors (superecliptic pHluorin), and nonsensitve fluorophores (mRuby3) were all taken from previously published papers. PROPS was a gift from Adam Cohen (pJMK001, Addgene plasmid 33780), GCaMP6f was a gift from Douglas Kim (pGP-CMV-GCaMP6f, Addgene plasmid 40755). CaPR was constructed by fusing PROPS and GCaMP6f while maintaining the His-tag linker at the end of PROPS. A second construct using separate ORFs was generated using the petDUET expression plasmid. Sequences for new constructs are provided in SI Appendix.
Imaging.
Imaging took place either on a Nikon NSTORM microscope equipped with a TIRF module or on a custom, widefield inverted microscope. A 100× 1.45 NA objective using laser excitation imaged onto an Andor 897 EMCCD. All measurements were conducted with a high illumination angle to maximize signal-to-noise ratio. For PROPS illumination, a 561-nm laser at 14 W/cm2 intensity with 200-ms exposure time was used. A quadband dichroic (405/488/561/637) with a laser blocking filter was used for imaging PROPS. GCaMP6f fluorescence was excited with a 488-nm laser at 1,200 mW/cm2 intensity for the CaPR construct and 620 mW/cm2 intensity for GCaMP6f alone with 200-ms exposure time using the same quadband dichroic. Additional microscope details are provided in SI Appendix.
Minimal media was made to a final concentration of 1× M9 salts (Sigma) with 0.4% wt/vol glucose then adjusted to a pH of 7.5 with sodium hydroxide. In the case of experiments occurring at pH other than 7.5, Hepes was added to a final concentration of 5 mM and pH was adjusted as indicated. In the case of experiments occurring at different osmolality, sorbitol was added to the final concentration indicated. All cells were imaged within 1 h of specified treatment unless otherwise noted.
Agarose Pads.
For 1% agarose pads, low-melt agarose was added to a given media above to a final concentration of 1% wt/vol, then heated until the agarose dissolved. Once in solution, the mixture was cooled in silicone molds and pads were cut from the agarose media gels. Unless otherwise noted, cells were placed directly on pads and imaged.
PLL Adhesion.
A 0.001% wt/vol solution of PLL was dried on glass. Cells were then adhered for 1 h in LB, then washed three times in the medium in which they were to be imaged. When comparing the adhered and pad conditions, the final wash in the pad condition was replaced by the addition of an agarose pad made with minimal medium on top of the adhered cells. PLL-coated glass was always prepared the same day.
YFP Fusion Lines.
To measure single cell, in vivo protein concentration, we imaged cells expressing a YFP tag fused to the endogenous gene (35). Strains were acquired from the E. coli Genetic Stock Center at Yale University via a transfer agreement from Harvard University. Growth and imaging conditions are provided in SI Appendix, SI Methods.
Computer Code.
All analysis was performed using custom scripts written in Matlab R2016b (Mathworks). Scripts and all datasets are available to researchers upon request. Details are provided in SI Appendix, SI Text.
Supplementary Material
Acknowledgments
Thanks to Tova Christensen for help with hydrogels; Cori Bargmann for the HisCl1 plasmid; Rob Phillips for the triple MSC knockout; Anjali Rao, Eric Burn, Amy Palmer, Ali Young, Corrie Detweiler, Michael Stowell, and Huen Jin Lee for helpful discussions; and Joe Dragavon and the BioFrontiers Advanced Imaging Core for help with imaging. NSTORM microscopy was performed on a Nikon Ti-E microscope acquired by the generous support of the Howard Hughes Medical Institute. This work is supported by the Searle Scholars Program and the NIH New Innovator Award (1DP2GM123458) (to J.M.K.) and a T32 Training Grant (T32GM065103) (to G.N.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703084114/-/DCSupplemental.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: Dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purvis JE, Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell. 2013;152:945–956. doi: 10.1016/j.cell.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domínguez DC, Guragain M, Patrauchan M. Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium. 2015;57:151–165. doi: 10.1016/j.ceca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, et al. Structures of Anabaena calcium-binding protein CcbP: Insights into Ca2+ signaling during heterocyst differentiation. J Biol Chem. 2011;286:12381–12388. doi: 10.1074/jbc.M110.201186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tisa LS, Adler J. Calcium ions are involved in Escherichia coli chemotaxis. Proc Natl Acad Sci USA. 1992;89:11804–11808. doi: 10.1073/pnas.89.24.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosch JW, Sublett J, Gao G, Wang Y-D, Tuomanen EI. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol Microbiol. 2008;70:435–444. doi: 10.1111/j.1365-2958.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbaud ML, Guiseppi A, Denizot F, Haiech J, Kilhoffer MC. Calcium signalling in Bacillus subtilis. Biochim Biophys Acta. 1998;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 9.Oomes SJCM, et al. The effect of calcium on the transcriptome of sporulating B. subtilis cells. Int J Food Microbiol. 2009;133:234–242. doi: 10.1016/j.ijfoodmicro.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Gode-Potratz CJ, Chodur DM, McCarter LL. Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus. J Bacteriol. 2010;192:6025–6038. doi: 10.1128/JB.00654-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persat A, et al. The mechanical world of bacteria. Cell. 2015;161:988–997. doi: 10.1016/j.cell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auer GK, et al. Mechanical genomics identifies diverse modulators of bacterial cell stiffness. Cell Syst. 2016;2:402–411. doi: 10.1016/j.cels.