Significance
Gamma delta T cells, a group of immune cells that exhibit features from both innate and adaptive immunity, possess significant potential in clinical applications such as treatment of microbial infections and cancer immunotherapy. To fully understand their biology and harness them in the clinic it is imperative to dissect the molecular mechanisms involved in their recognition of infected and tumor cells. In this paper we focus on Vγ9Vδ2 T cells, a major subset of human gamma delta T cells in blood and investigate the phosphoantigen-induced, MHC-independent molecular mechanisms governing their activation.
Keywords: human Vγ9Vδ2 T cells, butyrophilin 3A1, phosphoantigen, conformational change
Abstract
Human Vγ9Vδ2 T cells respond to microbial infections as well as certain types of tumors. The key initiators of Vγ9Vδ2 activation are small, pyrophosphate-containing molecules called phosphoantigens (pAgs) that are present in infected cells or accumulate intracellularly in certain tumor cells. Recent studies demonstrate that initiation of the Vγ9Vδ2 T cell response begins with sensing of pAg via the intracellular domain of the butyrophilin 3A1 (BTN3A1) molecule. However, it is unknown how downstream events can ultimately lead to T cell activation. Here, using NMR spectrometry and molecular dynamics (MD) simulations, we characterize a global conformational change in the B30.2 intracellular domain of BTN3A1 induced by pAg binding. We also reveal by crystallography two distinct dimer interfaces in the BTN3A1 full-length intracellular domain, which are stable in MD simulations. These interfaces lie in close proximity to the pAg-binding pocket and contain clusters of residues that experience major changes of chemical environment upon pAg binding. This suggests that pAg binding disrupts a preexisting conformation of the BTN3A1 intracellular domain. Using a combination of biochemical, structural, and cellular approaches we demonstrate that the extracellular domains of BTN3A1 adopt a V-shaped conformation at rest, and that locking them in this resting conformation without perturbing their membrane reorganization properties diminishes pAg-induced T cell activation. Based on these results, we propose a model in which a conformational change in BTN3A1 is a key event of pAg sensing that ultimately leads to T cell activation.
Gamma delta (γδ) T cells exhibit restricted T cell receptor (TCR) use and tissue-specific residence, and they play critical roles in immune responses to infection and tumorigenesis. In human peripheral blood, a major γδ T cell subset is called Vγ9Vδ2, based on the variable gene segments (TRGV9 and TRDV2) used in the recombined TCR. This population can be activated and expanded to about 50% of total blood T cells upon infection with certain microbes, including the bacterial pathogens Mycobacterium tuberculosis and Mycobacterium leprae and protozoal parasites such as Plasmodium falciparum (1). Vγ9Vδ2 T cells can quickly launch specific and effective immune responses toward these pathogens (1). Moreover, they show similar reactivity toward certain types of cancer cells, resulting in widening and intensifying interest in the use of gamma delta T cells for cancer immunotherapy (2). However, despite intensive research over the past 30 y, the molecular mechanisms governing Vγ9Vδ2 T cells’ recognition of infected and malignant cells are still poorly understood, thus impeding the overall understanding of Vγ9Vδ2 T cell immunity and development of its potential medical applications.
Vγ9Vδ2 T cells are specifically activated by a set of pyrophosphate metabolites collectively named phosphoantigens (pAgs), which are present in both infected and malignant target cells (3). These pAgs are sensed by the butyrophilin 3A1 (BTN3A1) protein, a member of the BTN3A family with three different isoforms (A1, A2, and A3) that confer pAg-mediated reactivity toward target cells by Vγ9Vδ2 T cells (4). Unrelated to MHC molecules, BTN3A proteins are type-I membrane proteins with two Ig-like extracellular domains with structural homology to the B7 superfamily of proteins (5). The antibody 20.1, specific to the BTN3A extracellular domains, is capable of activating Vγ9Vδ2 T cells even in the absence of pAgs (4, 5). Previous structural studies on the BTN3A Ig-like extracellular domains and their complex with 20.1 showed two possible conformations of extracellular domains: a “V-shaped” form, which is compatible with 20.1 binding and has the potential to oligomerize, and a “head-to-tail” form, of which the dimer interface overlaps with the 20.1 binding site (6). However, it is unknown whether these two dimer forms exist in the full-length BTN3A molecule in the cellular environment, and whether they play a role in pAg-induced T cell activation.
While it is still unclear how the extracellular domains of BTN3A contribute to T cell activation, the intracellular B30.2 domain of BTN3A1 has been proven to play a critical role in pAg detection (4, 7). pAgs bind directly to a positively charged pocket in the intracellular B30.2 domain of BTN3A1 (8, 9). Other proteins important for pAg-induced T cell activation, such as RhoB GTPase and periplakin, are also reported to interact with the intracellular domain (10, 11). Moreover, the BTN3A1 full-length intracellular domain (BFI), including the membrane proximal region located N-terminal to the B30.2 domain, undergoes a conformational change upon pAg binding (9). However, it is unknown how exactly pAg binding triggers a conformational change of BFI and how this ultimately leads to Vγ9Vδ2 TCR engagement and T cell stimulation.
Here we present structural, biophysical, computational, and functional data dissecting the pAg-induced conformational change of the intracellular domain of BTN3A1. Using NMR spectrometry and molecular dynamics (MD) simulations, we show that the BTN3A1 B30.2 domain undergoes a global conformational change upon pAg binding. We also reveal two distinct dimer interfaces of the BFI domain through crystallography. Mapping residues with significant chemical shift perturbation (CSP), obtained by NMR, onto the crystal structure of BFI reveals changes across the B30.2 domain, many of which are located in the dimer interfaces. Together with additional supporting data from MD simulations, we propose that the binding of pAg induces changes in the dimer interface of the intracellular domain that can potentially propagate to the extracellular domain of BTN3A1. Combining approaches such as EM, cross-linking, and functional assays, we then demonstrate that the extracellular domains of BTN3A1 adopt a V-shaped conformation at rest. We further found that locking the extracellular domains in this resting conformation without perturbing their membrane reorganization properties diminishes pAg-induced T cell activation, suggesting that rearrangement of BTN3A1 proteins is critical to Vγ9Vδ2 T cell activation. Altogether, our data strongly support a model in which pAg-triggered conformational change of BTN3A1 is an essential molecular event leading to Vγ9Vδ2 T cell activation.
Results
pAg Induces a Global Conformational Change of the BTN3A1 Intracellular B30.2 Domain.
Previous biophysical and structural studies have shown that pAgs bind directly to the BTN3A1 intracellular B30.2 domain (8, 9). In our attempts to obtain B30.2–pAg complex crystals through ligand soaking we observed that B30.2 apo crystals dissolve upon pAg addition, hinting that pAg binding may cause conformational changes of the B30.2 domain which disrupt crystal packing (8). To explore this possibility, we applied NMR techniques to the B30.2–pAg complex to reveal detailed structural information about the B30.2 domain upon pAg binding. Major CSP from multiple residues within the B30.2 domain were observed comparing the 15N, 1H- heteronuclear single quantum coherence (HSQC) spectra of 15N-single labeled B30.2 with increasing concentrations of 1-hydroxy-2-methylpent-2-enyl-pyrophosphonate (cHDMAPP), kindly provided by C. Belmant, Innate Pharma, Marseille, France, a synthetic paralog to the naturally occurring microbial pAg 1-hydroxy-2-methylpent-2-enyl-pyrophosphate (HMBPP) (Fig. 1A). The majority of these peaks exhibit fast-exchange behavior, whereas some peaks show intermediate to slow exchange behavior, suggesting a potential conformational change in addition to the ligand binding event. To understand where these CSPs reside on the 3D structure of the B30.2 domain, we performed 3D NMR spectroscopy for backbone resonance assignments of both B30.2 with and without pAg, using 2H, 15N, 13C triple-labeled B30.2 protein sample. About 80% of the peaks appearing in the HSQC spectra were assigned, and the assigned residues covered almost the entire structure of B30.2 (Figs. S1 and S2). For residues with assignment in both apo and complex forms, we calculated the weighted average CSP of 15N and 1H between the free and pAg-bound B30.2 domain (Fig. 1B). By mapping the residues undergoing large CSP (higher than SD) onto the crystal structure of B30.2 domain [Protein Data Bank (PDB) ID code 4N7I] we found that many of these residues are located near the pAg binding pocket, likely due to changes of the chemical environment in the presence of pAg (Fig. 1C). Interestingly, there were several residues residing in remote sites away from the binding pocket, indicating that there is a nonlocal conformational change of B30.2 domain upon pAg binding (Fig. 1C). Notably, some residues are either only assigned in apo or in pAg-bound form, likely due to the line-broadening effect from intermediate or slow chemical exchange (Fig. S2). Interestingly, these residues reside mostly away from the pAg-binding pocket, again suggesting a conformational change distant from the pAg-binding pocket.
