Significance
Sorghum’s ability to withstand harsh environmental conditions has placed it in the forefront of discussions regarding potential adaptation paths under climate change. While sorghum may indeed be a good candidate to substitute for other major row crops as warming materializes in areas where it has not traditionally been grown, an equally important consideration is whether its production can be sustained in the warmer areas where it has traditionally been grown. Our findings suggest limited potential for climate change adaption using currently available cultivars but do not preclude the overall role of genetic innovation and enhanced decision making in adapting to climate change. Successful adaptation could perhaps best be facilitated by expanding the scope of genetic stock within sorghum breeding programs.
Keywords: agriculture, climate change, crop, sorghum, warming
Abstract
Historical adaptation of sorghum production to arid and semiarid conditions has provided promise regarding its sustained productivity under future warming scenarios. Using Kansas field-trial sorghum data collected from 1985 to 2014 and spanning 408 hybrid cultivars, we show that sorghum productivity under increasing warming scenarios breaks down. Through extensive regression modeling, we identify a temperature threshold of 33 °C, beyond which yields start to decline. We show that this decline is robust across both field-trial and on-farm data. Moderate and higher warming scenarios of 2 °C and 4 °C resulted in roughly 17% and 44% yield reductions, respectively. The average reduction across warming scenarios from 1 to 5 °C is 10% per degree Celsius. Breeding efforts over the last few decades have developed high-yielding cultivars with considerable variability in heat resilience, but even the most tolerant cultivars did not offer much resilience to warming temperatures. This outcome points to two concerns regarding adaption to global warming, the first being that adaptation will not be as simple as producers’ switching among currently available cultivars and the second being that there is currently narrow genetic diversity for heat resilience in US breeding programs. Using observed flowering dates and disaggregating heat-stress impacts, both pre- and postflowering stages were identified to be equally important for overall yields. These findings suggest the adaptation potential for sorghum under climate change would be greatly facilitated by introducing wider genetic diversity for heat resilience into ongoing breeding programs, and that there should be additional efforts to improve resilience during the preflowering phase.
Sorghum (Sorghum bicolor) is predominantly grown in the arid and semiarid regions of the world, where heat stress is known to induce significant yield losses (1–4). Globally, sorghum is positioned as the fifth most economically important cereal and plays a critical role in providing food, fodder, and fuel (5, 6). Sorghum is considered to be relatively more hardy under extreme heat and drought conditions compared with other major crops and thus has received much attention as a potential adaptation strategy for farmers. In this context, farmer “adaptation” is a general term that involves many scenarios including the switching from one crop to another, switching among existing cultivars of a crop, and/or adopting a new production technology that might include newly developed cultivars that are not currently available.
The changing climate is pushing sorghum to endure harsher environments and it is unclear whether current production levels are sustainable. Although sorghum may be relatively more resilient to extreme conditions compared with other major row crops, it does not necessarily follow that sorghum is resilient in an absolute sense. One reason for this is that sorghum seed germination requires warmer soil temperatures compared with many other crops such as soybeans and maize, and thus is often sown in warmer seasons and exposed to relatively harsher growing conditions. Although future breeding efforts may allow other crops to “catch up” to sorghum by exploiting genetic variation in heat resilience within those species (7), it is unclear whether there exists potential for sorghum to become more resilient than it already is. Hence, the major focus of our investigation is to consider (i) whether there exists substantial variation in heat resilience among existing cultivars and (ii) if there are particular phases of sorghum plant growth that should be targeted for future genetic improvement. This study considers these questions using a long time series and cross-section of sorghum variety field-trial data produced under nonirrigated conditions.
Both controlled-environment studies (8–11) and more recent studies using custom-designed field-based structures (12, 13) have documented the susceptibility of sorghum to heat stress during the critical flowering and grain-filling stages. Hence, most empirical studies and current breeding efforts have been aimed toward reducing yield losses with stress coinciding with the sensitive flowering and grain-filling stages. Currently available sorghum cultivars require soil temperatures above 21 °C, while corn and soybeans can germinate at much lower soil temperatures of 13 °C and 10 °C, respectively (14–16). This effectively ensures that sorghum is exposed to higher temperatures during much of its growth cycle, not just the flowering and grain-filling phases. Of particular interest are phases just before flowering as heat exposures in this period have recently been found to be as stressful as those during latter stages (17). The physiological processes that could be impacted during this phase include panicle initiation, reproductive organ development (gametogenesis), and preflowering photosynthetic efficiency (18). Thus, ongoing research efforts focused on stress impacts at or after flowering alone are potentially short-sighted in that opportunities to further minimize stress damage may be overlooked by not considering improvement of heat-stress resilience during alternative crop growth stages.
