Significance
The therapeutic activity of checkpoint blockers and toll-like receptor (TLR) agonists, which show some efficacy against malignancies, appears to at least partially result from the secretion of type-I IFNs. Thus, we hypothesized that type-I IFN-inducible transcription factors, such as basic leucine zipper transcription factor ATF-like 2 (Batf2), might play a role in tumor immunity. Here, we investigated the role of Batf2, especially its positive transcriptional activities, and evaluated its antitumor effect. This study shows that Batf2 has an antitumor effect through the up-regulation of IL-12 p40 in tumor-associated macrophages, which eventually induces the activation of CD8+ T cells and their accumulation within the tumor. Batf2 may be an important target in anticancer treatment with immune checkpoint blockers and TLR agonists.
Keywords: Batf2, macrophage, IL-12 p40, NF-κB, TLR7
Abstract
The development of effective treatments against cancers is urgently needed, and the accumulation of CD8+ T cells within tumors is especially important for cancer prognosis. Although their mechanisms are still largely unknown, growing evidence has indicated that innate immune cells have important effects on cancer progression through the production of various cytokines. Here, we found that basic leucine zipper transcription factor ATF-like 2 (Batf2) has an antitumor effect. An s.c. inoculated tumor model produced fewer IL-12 p40+ macrophages and activated CD8+ T cells within the tumors of Batf2−/− mice compared with WT mice. In vitro studies also revealed that the IL-12 p40 expression was significantly lower in Batf2−/− macrophages following their stimulation by toll-like receptor ligands, such as R848. Additionally, we found that BATF2 interacts with p50/p65 and promotes IL-12 p40 expression. In conclusion, Batf2 has an antitumor effect through the up-regulation of IL-12 p40 in tumor-associated macrophages, which eventually induces CD8+ T-cell activation and accumulation within the tumor.
Malignant neoplasms are life-threatening diseases which urgently require the development of effective treatments. Recently, immune checkpoint blockers and toll-like receptor (TLR) agonists have proven effective as anticancer therapeutics (1, 2), demonstrating that the immune system plays a critical role in tumor suppression. The accumulation of CD8+ T cells within the tumor environment is a favorable prognostic factor (3) and is especially important for the efficacy of these treatments (4); however, the mechanisms underlying this process are still largely unknown. As key players that influence the number of CD8+ T cells within tumors, innate immune cells such as macrophages and dendritic cells (DCs) greatly affect tumorigenesis and immunotherapy via the secretion of various cytokines (5). The type-I IFNs may be involved in the therapeutic activity of checkpoint blockers (1). Furthermore, TLR7 stimulation in DCs also leads to the induction of IFN-inducible genes (6). Thus, we hypothesized that type-I IFN-inducible genes might be involved in tumor immunity. Using an s.c. inoculated mouse tumor model we investigated the potential antitumor effect of one of the type-I IFN-inducible transcription factors, basic leucine zipper transcription factor ATF-like 2 (Batf2).
The Batf2 gene is located on mouse chromosome 19 (human chromosome 11q), and the BATF2 (SARI) protein has 59% conservation between mice and humans (7). Along with BATF2, the BATF family includes BATF (SFA2) and BATF3 (JDP1; p21SNFT), and its members also belong to the AP-1 basic leucine zipper transcription factor family. Although BATF family members were initially thought to function only as inhibitors of AP-1 (8), recent studies have suggested that these factors additionally have positive and unique transcriptional activities (7).
Here, we assessed the role of Batf2, particularly its positive transcriptional activities via TLR signals, and evaluated its antitumor effect on tumor-associated macrophages (TAMs) because these cells subsequently affect the number of CD8+ T cells in the tumor environment.
Results
In an s.c. Inoculated Tumor Model, Significant Tumor Growth Was Observed in the Batf2−/− Mice.
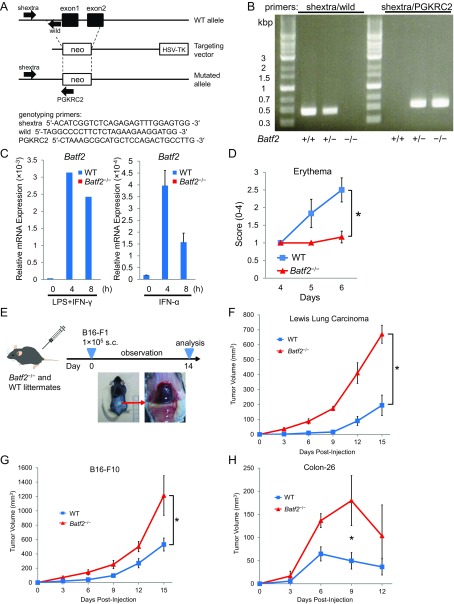
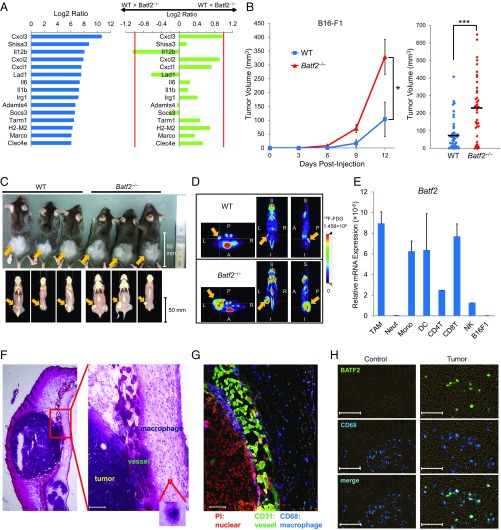
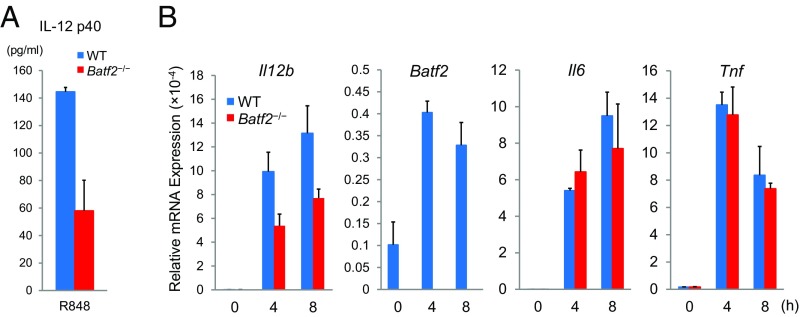
Because TLR7 agonists are clinically used against tumors and have proven effective as antitumor drugs, we first assessed effect of Batf2 on the in vivo responses to TLR7 ligands. Skin erythema induced by the topical application of imiquimod, a TLR7 ligand, was milder in Batf2−/− mice (Fig. S1 A–C) than in WT mice (Fig. S1D). Next, we performed microarray analyses using bone marrow-derived macrophages (BMDMs) from Batf2−/− and WT mice that had been stimulated with R848, another TLR7 ligand. The expression level of Il12b, one of the top 15 most highly expressed genes in the microarray analysis, was notably lower in BMDMs from Batf2−/− mice than in those from WT littermates (Fig. 1A). IL-12 is known as an important antitumor cytokine and rapid growth of B16-F1 nodules was reported in TLR7-deficient mice (9, 10). Therefore, we similarly established mouse models with s.c. injected tumor cells. Batf2−/− mice and WT littermate controls were injected s.c. with B16-F1 melanoma cells, and the resulting tumor growth was monitored (Fig. S1E). Significantly more tumor growth was observed in Batf2−/− mice than in WT littermates (Fig. 1B). These results were confirmed by PET–computed tomography (PET–CT) scans (Fig. 1 C and D). Similar results were obtained with other tumor cells: Lewis lung carcinoma, B16-F10, and Colon-26 (Fig. S1 F–H). Next, leukocyte subpopulations in the tumor tissues of WT mice were sorted, and Batf2 mRNA expression was quantified using qPCR. Batf2 was highly expressed in DCs, in CD8+ T cells, and especially in TAMs within tumor tissues (Fig. 1E). In the corresponding histological analysis many TAMs were observed in the stroma around the tumors (Fig. 1 F and G), and Batf2 was expressed in TAMs but not in the macrophages in normal skin (Fig. 1H).