Fig. 1.
(Upper) Binding of pAg cHDMAPP induces a conformational change in the BTN3A1 B30.2 domain. (A) 15N, 1H- HSQC spectra of 15N single-labeled B30.2 domain with increasing concentrations of cHDMAPP. The ligand-to-protein ratio is color-coded as indicated above the spectra. Representative enlarged views for six residues experiencing major CSP are also shown (Lower). (B) Histogram of weighted average CSP of 15N and 1H between free and ligand-bound (1:1) B30.2 domain. The SD and twofold SD are shown as gray lines on the histogram. (C) CSP mapping onto the B30.2 domain structure (PDB ID code 4N7I). The B30.2 domain is shown in yellow surface representation and cHDMAPP is shown in cyan stick representation in the pAg-binding pocket. The residues with major CSP located near the cHDMAPP-binding pocket (Left) or away from the ligand-binding pocket (Right, rotated 90° or 180° respectively from the left view) are shown in red.
Fig. S1.
Backbone assignments for the apo and pAg-bound B30.2 domain. Backbone assignments labeled onto the HSQC spectra of apo and pAg-bound B30.2 domain. The peaks in apo spectrum are shown in red whereas the peaks in pAg-bound spectrum are shown in purple. The residue number is labeled for each assigned peak in both spectra.
Fig. S2.
Sequence and structural view of residues in B30.2 domain with or without assignment. Both views show the residues assigned either only in apo or complex form of the B30.2 domain. Residues with assignment are shown in yellow and the ones without assignment are shown in black in both sequence and structure view. The residues assigned only in one of the spectra are colored in red both in the sequence and crystal structure. (Lower) The crystal structures are snapshots 180° apart showing regions near to (Left) or remote from (Right) the pAg-binding pocket, which is marked by pAg cHDMAPP in cyan.
To further investigate the putative pAg-induced conformational change, we performed all-atom MD simulations of the BTN3A1 intracellular domain in the presence or absence of pAg cHDMAPP, starting from the crystal structure model. Rmsd backbone analysis of these simulations, which reveal structural shifts in the protein backbone over time, show that overall the crystal structure is stable in solution, with a mean deviation between crystal structure and simulation of 1.81 Å2 for the apo systems and 1.57 Å2 for the pAg-bound systems. Notably, single-residue rmsd analysis of the backbone showed that the same residues that were identified in the CSP data accounted for the majority of this variation (Fig. 2A). The specific residues showing the most prominent shifts in silico were along two distinct flexible loops encompassing residues 393–397 and residues 410–419. Visualization of these trajectories show that pAg keeps the binding pocket of the intracellular domain in a more contracted state, while the apo systems are free to adopt a more open, flexible conformation which gives atomic-level insight in to the pAg proximal chemical shifts outlined above (Fig. 2B). Together, the NMR and computational data both strongly support the idea of a global conformational change of the B30.2 domain triggered by pAg binding.
Fig. 2.
All-atom MD simulations reveal dynamics and structural differences between B30.2 domain apo and pAg-bound state. (A) Single amino acid backbone rmsd of the residues identified to experience high CSP upon pAg binding. The thick horizontal line in each plot is the mean plus or minus SD of the backbone rmsd of the rest of the protein. (B) Coordinates of the BTN3A1 intracellular domain with (blue) or without (red) pAg cHDMAPP (shown in cyan stick) after 100 ns of MD simulations are shown in cartoon representation. The segments 393–397 and 410–419 that exhibit major backbone RMSD shift are shown as ribbon representation in red (apo) or blue (pAg-bound).
Tyrosine 352 Is Critical for pAg Binding to B30.2 Domain.
Among all of the residues with major CSP in the B30.2 domain, tyrosine 352 (Y352) showed the largest perturbation (Fig. 1B). Because of its location near the pAg-binding pocket, we postulated that it might be important for pAg binding. To test this hypothesis, we used isothermal titration calorimetry (ITC) to measure the binding between the B30.2 alanine mutant of Y352 (Y352A) and both cHDMAPP and the weaker-potency, native pAg isopentenyl pyrophosphate (IPP). Previous ITC measurements have measured the affinities (dissociation constant, Kd) of these pAgs for the B30.2 domain to be ∼1 μM and ∼1 mM, respectively (8). Compared with WT B30.2 protein, the mutant Y352A showed a significant defect in binding to both pAg species. In the case of cHDMAPP, the Kd for the protein–ligand interaction exhibited a sixfold increase (weaker affinity) whereas the mutant interaction with IPP was undetectable (Fig. 3A and Table S1). To determine if the Y352A mutant had similar effects on pAg-mediated Vγ9Vδ2 T cell activation, we transfected either full-length WT BTN3A1 or the Y352A mutant into HEK293 cells with the BTN3A genes (A1, A2, and A3) knocked out via CRISPR-Cas9 targeting. We then stimulated the Jurkat cells transduced with Vγ9Vδ2 G115 TCR by coculturing them with BTN3A1-transfected cells that were pretreated with either the microbial pAg HMBPP (the naturally occurring form of cHDMAPP) or the aminobisphosphate (NBP) pamidronate that causes intracellular IPP accumulation through inhibition of the enzyme farnesyl diphosphate synthase. Consistent with our biophysical measurements, the HMBPP-induced T cell activation was diminished for the Y352A mutant, but the defect was not as significant as the NBP-induced IPP activation, which was severely affected (Fig. 3B). The 20.1 antibody (20.1 mAb)-elicited Vγ9Vδ2 response for the Y352A mutant was reduced compared with WT, but the difference was not statistically significant (Fig. 3B). Together, these results indicate that residue Y352 in the B30.2 domain is critical for pAg binding.
Fig. 3.
Y352 in the B30.2 domain is important for pAg binding and pAg-induced T cell activation. (A) ITC binding isotherm traces of the BTN3A1 B30.2 domain WT protein and Y352 alanine mutant (Y352A) with either cHDMAPP or IPP. The binding measurements were all performed using 100 μM protein in the cell and 2 mM ligand in the syringe. The interactions between the WT/Y352A with cHDMAPP (Left) and their interactions with IPP (Right) are shown. Within the same group of ligand, the isotherm traces were plotted on the same y axis for comparison. The buffer controls are shown in open square, the WT traces in gray triangle (cHDMAPP) or square (IPP), and the Y352 traces in red triangle (cHDAMPP) or square (IPP). The binding curves shown in all plots were fitted assuming one-site binding. (B) Histogram showing CD69 up-regulation level of Jurkat G115 Vγ9Vδ2 TCR transductants in response to treatment of WT or Y352A BTN3A1 transfected HEK293 cells in BTN3A CRISPR KO background with either PBS, isotype control antibody, anti-CD277 agonist antibody (20.1 mAb), NBP pamidronate, or HMBPP. G115 transfectants treated with 1 μg/mL PHA or PBS served as positive or negative controls, and the CD69 level from all of the other experimental groups were normalized according to the controls. Means of three independent experiments with the SD are shown. P values indicated in the plot were calculated using the unpaired Student’s t test method [*P < 0.05, not significant (ns) P > 0.05].
Table S1.
Thermodynamic binding parameters determined by ITC of pAgs for the WT and mutant BTN3A1 B30.2 domain
| Group | Stoichiometry (N) | Kd | ΔH, kCal/mol | ΔS, cal/(mol⋅degree) |
| WT-cHDMAPP | 0.97 ± 0.0073 | 1.0 ± 0.7 μM | −12.1 ± 0.15 | −13.3 |
| WT-IPP | 1 (fixed value) | 4.8 ± 2.4 mM | −5.2 ± 0.4 | −1.69 |
| Y352A-cHDMAPP | 0.97 ± 0.023 | 6.2 ± 0.3 μM | −10.2 ± 0.33 | −10.5 |
| Y352A-IPP | 1 (fixed value) | N.A. | N.A. | N.A. |
N.A., not assessed.
The 1.9-Å Structure of BTN3A1 BFI Reveals Two Potential Dimer Interfaces.
To obtain a more complete view of the pAg-induced conformational change we performed crystallization of the BFI domain with cHDMAPP soaking, in light of recent works from Hsiao et al. (9) and Rhodes et al. (11) suggesting the importance of the membrane proximal region of BTN3A1 intracellular domain. Crystals diffracted to 1.9 Å and the structure was refined to an Rwork/Rfree of 20.5%/23.4% (Fig. 4A and Table S2). Unfortunately, the electron density for cHDMAPP was too weak to be interpreted unambiguously. However, the BFI crystalized in a different space group with two molecules in the asymmetric unit rather than one compared with the B30.2 domain structure published previously (8). The dimer interface in the asymmetric unit, referred to as Dimer I hereafter, involves more than 20 residues from both monomers (Fig. 4B, Upper). Among these, the side chain hydroxyl group of Y352, the residue that undergoes the largest CSP and is critical for pAg binding and T cell activation (Figs. 1B and 3), is one of the major contributors to this dimer interface, making hydrogen bonds with the backbone of E327 and D328 from the other monomer (Fig. 4B, Upper). Other important interface contacts are hydrophobic and van der Waals interactions made by W350, W391, and L471 from one monomer with a cluster of residues from the other monomer (Fig. 4B, Upper).