Sorghum and millets are often projected to be capable of sustaining productivity under harsher weather conditions accompanying climate change, based on their historical ability to produce in arid and semiarid regions. However, recent research using observational data has documented aggregate productivity reductions of 17% for both crops in sub-Saharan Africa during 2046–2065 relative to 1961–2000, compared with a 22% reduction for maize (2). Although irrigation or timely availability of rainfall are capable of reducing heat-stress impacts, neither of these options is a regular feature in the arid regions where sorghum is a dominant crop. Furthermore, a comprehensive crop modeling exercise using data on sorghum and millets obtained from 35 stations across sub-Saharan West Africa (Senegal, Mali, Burkina Faso, and Niger) indicated that negative impacts associated with a 2 °C temperature increase were not compensated by additional precipitation (19). Thus, even though sorghum might have some advantages relative to other crops for resisting extreme heat exposure, warming temperatures are still associated with large yield reductions in major growing regions, thereby suggesting an important need to investigate the potential for enhanced heat resilience. These possibilities are best analyzed using data containing observable variations among a large sample of cultivars, which typically is not possible when using aggregate regional yield data, controlled experimentation in growth chambers, or crop simulation models.
Our empirical approach is based on a statistical model that is estimated using nearly 9,000 field-level observations from 408 cultivars grown across 11 Kansas locations spanning 30 y (1985–2014). Our primary findings suggest that there are indeed significant heat-stress impacts occurring during the preflowering phase, and they are similar in size to the impacts that occur after flowering. This has important implications for climate change adaptation as it suggests that plant scientists can complement existing efforts to reduce heat susceptibility by focusing on the earlier plant growth phases that have not been previously emphasized. Other findings from our analysis include (i) identifying a temperature threshold of 33 °C beyond which yield starts to decline under field conditions spanning diverse environmental conditions and cultivars, (ii) evidence that breeding efforts have not improved heat-stress resilience within the past 30 y, and (iii) that there is little scope for producer adaptation to climate change via optimal selection among existing cultivars as we document that warming effects are quantitatively similar across the least and most resistant ones.
Results
The raw data include observed dryland sorghum yields matched by location with daily minimum/maximum temperatures and total precipitation. We only include cultivars that have appeared in at least three trial years, which results in 408 observed cultivars in the sample (SI Appendix, Table S1). The average number of trial years that each cultivar appears in is 5.5 y, and as one would expect newer cultivars have appeared in fewer years (SI Appendix, Fig. S1). Cultivars very often appear in multiple trial locations within each year as the average number of observations per cultivar is 21.8. The yield and weather data vary substantially in-sample (SI Appendix, Figs. S2 and S3 and Table S2). The growing season in each location–year is defined by the observed sowing and harvest dates, which vary across the 11 locations and 30 y of data (SI Appendix, Table S2). For some models we split the growing season into three phases: the first 35 d after sowing (early vegetative phase), 35 d after sowing to flowering (preflowering phase), and after flowering to the end of the growing season (postflowering phase). We define the end of the growing season to be 30 d after flowering. Importantly, flowering dates are observed and defined as the day at which 50% flowering occurs.
Degree days (hereafter DDs) are calculated using a sinusoidal interpolation of temperature exposure within each day. The DD variables are used to specify a piecewise linear temperature effect which allows us to quantify the nonlinear effects of extreme heat exposure (20). There exists substantial variation in these variables across both locations and years, and it is common for plants to be exposed to extreme heat above 30, 35, and 40 °C (SI Appendix, Fig. S4). It is not clear from the literature what the appropriate DD thresholds for sorghum are, as these can potentially vary by the method used for measuring temperature exposure. We follow the piecewise linear approach of ref. 20 and allow for two separate DD thresholds. Under this approach the upper and lower thresholds are used to construct three measures of temperature exposure: DDs between 0 °C and the lower threshold, DDs between the lower and upper thresholds, and DDs above the upper threshold. The optimal thresholds for our preferred model were estimated to be 10 and 33 °C (SI Appendix, Table S3). Hereafter extreme heat refers to temperatures above 33 °C. We find evidence of considerable uncertainty around this upper threshold as a block bootstrapping routine generates a 95% confidence interval of [28.04, 37.06]. As discussed below, the estimated warming impacts using 33 °C to define extreme heat are robust to alternative definitions based on the range of values within this confidence interval. We also considered whether the model should include a time-varying extreme heat effect to capture changes in heat resilience over the sample period, but we found that this extension was not warranted as the effect of an interaction between a linear (or quadratic) time trend and extreme heat was not statistically significant (P values of 0.55 and 0.52, respectively).
Sorghum Is More Resistant to Hotter Temperatures Compared with Other Crops, but Extreme Heat Is Still a Large Driver of Yield Loss.
The parameter estimates for the preferred regression model are reported in column 1 of SI Appendix, Table S4. This model regresses log sorghum yield on a cubic polynomial for precipitation and three DD variables for the piecewise linear temperature effect, in addition to location and cultivar dummy variables to account for fixed effects and a quadratic time trend to account for changes in management practices over time. We find that an additional DD above 33 °C is associated with a 1.3% yield reduction (SI Appendix, Table S4). This extreme heat threshold is above ones identified for corn (29 °C) and soybeans (30 °C) but similar to ones identified for cotton (32 °C), rice (33 °C), and wheat (34 °C) (20–22).
