Significance
Staphylococcus aureus secretes numerous proteins to evade our innate immune system, for example to evade opsonization and phagocytosis by neutrophils. Here we describe the discovery that S. aureus has evolved a protein, called SPIN, that specifically binds and inhibits the human myeloperoxidase enzyme (MPO). MPO is located inside the granules of neutrophils and is important in the oxidative burst against pathogens. We identify the molecular mode of action of SPIN inhibiting MPO, illustrate this with the cocrystal structure, and show that SPIN is important for bacterial survival by MPO-dependent killing. Our study shows that S. aureus fights back after it is engulfed by neutrophils, which will help our understanding of the complex nature of S. aureus infections.
Keywords: immune evasion, myeloperoxidase, neutrophil, phagocytosis, Staphylococcus aureus
Abstract
Staphylococcus aureus is highly adapted to its host and has evolved many strategies to resist opsonization and phagocytosis. Even after uptake by neutrophils, S. aureus shows resistance to killing, which suggests the presence of phagosomal immune evasion molecules. With the aid of secretome phage display, we identified a highly conserved protein that specifically binds and inhibits human myeloperoxidase (MPO), a major player in the oxidative defense of neutrophils. We have named this protein “staphylococcal peroxidase inhibitor” (SPIN). To gain insight into inhibition of MPO by SPIN, we solved the cocrystal structure of SPIN bound to a recombinant form of human MPO at 2.4-Å resolution. This structure reveals that SPIN acts as a molecular plug that prevents H2O2 substrate access to the MPO active site. In subsequent experiments, we observed that SPIN expression increases inside the neutrophil phagosome, where MPO is located, compared with outside the neutrophil. Moreover, bacteria with a deleted gene encoding SPIN showed decreased survival compared with WT bacteria after phagocytosis by neutrophils. Taken together, our results demonstrate that S. aureus secretes a unique proteinaceous MPO inhibitor to enhance survival by interfering with MPO-mediated killing.
The bacterium Staphylococcus aureus is a rising threat to human health. Thirty percent of healthy adults are colonized with this bacterium, resulting in an increased risk for infections ranging from abscesses to endocarditis (1). Neutrophils play a prominent role in fighting staphylococcal infections (2), as their intracellular granules contain numerous antimicrobial proteins and components for generating bactericidal reactive oxygen species (ROS). After S. aureus is phagocytosed, neutrophils’ azurophilic granules fuse with the phagosome and release their contents (3). The five essential components of NADPH oxidase then assemble in the phagosomal membrane and become active (4). Active NADPH oxidase produces superoxide from O2, which converts to hydrogen peroxide (H2O2) either spontaneously or by the action of superoxide dismutase. Myeloperoxidase (MPO) catalyses the reaction of H2O2 with chloride to generate hypochlorous acid (HOCl), which is a major effector in the oxidative defense of neutrophils (5). MPO also forms radicals by oxidizing a wide range of substrates, such as tyrosine, nitrite, nitric oxide, and phenols (6).
While the pathogen is taken up rapidly by phagocytes, mainly neutrophils and macrophages, not all bacteria are killed and these phagocytes can therefore act as so called “Trojan Horses” and distribute a pathogen away from the initial site of infection (7). To counteract the manifold antimicrobial defenses of neutrophils, S. aureus has evolved specific evasion molecules to inhibit intracellular killing (8). For example, the golden pigment staphyloxanthin serves as an antioxidant and is known to protect S. aureus against ROS (9). Catalase is yet another enzyme important for resistance against oxidative stress. This enzyme converts H2O2 into H2O and O2 and is considered to be a virulence factor. S. aureus also expresses an alkyl hydroperoxide reductase (ahpC) that contributes catalase-like activity. Whereas AhpC is believed to detoxify endogenously produced hydrogen peroxide, catalase appears more important for protection against external oxidative stress (10). Finally, S. aureus produces specific evasion proteins that disrupt phagosomal membranes, such as phenol-soluble modulins, hemolysin-α, and leukocidin AB (8). Together, these evasion molecules are believed to contribute to bacterial survival following phagocytosis.
Proteomic studies have shown that between 100 and 200 proteins are secreted from S. aureus, many with unknown functions (11). Consequently, known evasion molecules are likely to represent only a small fraction of the total repertoire. Therefore, we recently developed a phage display approach (12) as a nonbiased high-throughput method to screen for new potential staphylococcal immune evasion molecules. Since S. aureus can survive within the phagosome, but also because recent work suggests that SaeR/S regulated factors exist that inhibit neutrophil ROS production (13), we screened this staphylococcal phage library against several intracellular proteins of neutrophils.
Through this approach, we identified the hypothetical protein NWMN_0402 as an evasion factor. We have named this protein “staphylococcal peroxidase inhibitor” (SPIN), as it is able to bind and inhibit MPO. Here, we characterize SPIN and detail the structural basis for MPO inhibition by SPIN. We further show that the production of SPIN is up-regulated after phagocytosis of S. aureus by human neutrophils and that SPIN protects S. aureus from MPO-mediated killing.
Results
Identification of SPIN.
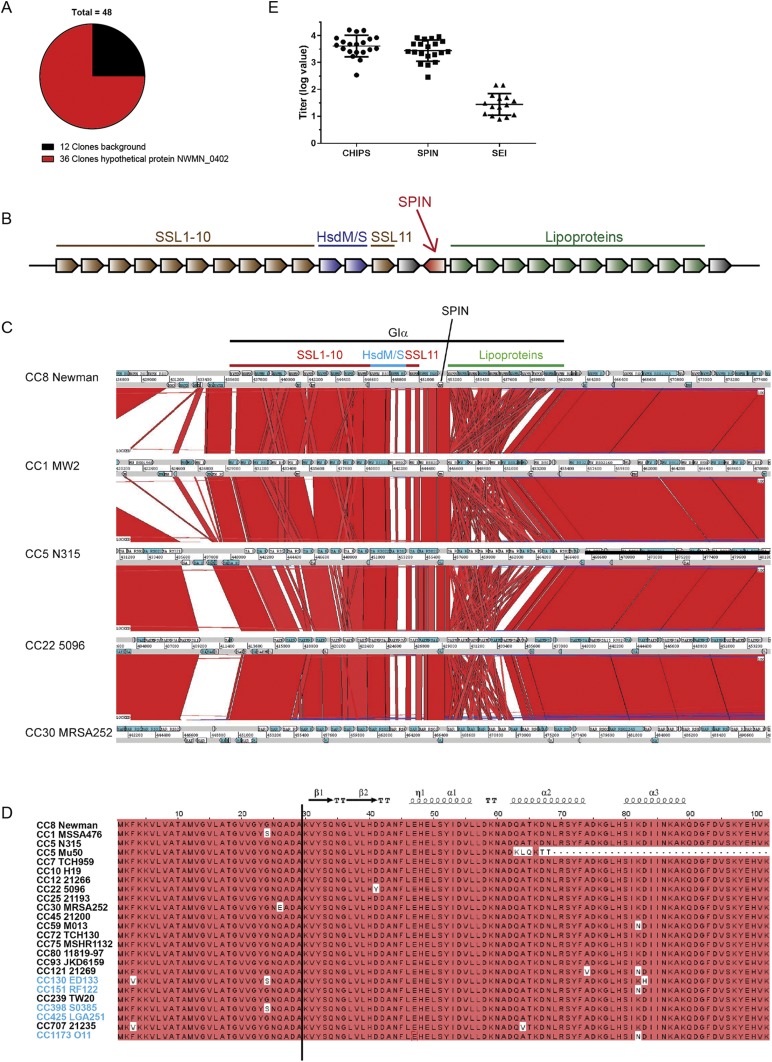
We designed a secretome phage display strategy to screen for unidentified evasion molecules that target neutrophil granule proteins, as described previously (12, 14). Isolated DNA of S. aureus strain Newman was randomly sheared and the resulting fragments were cloned in the pDJ01 phagemid vector for specific display of secretome proteins. Phages were produced upon addition of the VCSM13 helper phage. Screening was performed against total degranulate from TNF-primed, formylmethionine-leucyl-phenylalanine–stimulated neutrophils. After four rounds of selection, 48 clones were sequenced. The major hit was enriched in 36 clones and corresponded to the hypothetical ORF NWMN_0402 (accession no. BAF66674 strain Newman) (Fig. S1A). We named this protein SPIN due to its ability to inhibit MPO, as described in SPIN Binds and Inhibits MPO.
Fig. S1.
Identification of SPIN as a potential staphylococcal evasion protein. (A) Output from phages of secretome phage display after four rounds of selection against granular proteins of neutrophils showed enrichment of hypothetical ORF NWMN_0402. (B) Gene distribution of S. aureus genomic island α. The SPIN gene is located in the middle, downstream of the ssl cluster with the coding sequence oriented in the reverse direction. Proteins indicated in gray have an unknown function. (C) Genomic region of Giα (also known as νSaα) is compared between S. aureus strains Newman clonal complex (CC)8, MW2 (CC1), N315 (CC5), 5096 (CC22), and MRSA252 (CC30) using the Artemis Comparison Tool. Red blocks are indicative of homology between strains. GIα, SPIN (NWMN_0402), and the proteins encoded by genes in GIα are indicated at the top of the figure; these genes encode SSL proteins, type I restriction-modification system modification (HsdM), and specificity (HsdS) subunits and lipoproteins. (D) Amino acid sequence alignment of SPIN with signal sequence from Newman strain to 88 isolates, clustered by their clonal complex. The clonal complexes are both of human and animal origin (CC130, CC151, CC398, CC425, CC1173 are animal-associated and indicated in blue). The cleavage site that yields the mature protein is located between ADA-KV, and is indicated by a black line. Red indicates homology and the secondary structure of SPIN is indicated on top. (E) Anti-SPIN titers were determined from 20 healthy laboratory workers and compared with anti-CHIPS and anti-SEI titers by ELISA. Five donors for anti-SEI titers were below the detection limit. The values represent the logarithmic of the dilution factor that gave an OD450 of 0.2 after subtraction of the background. Each dot represents one individual in one experiment. The graph is representative of two trials.