Fig. S1.
A schematic of the mouse Batf2 gene, genotyping results, and the s.c. inoculated mouse tumor model. (A) A schematic of the mouse Batf2 gene, the targeting vector, and the targeted allele. (B) Genotyping PCR of Batf2+/+, Batf2+/−, and Batf2−/− mice. As indicated in A, primers shextra and wild together detect a fragment of the WT genomic locus, while primers shextra and PGKRC2 together detect a fragment of the mutant allele. (C) The relative expression levels of Batf2 mRNA in BMDMs from Batf2−/− or WT littermates following stimulation by LPS (100 ng/mL) and IFN-γ (30 ng/mL) were quantified using qPCR (Left). The Batf2 mRNA levels in BMDMs from Batf2−/− or WT littermates following stimulation with 103 U/mL of recombinant IFN-α were quantified using qPCR (Right). Error bars indicate ±SEM (n = 2). (D) WT and Batf2−/− mice were treated daily with imiquimod cream on shaved back skin for 6 d. The resulting erythema scores on days 4−6 are shown. Error bars indicate ±SEM (n = 6). *P < 0.05. (E) A schematic of the s.c. inoculated mouse tumor model. For this model, 1 × 105 B16-F1 cells were injected s.c. into Batf2−/− mice and WT littermates. The tumor volumes were calculated using the formula (width)2 × length × 0.52. (F–H) Tumor growth kinetics in Batf2−/− (triangle) and WT (square) littermates following an s.c. injection of 1 × 106 Lewis lung carcinoma cells (F), 1 × 106 B16-F10 melanoma cells (G), or 5 × 105 Colon-26 cells (H) per mouse. Average kinetics ± SEM of three (F), five (G), or three (H) mice per group. *P < 0.05.
Fig. 1.
Tumor growth in Batf2−/− mice and WT littermates. (A) BMDMs from WT and Batf2−/− littermates were stimulated with R848 for 8 h, and the total RNA from these cells was subjected to microarray analysis. The log2 ratios of the top 15 most highly expressed genes in WT BMDMs are shown (Left). The log2 ratio for each of the corresponding genes in Batf2−/− BMDMs was subtracted from that of WT, and the results were compared between WT and Batf2−/− (Right). (B) Tumor growth kinetics in Batf2−/− (triangle) and WT (square) littermates following their s.c. injection with 1 × 105 B16-F1 melanoma cells (Left). Average kinetics ± SEM of n = 6–7 mice per group. Tumor size on days 12–14 in Batf2−/− (n = 42) and WT (n = 44) littermates following their s.c. injection with B16-F1 cells (Right). Data are from 10 independent experiments. Bars show means. *P < 0.05; ***P < 0.001. (C) Photographic (Upper) and FDG PET–CT fusion (Lower) images of mice on day 17 after receiving an s.c. injection of B16-F1 cells (arrows). (D) Representative 18F-FDG PET images for tumor detection in Batf2−/− (Lower) and WT (Upper) mice on day 17 after receiving an s.c. injection of B16-F1 cells (arrows). A, anterior; I, inferior; L, left; P, posterior; R, right; S, superior. White cross-hairs indicate the locations of the other two cross-sections. (E) Leukocyte subpopulations in the tumor tissues of WT mice (n = 14) 2 wk post-B16-F1 implantation were sorted, and their relative Batf2 mRNA expression levels were quantified using qPCR. Data are expressed as mean ± SEM of triplicates. CD4T, CD4+ T cells; CD8T, CD8+ T cells; Mono, monocytes; Neut, neutrophils; NK, natural killer cells. (F and G) Histological observation of tumor tissues in a B16-F1 melanoma mouse model: H&E stain (F) and immunofluorescence detection (G). PI (red), CD31 (green), and CD68 (blue) are shown. (H) Immunofluorescence detection of BATF2 (green) and CD68 (blue) in the normal skin from naïve WT mice or in tumor tissues 2 wk after B16-F1 implantation in WT mice. (Scale bar, 100 μm in F–H.) All experiments were performed on male littermates.
There Were Fewer IL-12 p40+ Macrophages and Activated CD8+ T Cells Within the Tumors of Batf2−/− Mice.
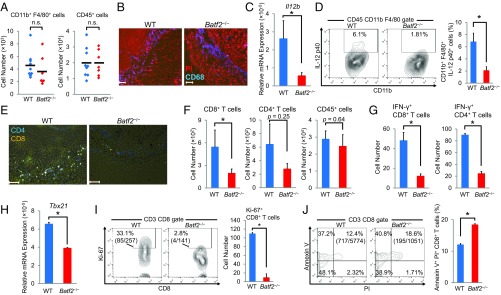
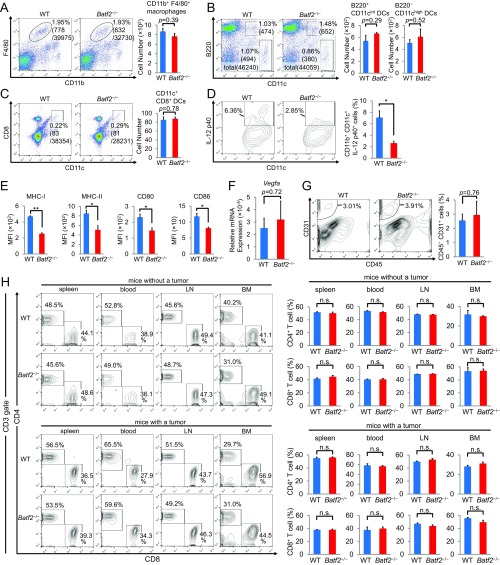
Although there were no significant differences in the number of TAMs, splenic macrophages, or DCs between Batf2−/− mice and WT littermates (Fig. 2 A and B and Fig. S2 A–C), the Il12b mRNA levels were lower in Batf2−/− TAMs than in WT TAMs (Fig. 2C). A flow cytometric analysis showed that the IL-12 p40 expression was also lower in Batf2−/− TAMs and DCs (Fig. 2D and Fig. S2D). Furthermore, the mean fluorescent intensities (MFIs) of MHC class I, class II, CD80, and CD86 were each lower in tumor-infiltrating DCs from Batf2−/− mice than in those from WT mice (Fig. S2E). We also measured the Vegfa expression in BMDMs from Batf2−/− and WT littermates and measured angiogenesis within the mouse tumors; however, we found no significant differences in these metrics between the two groups (Fig. S2 F and G).