Sorghum Does Not Exhibit Strong Sensitivity to Low Rainfall.
Yields for most crops have an inverted U-shape relationship with cumulative precipitation, indicating that both low- and high-precipitation years are associated with yield reductions. We find that sorghum exhibits a similar sensitivity to high precipitation; however, it tolerates low precipitation remarkably well (SI Appendix, Fig. S5). Low precipitation is likely, but not necessarily, linked to water-deficit conditions in the soil; however, we do not have soil moisture data available to confirm this. The precipitation variables are jointly statistically significant, as are the temperature variables and the location and cultivar fixed effects (SI Appendix, Table S5).
Increased Exposure to Extreme Heat Above 33 °C Leads to Large Yield Reductions Under Warming Temperatures.
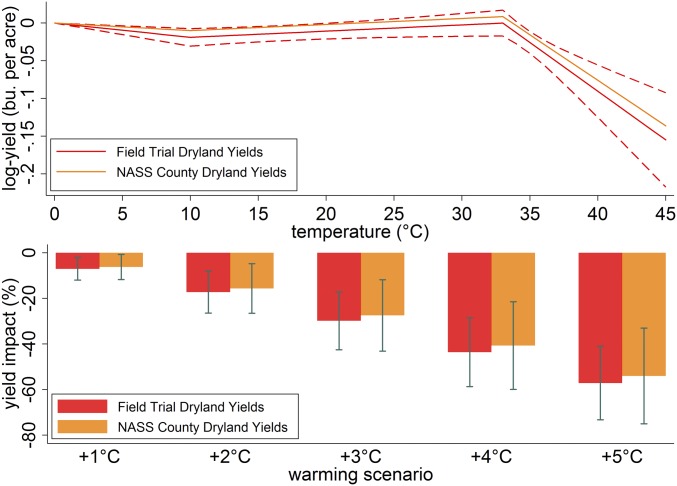
Fig. 1 shows that there are both beneficial and detrimental temperature exposures, those between 10–33 °C and those above 33 °C, respectively. To evaluate which effect dominates, we predict yield impacts across a range of uniform temperature changes from +1 and +5 °C. All scenarios suggest that warming is associated with net yield reductions and is statistically significant. The effects are highly nonlinear across warming scenarios, from a 7% reduction under 1 °C warming to a 57% reduction under 5 °C. The average effect is a 10% per degree Celsius across the five warming scenarios. The warming impact estimates are robust to alternative thresholds for defining extreme heat (SI Appendix, Fig. S6).
Fig. 1.
Nonlinear temperature–yield relationship and associated warming impacts across uniform changes in temperatures. (Top) Changes in log yield if the crop is exposed for 1 d (24 h) to a particular 1 °C temperature interval for both the field-trail data and the NASS county data. (Bottom) Estimated warming impacts as the percentage change in yield relative to historical climate. Dashed lines and error bars show 95% confidence intervals using spatially robust SEs clustered by year.
Recent research has suggested that one of the main channels by which warming temperatures can affect sorghum yields is through its influence on water-stress/drought conditions (23). This would suggest that our inability to control for soil moisture could be biasing the estimated effect of temperature on yield. While we cannot address this concern directly, we can include interactions between low- and high-precipitation events with our measure of extreme heat to see if the estimated effect varies. For example, if we focus on high precipitation years—when soil moisture is not likely to be a concern—and find that the estimated coefficient on extreme heat is very different from the one under our preferred model, then this would suggest biased heat estimates. A similar argument could be made using low precipitation years. To investigate this, we allow the effect of temperatures above 33 °C to vary by interacting it with dummy variables for precipitation outcomes below the 25th percentile and above the 75th percentile of the rainfall distribution for these data. The estimates suggest a slightly higher (lower) heat effect in the low- (high-) precipitation regimes; however, neither estimate is statistically significantly different from the estimate for the middle 50% of the rainfall outcomes. In addition, the implied warming estimates for the low- and high-precipitation regimes are very similar to the ones for our preferred model (SI Appendix, Fig. S7).
The Warming Effects Estimated from Field-Trial Yield Data Are Consistent with Effects Estimated from County-Level Aggregate Yield Data.
As discussed in ref. 22, the management practices for the Kansas field trials vary by location and year and are considered “best management practices” as they are designed to eliminate all yield-reducing factors such as nutrient deficiencies or toxicities, damage from insect pests and disease, and competition from weeds. These optimal growing practices potentially differ from the production practices of actual farmers, who base management decisions on profitability. To investigate the external validity of our results, we reestimate the preferred model (SI Appendix, Table S4, column 1) using county-level dryland yield data for sorghum from the National Agricultural Statistics Service (NASS). The 11 trial locations form a small but representative sample of all Kansas sorghum-producing counties (SI Appendix, Table S6). County-level farm yields are lower than field-trial yields on average, which is consistent with the existence of a yield gap between field-trial and on-farm production (24, 25). We match the NASS yield observations with the weather from the field-trial location in that county. The location and cultivar fixed effects from the preferred model are replaced with county fixed effects. We find that the temperature coefficient estimates and associated warming effects remain largely unchanged relative to our preferred model (Fig. 1). This provides evidence of external validity for the results based on field trial data reported here.