Fig. 4.
The 1.9-Å structure of BTN3A1 BFI reveals two potential dimer interfaces. (A) Overview of two dimer conformations observed in the crystal lattice of the BTN3A1 BFI domain. The domain architecture is presented above. One monomer is colored yellow with the extended membrane proximal region colored in orange, and the other is colored light blue. The dimer observed in the asymmetric unit (Left) is referred to as Dimer I. The other dimer (Right) similar to the previously published B30.2 structure is referred to as Dimer II. The pAg-binding pockets are indicated by cHDMAPP model colored in cyan. (B) Contact maps within the dimer interfaces of Dimer I (Upper) and Dimer II (Lower). Residues in purple and yellow panels are from different monomers within the dimer. The stars indicate residues that undergo major CSP. Dashed lines are hydrophobic or van der Waal interactions and solid lines are salt bridges or hydrogen bonds. Contact lines are color-coded by contact percentage calculated from MD simulations. Contact percentage is defined as the number of simulation frames in which two residues are separated by 5 Å or less divided by the total number of frames in the simulation. (C) Residues with major CSP and differential assignment in the apo and complex HSQC spectra are concentrated in two dimer interfaces of BFI. The residues located in or near either Dimer I or Dimer II interface are shown as bold red lines and labeled in the context of the whole dimer. The pAg-binding pockets are indicated by pAg cHDMAPP model colored in cyan.
Table S2.
BFI crystallographic data and refinement statistics
| Parameter | Value |
| Data collection | |
| Resolution range, Å | 72.92–1.93 (2.002–1.933) |
| Space group | P 1 21 1 |
| Unit cell | 37.43 145.84 44.91 90 105.57 90 |
| Total reflections | 587,610 (56,585) |
| Unique reflections | 34,081 (3,435) |
| Multiplicity | 17.1 (16.5) |
| Completeness, % | 98.73 (99.68) |
| Mean I/sigma, I | 30.48 (5.22) |
| Wilson B-factor | 17.22 |
| R-merge | 0.8229 (1.343) |
| R-meas | 0.8475 |
| CC1/2 | 0.821 (0.525) |
| CC* | 0.95 (0.83) |
| Refinement | |
| R-work | 0.1967 (0.3488) |
| R-free | 0.2271 (0.3376) |
| No. of nonhydrogen atoms | 3,439 |
| Macromolecules | 3,263 |
| Ligands | 36 |
| Water | 140 |
| Protein residues | 397 |
| Rms (bonds) | 0.011 |
| Rms (angles) | 1.49 |
| Ramachandran favored, % | 98 |
| Ramachandran allowed, % | 2 |
| Ramachandran outliers, % | 0 |
| Clashscore | 1.53 |
| Average B-factor | 24.9 |
| Macromolecules | 24.7 |
| Ligands | 39.9 |
| Solvent | 24.5 |
In addition to the Dimer I interface described above an additional dimer was identified, similar in orientation to that noted in the original B30.2 structure (PDB ID code 4N7I). In the BFI structure, the symmetrical N-terminal α-helical interface visualized in the original B30.2 domain structure is broken apart by the insertion of the membrane proximal region from residue K277 to S297. Unlike other parts of the membrane proximal region, this segment is well ordered in the BFI structure, clearly adopting an alpha helical structure. It interacts with the N terminus of the other monomer, mostly making hydrogen bonds and salt bridges, of which most are side-chain-specific (Fig. 4B, Lower). This interface involving 28 residues is hereafter referred to as Dimer II.
Using MD simulations we probed the stability of these dimer forms. With the two BFI dimer interfaces as starting points for our simulations each system was equilibrated and then run at 293.15 K and 303.15 K until an accumulated total trajectory time of 0.5 μs was reached (simulation details are given in Materials and Methods). In the case of both interfaces, multiple contacts persist throughout the entire 0.5-μs trajectory, suggesting that both Dimer I and Dimer II are stable over this time period (Fig. 4B). Visualizations of the simulated trajectories and analysis highlighting persistent contacts indicate that the hydrophobic interactions of the Dimer I interface and the interhelix hydrogen bonds and salt bridges of the Dimer II interface are both stable (Fig. 4B). Therefore, combined evidence from crystallographic and MD simulation experiments suggest that BFI can adopt two dimer configurations.
The dimer configurations revealed in the BFI structure reveal interesting features upon further analysis. Intriguingly, the Dimer I interface is very close to the pAg-binding pocket (Fig. 4). Moreover, in or near this dimer interface we also find a significant fraction of residues with major CSP or with differential assignments for the apo and the complex HSQC spectra. For example, Y352, a key residue in the Dimer I interface, showed the largest CSP as mentioned before (Fig. 1B). In addition, among the residues buried in the Dimer I interface, residues L324, W350, H351, C353, K393, M394, and T395 experience significant changes in chemical environment (Fig. 4C, Left). Notably, the same is true in the Dimer II interface for residues N300, L434, T438, S442, F443 and Y444 (Fig. 4C, Right). This indicates that the pAg-induced conformation change is concentrated at these putative BFI dimer interfaces.
The Extracellular Domains of Full-Length BTN3A Adopt a V-Shaped Conformation in Lipid Nanodiscs.
Since Vγ9Vδ2 T cells must detect signals on the extracellular side of target cells, the intracellular detection of pAg by BTN3A must be somehow transmitted to the extracellular space for detection. The conformation of the BTN3A extracellular domains has been speculated to be important for Vγ9Vδ2 T cell activation (6). As mentioned earlier, the extracellular domains of BTN3A1 can adopt two alternative dimer conformations, namely a “V-shaped” form and a “head-to-tail” form, according to crystal structure models (PDB ID code 4F80) (6). However, previous in vitro FRET assays on recombinant BTN3A1 extracellular domains provided evidence only for the existence of the V-shaped dimer (6).
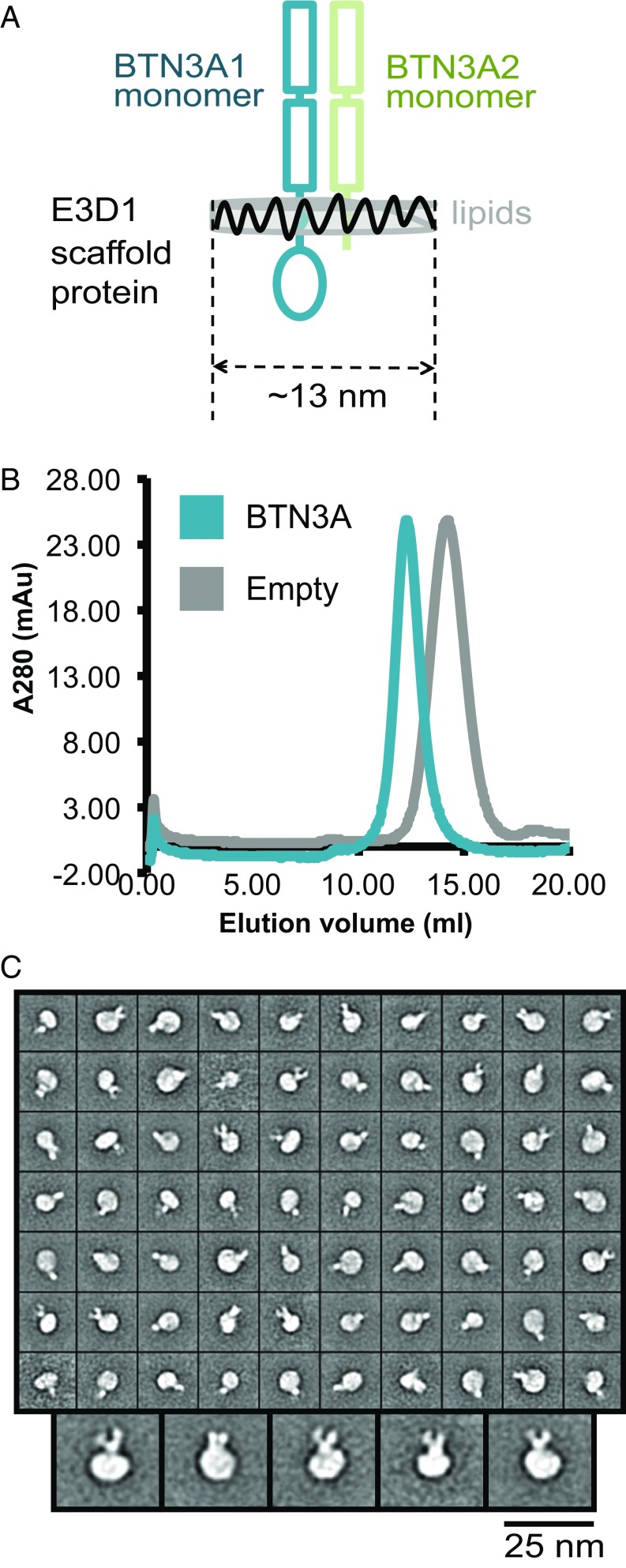
To fully examine the possibility of these two putative dimer conformations in a more physiologically relevant setting, we reconstituted full-length BTN3A dimers expressed in insect cells into nanodiscs, which are a synthetic model membrane system composed of a phospholipid bilayer surrounded by two amphipathic scaffold proteins (Fig. 5A). Interestingly, although the expression level was equivalent, the purification process of BTN3A1/A2 heterodimer was much more successful than the BTN3A1 homodimers, leading to significantly higher yield of the protein product with greater stability (Fig. 5B and Fig. S3). For this reason, nanodiscs of the BTN3A1/A2 heterodimer, instead of the BTN3A1 homodimer, were visualized by negative-stain EM followed by single-particle analysis to examine the conformation of the extracellular domains in the context of full-length BTN3A dimers. Consistent with the previously published FRET data, the V-shaped conformation was readily observable in several 2D class averages (Fig. 5C). In contrast, the head-to-tail conformation could not be unequivocally detected, even though the mean diameter of the reconstituted BTN3A dimer nanodisc is about 13 nm, which is large enough to accommodate the head-to-tail conformation (∼8 nm). We conclude that in the absence of other cellular components and/or exogenously added pAgs the extracellular domains of full-length BTN3A in a lipid bilayer form V-shaped dimers.