Fig. 2.
Analysis of immune cells within the tumors of WT and Batf2−/− mice. All samples were obtained from the tumor tissues of Batf2−/− and WT littermates 2 wk after B16-F1 implantation. (A) The numbers of tumor-infiltrating CD11b+ F4/80+ cells (Left) and CD45+ cells (Right). Data are from five independent experiments (n = 7–9). Bars show means. (B) Tumor tissue macrophages were examined by fluorescent staining of PI (red) and CD68 (blue). (C) Tumor tissue macrophages were sorted (n = 5–6), and their relative Il12b mRNA expression levels were quantified using qPCR. Data are expressed as mean ± SEM from three independent experiments. (D) The IL-12 p40 expressions of CD45+ CD11b+ F4/80+ macrophages from tumor tissues were analyzed by flow cytometry. Numbers represent the percentages of IL-12 p40+ cells within the CD45+ CD11b+ F4/80+ cell population. Representative plots are shown (Left). Data are expressed as mean ± SEM from three independent experiments (Right, n = 5). (E) Tumor-infiltrating T cells were examined by immunofluorescent staining of CD4 (blue) and CD8 (yellow). (F) Flow cytometric analysis of tumor-infiltrating CD8+ T cells, CD4+ T cells, and CD45+ cells. Numbers indicate the actual numbers of detected cells. Data are expressed as mean ± SEM from two independent experiments (n = 4). (G) Flow cytometric analyses of tumor-infiltrating IFN-γ+ CD4+ T cells and IFN-γ+ CD8+ T cells. Data are expressed as mean ± SEM from four independent experiments (n = 4). (H) Tumor-infiltrating CD4+ T cells were sorted (n = 2–3), and their relative mRNA expression levels of Tbx21 were quantified using qPCR. Data are expressed as mean ± SEM from two independent experiments. (I and J) Flow cytometric analyses of tumor-infiltrating, Ki-67+ CD8+ T cells (I) and Annexin V+ PI+ CD8+ T cells (J). Numbers represent the actual cell numbers and the percentages of these cells within the CD8+ T-cell population. Representative plots are shown (Left). Samples were obtained from one (I) or three pair(s) of mice (J). Data are expressed as mean ± SEM from two independent experiments (Right). All experiments were performed on male littermates. (Scale bar, 100 μm.) In all figures, *P < 0.05; n.s., not significant (P > 0.05).
Fig. S2.
Flow cytometric analyses of macrophages, DCs, and T cells. (A) A flow cytometric analysis of CD11b+ F4/80+ splenocytes of tumor-bearing mice. Numbers represent the percentage of cells within the total cell population or the actual cell number. Representative plots are shown (Left). Data are expressed as mean ± SEM from three independent experiments (Right, n = 4). (B–D) Flow cytometric analyses of DCs within the population of splenocytes from tumor-bearing mice (B and C) or within the B16-F1 tumors (D). Representative plots are shown (Left). Data are expressed as mean ± SEM from two independent experiments (Right). Numbers represent either the actual cell number or the percentage of cells within the total cell population (B, n = 3), CD45+ cell population (C, n = 3), or CD45+ CD11c+ cell population (D, n = 4). (E) Mean MFI of MHC class I, class II, CD80, and CD86 in tumor-infiltrating DCs (n = 3). Data are expressed as mean ± SEM from two independent experiments. (F) The Vegfa mRNA expression in BMDMs that had been stimulated with R848 for 4 h was quantified using qPCR. Data are expressed as mean ± SEM from two independent experiments (n = 3). (G) A flow cytometric analysis of CD45− CD31+ endothelial cells in the tumor tissues from Batf2−/− mice and WT littermates. Numbers represent the percentage of cells within the total tumor cell population. Representative plots are shown (Left). Data are expressed as mean ± SEM (Right, n = 2). (H) Flow cytometric analyses of the T-cell populations within the spleen, blood, lymph nodes (LN), and BM of naïve or tumor-bearing mice. Numbers represent the percentage of cells within the CD3+ T-cell population. Representative plots are shown (Left). Data are expressed as mean ± SEM (Right, n = 3). All experiments were performed on male littermates. In all figure parts, *P < 0.05; **P < 0.01; n.s., not significant (P > 0.05).
Next, we analyzed the T-cell populations in the tumors of Batf2−/− and WT mice. There were significantly fewer B16-F1 tumor-infiltrating CD8+ T cells in Batf2−/− mice than in WT mice (Fig. 2 E and F). The CD4+ T-cell number in tumor tissues also trended lower in Batf2−/− mice; however, this difference was not statistically significant. The total population of tumor-infiltrating CD45+ leukocytes was similar between Batf2−/− and WT littermates (Fig. 2F). The numbers of IFN-γ+ CD4+ T cells and IFN-γ+ CD8+ T cells were also lower in tumor tissues from Batf2−/− mice compared with those from WT littermates (Fig. 2G). Additionally, the mRNA levels of Tbx21 in tumor-infiltrating CD4+ T cells from Batf2−/− mice were lower than those from WT mice (Fig. 2H). Furthermore, the Ki-67 expression levels in CD8+ T cells were significantly lower in Batf2−/− mice (Fig. 2I). Because IFN-γ is an antitumor cytokine and Ki-67 is a proliferation marker, these data indicate that there were fewer activated and proliferating CD8+ T cells within the tumors of Batf2−/− mice. The population of annexin V+ and propidium iodide (PI)+ CD8+ T cells was significantly larger in Batf2−/− mouse tumor tissues, indicating that the percentage of tumor-infiltrating CD8+ T cells undergoing end-stage apoptosis and death was significantly higher in Batf2−/− mice (Fig. 2J). The populations of CD8+ T cells in the spleen, blood, lymph nodes, and bone marrow (BM) were similar between Batf2−/− and WT littermates with or without tumors (Fig. S2H).