The gene encoding SPIN, denoted as spn, is located on genomic island α, also known as νSaα. This island is found in all S. aureus genomes and contains a cluster of genes encoding known evasion molecules, most notably the staphylococcal superantigen-like (ssl) proteins (8). spn is located downstream of the ssl cluster with the coding sequence oriented in the reverse direction (Fig. S1B). The genes in νSaα are often variable and each S. aureus lineage has a unique combination of gene variants (15). However, spn is located in the conserved region of νSaα (Fig. S1C). It was present in 83 of 84 clinical strains isolated from intensive care unit patients as well as in all completed S. aureus genomes included in our analysis (15).
The amino acid sequence of SPIN is highly conserved among almost all S. aureus clonal lineages, both human- and animal-associated strains, with 92.4% of residues conserved (Fig. S1D). Only two strains of clonal complex (CC)5 lineage, Mu3 and Mu50, encoded a truncated version of SPIN, lacking 35 amino acids at the C terminus. To determine if SPIN is produced in vivo during colonization or infection of the human host, sera from 20 healthy individuals were tested for the presence of antibodies against SPIN. These sera showed substantial amounts of antibody, and elicited values comparable to CHIPS (chemotaxis inhibitory protein of S. aureus) (Fig. S1E). CHIPS is a known S. aureus immune evasion molecule (16) that yielded a mean fluorescence intensity value in a previous multiplex assay indicative of high IgG levels, whereas the superantigens (e.g., SEI) did not (17). Because SPIN is highly conserved in S. aureus strains, has a high prevalence, and is expressed in vivo, we further investigated this protein as a potential evasion molecule acting on one of the granular proteins of neutrophils.
SPIN Binds and Inhibits MPO.
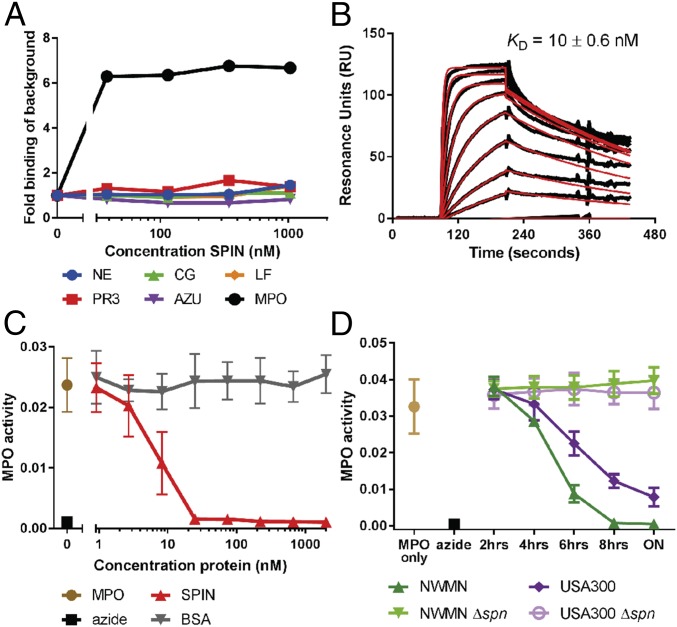
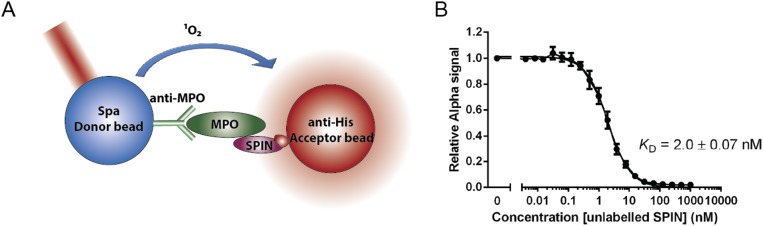
Since we identified SPIN as a potential evasion protein, recombinant SPIN was coupled to CNBr-activated Sepharose beads and used for affinity chromatography to probe neutrophil degranulate for its binding partner. Eluted proteins were visualized by silver-stained PAGE and identified by mass spectrometry. Although elastase, proteinase 3, cathepsin G, azurocidin, lactoferrin, and MPO were all identified as potential binding partners, a subsequent SPIN capture ELISA against these potential targets only showed evidence for SPIN binding to MPO (Fig. 1A). Next, we used conventional surface plasmon resonance (SPR) (Fig. 1B) as well as AlphaScreen equilibrium competitive binding assays (Fig. S2) to validate our ELISA result. Both SPR and AlphaScreen were consistent with low nanomolar affinity binding between SPIN and MPO, and yielded comparable KD values of 10 ± 0.6 nM and 2.0 ± 0.07 nM, respectively.
Fig. 1.
SPIN binds and inhibits MPO. (A) An ELISA-type binding assay of C-His SPIN to all candidate proteins. AZU, azurocidin; CG, cathepsin G; LF, lactoferrin; MPO, myeloperoxidase; NE, neutrophil elastase; PR3, proteinase 3. (B) Characterization of SPIN binding to MPO by SPR, where SPIN was injected over a surface of native MPO. (C and D) Recombinant SPIN and S. aureus supernatant inhibit MPO in a dose-dependent manner. ON is overnight culture. Bars express SD with n = 3 for B–D.
Fig. S2.
Determining KD of SPIN to MPO by AlphaScreen equilibrium competitive binding assay. An AlphaScreen equilibrium binding signal was observed by capturing MPO via antibodies to protein A donor beads and using C-His SPIN bound to anti-His acceptor beads (A). A concentration series of untagged SPIN was used to compete for MPO binding and a KD of 2 ± 0.07 nM was derived from nonlinear curve-fitting (B).
Since SPIN forms a high-affinity complex with MPO, we hypothesized that SPIN disrupts MPO function. Indeed, a colorimetric MPO activity assay demonstrated that SPIN inhibits MPO in a dose-dependent manner (Fig. 1C) with an apparent IC50 of ∼7 nM. Subsequently, we investigated whether SPIN was secreted as a functional protein in staphylococcal culture supernatant. Both WT strains Newman and USA300 accumulated increasing levels of MPO inhibitory activity in conditioned culture medium over time. Moreover, we also found that strain Newman supernatant showed a higher degree of inhibition compared with USA300. To determine whether this MPO inhibitory activity was caused by the SPIN protein, we generated isogenic KOs of the SPIN gene in both Newman and USA300 backgrounds. Conditioned culture medium from both KOs was completely devoid of MPO inhibitory activity (Fig. 1D).
We also performed a titration analysis on samples of conditioned culture supernatant from WT S. aureus strains Newman and USA300 (Fig. S3 A and B). The IC50 of these samples was 0.12% and 0.30% supernatant for Newman and USA300, respectively. By comparing this to the IC50 from recombinant SPIN (Fig. 1C), the amount of SPIN present in the overnight supernatant could be estimated. This appeared to be 5.9 µM for Newman and 2.4 µM for USA300. Interestingly, the supernatant from the Mu50 strain, which contains a truncated version of spn (Fig. S1D), showed no detectable MPO inhibitory activity in its supernatant (Fig. S3C), indicating a nonfunctional SPIN-variant. The slight inhibitory effect observed at low dilutions of the conditioned media is due to the inhibitory color of Todd Hewitt Broth (THB) itself (Fig. S3C).
Fig. S3.
MPO activity from overnight culture supernatant of different S. aureus strains. (A and B) Supernatant from USA300 (A) or Newman (B) overnight culture was diluted and preincubated with native MPO. WT strains show inhibition of MPO, whereas the KO strains (Δspn) do not. (C) Truncated SPIN of MU50 doesn’t inhibit MPO. MPO activity from supernatant of MU50 is on the THB control line. The lines of THB, Mu50, and both KOs show background inhibition at high concentration due to the color of the undiluted THB. Sodium azide is used as a positive control for complete MPO inhibition. Bars express SD with n = 3.
Structural Basis for MPO Inhibition by SPIN.
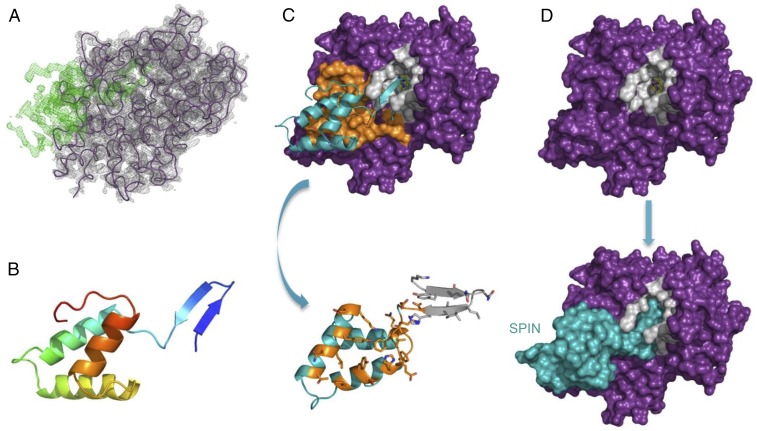
SPIN shares no significant sequence homology to other characterized proteins. Thus, we sought structural information to better understand the physical basis for SPIN binding to and inhibition of MPO. We succeeded in crystallizing a complex of SPIN bound to a recombinant form of MPO, collected X-ray diffraction data to 2.4-Å Bragg-limiting resolution, and solved the structure by molecular replacement (Table S1). Electron density maps calculated after the initial placement of a model for human MPO revealed a strong, contiguous feature that could not be explained by the presence of MPO (Fig. 2A). A model of the SPIN polypeptide was completed through a combination of automated chain tracing and manual building, and the structure of the complex was refined to final Rwork/Rfree values of 18.4% and 24.1%, respectively (Table S1). The SPIN protein consists of the common α-helix bundle fold found in other staphylococcal evasion proteins, with a unique addition of a prominent β-hairpin at its N terminus (Fig. 2B). As there are no biochemical features that would promote stability of this β-hairpin in the absence of ligand (e.g., a disulfide bond), we suspect that this region of SPIN is disordered in the unbound state.