Among Cultivars, There Exists a Positive Association Between Mean Yields and Heat Resilience and Mean Yields and First Trial-Year, but Not Heat Resilience and First Trial-Year.
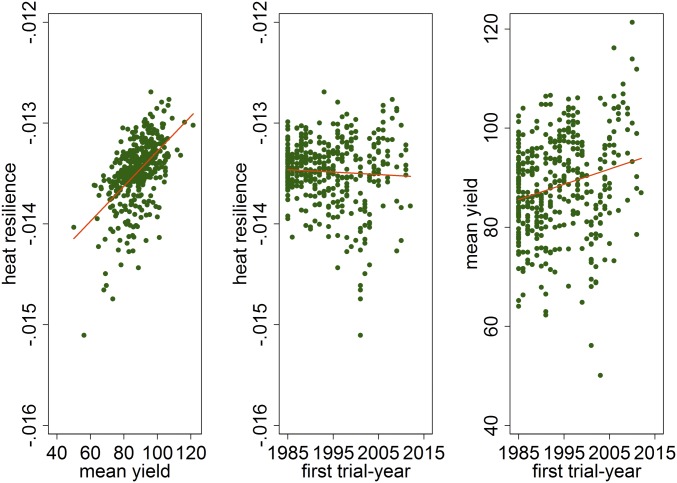
We test genotype × environment interactions to determine the ability of different sorghum cultivars to resist heat stress. We use a varying-slope multilevel model (26) where the fixed portion of the model takes the same form as our preferred model (SI Appendix, Table S4, column 1), but we allow the effect of DDs above 33 °C to vary across cultivars. Importantly, we have sufficient coverage in the data to do this as every cultivar was exposed to temperatures in excess of 33 °C in the data. If this was not the case, one might consider grouping the cultivars by genetic similarity (7). We compare these cultivar-specific resilience estimates to both mean yields, defined as the predicted yield under average weather conditions, and the year in which the cultivar first appeared in the trials (first trial-year). We find evidence of a positive relationship between heat resilience and mean yields (pairwise correlation of 0.55), indicating that cultivars with higher heat resilience also had higher mean yields (Fig. 2). Importantly, mean yields are defined here as yield potential—predicted yields under average/normal weather conditions—so high mean yields need not be associated with increased heat-stress resilience. This suggests that breeders have done an effective job of developing germplasm that enhance yield and simultaneously reduce risk. We also find that newer cultivars have mean yields that are higher than older ones as the pairwise correlation of mean yields and first trial-year is 0.23. Perhaps as a bit of a paradox we find no detectable association between heat resilience and the year that the developed cultivars entered testing (pairwise correlation of −0.06).
Fig. 2.
The trade-off between mean yield and heat resilience, as well as the innovation of breeding efforts over time. (Left) Mean yields and heat resilience are estimated for each cultivar and plotted against each other. (Middle) The resilience estimates against the year in which the cultivar first appeared in the field trial data. (Right) The same for mean yields. The reported value for heat resilience is the estimated coefficient from the regression model for extreme heat variable, which measures the percentage impact on yield from an additional DD above 33 °C. The smaller the number in absolute value the more heat-resilient the cultivar is. The mean yields are predicted under normal weather conditions, defined as the sample average of the observed weather variables, and are thus representative of yield potential. Lines indicate fitted values from a linear regression.
There Exists Limited Adaptation Potential for Reducing Warming Impacts by Switching from Less- to More-Heat-Resilient Cultivars.
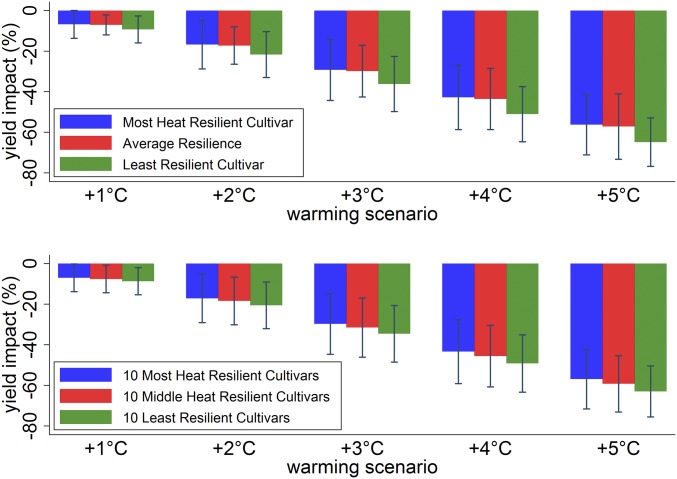
We use the cultivar-specific heat resilience estimates to simulate a switch from the least- to most-resilient cultivar, quantified by the change in the yield impact for the alternative warming scenarios. Fig. 2 shows that there exists a range of heat resilience across cultivars, which suggests that producers might be able to mitigate the negative effects of warming temperatures by switching cultivars. However, we find that there does not exist much scope for producer adaption to warming through alternative cultivar selection as the warming impacts across the most-, average-, and least-heat-resilient cultivars are very similar in magnitude (Fig. 3). This is robust to separately grouping the 10 least- and most-resistant cultivars.