The Batf2 Antitumor Effect Occurs via BM-Derived Immune Cells.
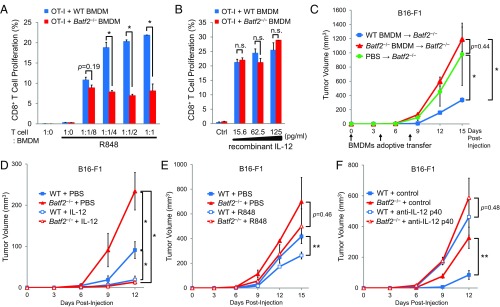
We performed the same tumor inoculation experiments described above using BM chimeric mice, which were reconstituted with BM cells isolated from Batf2−/− or WT littermates. As with conventional mice, the mean B16-F1 tumor volumes were significantly higher in Batf2−/− chimeric mice (Fig. S3A). The TAM and T-cell flow cytometric results from BM chimeric mice bearing B16-F1 tumors were similar to those in conventional mice (Fig. S3 B–D), as were results observed with Lewis lung carcinoma (Fig. S3 E and F). Thus, Batf2 depletion deteriorates the antitumor effect, which relies on BM-derived immune cells.
Fig. S3.
Tumor growth in BM chimeric mice. (A) Lethally irradiated WT mice received BM transplants from Batf2−/− or WT littermates. Eight weeks after BM transplant, the chimeric mice were each injected s.c. with 1 × 105 B16-F1 cells, and tumor growth was monitored (Left). Tumor sizes on day 18 are shown (Right). Error bars indicate ±SEM. (B) A flow cytometric analysis was performed on the tumor tissues of BM chimeric mice 2 wk after they received an implantation of B16-F1 cells. The populations of CD45+ CD11b+ F4/80+ macrophages from tumor tissues and their IL-12 p40 expressions were analyzed by flow cytometry. Numbers represent the percentages of F4/80+ cells within the CD45+ CD11b+ cell population (Upper) and of IL-12 p40+ cells within the CD45+ CD11b+ F4/80+ cell population (Lower). (C and D) An analysis of T cells within the tumor tissues of BM chimeric mice. Numbers represent the percentage of cells within the tumor-infiltrating CD45+ CD3+ cell (C) or CD8+ T-cell (D) populations. Representative plots from two independent experiments are shown. (E) BM chimeric mice were each injected s.c. with 1 × 106 Lewis lung carcinoma cells, and tumor growth was monitored (Left). Tumor sizes on day 12 are shown (Right). Error bars indicate ± SEM. (F) A photographic image of BM chimeric mice taken 15 d after the mice received an s.c. injection of Lewis lung carcinoma cells (E). Bars show means. In all figure parts, *P < 0.05; **P < 0.01; ***P < 0.001.
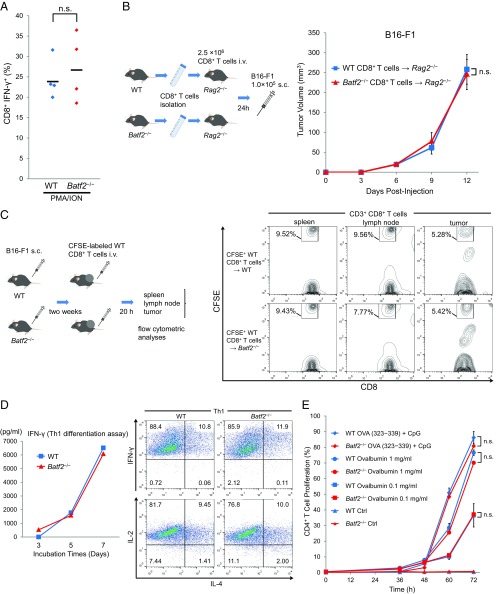
The defective IFN-γ expression by Batf2−/− CD8+ T cells suggests that Batf2−/− mice may have a CD8+ T-cell functional defect. To investigate this possibility, we first analyzed naïve CD8+ T cells by stimulation with phorbol myristate acetate (PMA) and ionomycin. However, we did not find any significant differences in the CD8+ T-cell IFN-γ expression (Fig. S4A). Next, we performed CD8+ T-cell adoptive transfer experiments in Rag2−/− mice. Compared with WT control transfers, the tumor growth kinetics were similar in Rag2−/− mice that received CD8+ T cells transferred from Batf2−/− mice (Fig. S4B). These data show that the higher levels of tumor growth in Batf2−/− mice described above likely result from a defect of other immune subpopulations, such as myeloid cells, rather than one of CD8+ T cells. The results of in vivo migration assays additionally demonstrate that the CD8+ T-cell chemoattractant activities in Batf2−/− tumors should be normal (Fig. S4C).
Fig. S4.
Analysis of the T-cell activities in Batf2−/− mice and WT littermates. (A) Splenic CD8+ T cells were isolated from Batf2−/− mice and WT littermates without tumors (naïve) and then stimulated with PMA and ionomycin (ION) for 4 h in the presence of monensin for the last 2 h. The IFN-γ expressions in these CD8+ T cells were analyzed by flow cytometry. Data are from two independent experiments (n = 4). (B) Naïve splenic CD8+ T cells isolated from Batf2−/− mice or WT littermates were transferred i.v. into Rag2−/− recipients. After 24 h, the recipient mice were injected s.c. with B16-F1 tumor cells (schematic, Left). Tumor growth was monitored (Right) in the mice with Batf2−/− CD8+ T cells (triangle) or WT CD8+ T cells (square). Average kinetics ± SEM of n = 3 mice per group. (C) In vivo migration assay. Naïve CD8+ T cells isolated from WT splenocytes (n = 14) were labeled with CFSE, and 2 × 107 of these CD8+ T cells per mouse were injected i.v. into Batf2−/− or WT littermates. After 20 h, lymphocytes were obtained from the spleen, lymph nodes, and tumor tissues and analyzed by flow cytometry (schematic, Left). Data are representative of three independent experiments (Right). Numbers represent the percentage of cells within the CD8+ T-cell population. (D) Th1 differentiation assay. Naïve CD4+ T cells from Batf2−/− mice or WT littermates were purified by cell sorting. The cells were cultured in an anti-CD3 antibody-coated plate in the presence of mIL-12, anti–IL-4, mIL-2, and anti-CD28. ELISAs were performed to assess the changes in the culture medium IFN-γ levels over time (Left). After 7 d, the cells were analyzed by flow cytometry (Right). Data are representative of two independent experiments. (E) MHC II-restricted antigen-presentation experiments. CFSE-labeled OT-II transgenic CD4+ T cells were incubated with the splenic CD11c+ DCs from Batf2−/− mice or WT littermates in the continuous presence of 1 mg/mL or 0.1 mg/mL chicken ovalbumin or with 1 μM Ova peptide (323-339) together with 1 µg/mL CpG1826. The percentages of CD4+ CFSElow cells were assessed by flow cytometry after 72 h. A time course of changes in the percentages of the proliferating CD4+ T cells is shown. Data are expressed as mean ± SEM of duplicates. All experiments were performed on male littermates. Bars show means. In all figures, n.s., not significant (P > 0.05).