Table S1.
Data collection and refinement statistics (molecular replacement)
| Data collection and refinement | SPIN/MPO |
| Data collection | |
| Space group | C 1 2 1 |
| Cell dimensions | |
| a, b, c, Å | 129.03, 92.94, 80.39 |
| α,β,γ, ° | 90, 120.14, 90 |
| Resolution, Å | 43.34–2.40 (2.49–2.40) |
| Rpim | 0.056 (0.357) |
| I/σI | 14.1 (2.0) |
| Completeness, % | 99.8 (98.3) |
| Redundancy | 7.5 (6.4) |
| Refinement | |
| Resolution, Å | 43.34–2.40 |
| No. reflections | 31,961 |
| Rwork/Rfree | 18.4/24.1 |
| No. atoms | |
| Protein | 5,171 |
| Ligand/ion | 85 |
| Water | 169 |
| B-factors | |
| Protein | 49.59 |
| Ligand/ion | 39.34 |
| Water | 45.03 |
| Rmsd | |
| Bond lengths, Å | 0.004 |
| Bond angles, ° | 0.605 |
Fig. 2.
Structural basis for inhibition of MPO by SPIN. (A) Electron density maps (2.4-Å resolution) calculated after initial placement of an MPO model (Rfree = 28%). 2Fo–Fc density contoured at 1.5 σ (gray cage) is shown for the MPO model (purple wire), and Fo–Fc density contoured at 3.0 σ (green cage) is attributable to SPIN. (B) Structure of the SPIN polypeptide depicted as a ribbon diagram. N terminus of the protein is indicated in indigo, the C terminus in red. The orientation of SPIN has been maintained across A and B for clarity. (C) Representation of the final model for the SPIN/MPO complex. (Upper) SPIN is shown as a cyan ribbon while MPO is depicted as a molecular surface. Residues comprising the first SPIN binding interface are colored orange, and residues lining the MPO active site channel are colored gray. (Lower) SPIN is drawn with the residues found at the first interface colored orange, and residues interacting with the MPO active site channel are colored gray. The sidechains of interfacing residues are depicted in ball-and-stick convention. Note the orientation of SPIN in the Lower panel is flipped 180° in the viewing plane relative to the Upper panel. (D) Surface representations provide insight into the physical basis for MPO inhibition by SPIN. (Upper) MPO is shown as a molecular surface with the residues lining the active site channel in gray. (Lower) SPIN is drawn as a cyan molecular surface according to its position in the final model of the SPIN/MPO complex. The location of the reactive site heme from native human MPO (33) is shown as a colored ball-and-stick. Note that the SPIN β-hairpin appears to completely occlude access of small molecules to the reactive site heme.
The interaction between SPIN and MPO buries 1,633 Å2 from the bacterial inhibitor and is comprised of two conceptually distinct interfaces (Fig. 2C). The first interface lies at the side of the peroxidase active site entrance, and accounts for 726 Å2 (∼45%) buried in the complex. Sixteen of the 20 SPIN residues associated with this site are found on either the first or second helix of the inhibitor. The sidechains of five such residues form either hydrogen bonds or salt-bridges with groups donated by MPO, and likely help impart specificity for this enzyme. The second interface accounts for 907 Å2 (∼55%) of the area buried in the complex, and is derived exclusively from residues within the SPIN β-hairpin. All positions in this region of SPIN form extensive contacts with residues that line the MPO active site channel. We find the Gly residue in the β-hairpin turn particularly noteworthy, since it not only allows the second strand to complete the hairpin but also dictates the extent to which this hairpin can penetrate the MPO active site. The overall effect of this structural feature allows SPIN to act as a molecular plug and thereby prevent access of the H2O2 substrate to the reactive heme located deep within the MPO active site channel (Fig. 2D).
Production of SPIN by S. aureus Is Up-Regulated After Phagocytosis by Neutrophils.
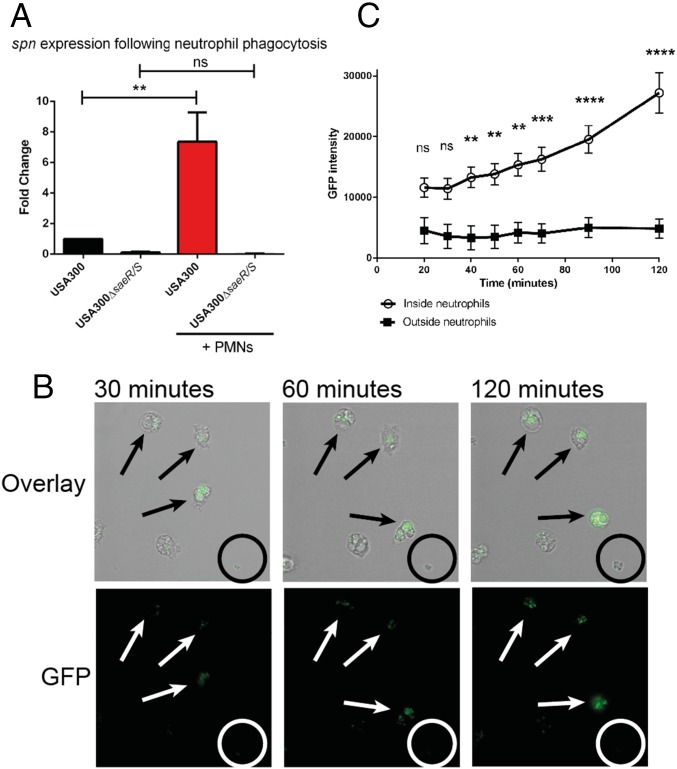
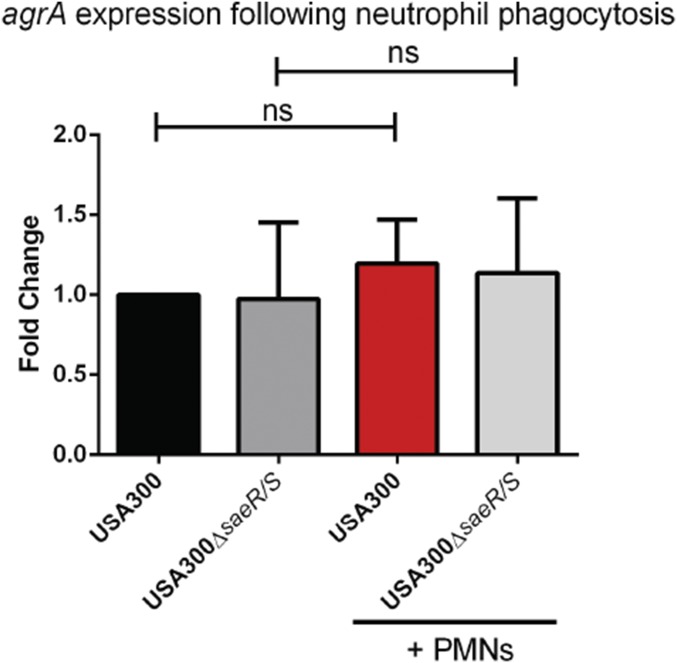
To better understand the biological context of SPIN production by S. aureus, we compared SPIN gene expression and promoter activity in bacteria that had been phagocytosed and not phagocytosed by neutrophils. We used strain USA300, since SPIN is constitutively expressed at low levels by strain Newman. SPIN gene expression over time was monitored using qPCR, either with or without phagocytosis. We observed a highly significant, eightfold increase in spn expression following phagocytosis compared with samples without neutrophils (Fig. 3A). This outcome is comparable to our earlier microarray study, where enhanced spn expression was detected after exposure to azurophilic granule proteins (18). Importantly, we did not detect a similar change in spn expression when we used bacteria lacking SaeR/S, which regulates production of multiple evasion factors (19). As a control, we found no significant change in agrA expression under any of these conditions (20) (Fig. S4).
Fig. 3.
Production of SPIN is up-regulated after phagocytosis inside neutrophils. (A) Analysis of spn expression following exposure to human neutrophils. Bars express SD with n = 3. Statistical significance was determined using one-way ANOVA. (B) Time-lapse analysis of SPIN expression shown as GFP fluorescence promoter-reporter USA300 in a fibrin-thrombin matrix gel. An overlay of bright-field and GFP (Upper) and GFP alone (Lower) is shown. (Magnification: 40×.) The time indicates the duration after start of phagocytosis. Arrows indicate bacteria inside neutrophils and the circle indicates a colony of bacteria growing outside neutrophils. One representative experiment is shown from three independent experiments. (C) Quantification of total GFP signal from neutrophil-resident or free bacteria. Three different experiments with 44 neutrophils and 26 colonies growing outside neutrophils were analyzed. Bars express SEM. Significance was determined by two-way ANOVA with Bonferroni posttest correction for multiple comparison. **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; ns, not significant.
Fig. S4.
Analysis of agrA expression following exposure to human neutrophils. Bars express SD with n = 3. Statistical significance was determined using one-way ANOVA; ns, not significant.