Fig. 3.
Predicted warming impacts on sorghum yields for the most and least heat resilient cultivars. This figure replicates Fig. 1 for various cultivars. Separate heat-resilience parameters are estimated for each cultivar and then used to predict warming impacts. (Top) Comparison of the most- and least-resilient cultivars to the average reported in Fig. 1. (Bottom) An average of the parameters within subgroups containing 10 cultivars each. Bars show 95% confidence intervals using spatially robust SEs clustered by year.
The Preflowering Growth Stage Is Susceptible to Extreme Heat Exposure, but Overall Warming Impacts Are Robust to the Allowance for Heterogeneous Intraseason Weather Effects.
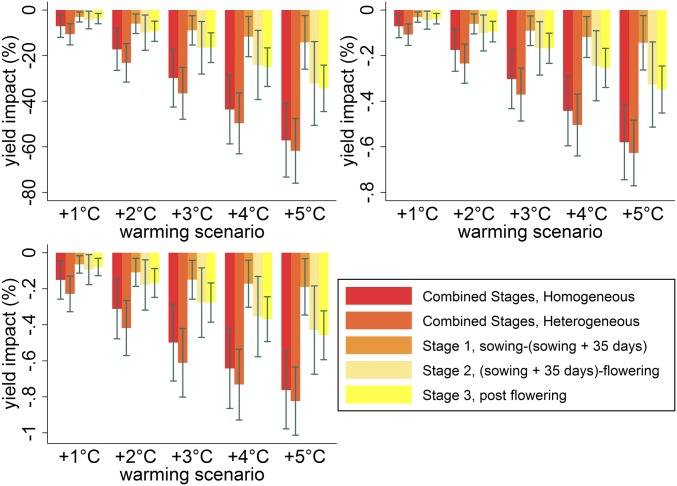
We next segment the growing season into three stages and allow the effects of the weather variables to vary across stages. We refer to this as the heterogeneous weather-effect model and separate the season as follows: (S1) sowing to 35 d after sowing, (S2) 35 d after sowing to flowering, and (S3) postflowering. The first stage captures plant growth and leaf determination before the beginning of the plant reproductive and ripening cycle, which spans the second and third stages, respectively (14). We find that precipitation and temperature are statistically significant drivers in all three stages, and there is no evidence that the weather effects are equivalent across stages (SI Appendix, Table S7). However, allowing the weather effects to vary by stage does not change the overall warming impacts reported above (Fig. 4). Although the combined effects are the same, this more general model does allow us to measure the relative contribution of each stage to the overall effect. We find that the contribution of the first 35 d to cumulative warming impacts is small relative to the later growth stages. More interestingly, we find that the contribution of the warming impacts across stages 2 and 3 are equivalent. This pattern of results is robust to normalizing the warming effects on a “per-day” basis defined by the effect in that stage divided by the number of days in that stage, and a similar normalization based on a “per-heat-day” measure where we instead divide by the number of days in which temperatures above 33 °C occurred (Fig. 4).
Fig. 4.
Predicted warming impacts on sorghum yields under alternative uniform temperature changes for a model that allows for heterogeneous weather effects. We extend the homogeneous weather-effect model from Fig. 1 to allow the effect of the weather variables to vary across three stages of the growing season. We report the warming effect associated with each stage as well as the cumulative effect of combining them (Combined Heterogeneous). We also include the impacts from Fig. 1 as a reference (Combined Homogeneous). The first panel reports the total warming impacts, and the next two divide the impact by the number of days within the growing season and the number of days temperature exceeded 33 °C within the season. Bars show 95% confidence intervals using spatially robust SEs clustered by year.
Discussion
Overall, the findings here suggest limited potential for climate change adaption using currently available sorghum cultivars but do not preclude the overall role of genetic improvement and enhanced decision making in adapting to climate change. The reported findings will help sorghum breeders to formulate or strategically revise their programs, physiologists and molecular biologists to explore additional mechanistic responses or traits to better understand the drivers of heat stress, crop modelers to refine their predictions for future climatic conditions, and economists to identify the most profitable and sustainable adaptation paths for producers.
Although sorghum is known to be highly heat-tolerant, it is prone to yield losses during the flowering and/or postflowering phases (12, 13, 27). Sorghum’s response to heat stress has been primarily quantified by imposing stress at predetermined stages using highly controlled environment facilities or field-based tents (8–13, 27) involving few cultivars. These findings under controlled conditions limit our ability to develop robust conclusions regarding sorghum’s heat-stress sensitivity (28). Here we use a diverse set of 408 cultivars grown under natural dryland field conditions over the last 30 y and identify a temperature threshold of 33 °C, beyond which significant yield reductions are observed. The magnitude of heat stress for temperature exposures above 33 °C was found to be the same for both field-trial and farmer-managed fields, which represents an important validity check for our results and fills a major knowledge gap in sorghum research.