We also performed Th1 differentiation assays to examine the possibility that Batf2−/− mice have a functional defect of CD4+ T-cell differentiation into Th1 cells. However, we did not find any significant differences in Th1 cell differentiation between Batf2−/− and WT littermates (Fig. S4D). Additionally, MHC II-restricted antigen presentation was normal in CD4+ T cells from Batf2−/− mice (Fig. S4E).
In Vitro IL-12 p40 Levels Are Significantly Lower in Batf2−/− Macrophages than in WT Macrophages.
We performed ELISAs to examine the levels of TNF-α, IL-6, and IL-12 p40 secreted by Batf2−/− and WT BMDMs following their stimulation by R848. Although there were no significant differences in the TNF-α and IL-6 levels, the IL-12 p40 levels were significantly lower in the culture medium of Batf2−/− BMDMs than in that of WT BMDMs (Fig. 3A). Additionally, the Il12b mRNA expression level was significantly lower in Batf2−/− mice than in WT mice (Fig. 3B). Similar results were obtained in Batf2-deficient BM-derived DCs (BMDCs) (Fig. S5).
Fig. 3.
In vitro protein expression and mRNA levels in BMDMs from WT or Batf2−/− mice. (A) Levels of IL-12 p40, TNF-α, and IL-6 secreted by BMDMs following stimulation by serially diluted concentrations of R848 for 24 h were measured by ELISA. Data are from five independent experiments (n = 10). (B) Relative expression levels of Il12b, Batf2, Tnf, Il6, Ifnb1, and Nos2 in BMDMs stimulated by R848 (100 ng/mL) for 4 h were quantified using qPCR. Data are expressed as mean ± SEM from two independent experiments (n = 3). (C) The mRNA levels of Batf2 and Il12b in BMDMs that had been treated with DOTAP for 4 h were quantified using qPCR. BMDMs were treated with either DOTAP alone, DOTAP formulations of RNA, or RNA alone. RNA was isolated from B16-F1 cells. Data are from three independent experiments (n = 3) and expressed as mean ± SEM. All experiments were performed on male littermates. Bars show means. In all figures, *P < 0.05; **P < 0.01; n.s., not significant (P > 0.05).
Fig. S5.
The gene expression levels in BMDCs. (A) Secreted IL-12 p40 levels of BMDCs that had been stimulated by R848 (100 ng/mL) for 24 h were measured by ELISA. Data are expressed as mean ± SEM of duplicates (n = 2). (B) The relative expression levels of indicated genes in BMDCs that had been stimulated by R848 for 4 h were quantified using qPCR. Data are expressed as mean ± SEM (n = 2). Similar results were obtained in two independent experiments.
Given that TLR7 ligands are single-stranded RNA molecules, these data suggest that the Batf2 antitumor effect may also be induced by tumor-derived RNA within the tumor environment. Exosomes are vesicles that contain various proteins and RNA, mediating communication between cells (11). To mimic the cancer exosomes in which RNA is enclosed, we used DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N, N, N-trimethylammonium methyl-sulfate) liposomal formulations of RNA as previously reported (12). With this method, we found that the Il12b and Batf2 mRNA levels in the BMDMs of WT mice were highly induced by DOTAP plus RNA, while the induced Il12b mRNA levels were lower in the BMDMs of Batf2−/− mice (Fig. 3C).
Batf2−/− Macrophages Cause Less CD8+ T-cell Proliferation and Have Deteriorated Antitumor Responses.
The observed deterioration of the antitumor response in Batf2−/− mice might be due to the lower IL-12 p40 expression by macrophages in these mice. Therefore, we performed antigen-presentation assays with OT-I transgenic mouse cells. Under R848 stimulation, a significantly lower proliferation level was observed in the OT-I CD8+ cells cultured with BMDMs from Batf2−/− mice compared with those cultured with WT BMDMs (Fig. 4A). In contrast, the OT-I T-cell proliferation levels were similar when these cells were cocultured with recombinant IL-12 instead of R848 (Fig. 4B), suggesting that the lower amount of CD8+ T-cell proliferation observed was due to the defective IL-12 p40 expression by Batf2−/− macrophages. To investigate the in vivo implications we performed BMDM adoptive transfers. Significantly more tumor growth was observed in the Batf2−/− recipient mice that received Batf2−/− BMDMs than in those that received WT BMDMs (Fig. 4C). Additionally, tumor growth was significantly suppressed by treatment with recombinant IL-12 in both Batf2−/− mice and WT littermates (Fig. 4D). In contrast, the R848 antitumor efficacy against B16-F1 was smaller than that of PBS treatments in Batf2−/− mice (Fig. 4E). Furthermore, the significant difference in tumor growth between WT and Batf2−/− mice disappeared following treatment with an anti-IL-12 p40 neutralizing antibody (Fig. 4F).
Fig. 4.
Analysis of the role of Batf2 in CD8+ T-cell proliferation and antitumor responses. (A) Carboxyfluorescein succinimidyl ester (CFSE)-labeled CD8+ T cells from OT-I transgenic mice and BMDMs from Batf2−/− or WT littermates pulsed with Ova 257–264 peptide (SIINFEKL) were cocultured at decreasing dilutions (OT-I:BMDM = 1:1–1:1/8) together with R848. Proliferation of OT-I cells was assessed after 60 h by flow cytometry. (B) CFSE-labeled CD8+ T cells and BMDMs from Batf2−/− or WT littermates with SIINFEKL peptide were cocultured with recombinant IL-12 at decreasing dilutions for 60 h (OT-I:BMDM = 1:1/2). (C) BMDMs (1 × 106) from Batf2−/− or WT littermate donor mice were transferred into Batf2−/− recipient mice on days 0, 4, and 8. Control mice were injected with PBS in the same protocols. Tumor growth was monitored in mice that had received Batf2−/− BMDMs (triangle), WT BMDMs (square), or PBS (circle). (D) Batf2−/− (open triangle) and WT (open square) littermates were injected intraperitoneally with recombinant IL-12 on days 4, 3, and 1 before tumor implantation as well as 24 h after tumor inoculation. Control Batf2−/− (closed triangle) and WT (closed square) mice were injected with PBS in the same protocols. (E) Batf2−/− (open triangle) and WT (open square) littermates were injected intraperitoneally with R848 on days 4, 3, and 1 before tumor implantation and then twice per week after tumor inoculation. Control Batf2−/− (closed triangle) and WT (closed square) mice were injected with PBS in the same protocols. (F) Batf2−/− (open triangle) and WT (open square) littermates were injected intraperitoneally with anti–IL-12 p40 neutralizing antibody at 1 mg/mouse, with follow-up doses of 0.5 mg every 5 d. Control Batf2−/− (closed triangle) and WT (closed square) mice were injected with control IgG in the same protocols. After 1 × 105 B16-F1 cells per mouse were injected, the tumor growth was monitored (C–F). Average kinetics ± SEM of 5 (C), 3–4 (D), 3–4 (E), or 6 (F) mice per group. All experiments were performed on male littermates. In all figures, *P < 0.05; **P < 0.01; n.s., not significant (P > 0.05).