Fig. 5.
The extracellular domains of BTN3A full-length protein reconstituted in lipid nanodiscs form a V-shaped conformation. (A) A schematic representation of the BTN3A1/A2 heterodimer nanodisc. All components are labeled in the corresponding colors. (B) Gel filtration chromatographs of the reconstituted BTN3A1/A2 heterodimer nanodiscs (blue) overlaid with the empty nanodiscs control (gray). (C) Negative-staining EM images of the BTN3A1/A2 heterodimer in nanodiscs. On the left are the class averages of 3,968 out of the 4,359 picked particles. A close-up view of the selected class averages is shown at the bottom.
Fig. S3.
BTN3A1/A2 heterodimer purification and negative staining imaging. (A, Left) The gel filtration curve for BTN3A1/A2 heterodimer in detergent Triton X-100 and (A, Right) the SDS/PAGE of BTN3A1/A2 heterodimer before (input) and after gel filtration purification. (B) Raw image of BTN3A1/A2 heterodimer reconstituted in nanodiscs.
The Extracellular Domains of BTN3A1 in the Native Cellular Environment Adopt the V-Shaped Conformation at Rest.
We next investigated whether the V-shaped conformation of BTN3A1 exists in the native cellular environment. To this end, we performed disulfide trapping by designing a pair of cysteine residues in BTN3A1 that should form an intermolecular disulfide bond if the protein adopts the V-shaped conformation (Fig. 6A). Residues D124 and S207 in the V-shaped dimer interface were mutated to cysteines in the context of a mCherry-tagged full-length BTN3A1, and this construct was transfected into the BTN3A-KO HEK293 cells described previously. Cells expressing the engineered construct were either treated with the pAg HMBPP or left untreated and then subjected to flow cytometry to verify proper expression on the cell surface (Fig. S4) and Western blot analysis to detect dimerization (Fig. 6B). As expected, all constructs appear as monomeric species when reducing agent is included in the protein-loading buffer to disrupt disulfide bonds (Fig. 6B, Upper). However, under nonreducing conditions, which leave disulfide bonds intact, WT BTN3A1 appears as a monomer, whereas the D124/S207C mutant migrates as a homogenous high-molecular-weight species, with a molecular weight consistent with a BTN3A1 dimer (Fig. 6B, Lower). Notably, dimerization of the D124/S207 was observed both with and without HMBPP treatment. These results demonstrate that BTN3A1 forms V-shaped dimers on the cell surface and are consistent with our in vitro data.
Fig. 6.
A proper conformation of the BTN3A1 extracellular domains is important for pAg-induced Vγ9Vδ2 T cell activation. (A) Positions of the residues (shown in stick representation) that were mutated to cysteine for disulfide bond formation in the putative dimer interface of the BTN3A1 extracellular domains. The proposed full-length cross-linked BTN3A1 in the cell membrane is also shown in cartoon representation. (B) Western blots revealing the BTN3A1 protein species from WT BTN3A1 and S207A/D124A BTN3A1 mutant transfected HEK293 cells in BTN3A CRISPR KO background, either treated with HMBPP or left untreated. (Upper) A PM fraction sample from each group was treated with reducing protein loading buffer with 5 min heating at 95 °C. (Lower) The PM fraction samples were treated with nonreducing protein loading buffer. “M” marks the monomer species and “D” marks the dimer species. (C) Histogram shows CD69 up-regulation level of Jurkat G115 transductants in response to treatment of WT or S207/D124C mutant BTN3A1 transfected HEK293 cells in BTN3A CRISPR KO background with either PBS, isotype control antibody, anti-CD277 agonist antibody (20.1 mAb), pamidronate (NBP), or HMBPP. G115 transfectants treated with 1 μg/mL PHA or PBS served as positive or negative control, and the CD69 level from all of the other experimental groups was normalized according to the controls. Means of three independent experiments with the SD are shown. P values indicated in the plot were calculated using the unpaired Student’s t test method (*P < 0.05, **P < 0.01). (D) FRAP analysis of cells expressing BTN3A1 (WT) or S207/D124C mutant after incubation for 30 min with anti-CD277 agonist antibody or PBS. Data are presented as the value for immobile fraction, measured as described under “FRAP Analysis” in SI Materials and Methods. WT-PBS: n = 19; WT-20.1mAb: n = 24; S207/D124C-PBS: n = 22; S207/D124C-20.1mAb: n = 20. Bars represent mean values and SD. P values indicated in the plot were calculated using the unpaired Student’s t test method [****P < 0.0001, not significant (ns) P > 0.05].
Fig. S4.
Surface expression of BTN3A1 constructs in HEK293 cells with BTN3A KO background. (A) FACS plots of the WT, Y352A, S207/D124C, and a control mutant BTN3A1 expression in HEK293 cells with BTN3A KO background are shown. BTN3A KO cells serve as a control. For all of the plots, the y axis is the APC-conjugated 103.2 antibody tetramer staining level detected by APC channel (640-nm laser with 670/30-nm filter), and the x axis is the mCherry level detected by PE channel (561-nm laser with 585/15-nm filter). The APC/PE double-positive population of each group is also indicated in the FACS plots (dark purple gate). (Lower Left) The percentile of double-positive population in each group is summarized in a histogram. (Lower Right) The MFI of 103.2 antibody tetramer staining level for each group is depicted in a histogram. (B) Histogram shows CD69 up-regulation level of Jurkat G115 transductants in response to treatment of WT, S207/D124C, or control mutant BTN3A1 transfected HEK293 cells in BTN3A CRISPR KO background with either PBS, isotype control antibody, anti-CD277 agonist antibody (20.1 mAb), pamidronate (NBP), or HMBPP. G115 transfectants treated with 1 μg/mL PHA or PBS served as positive or negative control, and the CD69 level from all of the other experimental groups was normalized according to the controls. Means of three independent experiments with the SD are shown.
Locking BTN3A1 at the V-Shaped Dimer Interface Diminishes pAg-Induced T Cell Activation.
To test whether the conformation of the extracellular domains of BTN3A1 plays a role in pAg-induced T cell activation we assayed the disulfide-linked BTN3A1 mutant for its ability to stimulate Vγ9Vδ2 T cells in their basal state and upon addition of pAgs (HMBPP or NBP) or the 20.1 antibody. Intriguingly, we observed that locking BTN3A1 together at the V-shaped dimer interface (D124/S207C) significantly impaired both pAg-induced and 20.1-induced T cell activation (Fig. 6C). This observation is consistent with the notion that the V-shaped conformation is the resting state of BTN3A1 and is not in itself sufficient for T cell activation.