Our findings have important implications for producer adaptation to climate change and suggest an avenue for future genetic improvement. We find that a moderate warming scenario of 2 °C resulted in a 17% yield reduction, and the average effect across warming scenarios from +1 °C to +5 °C resulted in a 10% yield reduction per degree Celsius. These impacts are similar to those found for sorghum in other production areas, as well as other major row crops (2–4, 20), thereby suggesting that sorghum’s ability to withstand extreme heat exposure will not likely be sustained under future warming conditions. While this does not limit sorghum’s role as an alternative crop in current temperate production environments where it has not traditionally been grown, such as the US corn belt, it does suggest a more limited capability within the warmer climates where it is currently being produced. Our data include information on sowing, flowering, and harvest dates, which allows us to disentangle heat-stress impacts across alternative plant growth phases. While we find that exposure to temperatures above 33 °C produces statistically significant yield reductions in the early-vegetative and pre- and postflowering phases, the effects in the latter two stages are much larger compared with the early-vegetative phase. The impacts in both the pre- and postflowering phases are found to be very similar, which indicates that increased heat resilience in either phase could provide much-needed genetic improvements. This is particularly interesting because both public and private sorghum breeding programs have tended not to focus on heat resilience during the early reproductive period that includes panicle initiation (29) and reproductive organ development (gametogenesis) (17). The quantification of these heat-stress impacts across the various growing phases provides breeders a foothold for identifying genetic traits associated with heat resilience, which is important given the overall susceptibility of sorghum production to climate change documented here.
Another important consideration for future modifications of sorghum cultivars is the genetic potential among currently available cultivars. If the current stock of cultivars does not possess sufficient heat resilience to reduce the impacts of warming temperatures, as our results suggest, then increased efforts to strategically introduce greater genetic diversity into the breeding population would seem warranted. We find that while average yields for newly released cultivars have been increasing since the mid-1980s, thereby suggesting that breeding efforts have been yield-enhancing for commercial producers in general, there exists no detectable increase in heat resilience over this same time period—the implication being that warming temperatures could potentially offset the previous gains made by breeders. Two competing scenarios could explain this result: (i) genetic traits associated with improved heat resilience have not been fully captured through breeding efforts or (ii) programs are not fully using the wide range of genetic diversity available outside of the current breeding stock. Our results suggest that there is limited potential among currently grown cultivars, and thus more effort should be placed on expanding the scope of genetic stock within US breeding programs. This seems plausible given sorghum’s reputation as a versatile crop with a large amount of genetic diversity (30).
Historically, crop productivity has often been increased and sustained across challenging growing conditions by incorporating genetic diversity from landraces or wild accessions (31, 32). The wide range in temperatures observed in this study is representative of the growing conditions for other sorghum production regions both in the United States and globally. Therefore, we suggest that there is a need for systematic and sustained introduction of wider genetic sources into ongoing breeding programs. An alternative route to minimize future heat-stress-induced yield losses could entail the introduction of greater chilling tolerance during germination and early seedling establishment, thereby facilitating earlier planting at lower soil temperatures to escape the more extreme heat stress that is currently coinciding with sensitive growth phases. Our findings are relevant for moderate- to high-yielding nonirrigated production environments, and we do not account for potential fertilization effects of increased CO2 concentrations associated with global warming (33–38). Future studies focusing on long-term climatic responses of crops under severe stress conditions would need to exercise appropriate measures to account for increasing atmospheric CO2 and its interactions with other factors influencing yield response.
Methods
Data.
Weather data were taken from Kansas Weather Library. Daily temperature observations correspond to each field trial location. Following ref. 39, a sinusoidal distribution was fit between daily minimum and maximum temperatures to estimate exposure for each degree Celsius in 30-min increments. This approach is slightly different from that of ref. 20 as it makes use of sunrise and sunset times but generates the same measures of temperature exposure, model performance, and results (SI Appendix, Fig. S8 and Tables S8 and S9). We also collect daily precipitation, which along with the temperature exposures is summed across days up to a cumulative measure for the growing season. Sorghum harvest can sometimes be delayed for various reasons unrelated to yield, so we restrict the end of the growth season to be 30 d after observed flowering dates. This restriction wherein the physiological maturity is reached effectively increases growing season average temperatures as it removes colder days from the period of observation, and reduces cumulative precipitation as it restricts the number of days in the season (SI Appendix, Fig. S9). This purges uninformative noise from the data and improves model performance (SI Appendix, Table S8). A potential concern is that measuring weather based on flowering dates could generate biased estimates of the temperature effects if there is an omitted variable affecting both yield and flowering time. We find that this is not a concern here as the warming effects for our preferred model are very similar to an alternative model that normalizes the temperature variables by the number of days in the season (SI Appendix, Fig. S10). Sorghum yield data are from Kansas Grain Sorghum Performance Tests for the years 1985–2014, from 11 sites, each from a different county in Kansas. All yield data used are for dryland (nonirrigated, rainfed) sorghum. Some data from plots/locations/years that were exposed to severe biotic or abiotic (primarily drought) factors are missing as the trials were abandoned midseason.