Overall, compared with WT macrophages, Batf2-deficient macrophages cause less CD8+ T-cell proliferation and have a deteriorated antitumor response.
BATF2 Interacts with NF-κB p50 and p65 and Promotes IL-12 p40 Expression Through the Il12b Promoter NF-κB Binding Site.
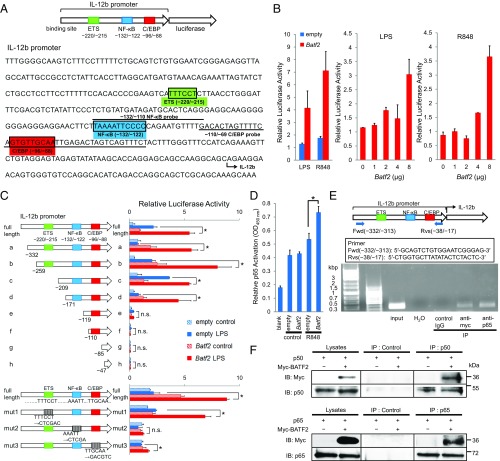
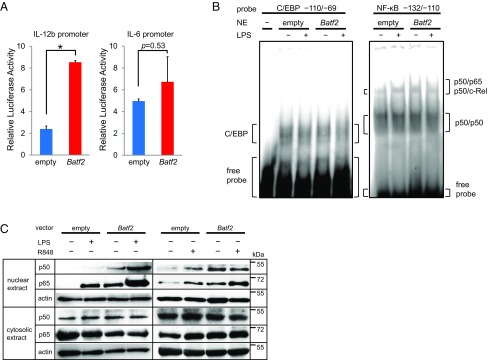
Because recent studies have suggested that Batf2 may function as a positive transcription factor we hypothesized that Batf2 might be a positive regulator of IL-12 p40 induction. We first performed luciferase-reporter assays with a full-length Batf2 construct, using an Il12b promoter linked to a luciferase reporter gene (Fig. 5A). Overexpression of Batf2 in RAW 264.7 cells enhanced the luciferase reporter activity in a dose-dependent manner (Fig. 5B). In contrast, luciferase enhancement was not observed in a similar construct using the Il6 promoter (Fig. S6A). These results suggest that BATF2 recruitment to the Il12b promoter region is responsible for its transcriptional activation. Next, we performed serial-deletion and substitution mutant analyses to identify the Il12b promoter elements that are important for transcriptional activation by Batf2. Deletions of the NF-κB binding site, but not of the E26 transformation-specific (ETS) binding site, diminished the significant differences in luciferase reporter activity between Batf2 and empty vector. The Batf2 luciferase enhancement was not observed when only the CCAAT/enhancer-binding protein (C/EBP) binding element remained (Fig. 5C). Similarly, while ETS or C/EBP binding site substitutions did not diminish the significant differences between Batf2 and empty vector, the significant luciferase enhancement by Batf2 disappeared with the NF-κB binding site substitution (Fig. 5C). These results indicate that the NF-κB binding site is important for Il12b transcriptional activation by BATF2.
Fig. 5.
Analysis of the mechanism responsible for the effect of BATF2 on IL-12 p40 expression. (A) A schematic of the luciferase reporter construct with the mouse IL-12 p40 promoter and its sequence are shown. Underlined sequences indicate the locations of oligonucleotide probes used in DNA-binding assays and EMSAs. Potential transcription factor binding consensus sequences mentioned in the text are boxed and labeled. (B) The mouse IL-12 p40 promoter luciferase reporter construct was transfected into RAW 264.7 cells along with a Batf2 construct. Cells were activated with LPS or R848 at 24 h posttransfection, and luciferase activity was measured 24 h after stimulation (Left). The mouse IL-12 p40 promoter luciferase reporter construct was cotransfected with increasing amounts of Batf2 construct, and cells were activated with LPS (Middle) or R848 (Right). Luciferase activities relative to empty vector control are shown as a representative mean ± SEM. (C) Deletion-mutant (Upper) and substitution-mutant (Lower) analyses of the IL-12 p40 promoter. Mutants were transfected into RAW 264.7 cells with a Batf2 or empty construct. Twenty-four hours after transfection, cells were activated with LPS for 24 h and compared with nonactivated control cells. The result shown for each mutant represents the mean ± SEM of data from three independent experiments. (D) DNA-binding activities of p65 in RAW 264.7 cells transfected with Batf2 or empty constructs were measured with a TransAM Flexi NF-κB Transcription Factor Assay Kit. (E) ChIP analysis was performed using RAW 264.7 cells overexpressing Myc-tagged Batf2. Immunoprecipitation was performed using the following antibodies: normal rabbit IgG (control IgG) and anti–c-Myc or anti–NF-κB p65. Sample DNA was analyzed by PCR using primers that detect sequences containing the Il12b promoter. (F) Data from coimmunoprecipitation experiments assessing BATF2 binding to p50 and p65. For the p50 analysis, cell extracts from RAW 264.7 cells overexpressing both Nfkb1 (p50) and Myc-tagged Batf2 were prepared. After immunoprecipitation with anti-p50 antibody or normal IgG (Control), samples were analyzed with anti–c-Myc antibody by Western blotting. The interactions between Myc-tagged BATF2 and p65 were analyzed similarly. Data are representative of at least two independent experiments. In all figures, *P < 0.05; n.s., not significant (P > 0.05).
Fig. S6.
Analysis of the mechanism responsible for the effect of BATF2 on IL-12 p40 expression. (A) A luciferase reporter construct of the mouse IL-12 p40 (Left) or IL-6 (Right) promoter was transfected into RAW 264.7 cells along with a Batf2 construct. The cells were activated with R848 at 24 h posttransfection, and the luciferase activity was measured 24 h after stimulation. Data are shown as a mean ± SEM, *P < 0.05. (B) EMSAs were performed with nuclear extracts from RAW 264.7 cells transfected with Batf2 or empty constructs. After cells were activated with LPS for 1 h, nuclear extracts (NE) were obtained. The radiolabeled probes contained positions −110 to −69 of the gene sequence, to detect the C/EBP binding site (Left), or positions −132 to −110, which included the NF-κB half-site (Right). Similar results were obtained from two independent experiments. (C) The p50 and p65 expressions in nuclear and cytosolic extracts of RAW 264.7 cells transfected with Batf2 or empty control vector and activated with LPS or R848 for 1 h were analyzed by Western blotting. Data are representative of at least two independent experiments.