It has been reported previously that BTN3A1 becomes immobilized on the cell surface in the presence of 20.1 antibody, suggesting that BTN3A1 membrane reorganization is also important for Vγ9Vδ2 T cell activation (4). Thus, we speculated that the D124/S207C mutant might prevent T cell activation by disrupting proper immobilization of BTN3A1 on the cell surface. To formally test this prediction, we performed fluorescence recovery after photobleaching (FRAP) experiments, wherein mCherry-tagged BTN3A1 is photobleached within a small area of the cell surface and the recovery of fluorescence is observed as an indicator of BTN3A1 mobility within the membrane. Contrary to our prediction, we observed that the D124/S207C mutant exhibited no defect in immobilization in the presence of the 20.1 antibody (Fig. 6D and Fig. S5). This is evidence that even though important to Vγ9Vδ2 T cell activation, simply immobilizing BTN3A1 at the cell membrane is insufficient to stimulate Vγ9Vδ2 T cells. Based on the cumulative work presented here, we propose that the activation of Vγ9Vδ2 T cells requires additional features beyond membrane reorganization on the target cell, including a conformational rearrangement of the BTN3A1 extracellular domain.
Fig. S5.
FRAP analysis of BTN3A KO HEK293 cells expressing BTN3A1 constructs. (A) Mean FRAP and fit curves in mCherry WT or D124/S207C BTN3A1-expressing BTN3A KO HEK293 cells, after treatment with anti-CD277 agonist antibody 20.1 mAb (0.5 μg/mL) for 30 min, NBP pamidronate (250 μM) for 12 h, or PBS. The symbols correspond to the mean and SEM of FRAP collected every 2.063 s. The curves were fitted by one-phase exponential equations. The mean fluorescence of whole cell before photobleaching corrected with fluorescence decay over imaging time was counted as one. (B) FRAP analysis of cells expressing BTN3A1 WT or S207/D124C mutant after incubation for 30 min with anti-CD277 agonist antibody or PBS. Data are presented as the value for τ value (Left) or for mobile fraction (Right), measured as described under “FRAP Analysis” in SI Materials and Methods. Bars represent mean values and SD. P values indicated in the plot were calculated using the unpaired Student’s t test method [****P < 0.0001, not significant (ns) P > 0.05]. WT-PBS: n = 19; WT-NBP: n = 29; WT-20.1mAb: n = 24; D124/S207C-PBS: n = 22; D124/S207C-NBP: n = 19; D124/S207C-20.1mAb: n = 2.
Discussion
Human Vγ9Vδ2 T cell recognition of infection and tumorigenesis is of great interest in immunology and directly relevant for immunotherapeutic applications. Despite the significant potential of these cells, a clear understanding of the molecular mechanisms underlying pAg-induced T cell activation still remains elusive. The structural, biophysical, and functional data presented here provide insights into the events after intracellular pAg sensing critical for Vγ9Vδ2 T cell activation.
First, we showed by NMR spectrometry that upon pAg binding the BTN3A1 intracellular B30.2 domain undergoes a conformational change. A significant fraction of residues experiencing major CSP lie in or near the ligand-binding pocket but also include residues located in other regions of the B30.2 domain. Complementary to the experimental data, all-atom MD simulations of the intracellular domain monomer with and without pAg cHDMAPP provide insight into the structural changes that are causing these chemical shifts. Backbone rmsd analysis derived from the MD simulations are consistent with the NMR data and also suggest that the pAg-proximal CSPs, namely those of residues 393–397 and 410–419, are due to a decrease in flexibility of the binding pocket upon pAg binding.
These backbone motions, which show overall flexibility in the apo simulations, can be seen in a relatively short (100 ns) timescale but do not rule out the possibility of slower, global changes in the protein backbone. Consistent with this idea, our NMR data revealed many residues exhibiting broadened signals either in the apo or the complex spectra possibly due to intermediate or slow chemical exchange, thus hindering their assignments of both spectra. Although these residues cannot be subjected to CSP analysis due to missing assignments and likely cannot be probed with all-atom MD simulations, they still reveal valuable information on where the chemical environment change occurs during ligand binding, and intriguingly, most are located remotely from the pAg-binding pocket (Fig. S2).
What could be the main consequence of this conformational change? By mapping residues with major CSP and residues with differential assignments in the newly solved crystal structure of the BFI domain we found that many of these residues concentrated in two dimeric interfaces revealed in the structure. According to the MD simulations, the clusters of residues with major CSP and rmsd shifts reside in or near the Dimer I interface of the BFI. This overlap between interfacial residues and those that shift upon the addition of pAg, as well as the involvement of a residue required for pAg binding and T cell activation, Y352, further implicates a connection between pAg binding and intracellular conformational change (12).
Y352 makes a major contribution to the Dimer I interface interaction through two hydrogen bonds via the hydroxyl group, and the contacts are shown to be long-lived (they persist for nearly the entire simulated trajectory). Based on the location of this residue in relation to the orientation of pAg in the binding site (8), it might engage the isopentenyl chain of pAgs with its hydroxyl group, which could potentially compete with its dimer interface interactions. Indeed, Y352 experiences the largest CSP upon pAg binding, implying that its surrounding chemical environment changes substantially in the presence of pAg. These observations suggest that Y352 might act as a switch that directly links the pAg binding to the change of BTN3A1 intracellular domain dimer conformation. Since other residues within the same dimer interface also experience major change in their chemical environment in the presence of pAg, this pAg-sensing switch is likely to be a synergic effect among all these residues including Y352. This is consistent with our observation that single mutation of Y352 is not sufficient to completely abrogate the pAg-induced T cell activation.
The membrane proximal region of BFI is an important addition to the previously published B30.2 domain structure (8). It is involved in a new, N-to N-terminal dimer interface (Dimer II) and adds stability to this particular interface based on the result from MD simulations. While the BFI interface is stable in simulation for the full 0.5-μs trajectory, the truncated B30.2 interface quickly dissociates (∼100 ns). Although we were unable to resolve the entire membrane proximal region in the crystal structure, previously published work from our laboratory and others suggests that this region interacts with other protein(s) that are important for pAg-induced T cell activation and may adopt a more structured conformation when bound to its partner(s) (10, 11). Consistent with the idea that a conformational change likely occurs in this dimer interface, residues in this region were shown to undergo major CSP in previous studies (9). Because of its location in between the intracellular and the extracellular domains, this region might be critical in translating the pAg-induced conformational change of BTN3A1 from inside to outside of the target cells, which could then be detected by Vγ9Vδ2 T cells. Structural determination of the full stem region with both apo and pAg-bound B30.2 domain could potentially reveal more detailed structural information on how this process would occur.
How is a conformational change of the intracellular domain of BTN3A1 transduced to the extracellular region to be sensed by Vγ9Vδ2 T cells? A critical role of BTN3A extracellular domains in the activation process has been proposed, since the antibody 20.1 against the BTN3A extracellular domains can stimulate Vγ9Vδ2 T cells independent of pAgs. Two distinct dimer conformations, namely a V-shaped and a head-to-tail form, were observed in the crystal structure model of the BTN3A1 extracellular domains (6). We originally speculated that the binding of 20.1 or binding of pAg in the BTN3A1 intracellular domain drives a conformation conversion between the two. Previous investigations of soluble versions of the BTN3A1 extracellular domains via FRET revealed evidence for only the V-shaped form (6). Here we examined the dimer conformations in the context of the full-length BTN3A1 protein in a reconstituted lipid system as well as in the native cellular environment. Consistent with our previous data, we found direct evidence for the existence of the V-shaped dimer form. The presence of the V-shaped extracellular domains in solution, in lipid-like nanodiscs, and in cellular membranes without any exogenously added pAg stimulants indicates that this form is a resting state of the BTN3A1 molecule. Furthermore, locking this dimer interface with engineered disulfide bonds produced BTN3A1 that is defective in its ability to mediate pAg-induced and 20.1-induced T cell activation. Interestingly, the Y352A mutant also showed a defect in both pAg-mediated and 20.1-mediated T cell stimulation. These results suggest that compromised dimer interfaces, either in the extracellular or the intracellular domains, can affect the global configuration of BTN3A1, and thus affect Vγ9Vδ2 T cell stimulation. We also assessed the membrane mobility of this mutant under different conditions using FRAP and found that it behaves much like the WT BTN3A1. Of note, in our FRAP analysis we were unable to observe a significant difference between PBS-treated and pamidronate-treated BTN3A1, both for the WT and the mutant BTN3A1 (Fig. S5). Since pAg-induced membrane reorganization was not impaired in the locked V-shaped mutant, these results suggest that even though membrane reorganization is important for pAg-induced T cell activation, itself alone is not sufficient to stimulate Vγ9Vδ2 T cells, and a conformational rearrangement of the extracellular domain is indeed critical for the activation process.