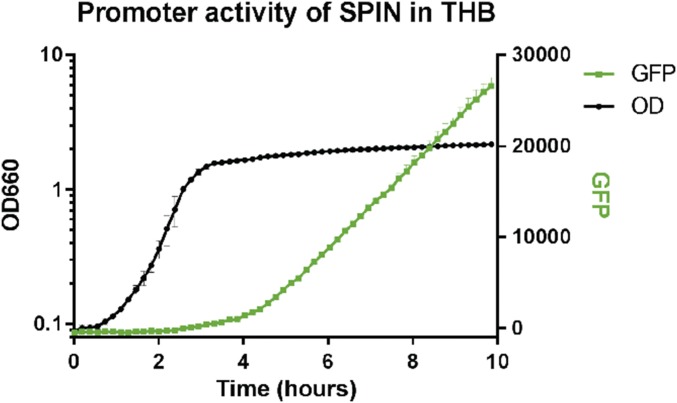
Next, we used GFP promoter-reporter USA300 bacteria in a fibrin-thrombin matrix gel to immobilize cells. Time-lapse imaging of the bacteria revealed up-regulation of the spn promoter-driven GFP signal following phagocytosis by neutrophils (arrows, Fig. 3B). In contrast, the spn promoter appeared inactive in those bacteria that had not been phagocytosed (circles, Fig. 3B); this observation correlates with spn promoter activity for THB-grown bacteria, which showed activity only after 2 h of growth (Fig. S5). We further quantified the total GFP signal for either free or neutrophil-resident bacteria for 2 h, beginning at 20 min. We found that GFP intensity increased more than twofold over time for phagocytosed, but not free-living bacteria (Fig. 3C).
Fig. S5.
Promoter expression of SPIN after 2 h in THB culture. Bacteria were grown overnight in THB where GFP fluorescence and absorbance at OD660 was both measured in 10-min intervals, GFP signal was subtracted from the background of USA300 WT strain. The promoter of SPIN is activated after 2 h, when bacteria are within the late exponential phase. Data are representative of three independent experiments with duplicate measurements.
Production of SPIN Promotes Survival Against Neutrophil-Derived ROS.
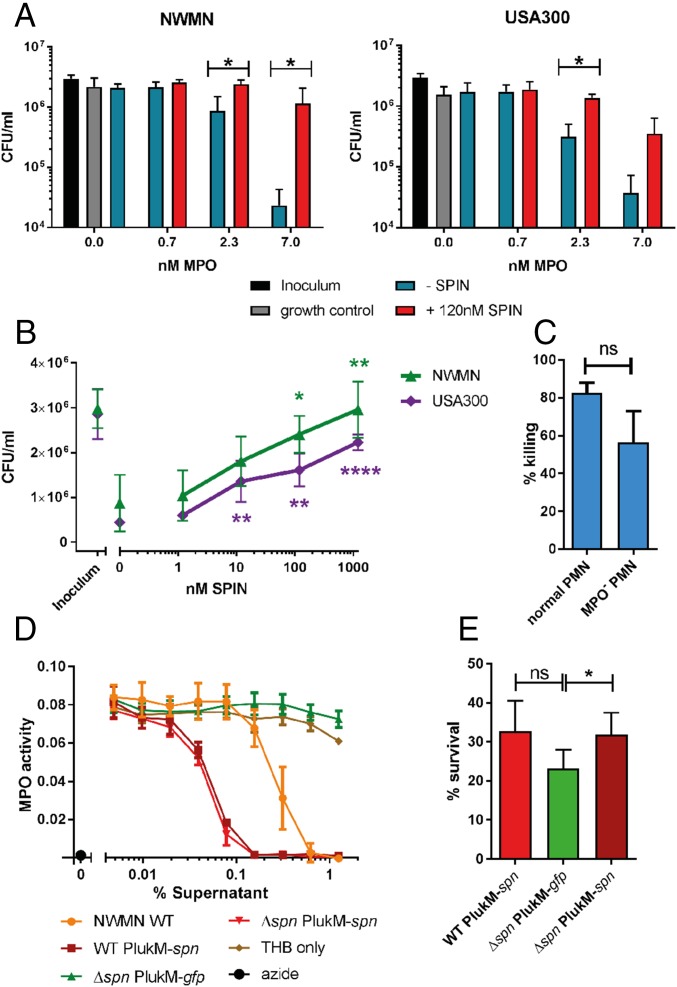
Since SPIN inhibits MPO and is up-regulated following phagocytosis, we next investigated the relevance of SPIN to S. aureus evasion of neutrophil killing mechanisms. First we used an artificial system that mimics phagosomal ROS production using glucose oxidase (GO), which forms H2O2 in the presence of glucose. We evaluated bacterial survival after the addition of MPO and NaCl, which generates the bactericidal reagent HOCl (Fig. 4 A and B). Excessive quantities of GO result in bactericidal concentrations of H2O2, which makes it impossible to evaluate MPO-mediated killing. In limiting concentrations of GO, increasing MPO concentrations led to killing of S. aureus Newman (Fig. 4A, Left) and USA300 (Fig. 4A, Right). Addition of SPIN restored the survival of bacteria (Fig. 4A). Moreover, adding increasing concentrations of SPIN (Fig. 4B) resulted in a dose-dependent rescue from HOCl killing for both Newman and USA300. This demonstrates clearly that the presence of SPIN is important for protection from MPO-generated HOCl.
Fig. 4.
SPIN is important for evasion of MPO-dependent neutrophil killing. (A) S. aureus strains Newman (Left) and USA300 (Right) were killed in a dose-dependent manner by adding 0.7–7.0 nM MPO in a coupled glucose oxidase-MPO system. Addition of 120 nM SPIN prevented ROS killing. Statistical significance was determined by two-way ANOVA with Bonferroni posttest correction for multiple comparison. (B) SPIN provides dose-dependent protection from HOCl-mediated killing for strains Newman and USA300 relative to 0 nM SPIN. Statistical significance was determined by one-way ANOVA with Bonferroni posttest correction for multiple comparison. (C) Differential bacterial survival of Newman WT from neutrophils with active or membrane-permeable AZM198-inactivated MPO. (D) Supernatants of overnight culture of strains WT PlukM-spn, Δspn PlukM-spn, Δspn PlukM-gfp were diluted, tested for MPO activity, and compared with Newman WT. Bars express SD with n = 3 (A–D). (E) Effect of S. aureus survival from isolated neutrophils after 60 min of phagocytosis. Statistical analysis determined by paired Student t test for C and E. Bars express SEM with n = 6. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001; ns, not significant.
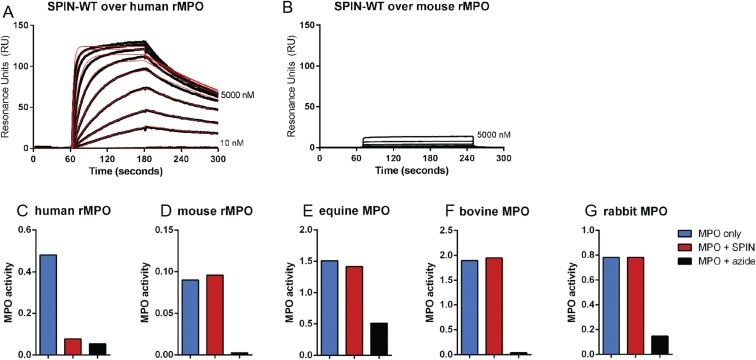
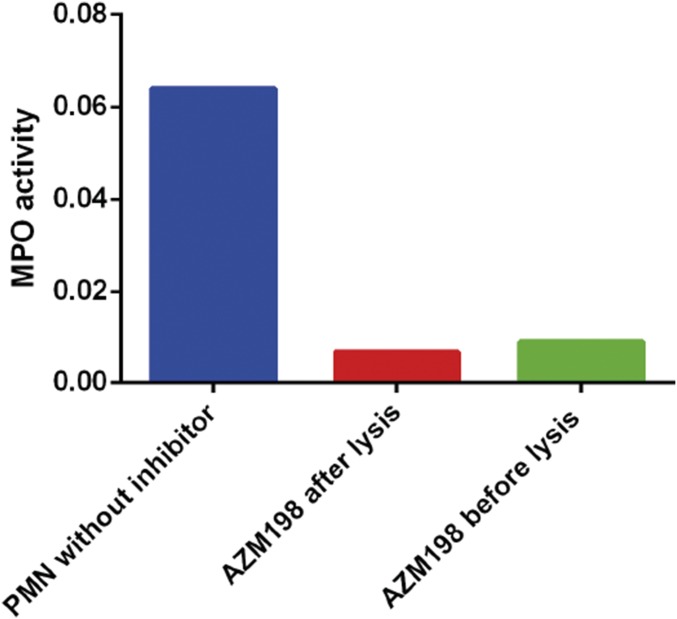
SPIN is neither able to bind nor inhibit mouse, horse, cow, or rabbit MPOs, hampering the development of an in vivo animal model system (Fig. S6). We therefore set out to investigate how the presence or absence of the SPIN gene influences S. aureus survival following phagocytosis by human neutrophils. First, the contribution of MPO to staphylococcal killing by neutrophils was investigated using the membrane-permeable MPO inhibitor AZM198 (21). Incubation of intact neutrophils with this inhibitor before washing and lysis was equally as effective as direct treatment of lysates, indicating that AZM198 is membrane-permeable and completely inhibits MPO (Fig. S7). We quantified bacterial killing by neutrophils with and without AZM198 pretreatment, and observed a difference of 26% between neutrophils with active and inactive MPO. This illustrates the modest extent of MPO-dependent staphylococcal killing by neutrophils in our experiments (Fig. 4C).
Fig. S6.