Regression Models.
The data vary temporally across growing seasons and cross-sectionally across field trial locations and cultivars. Time-invariant factors such as soil quality may vary across locations. While we do not directly observe these invariant factors in the data, we can control for them using location fixed effects. We also include seed cultivar fixed effects as the mean yields will vary across this dimension. These cultivar fixed effects directly control for genetic gains over time from breeding efforts. A quadratic time trend is included to capture changes in the experimental design of the field trials over time, which could result from changes in best management practices. The trend parameters suggest an increase in yields over time at a decreasing rate; however, the parameters are not statistically significant (P > 0.10).
We used multiple regression to estimate the following statistical model:
where is log yield for cultivar i at location j in trial year t, and capture fixed effects across cultivars and locations, captures the trend component, and captures the (potentially nonlinear) effects of weather on yields. Note here that although weather is the same at location j in year t, variation in flowering dates across cultivars induces variation in weather at the location-year-cultivar level. It is likely that the error terms are heteroskedastic and spatially correlated, so we cluster SEs by year.
The preferred specification for the weather effects is given by
The first three variables provide a piecewise linear effect of temperature on yields; measures DDs between zero and the lower threshold, measures DDs between the lower and upper threshold, and measures DDs above the upper threshold. The final three variables provide a cubic effect of cumulative precipitation on yields, which improved model performance relative to the more typically used quadratic effect.
We also estimate a heterogeneous weather-effects model where the effects of the weather variables are allowed to vary across the three segmented growth stages: sowing to 35 d after sowing, 35 d after sowing to flowering, and postflowering. Indexing these by s = 1, 2, 3, the specification of the weather effects for this model is
The predicted yield impacts when the average weather variables change from the 1985–2014 average to the new values are derived as the percentage change in yield relative to baseline climate, . We simulate new values for each 1 °C increase up to 5 °C by increasing the observed daily maximum and minimum temperatures and then recalculating the appropriate weather variables. The regression estimates from SI Appendix, Table S4 are used as values for .
To investigate heterogeneous effects of high temperatures across cultivars we modify the preferred specification using the the multilevel model
where is the same as above. The varying intercepts for cultivars and locations are captured by and , and the effect of DDs above 33 °C is allowed to vary across each cultivar i through .
We also consider models that replace the piecewise linear temperature specification with measures of average, minimum, and maximum temperatures. Specifically, the average temperature model is defined as
where is the average of the observed daily temperatures, while the minimum and maximum model is defined as
where and are the average of the observed daily minimum and maximum temperatures, respectively. We find that both alternatives reduce prediction accuracy relative to the preferred model (SI Appendix, Table S8). Both models produce warming impacts similar to those of the preferred model (SI Appendix, Fig. S11).
Supplementary Material
Acknowledgments
We thank the Kansas Sorghum Grain Commission for supporting the stress physiology at Kansas State University. We are grateful to Mary Knapp and Christopher Redmond for their assistance with the weather data; Contribution 18-015-J from the Kansas Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706383114/-/DCSupplemental.
References
- 1.Maiti RK. Sorghum Science. Science Publishers; Enfield, NH: 1996. [Google Scholar]
- 2.Schlenker W, Lobell DB. Robust negative impacts of climate change on African agriculture. Environ Res Lett. 2010;5:014010. [Google Scholar]
- 3.Sultan B, et al. Robust features of future climate change impacts on sorghum yields in West Africa. Environ Res Lett. 2014;9:104006. [Google Scholar]
- 4.Liu B, et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat Clim Change. 2016;6:1130–1136. [Google Scholar]
- 5.Maikasuwa MA, Ala AL. Trend analysis of area and productivity of sorghum in Sokoto state, Nigeria, 1993-2012. Eur Sci J. 2013;9:69–75. [Google Scholar]
- 6.Tari I, Laskay G, Takacs Z, Poor P. Response of sorghum to abiotic stresses: A review. J Agron Crop Sci. 2013;199:264–274. [Google Scholar]
- 7.Tack J, Barkley A, Rife TW, Poland JA, Nalley LL. Quantifying variety-specific heat resistance and the potential for adaptation to climate change. Glob Change Biol. 2016;22:2904–2912. doi: 10.1111/gcb.13163. [DOI] [PubMed] [Google Scholar]
- 8.Prasad PVV, Boote KJ, Allen LH. Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric For Meteorol. 2006;139:237–251. [Google Scholar]
- 9.Prasad PVV, Pisipati SR, Muthava RN, Tuinstra MR. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008;48:1911–1917. [Google Scholar]
- 10.Djanaguiramana M, Prasad PVV, Murugan M, Perumal R, Reddy UK. Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environ Exp Bot. 2014;100:43–54. [Google Scholar]
- 11.Singh V, et al. Genotypic differences in effects of short episodes of high-temperature stress during reproductive development in sorghum. Crop Sci. 2016;56:1561–1572. [Google Scholar]
- 12.Prasad PVV, Djanaguiraman M, Perumal R, Ciampitti IA. Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: Sensitive stages and thresholds for temperature and duration. Front Plant Sci. 2015;6:820. doi: 10.3389/fpls.2015.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunoj VSJ, et al. Resilience of pollen and post-flowering response in diverse sorghum genotypes exposed to heat stress under field conditions. Crop Sci. 2017;57:1658–1669. [Google Scholar]
- 14.Shroyer J, Kok H, Fjell D. 1998 Seedbed preparation and planting practices. Grain sorghum production handbook (Kansas State Univ, Manhattan, KS). Available at https://www.bookstore.ksre.ksu.edu/pubs/C687.pdf.