Outside of the crystallographic data where we observed the head-to-tail dimer we have no data supporting the existence or the functional relevance of this dimer in full-length, cell-surface expressed BTN3A. We originally speculated this could be the resting-state dimer. This is because the dimer interface of the head-to-tail dimer overlaps with the 20.1-binding site, and thus binding of the agonist 20.1 is incompatible with this dimer conformation and would presumably destabilize it on the cell surface (6). However, the data presented here do not support the aforementioned hypothesis. Alternatively, based on the 20.1–BTN3A1 extracellular domain complex structure where we observed a subtle rotational shift in the V-shaped dimer when in complex with 20.1 (6), we hypothesize that a similar shift to BTN3A1 extracellular domains may be induced by pAg binding to the intracellular domain. This “rotational shift” model is consistent with the data we present here. Additional studies will be needed to determine whether such a state exists and, if so, to uncover the unique structural features that enable it to activate Vγ9Vδ2 T cells.
Overall, clarifying the nature of the BTN3A1 conformational change induced by pAgs is essential for understanding how an intracellular pAg-sensing event is transduced to outside of the target cells for possible detection by Vγ9Vδ2 T cells. Elucidation of the molecular mechanisms of pAg-induced T cell activation not only broadens and deepens our understanding of the myriad ways in which γδ T cells can be activated but also lays a foundation for practical applications such as the development of therapeutics targeting certain infectious diseases and cancers.
Materials and Methods
NMR Spectrometry.
NMR spectra were acquired with Bruker AVANCE IIIHD 600-MHz NMR spectrometer equipped with a room-temperature TXI probe. All spectra were acquired on partially deuterated samples at a temperature of 25 °C. It was found that at 600 MHz, HN transverse relaxation-optimized spectroscopy (TROSY) showed higher resolution than the normal HN HSQC. Assignments were thus obtained by analysis of TROSY versions of the standard triple resonance experiments including HNCO, HNcaCO, HNCA, HNCACB, HNcoCA, HNcoCACB, and NcocaNH. Spectra were processed in NMRpipe (12) and then assignments were done with CARA (13). The average CSPs were calculated according to the formula for the residues assigned both in the free and ligand-bound B30.2 domain.
ITC.
Proteins samples for ITC were purified over a Superdex 200 into 20 mM Hepes, pH 7.4, and 150 mM NaCl. Concentration of peak fractions was determined from A280 using the theoretical extinction coefficient. The fractions were pooled and diluted to 50 μM. IPP was obtained from Echelon Biosciences, and cHDMAPP was obtained from Innate Pharma. ITC data were collected using 50 μM B30.2 WT and Y352A mutant in the cell and 2 mM pAg in the syringe on a MicroCal iTC200 (GE Healthcare Life Sciences) at 25 °C using an initial injection of 0.4 μL followed by 19 2-μL injections. ITC binding fits were determined after reference subtraction of injections of the ligand into buffer only from the experimental results. Fits were determined using the Microcal Origin software. The stoichiometry (N) of the IPP-B30.2 interaction was set to one during fitting due to weak affinity.
Crystallization.
Large rod-shaped crystals of apo BTN3A1 BFI were obtained using hanging drops consisting of 1 μL of 12.5 mg/mL protein purified as above with 4 mM β-mercaptoethanol in the protein solution and 1 μL of mother liquor containing 0.1 M Hepes, pH 7.0, 0.2 M MgCl2, and 22% PEG3350. Crystals were transferred into drops containing mother liquor with either an additional 1 mM, 5 mM, or 10 mM cHDMAPP and incubated at room temperature overnight. Of note, these crystals did not show any visible damage. Crystals were then cryoprotected by sequentially transferring to drops containing the mother liquor with an additional 5%, then 10%, and finally 20% glycerol before freezing. The cryoprotectant for each crystal contained 1 mM, 5 mM, or 10 mM cHDMAPP according to pAg-soaking condition of the crystal.
Data Collection and Processing.
X-ray datasets for the BFI-pAg crystals were collected at the Advanced Photon Source (APS) beamline 23-ID-B on a MAR300 CCD at 100 °K. A complete dataset was collected over 360 images using a 0.5° oscillation at a wavelength of 1.033 × 10−10 m. HKL2000 and Mosflm were used to index, integrate, and scale the data (14). An initial molecular replacement solution was obtained using PHASER with PDB ID code 4N7I as a search model (15, 16). The initial model was built using ARP/wARP and improved using Coot with manual building followed by automated individual site, NCS, and B-factor refinement using Refmac5. All structural figures were generated using Pymol (DeLano Scientific).
All-Atom MD Simulations.
All simulations performed were prepared using the CHARMM-GUI Input Generator (17–19). All systems, starting from the BFI crystal structure, were fully hydrated with TIP3P water molecules and neutralized with 0.15 M KCl. All simulations were carried out in simulation boxes with periodic boundary conditions (20) using the additive PARAM36 force field from the CHARMM (Chemistry at HARvard Macromolecular Mechanics) (19). pAg, which has not been parameterized and is not found in the BFI structure, was modeled in based on the binding location determined by Sandstrom et al. (8) with custom parameter files based upon other well-characterized diphosphate parameters. Simulation specifics varied for each system and are described in the SI Materials and Methods but used a combination of NAMD, OpenMM, and AMBER (18, 21–24). For all simulated systems run on the Midway Computing Cluster at the University of Chicago at least two replicas were run to confirm independence of results on initial velocity assignments.
BTN3A Nanodisc Production.
Reconstitution of detergent-solubilized His-tagged BTN3A1/A2 dimers was performed according to the protocols described by the Sligar laboratory with minor modifications (25). BTN3A1/A2 dimer was reconstituted into nanodiscs using either MSP 1D1 or E3D1 (Addgene) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC): TX-100 mixed micelles described in the SI Materials and Methods. Excess of detergent was removed by overnight incubation at 4 °C with Bio-Beads SM-2 Adsorbents (Bio-Rad). BTN3A nanodiscs were separated from proteoliposomes and empty nanodiscs first by Ni-NTA resin pull-down (Qiagen) followed by Superdex 200 10/300 GL column (GE Healthcare) in 50 mM Hepes, pH 7.5, with 50 mM NaCl. As a control, empty nanodiscs were prepared using the same procedure. Gel filtration fractions corresponding to BTN3A1/A2 nanodiscs were concentrated and an aliquot was shipped on ice to G.S. for negative-stain EM. The remainder sample was supplemented with 5% (wt/vol) sucrose, flash-frozen in liquid nitrogen, and stored at −80 °C.
Negative-Stain EM.
BTN3A1/A2 nanodiscs, produced as described above, were prepared for EM using the conventional negative staining protocol (26) and imaged at room temperature with a Tecnai T12 electron microscope operated at 120 kV using low-dose procedures. Images were recorded at a magnification of 71,138× and a defocus value of ∼1.5 μm on a Gatan US4000 CCD camera. All images were binned (2 × 2 pixels) to obtain a pixel size of 4.16 Å on the specimen level. Particles were manually excised using e2boxer (part of the EMAN 2 software suite) 200 (27). Two-dimensional reference-free alignment and classification of particle projections was performed using ISAC (28); 4,359 projections of BTN3A1/A2 nanodisc were subjected to ISAC, producing 70 classes consistent over two-way matching and accounting for 3,968 particle projections.
In Vivo Cross-Linking.
The BTN3A1 S207/D124C-RFP mutant cell lines were cultivated in a 150-mm TC-treated cell culture dish (Falcon) with 25 mL Dulbecco’s modification of Eagle’s medium media with 4.5 g/L glucose, l-glutamine, sodium pyruvate, and 10% FBS (Corning). The cells were either treated with 5 μM HMBPP (Sigma) for 2 h at 37 °C or left untreated before harvesting using a large cell scraper (Corning). The cells were then washed three times with 1× PBS and incubated in PBS, pH 8.0, contained with 0.1 M iodoacetamide (Thermo Scientific) in the dark at room temperature for 30 min. After incubation, the cells were lysed and the plasma membrane (PM) fraction was isolated using the Minute PM protein isolation kit (Invent Biotechnologies, Inc.). The PM fraction was then split into two samples: one was treated with NuPAGE LDS sample buffer (Novex) supplemented with 600 mM β-mercaptoethanol and heated at 95 °C for 5 min, and the other was treated with NuPAGE LDS sample buffer (Novex) alone without heating. All samples were then subjected to SDS/PAGE and Western blot analysis. The primary antibody used was a rabbit polyclonal antibody against residues 344–374 of BTN3A1 (LSBio) and the secondary antibody was a horseradish peroxidase-conjugated goat polyclonal antibody against rabbit IgG (EMD; Millipore). The blot was detected using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
Functional Assays.