SPIN does not inhibit murine, bovine, equine, or rabbit MPO. (A and B) Direct binding of SPIN to human recombinant MPO (rMPO) (A) and mouse rMPO (B) determined by SPR. SPIN is able to bind to human rMPO, but not to mouse rMPO. (C) SPIN inhibits human rMPO. “MPO only” shows MPO activity; adding 1 µg/mL SPIN inhibits the activity of MPO similar to azide control. (D) SPIN cannot inhibit mouse rMPO. Adding 50 µg/mL SPIN does not change the activity. Azide is able to completely inhibit the activity of the mouse rMPO. (E–G) SPIN cannot inhibit bovine, equine, and rabbit MPO. Neutrophils from cow, horse, and rabbit were lysed and incubated with 10 µg/mL SPIN (cow and horse) or 9 µg/mL SPIN (rabbit).
Fig. S7.
MPO inhibitor AZM198 is able to cross neutrophil membrane and inhibit intracellular MPO. Polymorphonuclear (PMN) lysate without the inhibitor shows MPO activity. PMNs incubated with 10 µM inhibitor for 1 h at room temperature, washed, and then lysed, are devoid of MPO activity. The activity was comparable to samples which were incubated with the inhibitor after lysis. Thus, the MPO inhibitor AZM198 crosses the neutrophil membrane and is suitable to use for bactericidal assays to test the MPO-dependent killing of bacteria.
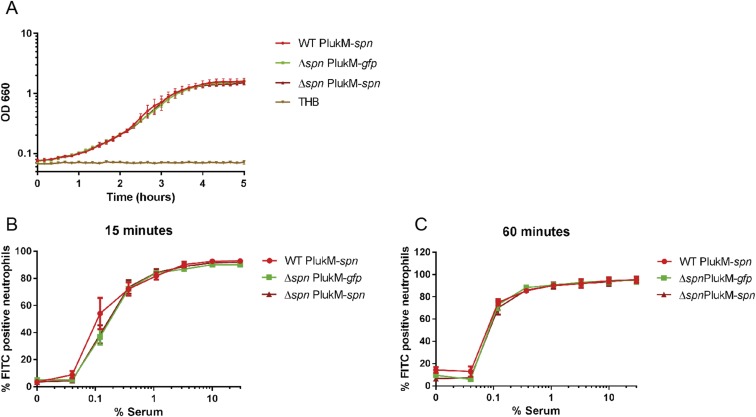
Given the contribution of MPO in killing of phagocytosed S. aureus, we used the strong lukM promoter (22) and used the Newman strain to increase intraphagosomal SPIN production (WT PlukM-spn), thereby ensuring maximal levels of MPO inhibition in our bactericidal assays (Fig. 4D). We used the same plasmid to complement the isogenic spn KO (Δspn PlukM-spn) and prepared a strain of the spn KO expressing GFP from the same plasmid (PlukM-gfp) to serve as a negative control. Supernatants from overnight cultures of these strains were compared with Newman WT for their ability to inhibit MPO (Fig. 4D). As predicted, the amount of SPIN produced in the WT PlukM-spn and Δspn PlukM-spn was increased approximately fivefold relative to Newman WT, as judged by IC50 values. Importantly, these strains grew with similar kinetics in THB (Fig. S8A) and showed comparable phagocytosis by neutrophils at both 15- and 60-min time points (Fig. S8 B and C). Therefore, we used these three strains to detect differences in bacterial survival at 60 min following phagocytosis (Fig. 4E). Indeed, we observed a spn-dependent, significant difference in killing between the negative control strain Δspn and the complemented strain that overexpressed spn. Although the difference between the Δspn strain and the overexpressing WT strain was not statistically significant, it nevertheless showed a trend towards increased survival similar to the complemented strain. Taken together, these data show that SPIN is an important component to S. aureus survival of the oxidative killing mechanisms deployed within the neutrophil phagosome.
Fig. S8.
No difference in growth or phagocytosis between mutant strains WT PlukM-spn, Δspn PlukM-gfp, and Δspn PlukM-spn. (A) Newman mutants show growth with similar kinetics in THB. Absorbance at OD660 was measured every 10 min. (B and C) No difference in phagocytosis of WT PlukM-spn, Δspn PlukM-gfp, and Δspn PlukM-spn by neutrophils. The three Newman strains were labeled with FITC, washed, and treated with indicated dilutions of NHS. The percentage FITC-positive neutrophils was measured after 15 or 60 min of phagocytosis by FACS. All strains were phagocytosed by neutrophils at comparable levels. Bars express SD with n = 3 for A–C.
Discussion
Here we describe SPIN as a component of the S. aureus immune evasion arsenal that impairs bacterial killing by neutrophils. The phagocytosed bacteria must confront numerous antibacterial systems within the phagosome. Considering this as a potentially important site for execution of an immune evasion strategy, we set out to identify any previously unknown S. aureus proteins that inhibit components within neutrophil granules. By using an innovative phage-display approach that is limited to S. aureus secreted proteins (Fig. S1), we discovered that SPIN binds MPO with low nanomolar affinity and inhibits its activity (Fig. 1). Thus, together with the recently characterized neutrophil serine protease inhibitors, Eap, EapH1, and EapH2 (23), SPIN constitutes part of an elaborate staphylococcal innate immune evasion program that specifically targets the antibacterial enzymes stored within the azurophilic granules of neutrophils.
From experiments using promoter-GFP reporter bacteria, we observed that the promoter of spn is stimulated following uptake of S. aureus by neutrophils compared with bacteria outside neutrophils or in the first 2 h of growth in THB (Fig. 3 B and C and Fig. S5). Thus, SPIN is specifically up-regulated intracellularly, where MPO is located. A similar result was also observed when looking at mRNA levels (Fig. 3A), where the production of SPIN was increased over eightfold 30 min after phagocytosis. Moreover, there was no up-regulation observed when bacteria lacking the SaeR/S two-component regulatory system were used. The SaeR/S system plays an important role in neutrophil evasion (19) and regulates factors that reduce neutrophil ROS (13). SaeR/S is up-regulated comparably to spn (≈3- to 10-fold 30 min after phagocytosis) (20) and the promoter of SPIN contains the binding consensus sequence of SaeR (24). At least two of the 11 ssl genes (ssl5 and ssl8) are regulated via the Sae system (25) and are located directly upstream of the SPIN gene. Thus, we propose that SPIN is largely regulated via the SaeR/S regulon and likely contributes to the SaeR/S-dependent reduction of neutrophil ROS (13).
By using a simplified system consisting of an enzymatic H2O2-generating system and Cl− as a substrate for MPO, we found that SPIN had a clear dose-dependent and statistically significant effect on S. aureus survival (Fig. 4 A and B). However, in the complex and necessarily redundant process of bacterial killing within neutrophils, a multitude of oxygen radicals, proteases, and other enzymes must work in concert to ensure effective elimination of S. aureus cells. Although the contribution of MPO and its main product, HOCl, to S. aureus killing has been studied since the early 1970s (5), we and others believe this process remains incompletely understood (26). For example, it was previously believed that MPO is the main component for killing S. aureus, because neutrophils from MPO-deficient patients showed a log difference in bacterial survival after phagocytosis (5). Using the potent and cell-permeable MPO inhibitor, AZM198, we found that while untreated neutrophils kill 82 ± 5% of bacteria, MPO-depleted neutrophils are still able to kill 56 ± 16% (Fig. 4C). Thus, under the specific test conditions in our assays, roughly one-fourth of the staphylococcal killing is due to MPO. Nevertheless, when a strain of S. aureus overexpressing spn was compared with a spn-deleted counterpart (22) (Fig. 4D), we observed a statistically significant difference in bacterial survival dependent on SPIN (Fig. 4E). This not only confirms the relevance of SPIN-mediated inhibition of MPO in the physiological context, it also provides a starting point for reanalysis of previous studies where the presence of SPIN may have adversely affected data interpretation.
SPIN consists of a three α-helix bundle fold, which is commonly found among S. aureus innate immune evasion proteins, including Efb, Ecb (also called Ehp), Sbi, and SCINs (27). The triple-helix motif is a thermodynamically stable structure which can accommodate high sequence diversity and adapt to binding a wide variety of targets (28). However, this simple structural motif is typically modified by an N-terminal extension that imparts specific function to the protein in question (27). Indeed, this appears to be the case for SPIN as well, as is suggested by the cocrystal structure of SPIN/MPO (Fig. 2). To our knowledge, SPIN is unique as an example of a pathogen-derived protein whose role is to specifically bind and inhibit MPO. SPIN is not the only known inhibitor of MPO, however, as it has previously been shown that the host serum protein ceruloplasmin (CP) binds MPO with a KD of ∼30 nM (29) and also blocks MPO activity (30). Examination of the crystal structure of CP bound to MPO (31) indicates that the mechanism through which CP inhibits MPO is conceptually distinct from that of SPIN. Indeed, even though the MPO active site appears to remain partly solvent accessible in the CP/MPO complex, CP attenuates MPO activity by disturbing essential transitions between the redox states of the peroxidase catalytic heme (32). In contrast, our structure of SPIN/MPO shows that SPIN forms a molecular plug that is likely to completely occlude access of substrates to the MPO active site (Fig. 2). Separately, while CP and SPIN share partial overlap in their binding sites on MPO and recognize their target with similar affinities, they display no obvious homology to one another at the sequence or structural levels. This suggests that the MPO-inhibitory capacity of SPIN has arisen through independent evolutionary processes from that of CP.