- 15.Ciampitti IA, et al. 2017 Kansas corn management (Kansas State Univ, Manhattan, KS). Available at https://www.bookstore.ksre.ksu.edu/pubs/MF3208.pdf.
- 16.Ciampitti IA, et al. 2017 Kansas soybean management (Kansas State Univ, Manhattan, KS). Available at https://www.bookstore.ksre.ksu.edu/pubs/MF3154.pdf.
- 17.Prasad PVV, Bhemanahalli R, Jagadish SVK. Field crops and the fear of heat stress–Opportunities, challenges and future directions. Field Crops Res. 2017;200:114–121. [Google Scholar]
- 18.Lemoine R, et al. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 2013;4:272. doi: 10.3389/fpls.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultan B, et al. Assessing climate change impacts on sorghum and millet yields in the Sudanian and Sahelian savannas of West Africa. Environ Res Lett. 2013;8:014040. [Google Scholar]
- 20.Schlenker W, Roberts MJ. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proc Natl Acad Sci USA. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bheemanahalli R, et al. Temperature thresholds for spikelet sterility and associated warming impacts for sub-tropical rice. Agric For Meteorol. 2016;221:122–130. [Google Scholar]
- 22.Tack J, Barkley A, Nalley LL. Effect of warming temperatures on US wheat yields. Proc Natl Acad Sci USA. 2015;112:6931–6936. doi: 10.1073/pnas.1415181112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobell DB, et al. The shifting influence of drought and heat stress for crops in northeast Australia. Glob Change Biol. 2015;21:4115–4127. doi: 10.1111/gcb.13022. [DOI] [PubMed] [Google Scholar]
- 24.Tack J, Barkley A, Nalley LL. Estimating yield gaps with limited data: An application to United States wheat. Am J Agric Econ. 2015;97:1464–1477. [Google Scholar]
- 25.Lobell DB, Cassman KG, Field CB. Crop yield gaps: Their importance, magnitudes, and causes. Annu Rev Environ Resour. 2009;34:179–204. [Google Scholar]
- 26.Hill J, Gelman A. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge Univ Press; New York: 2007. [Google Scholar]
- 27.Singh V, et al. Sorghum genotypes differ in high temperature responses for seed set. Field Crops Res. 2015;171:32–40. [Google Scholar]
- 28.Bahuguna RN, et al. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol Plant. 2015;154:543–559. doi: 10.1111/ppl.12299. [DOI] [PubMed] [Google Scholar]
- 29.Quinones C, Mattes N, Faronilo J Sudhir-Yadav, Jagadish SVK. Drought stress reduces grain yield by altering the floral meristem development and sink size under dry-seeded rice cultivation. Crop Sci. 2017;57:1–11. [Google Scholar]
- 30.Lasky JR, et al. Genome-environment associations in sorghum landraces predict adaptive traits. Sci Adv. 2015;1:e1400218. doi: 10.1126/sciadv.1400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mickelbart MV, Hasegawa PM, Bailey-Serres J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet. 2015;16:237–251. doi: 10.1038/nrg3901. [DOI] [PubMed] [Google Scholar]
- 32.Ishimaru T, et al. A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Ann Bot (Lond) 2010;106:515–520. doi: 10.1093/aob/mcq124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 34.McGrath GM, Lobell DB. Regional disparities in the CO2 fertilization effect and implications for crop yields. Environ Res Lett. 2013;8:031001. [Google Scholar]
- 35.Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- 36.Leakey AD, Bishop KA, Ainsworth EA. A multi-biome gap in understanding of crop and ecosystem responses to elevated CO2. Curr Opin Plant Biol. 2012;15:228–236. doi: 10.1016/j.pbi.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Osborne CP. Crop yields: CO2 fertilization dries up. Nat Plants. 2016;2:16138. doi: 10.1038/nplants.2016.138. [DOI] [PubMed] [Google Scholar]
- 38.Gray SB, et al. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat Plants. 2016;2:16132. doi: 10.1038/nplants.2016.132. [DOI] [PubMed] [Google Scholar]
- 39.Cesaraccio C, Spano D, Duce P, Snyder RL. An improved model for determining degree-day values from daily temperature data. Int J Biometeorol. 2001;45:161–169. doi: 10.1007/s004840100104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.