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsharif G, et al. Host attachment and fluid shear are integrated into a mechanical signal regulating virulence in Escherichia coli O157:H7. Proc Natl Acad Sci USA. 2015;112:5503–5508. doi: 10.1073/pnas.1422986112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginocchio CC, Olmsted SB, Wells CL, Galán JE. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 15.Siryaporn A, Kuchma SL, O’Toole GA, Gitai Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci USA. 2014;111:16860–16865. doi: 10.1073/pnas.1415712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersson J, et al. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 17.Booth IR. Bacterial mechanosensitive channels: Progress towards an understanding of their roles in cell physiology. Curr Opin Microbiol. 2014;18:16–22. doi: 10.1016/j.mib.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Lengweiler UD, Seebach D, Reusch RN. Proof for a nonproteinaceous calcium-selective channel in Escherichia coli by total synthesis from (R)-3-hydroxybutanoic acid and inorganic polyphosphate. Proc Natl Acad Sci USA. 1997;94:9075–9079. doi: 10.1073/pnas.94.17.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T-W, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 2011;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dempsey GT, et al. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J Pharmacol Toxicol Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333:345–348. doi: 10.1126/science.1204763. [DOI] [PubMed] [Google Scholar]
- 24.Naseem R, Wann KT, Holland IB, Campbell AK. ATP regulates calcium efflux and growth in E. coli. J Mol Biol. 2009;391:42–56. doi: 10.1016/j.jmb.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Jones HE, Holland IB, Campbell AK. Direct measurement of free Ca(2+) shows different regulation of Ca(2+) between the periplasm and the cytosol of Escherichia coli. Cell Calcium. 2002;32:183–192. doi: 10.1016/s0143416002001537. [DOI] [PubMed] [Google Scholar]
- 26.Bajar BT, et al. Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Sci Rep. 2016;6:20889. doi: 10.1038/srep20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou JH, Kralj JM, Douglass AD, Engert F, Cohen AE. Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front Physiol. 2014;5:344. doi: 10.3389/fphys.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The use of pHluorins for optical measurements of presynaptic activity. Biophys J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weekley RA, Goodrich RK, Cornman LB. An algorithm for classification and outlier detection of time-series data. J Atmos Ocean Technol. 2010;27:94–107. [Google Scholar]
- 30.Campbell AK, Naseem R, Holland IB, Matthews SB, Wann KT. Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ transients and inhibit growth in E. coli. Arch Biochem Biophys. 2007;468:107–113. doi: 10.1016/j.abb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards MD, et al. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels (Austin) 2012;6:272–281. doi: 10.4161/chan.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levina N, et al. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: Identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floyd KA, et al. The UbiI (VisC) aerobic ubiquinone synthase is required for expression of type 1 pili, biofilm formation, and pathogenesis in uropathogenic Escherichia coli. J Bacteriol. 2016;198:2662–2672. doi: 10.1128/JB.00030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi Y, et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendall MM, Gruber CC, Rasko DA, Hughes DT, Sperandio V. Hfq virulence regulation in enterohemorrhagic Escherichia coli O157:H7 strain 86-24. J Bacteriol. 2011;193:6843–6851. doi: 10.1128/JB.06141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaim G, Dimroth P. ATP synthesis by the F1F0 ATP synthase of Escherichia coli is obligatorily dependent on the electric potential. FEBS Lett. 1998;434:57–60. doi: 10.1016/s0014-5793(98)00969-7. [DOI] [PubMed] [Google Scholar]
- 39.Strahl H, Hamoen LW. Membrane potential is important for bacterial cell division. Proc Natl Acad Sci USA. 2010;107:12281–12286. doi: 10.1073/pnas.1005485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verstraeten N, et al. Obg and membrane depolarization are part of a microbial bet-hedging strategy that leads to antibiotic tolerance. Mol Cell. 2015;59:9–21. doi: 10.1016/j.molcel.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Johnson MDL, et al. Pseudomonas aeruginosa PilY1 binds integrin in an RGD- and calcium-dependent manner. PLoS One. 2011;6:e29629. doi: 10.1371/journal.pone.0029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orans J, et al. Crystal structure analysis reveals Pseudomonas PilY1 as an essential calcium-dependent regulator of bacterial surface motility. Proc Natl Acad Sci USA. 2010;107:1065–1070. doi: 10.1073/pnas.0911616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broder UN, Jaeger T, Jenal U. LadS is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat Microbiol. 2016;2:16184. doi: 10.1038/nmicrobiol.2016.184. [DOI] [PubMed] [Google Scholar]
- 44.Prindle A, et al. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphries J, et al. Species-independent attraction to biofilms through electrical signaling. Cell. 2017;168:200–209.e12. doi: 10.1016/j.cell.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Barrila J, Roland KL, Ott CM, Nickerson CA. Physiological fluid shear alters the virulence potential of invasive multidrug-resistant non-typhoidal Salmonella Typhimurium D23580. NPJ Microgravity. 2016;2:16021. doi: 10.1038/npjmgrav.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.