We next hypothesized that BATF2 might interact with the NF-κB half-site, a NF-κB binding element (13). We performed DNA-binding ELISAs, using probes against position −132 to −110 (NF-κB sites). Compared with extracts from empty vector-transfected cells, an up-regulation of NF-κB p65 was observed in nuclear extracts overexpressing Batf2 (Fig. 5D). Similar results were also obtained with LPS stimulation in EMSAs (Fig. S6B). Furthermore, ChIP analyses also demonstrated that BATF2 can interact with the Il12b promoter (Fig. 5E). Because these data suggest that BATF2 interacts with NF-κB, we performed coimmunoprecipitation experiments with Nfkb1 (p50) or Rela (p65) using cell extracts from RAW 264.7 cells overexpressing Myc-tagged Batf2. We found that BATF2 interacts with both p50 and p65 (Fig. 5F). We also performed Western blotting using nuclear and cytosolic extracts from RAW 264.7 cells overexpressing Batf2 or empty vectors. Although p50 and p65 up-regulation were observed in the nuclear extracts Batf2-overexpressing cells compared with empty vector-transfected cells, there were no corresponding significant differences in the cytosolic extracts (Fig. S6C). Together, these results indicate that BATF2 interacts with p50 and p65 and up-regulates them in the nucleus, eventually promoting IL-12 p40 expression through the NF-κB binding site of the Il12b promoter.
Discussion
The BATF family has three members: BATF, BATF2, and BATF3. Batf3 is essential for the development of CD8+ classical DCs and related CD103+ DCs (14, 15). Batf2 can compensate for Batf3 in CD8+ and CD103+ DC development during Toxoplasma gondii infection, and Batf2-deficient mice have an increased mortality (15). During chronic infection with pathogens such as Mycobacterium avium, BATF2 can also be a potential mediator of IFN-γ–induced hematopoietic stem and progenitor cells (16). Although it was initially thought that BATF family members function only as inhibitors of AP-1 (17, 18), recent studies have suggested that an interaction with a non-AP-1 factor is likely involved in BATF-specific positive transcriptional activities (7). Although BATF family proteins lack a transcriptional activation domain, they support other transcription factors and can exert unique positive transcriptional activities. For example, BATF/IRF4 and BATF/IRF8 interactions are important for compensatory DC development through the AICE motif (15). Additionally, BATF2 interacts with IRF1 to induce inflammatory responses during Mycobacterium tuberculosis infection (19, 20). Although IRF1 is a positive regulatory factor through the ETS binding site on the Il12b promoter following IFN-γ stimulation (9), ETS binding site deletions did not diminish the significant LPS-stimulated luciferase enhancement by BATF2 in our reporter assays (Fig. 5C). These results suggest that BATF2 interacts with other factors, especially following stimulation by TLR ligands.
We demonstrated that BATF2 interacts with p50 and p65 and up-regulates them in the nuclei of macrophages, eventually promoting IL-12 p40 expression through the NF-κB binding site in the Il12b promoter (Fig. 5 and Fig. S6). The interaction between BATF2 and p65 was reported previously (21), a finding that is consistent with our results. Notably, our data indicate that this up-regulation is specific for Il12b expression because the expression levels of other cytokines, such as Tnf, Il6, and Nos2, were similar between BMDMs from Batf2−/− and WT mice (Fig. 3B and Fig. S6A). Unfortunately, a clear reason for this specificity remained elusive, suggesting that BATF2 has a complicated effect due to its interactions with other transcription factors. While our results show that BATF2 is an up-regulator of NF-κB, BATF family members including BATF2 also function as AP-1 inhibitors (8). Therefore, BATF2 can function as either an inhibitor or an up-regulator depending on the promoters of various cytokines. AP-1 is thought to bind to the C/EBP binding site on the Il12b promoter (9). Notably, the luciferase activities were similar between the Batf2 and control vectors when only the C/EBP binding element remained (Fig. 5C), suggesting that the effect of NF-κB up-regulation by BATF2 is relatively more important than the inhibitory effect of BATF2 on AP-1 on the Il12b promoter. Additionally, BATF was recently proposed to be a pioneer factor (22), a type of molecule that binds to a target site in condensed chromatin and allows the rapid recruitment of other transcription factors; BATF2 may have some similar epigenetic functions.
Batf2 and Il12b were induced by TLR7 agonists, not only R848 but also tumor-derived RNA with DOTAP, which mimics cancer exosomes (Fig. 3C). TLR7 can recognize both RNA derived from microbes and self-derived RNA and subsequently activate immune responses. Furthermore, augmented TLR7 expression can promote autoimmunity (23), while B16-F1 melanoma can grow rapidly in TLR7-deficient mice (10). Therefore, TLR7 signaling by self-derived RNA may be important as an antitumor factor.
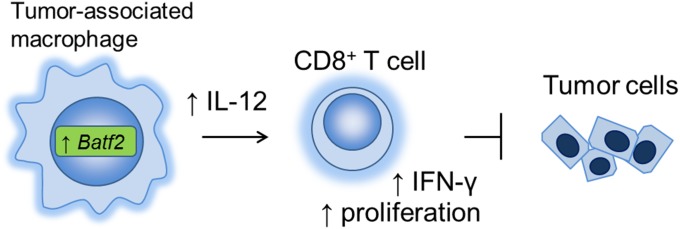
Collectively, our data indicate that NF-κB up-regulation and Batf2 induction are important for IL-12 p40 expression in TAMs, which leads to CD8+ T-cell activation and accumulation within the tumor (Fig. 6). However, a multifaceted role has been described previously for NF-κB in TAMs depending on the cancer type and its tumor environment (24, 25). Here, we used an s.c. inoculated mouse tumor model, predominately B16-F1 melanoma, to examine the role of Batf2 as an antitumor factor. Therefore, this theory may not be adaptable to all types of tumors, especially inflammation-induced cancers in which tumor-infiltrating myeloid cells contribute to carcinogenesis in various ways, such as by secreting proinflammatory cytokines.
Fig. 6.
A schematic model of the antitumor effect of Batf2 through IL-12 p40 up-regulation in TAMs.