For CD69 expression assays target cells were plated at 70% confluence in a 96-well plate (∼10,000 cells in total) and pretreated for 2 h at 37 °C with either CD277-specific mAb (BioLegend) at 5 μg/mL, isotype control mAb (BioLegend) at 5 μg/mL, NBP pamidronate (Sigma) at 125 μM, or HBMPP (Sigma) at 5 μM per well. Treated cells were next extensively washed and cocultured together with Jurkat J.RT3-T3.5 T cells transfected with Vγ9Vδ2 G115 TCR (100,000 cells in total) at 37 °C in complete RPMI-1640 Medium. G115 transductants alone treated with 1 μg/mL phytohemagglutinin (PHA) served as the positive control whereas transductants left untreated served as the negative control. After 4 h, cells were harvested, stained with fluorochrome-labeled Vδ2 TCR-specific mAb (BioLegend) and CD69-specific mAb (BioLegend) analyzed by flow cytometry. Data were collected on a Fortessa 4-15 or a LRII cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar). All of the experimental data were normalized to the positive control (100%) and the negative control (0%) using the following equation:
where MFI is the mean fluorescence intensity of each experimental group or the controls.
More details are provided in SI Materials and Methods.
FRAP.
mCherry-fused WT and D124/S207C mutant BTN3A1 transfected cells were cultivated and imaged in 35-mm, no. 1.5 coverslip, 10-mm glass-bottom dish (MatTek). The cells were subjected to pretreatment either with PBS, 250 μM pamidronate (Sigma), or 5 μg/mL purified anti BT3.1 20.1mAb (Biolegend) for 2 h at 37 °C. Then the cells were imaged under Leica SP5II STED-CW supperresolution laser scanning confocal microscope (with 63×/1.4 UV oil objective). A 561-nm DPSS laser was used at 15% intensity for imaging and a 592-nm depletion laser was used at 80% intensity for photobleaching. The selected rectangular areas within the cell membrane were photobleached for five frames (200 ms per frame, 2-s interval). Images were collected every 5 s for 15 s (three frames, 200 ms per frame) before bleaching and every 2 s for 180 s (90 frames, 200 ms per frame) after bleaching. The images were processed using ImageJ software and the data were analyzed using PRISM6. More details are provided in SI Materials and Methods.
SI Materials and Methods
Protein Expression and Purification.
The BTN3A1 B30.2 domain and the Y352 mutant were cloned, expressed, and purified as previously described (8). The 15N-labeled version of B30.2 domain was expressed in BL21 strain Escherichia coli. The freshly transformed cells were inoculated into LB as a preculture at 37 °C and were grown to a high OD600. The cells were then transferred to a preculture of M9 media (15NH4Cl from Cambridge Isotope Laboratories, Inc.; trace elements: Na2HPO4, KH2PO4, MgSO4, CaCl2, glucose, antibotics, and vitamins) with a 1:100 inoculum. The M9 preculture was incubated overnight at 37 °C with agitation and inoculated into a M9 main culture with a 1:100 inoculum. The cells were grown to OD600 = 0.6 and then induced with 1 mL 1 M isopropyl β-d-1-thiogalactopyranoside (IPTG) per liter of culture for 4 h at room temperature. Protein was harvested and purified using Ni-NTA (Qiagen) immobilized metal affinity chromatography (IMAC) in 20 mM Tris pH, 8.0, 400 mM NaCl, and 20 mM imidazole, eluted with 20 mM Tris, pH 8.0, 400 mM NaCl, and 250 mM imidazole, and desalted into 10 mM Hepes, pH 7.2, 150 mM NaCl, and 0.02% azide using an Econo-Pac 10DG column (Bio-Rad). Protein was cleaved overnight with carboxypeptidase A (Sigma) at room temperature. Protein was further purified by gel filtration over a Superdex 200 column (GE Healthcare) into 10 mM Hepes, pH 7.2, 150 mM NaCl, and 0.02% azide. Protein concentration was initially determined by bicinchoninic acid test and matched A280 measurements using the theoretical extinction coefficient. The 2H, 15N, 13C triple-labeled B30.2 domain was expressed in BL21 strain E. coli. The cells were grown to OD600 = 0.6 in 4 L of LB at 37 °C and spin down at 4,500 rpm for 10 min. The pellet was collected and resuspended in 1 L of M9-D2O media (15NH4Cl and 13C-labeled glucose from Cambridge Isotope Laboratories, Inc.; trace elements: Na2HPO4, KH2PO4, MgSO4, CaCl2, antibotics, vitamins, and thiamine and additive 15N, 13C-labeled Isogro in D2O from Sigma). The cells were incubated for 1 h at room temperature with agitation before inducing with 1 mL 1 M IPTG (dissolved in D2O) for 4 h at room temperature. Protein was harvested and purified as mentioned above.
The BTN3A1 full-length intracellular domain was cloned into pET-28a with a 3C protease site followed by a C-terminal 6x-polyhistidine-tag (6xHis). The construct was expressed in BL21 strain E. coli. Cells were grown to OD600 = 0.6 in LB at 37 °C and induced with 1 mL 1 M IPTG per liter of culture for 4 h at room temperature. Protein was harvested and purified using Ni-NTA (Qiagen) IMAC in 20 mM Tris, pH 8.0, 400 mM NaCl, 20 mM imidazole, 4 mM β-mercaptoethanol (BME), and eluted with 20 mM Tris, pH 8.0, 400 mM NaCl, 250 mM imidazole, and 4 mM BME and desalted into 10 mM Hepes, pH 7.2, 150 mM NaCl, 0.02% azide, and 4 mM BME using an Econo-Pac 10DG column (Bio-Rad). Protein was cleaved overnight using 3C protease at 4 °C. Protein concentration was measured as mentioned above.
The full-length BTN3A1 was subcloned into the pAcGP67A baculovirus transfer vector (BD Biosciences) encoding C-terminal 12x-polyhistidine-tag (12xHis). The full-length BTN3A2 was subcloned into a variant of pAcGP67A with a stop codon introduced upstream of the 12xHis allowing for the expression of tagless form of BTN3A2. Constructs were used for the production of recombinant baculoviruses using BestBac linearized baculovirus DNA (Expression Systems). Proteins were expressed through the infection or coinfection of High Five insect cells and incubation for 72 h at 27 °C and 125 rpm. Surface expression of BTN3A1-12xHis and BTN3A2 was confirmed by flow cytometry using the APC-labeled tetramers of single-chain Fv 20.1 agonist antibody specific to extracellular domains of BTN3 isoforms.
High Five insect cell culture was coinfected with baculoviruses encoding BTN3A1-12xHis and BTN3A2, incubated as described above and spun down for 15 min at 4 °C and 1,700 × g. Cells were washed in 20 mM Hepes buffer, pH 7.2, with 150 mM NaCl and spun down for 15 min at 4 °C and 1,700 × g. Pellet was resuspended in 10 mM Tris buffer, pH 7.9, with 1 mM EDTA and protease inhibitors mixture (PIC; Sigma). Cells were lysed in a glass homogenizer. Lysate was spun down for 30 min at 4 °C and 40,000 × g and the pellet containing membrane fraction was collected. BTN3A1-12xHis/A2 was extracted from the membrane with 50 mM Tris buffer, pH 7.9, with 150 mM NaCl, 1% (vol/vol) Triton X-100 (TX-100; Acros Organics) and PIC, rotated at 4 °C for 1 h. The suspension was spun down for 30 min at 4 °C and 40,000 × g. Detergent soluble fraction was collected and incubated with Ni-NTA resin (Qiagen) in the presence of 30 mM imidazole and 5 mM 2-mercaptoethanol for 1 h at 4 °C. The resin was washed with 50 mM Tris buffer, pH 7.9, with 150 mM NaCl, 30 mM imidazole, 0.1% (vol/vol) TX-100, and 5 mM 2-mercaptoethanol. Protein was eluted with 50 mM Tris buffer, pH 7.9, 150 mM NaCl, 500 mM imidazole, 0.1% (vol/vol) TX-100, and 5 mM 2-mercaptoethanol. Sample containing BTN3A1-12xHis/A2 heterodimer was concentrated using Amicon Ultra filter with 100-kDa molecular cutoff (Millipore) and purified by gel filtration on Superdex 200 10/300 GL column (GE Healthcare) in 10 mM Tris buffer, pH 7.9, with 50 mM NaCl and 0.03% (vol/vol) TX-100.
Preparation of Phospholipids.
The POPC (Avanti) lipids purchased in chloroform were dried in glass vials under a stream of a nitrogen gas. Residual chloroform was additionally removed by overnight incubation under vacuum desiccator; 10 mM of lipids were then dissolved in buffer A (50 mM Hepes, pH 7.5, and 200 mM NaCl) supplemented with 20 mM Triton X-100 (TX-100) and sonicated until clear using a digital sonicator (Branson) with a tapered microtip. A stock of mixed lipid-detergent micelles was stored at −80 °C and thawed directly before reconstitution of nanodiscs.