In conclusion, we identified an evasion protein called SPIN which is able to bind and inhibit human MPO. By solving the cocrystal structure of SPIN/MPO, we demonstrate the molecular mechanism of inhibition, via insertion of an N-terminal hairpin into the active site of MPO to block substrate access. We further show the expression of SPIN is up-regulated inside the neutrophil, where MPO is located, and that its presence helps in evading MPO-mediated killing. Various proteins secreted by S. aureus are known to be human-specific, which leads to restrictions in investigating this pathogen in animal models (8, 16). This seems to account for SPIN as well since it inhibits only human MPO and not MPO of other animals we have tested (Fig. S6). By adding this evasion molecule to the arsenal of previously identified evasion molecules, we can better understand the elaborate mechanism by which S. aureus evades killing through a multifaceted strategy that acts within the phagosomal compartment.
Materials and Methods
Bacterial Strains and Constructs.
The bacterial strains and constructs used in this study are described in detail in SI Materials and Methods.
Identification, Expression, and Purification of SPIN.
SPIN was subsequently produced in Escherichia coli and purified according to procedures described in SI Materials and Methods. Primers used in this study are listed in Table S2.
Table S2.
Primers used in this study
| Primer | Sequence |
| spn_NotI_stopcodon_fwd | 5′-ATATGCGGCCGCTTATTTTACGTGTTCATATTTTG-3′ |
| spn_NotI_fwd | 5′-ATATGCGGCCGCTTTTACGTGTTCATATTTTG-3′ |
| spn_enterokinase_BamHI_rvs | 5′-CGGGATCCGACGATGACGACAAGAAAGTTTATTCTCAAAATGGAC-3′ |
| spn_BamHI_rvs | 5′-CGGGATCCAAAGTTTATTCTCAAAATGGAC-3′ |
| PSPIN_XbaI_fwd | 5′-ATCGTCTAGATTCTCTTTTACGTTACCAAAATATACATATAC-3′ |
| PSPIN_KpnI_rvs | 5′-ATTAAGGTACCTAACTCCATCATAACACTGAATATTAAGAAAAT-3′ |
| KO_Gibson_EcoRI_UP_fwd | 5′-GAGGCCCTTTCGTCTTCAAGAATTCCCTACAGAACTGCCTTTCAG-3′ |
| KO_Gibson_KpnI_DN_rvs | 5′-GACTCTAGAGGATCCCCGGGTACCAAAAGATGGACATCACCAAG-3′ |
| KO_UP_rvs | 5′-GCATTATTGAATATCTATCACTCCTCTAAAAATTG-3′ |
| KO_DN_fwd | 5′-AGAGGAGTGATAGATATTCAATAATGCTTTGTAAC-3′ |
| pLukM_spn_rvs | 5′-GCTACTAAAACCTTTTTAAATTTCATAGTTTCACTTTCTTTCTCTTTATA-3′ |
| pLukM_spn_fwd | 5′-TATAAAGAGAAAGAAAGTGAAACTATGAAATTTAAAAAGGTTTTAGTAGC-3′ |
| pCM29_pLukM_fwd | 5′-CATGCCTGCAGGTCGACTCTAGAAAACGCGCAGTTAATAAAAAG-3′ |
| spn_pCM29_rvs | 5′-GAAACAGCTATGACATGATTACGAATTCTTATTTTACGTGTTCATATTTTG-3′ |
| spn_qPCR_fwd | 5′-GATGATGCAAACTTCTTAGAACATGAATTAAGT-3′ |
| spn_qPCR_rvs | 5′-GTCCTTTATCGGCGAAGTATGATCT-3′ |
| spn_qPCR_probe | 5′-CTTGGTCAGCATTCTT-3′ |
| agrA_qPCR_fwd | 5′-GTTACGAGTCACAGTGAACTTACCT-3′ |
| agrA_qPCR_rvs | 5′-CGAGTTCTTAATTCAGCTGGATCATCT-3′ |
| agrA_qPCR_probe | 5′-CATCGCTGCAACTTT-3′ |
| gyrB_qPCR_fwd | 5′-CGCACGTACAGTGGTTGAAAA-3′ |
| gyrB_qPCR_rvs | 5′-CGTGTTACTTCACGCGCTT-3′ |
| gyrB_qPCR_probe | 5′-ACGTGCCGCCATAATA-3′ |
Restriction sites are underlined.
Binding Studies.
Detailed procedures about ELISAs, SPR, and the AlphaScreen assay are described in SI Materials and Methods.
MPO Activity Assay.
SPIN activity was determined by measuring the MPO activity via the redox indicator o-Dianisidine. For detailed procedures, see SI Materials and Methods.
Crystallization, Structure Determination, Refinement, and Analysis.
A description of crystal cell constants, diffraction data quality, and properties of the final model for the SPIN/MPO complex can be found in Table S1. See SI Materials and Methods for detailed information about the SPIN/MPO crystal structure.
Analysis of spn and agrA Expression Following Exposure to Human Neutrophils.
Gene expression was assessed using TaqMan RNA-to-CT 1-Step Kit (Life Technologies). Informed consent for blood draw was obtained from all subjects, in accordance with the Declaration of Helsinki. Approval from the medical ethics committee of the University Medical Center Utrecht was attained (METC-protocol 07–125/C approved on 1 March 2010). For detailed procedures, see SI Materials and Methods.
Time-Lapse Microscopy and Phagocytosis Assay.
Detailed information about time-lapse microscopy of promoter reporter USA300 bacteria and phagocytosis is described in SI Materials and Methods.
Bactericidal Assays.
Bactericidal assays with MPO and GO or neutrophils is described in detail in SI Materials and Methods.
Graphical and Statistical Analyses.
MPO activity analyses and statistical analyses were performed with Graphpad Prism v6. Statistical significance was calculated using ANOVA and Student’s t test.
SI Materials and Methods
Bacterial Strains and Constructs.
In this study the Staphylococcus aureus strains USA300, MU50 (34), and Newman (provided by T. Foster, Dublin) were used. The genomes of 88 isolates representing 25 clonal complexes were analyzed for the presence of the SPIN gene (15) in Jalview (35). The presence of the SPIN gene from 84 S. aureus clinical strains isolated from University Medical Center Utrecht patients was checked by PCR. The Artemis Comparison Tool was used to compare the genomic region GIα (36). The KO of spn in the Newman strain was generated by cloning ∼1,000-bp upstream and ∼1,000-bp downstream of the gene into the temperature-sensitive vector pJB38, as described previously (37). Deletion of the SPIN gene in the KO was confirmed by sequencing. The promoter reporter construct was modified from pCM29 vector (38) where the sarA P1 promoter was changed to the spn promoter region and this vector was electroporated into USA300 strain. The superproducing SPIN strain was generated by fusing the promoter region of lukM (22) upstream of spn in pCM29 vector. This vector was electroporated in Newman WT and for complementation in the Δspn Newman. To compare the superproducing SPIN strain and the superproducing complemented strain, the same vector was electroporated in Δspn Newman, where the spn-ORF was replaced by GFP, resulting in the strain Δspn PlukM-gfp.
Identification, Expression, and Purification of SPIN.
SPIN was identified by using a secretome phage display. The library used was identical to the previously described (12) staphylococcal library. Briefly, isolated DNA from S. aureus strain Newman was randomly sheared into fragments ranging 0.3–3 kb in size. These fragments were cloned into the pDJ01 phagemid vector for specific display of secreted proteins. Constructs were transformed into TG1 Escherichia coli yielding 7.2 × 107 clones. The VCSM13 helper phage was used to create a S. aureus secretome phage library. This library was used to identify S. aureus proteins interacting with neutrophil degranulate. Panning was performed on coated total neutrophil degranulate obtained by stimulating TNF-α (10 ng/mL) and cytochalasin B (5 µg/mL) -treated neutrophils (3 × 107 cells/mL) at 37 °C with 1 µM formyl-methionyl-leucyl phenylalanine, as described previously (39). After four rounds of selection, 48 colonies were sequenced and three different phage clones containing SPIN were identified in 36 colonies. The primary phage-identified ORF (hypothetical protein NWMN_0402) was cloned and expressed (see below) and 2.5 mg of C-His SPIN was coupled to 1 mL of CNBr-activated Sepharose 4B (GE Healthcare) according to the manufacturer’s instructions, with a couplings percentage of 56% and loaded on an empty Tricorn 5/50 column (GE Healthcare). Neutrophil degranulate was treated with 1 mM EDTA (Merck) and 5 mM DFP (Sigma-Aldrich) to inhibit metalloproteases and serine proteases, respectively. This degranulate was subsequently loaded on the SPIN column, washed with PBS, and eluted with 0.1 M glycine buffer pH 2.7 (Serva) on an AKTA Explorer (GE Healthcare). The protein content of complete degranulate and eluate after affinity chromatography was analyzed by SDS/PAGE and silver stain. A protein species eluted from the SPIN-column was cut from the SDS/PAGE gel and analyzed by MALDI MS/MS (Alphalyse).
The SPIN gene, without the signal sequence (cleavage site between ADA-KV), of S. aureus strain Newman (hypothetical protein NWMN_0402) was used to make recombinant SPIN. PCR products were amplified using Phusion polymerase (Thermo Scientific) and ligated with NotI and BamHI restriction sites in the modified expression vector pRSETB (Invitrogen Life Technologies), containing a cleavable N-terminal 6xHis tag (N-His SPIN) or a noncleavable C-terminal 6xHis tag (C-His SPIN), leaving three alanines between SPIN and the C-His tag. Expression was performed in E. coli Rosetta-gami(DE3)pLysS (Novagen; Merck Biosciences). The bacterial pellets were lysed with 10 µg/mL lysozyme and three freeze–thaw sonication cycles in 20 mM sodium phosphate (pH 7.8). After centrifugation, the His-tagged proteins were purified using nickel-affinity chromatography (HiTrap chelating, HP; GE Healthcare) with an imidazole gradient (10–250 mM; Sigma-Aldrich). The cleavable N-His SPIN was cleaved with enterokinase (Invitrogen) and subsequently untagged SPIN was obtained after a second nickel-column passage. Purified C-His SPIN and untagged SPIN were stored in PBS at −20 °C.