BTN3A CRISPR KO.
The targeting single-guide RNA component was designed using an online CRISPR design tool (crispr.mit.edu). The target genomic DNA sequence selected was AGGCAGAGTGCACCGTATCG, which locates inside the exon 2 of all BTN3A isoform sequences. The target sequence was cloned into the plasmid pX458 for coexpression of Cas9 as well as GFP as a selection marker. The CRISRP plasmid was then transfected into WT HEK293 cells (ATCC) using Lipofectamine 2000 reagent (Thermo Fisher Scientific). Cell clones were established through single-cell cloning from a sorted pool of successfully transfected cells based on the GFP level. The cell clones were then validated based on the BTN3A expression level and genomic sequencing. One of the successful cell clones was selected for subsequent experiments.
Expression of Full-Length BTN3A1-RFP Proteins in BTN3A KO Background HEK293 Cells.
Carboxyl terminus mCherry-fused BTN3A1 proteins, including the WT, Y352A, D124/S207C, and S61/V161C mutants, were cloned into pcDNA3.1+/Zeo vector (Thermo Fisher Scientific). A16-residue-long serine/glycine linker was also cloned in between the BTN3A1 protein and mCherry. The plasmids were then transfected into the BTN3A KO HEK293 cell clone described above using Lipofectamine 2000 reagent (Thermo Fisher Scientific). The cells were then treated with 200 μg/mL Zeocin reagent (Thermo Fisher Scientific) to establish stably transfected cell lines. After 2 wk of selection, the cells were sorted based both on the mCherry expression level and allophycocyanin (APC)-conjugated 103.2 antibody tetramer staining level. Only the double-positive populations from these cells are used for subsequent experiments. All cells including the HEK293 parental cells and the BTN3A KO HEK293 cell clone were cultivated in Dulbecco’s modification of Eagle’s medium media with 4.5 g/L glucose, l-glutamine, sodium pyruvate, and 10% FBS (Corning) under a 37 °C and 5% CO2 condition.
FRAP Analysis.
The mean fluorescence intensity (I) of bleached areas (FRAP), whole-cell areas (W), and background areas (B) of prebleached (pre) and postbleached (post) cells were measured and the normalized FRAP traces were generated using the following equation:
where “i” denotes each time point during the postbleached imaging period (every 2.063 s during a180-s period). The data points were then plotted and fitted using GraphPad PRISM5 software. The curves were fitted using the one-phase association model
where “t” denotes each time point during the postbleached imaging period (every 2.063 s during a 180-s period). The immobile fraction is defined by the following equation:
where “Plateau” is the fitted normalized recovery value at 185.67 s. The mobile fraction is defined by the following equation:
where “Plateau” is the fitted normalized recovery value at 185.67 s and “Y0” is the fitted normalized recovery value at 0 s. For the τ value, the curves were first fitted using the one-phase association model as described above, but under the constraint that Y0 is shared by all groups and is less than 1. The τ then is defined by the following equation:
All-Atom MD Simulations.
BFI monomer simulations with and without pAg were run in cubic simulation boxes on graphical processing units (GPUs) using the python-based OpenMM toolkit (version 6.2) with a 2-fs time step at 293.15 K (22, 23). Each replica was equilibrated with a 1-fs time step for 250 ps and run for 100 ns of simulation time. Data were analyzed using MATLAB once the simulation was fully equilibrated, when the backbone rmsd reached a stable value.
BFI Dimer I and Dimer II simulations were run in rectangular simulation boxes to minimize the number of atoms in the system and were run using the AMBER16 GPU software (24). To get fast and robust sampling, hydrogen mass repartitioning of both the protein and solvent, a frictional coefficient of 0.3 to lower effective viscosity, and a 4-fs time step were used (29). To ensure the dimers did not float out of the rectangular simulation box and interact with periodic images a spatial constraint was applied to noninterfacial residues on one of the monomers in the simulation box. A third, unconstrained replica set of dimer simulations was run using the NAMD software package with a 2-fs time step for 100 ns to confirm that the steps taken to enhance sampling did not affect the outcome of the simulations (21).
Equilibrated systems use an NVT ensemble and production runs use an NPT ensemble, with the temperature kept constant using Langevin dynamics (30). The simulations were kept at constant pressure at one bar with the Nosé–Hoover Langevin piston by allowing the cell box size to change semiisotropically (31). van der Waals interactions were computed using a Lennard-Jones force-switching function over 10–12 Å while long-range electrostatics used particle mesh Ewald (32). Production runs used a 2-fs time step and the SHAKE algorithm to constrain the bonds having hydrogen atoms (33).
Acknowledgments
We thank the staff of the APS at GM/CA-CAT (23-ID) for their use and assistance with X-ray beamlines and Dr. Ruslan Sanishvili in particular for help and advice during data collection, Dr. John Leonard and Dr. Sobhan Roy for advice and insightful discussion, Dr. C. Belmant from Innate Pharma for providing cHDMAPP, Dr. Caitlin Castro for help and advice during CRISPRi KO cell line generation and cellular functional assays, and Dr. Christine Labno at the Integrated Light Microscopy Core Facility, University of Chicago, for help and advice in conducting FRAP experiments. This work was supported by NIH Grant R01 AI115471. The crystallographic data were collected, processed and interpreted during the 2015 CCP4/APS School in macromolecular crystallography held in APS. All NMR spectra were collected in the Biomolecular NMR Core Facility at the University of Chicago.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID code 5HM7).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707547114/-/DCSupplemental.
References
- 1.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: Discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 2.Gober HJ, et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang F, et al. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 4.Harly C, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abeler-Dörner L, Swamy M, Williams G, Hayday AC, Bas A. Butyrophilins: An emerging family of immune regulators. Trends Immunol. 2012;33:34–41. doi: 10.1016/j.it.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Palakodeti A, et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J Biol Chem. 2012;287:32780–32790. doi: 10.1074/jbc.M112.384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vavassori S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol. 2013;14:908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 8.Sandstrom A, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao CH, et al. Synthesis of a phosphoantigen prodrug that potently activates Vγ9Vδ2 T-lymphocytes. Chem Biol. 2014;21:945–954. doi: 10.1016/j.chembiol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Sebestyen Z, et al. RhoB mediates phosphoantigen recognition by Vγ9Vδ2 T cell receptor. Cell Rep. 2016;15:1973–1985. doi: 10.1016/j.celrep.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes DA, et al. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol. 2015;194:2390–2398. doi: 10.4049/jimmunol.1401064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaglio F, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 13.Keller RLJ, Meier BH, Pervushin K. 2005. Optimizing the process of nuclear magnetic resonance spectrum analysis and computer aided resonance assignment. PhD dissertation (Naturwissenschaften, Eidgenössische Technische Hochschule, ETH Zürich, Zurich), no. 15947.
- 14.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 15.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinert C, Grütter C, Roschitzki-Voser H, Mittl PR, Grütter MG. The crystal structure of human pyrin b30.2 domain: Implications for mutations associated with familial Mediterranean fever. J Mol Biol. 2009;394:226–236. doi: 10.1016/j.jmb.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Jo S, Kim T, Im W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS One. 2007;2:e880. doi: 10.1371/journal.pone.0000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J Chem Theory Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 21.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eastman P, et al. OpenMM 4: A reusable, extensible, hardware independent library for high performance molecular simulation. J Chem Theory Comput. 2013;9:461–469. doi: 10.1021/ct300857j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedrichs MS, et al. Accelerating molecular dynamic simulation on graphics processing units. J Comput Chem. 2009;30:864–872. doi: 10.1002/jcc.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomon-Ferrer R, Götz AW, Poole D, Le Grand S, Walker RC. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J Chem Theory Comput. 2013;9:3878–3888. doi: 10.1021/ct400314y. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie TK, et al. Chapter 11 - Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–231. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filizola M. G Protein-Coupled Receptors in Drug Discovery: Methods and Protocols. 2nd Ed. Springer; New York: 2015. [DOI] [PubMed] [Google Scholar]
- 27.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Fang J, Chittuluru J, Asturias FJ, Penczek PA. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure. 2012;20:237–247. doi: 10.1016/j.str.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins CW, Le Grand S, Walker RC, Roitberg AE. Long-time-step molecular dynamics through hydrogen mass repartitioning. J Chem Theory Comput. 2015;11:1864–1874. doi: 10.1021/ct5010406. [DOI] [PubMed] [Google Scholar]
- 30.Hoover WG. Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A Gen Phys. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 31.Nose S, Klein ML. A study of solid and liquid carbon tetrafluoride using the constant pressure molecular-dynamics technique. J Chem Phys. 1983;78:6928–6939. [Google Scholar]
- 32.Darden T, York D, Pedersen L. Particle mesh Ewald - An N.Log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 33.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-integration of cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]