Binding Studies.
ELISAs were performed to show binding of C-His SPIN to MPO (Elastin Products), neutrophil elastase, proteinase 3 (Elastin Products), cathepsin G (Biocentrum), azurocidin (ITK Diagnostics), and lactoferrin (Sigma-Aldrich). The granule proteins were coated at 250 ng per well in PBS on a Nunc maxisorp plate and, after blocking with 4% (wt/vol) BSA (Serva) in PBST (or TBST for MPO), a concentration range of 0–1,000 nM C-His SPIN was added. Bound His6 protein was detected by monoclonal His mouse antibody (Hytest) and peroxidase-conjugated goat anti-mouse IgG (Bio-Rad) and visualized using tetramethylbenzidine for binding to other granular proteins. Alkaline phosphatase-conjugated goat anti-mouse IgG (Novex Life Technologies) and visualization by using para-Nitrophenylphosphate (pNPP) was used for MPO detection.
Direct binding of WT SPIN to native MPO was assessed by SPR using a Biacore 3000 instrument (GE Healthcare) at 25 °C. A running buffer of 20 mM Hepes (pH 7.4), 140 mM NaCl, and 0.005% (vol/vol) Tween-20 and a flowrate of 20 µL min−1 was used for all experiments. A native MPO biosensor was created by immobilizing MPO on a CM5 sensor chip (GE Healthcare) using standard amine coupling chemistry to a final immobilization density of 3,330 RU. A reference surface was generated by activation and subsequent quenching of a separate flow cell. A variable concentration series of SPIN (0–1,000 nM) was injected in triplicate over the MPO surface for 2 min and allowed to dissociate for 4 min. Regeneration of the MPO surface to baseline was achieved by three injections of glycine (pH 10.0) for 3 min. Kinetic analysis of each reference subtracted injection series was performed using BIAEvaluation software v4.1.1 (GE Healthcare) using a 1:1 (Langmuir) binding model and fitting Rmax locally.
Anti-SPIN IgG antibodies were determined in normal human serum (NHS) from 20 healthy donors, as described previously (40), using Nunc maxisorp microtiter plates (Thermo Scientific) coated with 250 ng recombinant SPIN, CHIPS, or SEI in PBS. The titers were determined as logarithmic value of the dilution factor that gave an OD450 of 0.2 after subtraction of the background.
The AlphaScreen assay (PerkinElmer) (41) was used to determine the binding interaction between SPIN and MPO in PBS + 1 mg/mL BSA buffer. Human MPO (final concentration 0.6 nM), C-His SPIN (final concentration 1 nM), and untagged (shown as unlabeled) SPIN (final concentration ranging from 0 to 1,000 nM) were mixed together and incubated at room temperature for 1 h. Next, rabbit IgG against MPO (Dako) was added in a final concentration of 0.6 nM and incubation was continued for another hour. Finally protein A donor beads and anti-His acceptor beads, each at a final concentration of 20 µg/mL, were added and incubated in the dark for 1 h. Binding was then measured in a CLARIOstar microplate reader (BMG Labtech). The KD was calculated after fitting nonlinear regression, one-site specific binding using Graphpad Prism v6.
MPO Activity Assay.
The redox indicator o-Dianisidine was used at OD450 to measure MPO activity in the presence of H2O2. 0.2 U/mL MPO isolated from human sputum (Elastin Products), 18 µg/mL human rMPO (R&D Systems), or 18.8 µg/mL mouse rMPO (R&D Systems) were incubated with several dilutions of supernatant from different S. aureus strains, or with purified protein for 1 h at 37 °C in 96-well plates. Supernatant of WT and Δspn strains from Newman and USA300 were taken at different time points and diluted 16-fold in PBS. THB and 0.3 mM azide were used as controls. A substrate mixture composed of 45 mM phosphate buffer pH 6.0, 0.5 mM H2O2, and 15 µg of o-Dianisidine dihydrochloride (tablet from Sigma-Aldrich) was used. OD450 nm was measured continuously every 45 s for 1 h at 37 °C using a Fluostar Omega microplate reader. The slope before saturation was calculated via Prism 6 and set as “MPO activity.” Next, 10 µM AZM198 (a kind gift from Innovative Medicines and Early Development Cardiovascular and Metabolic Diseases, AstraZeneca) was added for 1 h at room temperature to 2 × 106 neutrophils and washed afterward to test the intracellular effect on intact cells. To prepare lysates, neutrophils were lysed with 0.1% (vol/vol) Triton-X100 in PBS, centrifuged for 5 min at 10,000 × g and cell-free supernatant was tested for MPO activity. As a positive control for AZM198, 10 µM of the inhibitor was added to the cell-free supernatant after lysis.
Crystallization, Structure Determination, Refinement, and Analysis.
A sample of untagged SPIN bound to recombinant human MPO (R&D Systems) was prepared by mixing stoichiometric amounts of each protein and concentrating to 5 mg/mL total protein in a buffer of 5 mM Tris (pH 7.4), 50 mM NaCl. Crystals of the SPIN/MPO complex were obtained by vapor diffusion of hanging drops at 20 °C. Drops were established by mixing 1 µL of complex with 1 µL of precipitant solution [0.2 M triammonium citrate (pH 7.0), 20% (wt/vol) peg-3350], and incubating over 500 µL precipitant solution. Rhomboid-shaped crystals generally appeared within 2–3 d, although microseeding was occasionally necessary to facilitate nucleation of crystals. Single crystals were harvested and cryopreserved in precipitant solution supplemented with an additional 10% (vol/vol) peg-400. Monochromatic X-ray diffraction data were collected at 1.0-Å wavelength using beamline 22-BM of the Advanced Photon Source (Argonne National Laboratory). Diffraction data were integrated, scaled, and reduced using the HKL2000 software suite (42). Crystals grew in the space group C2 and contained a single copy of the complex in the asymmetric unit. Structure solution and refinement were carried out by individual programs as implemented in the PHENIX software package (43). Initial phases were obtained by molecular replacement using a single copy of native human MPO as a search model (PDB ID code 1CXP) (33). Following an initial round of refinement, a model for the SPIN polypeptide was completed by a combination of automated chain tracing using PHENIX.AUTOBUILD (44) and manual building into electron density maps using COOT (45). The final model was completed upon iterative cycles of manual rebuilding and refinement using PHENIX.REFINE. The recombinant MPO preparation used here is characterized by a lower occupancy of the heme prosthetic group compared with MPO isolated from human sources; this resulted in weak electron density for the heme in the cocrystal structure, and prevented inclusion of heme in the final model. Refined coordinates and structure factors have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University (www.rcsb.org/) under PDB ID code 5UZU. A description of crystal cell constants, diffraction data quality, and properties of the final model for the SPIN/MPO complex can be found in Table S1. All structural analyses, including calculation of buried surface areas and identification of potential hydrogen bonds, were performed using the EBI-PISA server (www.ebi.ac.uk/pdbe/prot_int/pistart.html) (46). Representations of protein structures and electron density maps were generated by PyMol (www.pymol.org/).
Analysis of spn and agrA Expression Following Exposure to Human Neutrophils.
Overnight bacterial cultures grown in tryptic soy broth (TSB) were diluted 1:100 in fresh TSB and grown to OD600 = 1.5. Bacteria were washed in DPBS and resuspended in RPMI supplemented with 5 mM Hepes. Human neutrophils were purified from human venous blood, as previously described (20). Informed consent was obtained from all subjects, in accordance with the Declaration of Helsinki. Approval from the medical ethics committee of the University Medical Center Utrecht was attained (METC-protocol 07-125/C approved on 1 March, 2010). Human neutrophils (1 × 107) were exposed to strain USA300 (1 × 108) in human serum-coated 24-well plates and phagocytosis was synchronized by centrifugation, as described previously (47). After 30 min of incubation at 37 °C in 5% (vol/vol) CO2, RNA was extracted using RNeasy Mini Kit (Qiagen) (47). Gene expression was assessed using TaqMan RNA-to-CT 1-Step Kit (Life Technologies) and amplification was measured using a 7,500 Fast Real-Time PCR System (Applied Biosystems). gyrB expression was used as the endogenous control.
Time-Lapse Microscopy and Phagocytosis Assay.
USA300 bacteria were electroporated with modified pCM29 GFP plasmid, where the promoter SarA was changed to the SPIN promoter from strain Newman. The promoter regions of USA300 and Newman are 100% identical. These bacteria were phagocytized by neutrophils (10 bacteria: 1 neutrophil with 10% NHS) for 10 min at 37 °C and monitored by time-lapse microscopy every 10 min for 2 h. After that time point bacteria started to proliferate and therefore showed an increase in GFP signal. The experiment was performed in a fibrin-thrombin matrix as previously described (22) with a Leica TSC SP5 inverted microscope equipped with a HCX PL APO 40× 0.85 objective (Leica Microsystems). Total GFP was quantified by defining individual regions of interest from single neutrophils or bacterial colonies outside neutrophils after background subtraction (region of interest outside cells) using ImageJ.
Phagocytosis assays were performed using FITC- (Sigma-Aldrich) labeled bacteria and phagocytosis by isolated neutrophils in the presence of varying concentrations of sera. The percentage of FITC-positive neutrophils was calculated after 15 min and 60 min of phagocytosis by FACS, as described previously (40).
Bactericidal Assays with MPO and GO.
This experiment was adapted from Denys et al.(48). In short, bacteria were grown to logarithmic phase (OD660 ∼ 0.5) from an overnight culture, washed twice, and resuspended to OD660 ∼ 0.5 in sterile HBSS. Next, 100 µL of bacteria were diluted into 10 mL of substrate solution containing 300 mM glucose (Merck) in HBSS. Fifty microliters of this bacterial stock solution was diluted 1:1 (vol/vol) with an enzyme solution containing 4 ng/mL GO (from Aspergillus; Sigma-Aldrich), and MPO concentrations ranging from 0 to 7 nM with or without the addition of 120 nM recombinant SPIN. Additional experiments were performed by titrating SPIN (ranging from 0 to 1,200 nM) with 2.3 nM MPO. The enzyme/bacteria mixture was incubated for 1 h at 37 °C and peroxidase activity was stopped by addition of 10 mg/mL catalase from bovine liver (Sigma-Aldrich). Samples were serially diluted in PBS and plated onto Todd Hewitt Agar (THA) plates. CFUs were counted the next day after overnight incubation at 37 °C.
Bactericidal Assays by Neutrophils.
Neutrophils were purified from healthy volunteers by Ficoll/Histopaque centrifugation as described previously (49). Bacteria were grown to logarithmic phase (OD660 ∼ 0.5) from an overnight culture in THB, washed twice, and diluted in HBSS supplemented with 0.05% (wt/vol) human serum albumin (HSA; Sanquin). To inhibit MPO activity, 10 µM AZM198 was added for 1 h at room temperature with gentle shaking, after which neutrophils were washed once in HBSS + 0.05% (wt/vol) HSA. Per condition, 8.5 × 105 neutrophils with 1.70 × 104 bacteria (8.5 × 105 neutrophils with 5.1 × 105 bacteria for AZM198 experiment) and 5% (wt/vol) NHS, were added in sterile siliconized tubes and incubated various times with shaking in a 5% (vol/vol) CO2 incubator. After 60 min of incubation, the neutrophils were lysed for 5 min with ice cold 0.3% (wt/vol) saponin (Sigma-Aldrich) in water. Samples were then serially diluted in PBS and plated onto THA plates in duplicate. CFUs were counted after overnight incubation at 37 °C, and percentage survival was calculated and compared with inoculum.
Growth Curves.
Bacteria were grown overnight in THB, diluted 1:100 the next morning, and grown until they reached logarithmic phase. The bacteria were diluted again to OD660 0.01 and placed in 96-wells flat bottom plates in 150-µL volume, four wells per strain. Absorbance at OD660 was measured in a plate reader (Fluostar Omega; BMG Labtech) every 10 min at 37 °C with 600 RPM intermittent shaking.
Acknowledgments
We thank Franke A. Quee, Lindert Benedictus, Ruben Shrestha, Ping Li, and Kyler B. Pallister for experiments and statistical analysis. This research was supported by NIH Grants AI111203 and GM121511 (to B.V.G.), R01AI1090046 and PAR98-072 (to J.M.V.), and RR020185 for a fellowship award (to F.E.G.); and ZonMw Grant 205200004 from the Netherlands Organization for Health Research and Development (to J.A.G.v.S.). X-ray diffraction data were collected at Southeast Regional Collaborative Access Team 22-BM beamline at the Advanced Photon Source, Argonne National Laboratory. Supporting institutions may be found at www.ser-cat.org/members.html. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract W-31-109-Eng-38.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID code 5UZU).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707032114/-/DCSupplemental.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 5.Klebanoff SJ, Hamon CB. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972;12:170–196. [PubMed] [Google Scholar]
- 6.Kettle AJ, Winterbourn CC. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 7.Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9:215–222. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 8.Spaan AN, Surewaard BGJ, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: A biological tug of war. Annu Rev Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 9.Liu GY, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove K, et al. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol. 2007;189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusch H, Engelmann S. Secrets of the secretome in Staphylococcus aureus. Int J Med Microbiol. 2014;304:133–141. doi: 10.1016/j.ijmm.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Fevre C, et al. Staphylococcus aureus proteins SSL6 and SElX interact with neutrophil receptors as identified using secretome phage display. Cell Microbiol. 2014;16:1646–1665. doi: 10.1111/cmi.12313. [DOI] [PubMed] [Google Scholar]
- 13.Guerra FE, et al. Staphylococcus aureus SaeR/S-regulated factors reduce human neutrophil reactive oxygen species production. J Leukoc Biol. 2016;100:1005–1010. doi: 10.1189/jlb.4VMAB0316-100RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fevre C, Scheepmaker L, Haas PJ. Identifying bacterial immune evasion proteins using phage display. Methods Mol Biol. 2017;1535:43–61. doi: 10.1007/978-1-4939-6673-8_4. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy AJ, Lindsay JA. Staphylococcus aureus innate immune evasion is lineage-specific: A bioinfomatics study. Infect Genet Evol. 2013;19:7–14. doi: 10.1016/j.meegid.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 16.de Haas CJC, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verkaik NJ, et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis. 2009;199:625–632. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 18.Palazzolo-Ballance AM, et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J Immunol. 2008;180:500–509. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- 19.Voyich JM, et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis. 2009;199:1698–1706. doi: 10.1086/598967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voyich JM, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 21.Björnsdottir H, et al. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic Biol Med. 2015;89:1024–1035. doi: 10.1016/j.freeradbiomed.2015.10.398. [DOI] [PubMed] [Google Scholar]
- 22.Vrieling M, et al. Bovine Staphylococcus aureus secretes the leukocidin LukMF’ to kill migrating neutrophils through CCR1. MBio. 2015;6:e00335. doi: 10.1128/mBio.00335-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stapels DA, et al. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc Natl Acad Sci USA. 2014;111:13187–13192. doi: 10.1073/pnas.1407616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nygaard TK, et al. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis. 2010;201:241–254. doi: 10.1086/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantrangi M, Singh VK, Wolz C, Shukla SK. Staphylococcal superantigen-like genes, ssl5 and ssl8, are positively regulated by Sae and negatively by Agr in the Newman strain. FEMS Microbiol Lett. 2010;308:175–184. doi: 10.1111/j.1574-6968.2010.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serruto D, Rappuoli R, Scarselli M, Gros P, van Strijp JA. Molecular mechanisms of complement evasion: Learning from staphylococci and meningococci. Nat Rev Microbiol. 2010;8:393–399. doi: 10.1038/nrmicro2366. [DOI] [PubMed] [Google Scholar]
- 28.Koymans KJ, Vrieling M, Gorham RD, Jr, van Strijp JAG. Staphylococcal immune evasion proteins: Structure, function, and host adaptation. Curr Top Microbiol Immunol. 2016;6:23–27. doi: 10.1007/82_2015_5017. [DOI] [PubMed] [Google Scholar]
- 29.Bakhautdin B, Goksoy Bakhautdin E, Fox PL. Ceruloplasmin has two nearly identical sites that bind myeloperoxidase. Biochem Biophys Res Commun. 2014;453:722–727. doi: 10.1016/j.bbrc.2014.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segelmark M, Persson B, Hellmark T, Wieslander J. Binding and inhibition of myeloperoxidase (MPO): A major function of ceruloplasmin? Clin Exp Immunol. 1997;108:167–174. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samygina VR, et al. Ceruloplasmin: Macromolecular assemblies with iron-containing acute phase proteins. PLoS One. 2013;8:e67145. doi: 10.1371/journal.pone.0067145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman ALP, et al. Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem. 2013;288:6465–6477. doi: 10.1074/jbc.M112.418970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiedler TJ, Davey CA, Fenna RE. X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 A resolution. J Biol Chem. 2000;275:11964–11971. doi: 10.1074/jbc.275.16.11964. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg S, et al. A human monoclonal antibody targeting the conserved staphylococcal antigen IsaA protects mice against Staphylococcus aureus bacteremia. Int J Med Microbiol. 2015;305:55–64. doi: 10.1016/j.ijmm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carver TJ, et al. ACT: The Artemis comparison tool. Bioinformatics. 2005;21:3422–3423. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- 37.Bose JL, Fey PD, Bayles KW. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol. 2013;79:2218–2224. doi: 10.1128/AEM.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang YY, et al. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun. 2010;2:546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Kessel KPM, van Strijp JAG, Verhoef J. Inactivation of recombinant human tumor necrosis factor-alpha by proteolytic enzymes released from stimulated human neutrophils. J Immunol. 1991;147:3862–3868. [PubMed] [Google Scholar]
- 40.Rooijakkers SHM, van Wamel WJB, Ruyken M, van Kessel KPM, van Strijp JA. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005;7:476–484. doi: 10.1016/j.micinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Bosse R, Illy C, Chelsky D. Application Note. Principles of AlphaScreen. PerkinElmer Life Sciences; Boston: 2002. [Google Scholar]
- 42.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 43.Zwart PH, et al. Automated structure solution with the PHENIX suite. In: Kobe B, Guss M, Huber T, editors. Structural Proteomics High-Throughput Methods. Humana Press; Totowa, NJ: 2008. pp. 419–435. [Google Scholar]
- 44.Afonine PV, et al. Joint X-ray and neutron refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr. 2010;66:1153–1163. doi: 10.1107/S0907444910026582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Voyich JM, Sturdevant DE, DeLeo FR. Analysis of Staphylococcus aureus gene expression during PMN phagocytosis. Methods Mol Biol. 2008;431:109–122. doi: 10.1007/978-1-60327-032-8_9. [DOI] [PubMed] [Google Scholar]
- 48.Denys GA, Grover P, O’Hanley P, Stephens JT., Jr In vitro antibacterial activity of E-101 Solution, a novel myeloperoxidase-mediated antimicrobial, against Gram-positive and Gram-negative pathogens. J Antimicrob Chemother. 2011;66:335–342. doi: 10.1093/jac/dkq429. [DOI] [PubMed] [Google Scholar]
- 49.Bestebroer J, et al. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood. 2007;109:2936–2943. doi: 10.1182/blood-2006-06-015461. [DOI] [PubMed] [Google Scholar]