Interestingly, Batf2 in cancer cells has also been reported to be involved in cancer cell growth inhibition. For example, lower BATF2 expression is associated with a poor prognosis in hepatocellular carcinoma (26), oral tongue squamous cell carcinoma (27), esophageal squamous cell carcinoma (28), and medulloblastoma (29). BATF2 is down-regulated in various types of cancers and induces apoptosis (8). In addition, BATF2 regulates CCN1-induced cell proliferation (30) and is necessary for antitumor effects by MDA-7 and IL-24 (31). Furthermore, the loss of BATF2 expression initiates epithelial–mesenchymal transition (32), and BATF2 regulates hepatocyte growth factor/MET signaling (33). Recently, BATF2 was shown to be an inhibitor of colon tumor growth and angiogenesis (34). Notably, we measured the Vegfa expression in BMDMs of Batf2−/− and WT littermates and measured angiogenesis within the Batf2−/− and WT mouse tumors; however, we found no significant differences between these groups (Fig. S2 F and G). Therefore, the antitumor effect of Batf2 may result from not only its effects on immune cells but also its effects on cancer cells.
In this study, we demonstrated that Batf2 has an antitumor effect through IL-12 p40 up-regulation in TAMs, which eventually induces CD8+ T-cell activation and accumulation within tumors. Batf2 could be a new target for stimulating antitumor immunity and may also be important in cancer treatment with immune checkpoint blockers and TLR agonists. Further studies are needed to elucidate the full role of Batf2 within tumors.
Materials and Methods
Detailed information on the materials, methods, and associated references can be found in SI Materials and Methods.
Mice.
Batf2−/− mice were generated as previously described (35). OT-I transgenic mice were provided by W. R. Heath, The Walter and Eliza Hall Institute, Parkville, VIC, Australia (36). OT-II transgenic mice and RAG2-deficient mice have been described previously (37, 38). C57BL/6J mice were obtained from CLEA Japan. All animal experiments were performed with approval from the Animal Research Committee of the Research Institute for Microbial Diseases (Osaka University, Osaka, Japan).
Cells.
B16-F1 (ATCC), B16-F10 (Riken BRC), colon-26 (Riken BRC), and Lewis lung carcinoma (Riken BRC) cell lines were grown in DMEM containing 10% FBS (Gibco; Life Technologies). RAW 264.7 cells, which were described previously (39), were grown in RPMI-1640 containing 10% FBS.
BMDM and BMDC Generation and Cell Stimulation.
BMDMs were generated from male mice as described previously (19). BM cells were harvested from femurs and differentiated into macrophages in 15-cm noncoated sterile tissue culture dishes in RPMI-1640 with 10% FBS containing 3 ng/mL macrophage colony-stimulating factor (M-CSF; PeproTech). Medium was changed on day 3. On day 5, macrophages were harvested and stimulated. BMDCs were also generated as described previously (40). BM cells were obtained and incubated in RPMI-1640 with 10% FBS containing 10 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF; PeproTech). Medium containing GM-CSF was changed every 2 d. On day 6, cells were collected. BMDMs and BMDCs were stimulated with IFN-α (BioLegend), IFN-γ (R&D), or TLR ligands. R848 (InvivoGen), LPS (LPS-SM ultrapure; InvivoGen), and CpG1826 (InvivoGen) were used as TLR ligands.
Plasmids.
Batf2 cDNA was obtained by PCR from a mouse cDNA library. Myc-tagged Batf2 was purchased from OriGene technologies (MR203728). Il12b 400-bp pro-Luc, p50 cFlag pcDNA3, and RelA cFlag pcDNA3 were gifts from Stephen Smale, Howard Hughes Medical Institute and Department of Microbiology and Immunology, UCLA School of Medicine, Los Angeles (Addgene plasmids 20020, 20018, and 20012, respectively) (41, 42), and pmIL-6 FL was a gift from Gail Bishop, Department of Microbiology, University of Iowa, Iowa City, IA (Addgene plasmid 61286) (43).
Microarray Analysis.
BMDMs from WT and Batf2−/− littermates were stimulated with R848 for 8 h. Total RNA was then isolated using a High Pure RNA Isolation Kit (Roche) and subjected to an Agilent Expression microarray analysis. The microarray was performed by Takara Bio, and the resulting data were analyzed by Aqua (Takara).
Tumor Model.
Mice were s.c. implanted with B16-F1 melanomas, Lewis lung carcinomas, B16-F10 melanomas, or Colon-26 carcinomas. For the B16-F1 implantations, B16-F1 cells, grown in DMEM culture media with 10% FBS, were washed with PBS, dispersed in a 0.25% solution of trypsin, and resuspended in fresh culture media. After centrifugation, the cell pellet was resuspended in PBS, and the concentration was adjusted to 106 cells per mL. Mice were then injected with 0.1 mL of the suspension. Tumors were measured with a dial caliper every 3 d, and volumes were determined using the formula (width)2 × length × 0.52, as previously described (44, 45). When the mean tumor diameter reached 1.2 cm, experiments were terminated, and mice were killed and autopsied.
PET–CT Imaging Experiments.
PET–CT imaging experiments were performed with a small animal-dedicated micro-PET/CT system (Inveon; Siemens). WT and Batf2−/− littermates with B16-F1 melanoma were treated on day 17 under fasting conditions for a duration of 18 h before they received 17.4 ± 1.2 Mbq of 18F-fluorodeoxyglucose (FDG) per mouse. One hour after injection mice were maintained under 1–2 L/min isoflurane anesthesia and scanned with the micro-PET/CT system. The acquired PET images were coregistered with the CT images for anatomical reference and analyzed for FDG uptake with a Medical Image Data Analysis Tool (AMIDE).
Tissue Digestion for Population Analyses and Cell Sorting.
Tissues were isolated from the mice and placed in 10 mL of RPMI-1640 containing 1 mg/mL collagenase type II (C6885; Sigma-Aldrich). Tissues were disrupted with surgical scissors and were incubated at 37 °C for 30 min. Samples were filtered through 40-µm filters, and erythrocytes were removed by incubation for 3 min with ACK Lysing Buffer (Gibco; Life Technologies). After being washed twice with magnetic-activated cell sorting (MACS) buffer (0.5% BSA and 2 mM EDTA in PBS, pH 7.2), samples were then stained for analysis by flow cytometry (FACSCanto II) and cell sorting (BD FACSAria II).
Flow Cytometry.
All data were collected on a FACSCantoII instrument (BD Biosciences) or Attune NxT Flow Cytometer (Thermo Fisher Scientific) and analyzed using FlowJo analysis software (TreeStar). After being washed with MACS buffer, the cells were incubated with antibodies for 15 min and washed twice. For intracellular cytokine staining, cells were generally incubated for 4 h in the presence of monensin and analyzed by using a BD Cytofix/Cytoperm Kit.
Histological Analysis.
Samples were frozen in Tissue-Tek optimal cutting temperature compound (Sakura Finetek). Frozen 10-μm tissue sections were fixed in 4% paraformaldehyde and blocked with 3% BSA, after which H&E or immunofluorescent staining was performed. The antibodies and reagents for immunofluorescence analyses were purchased as follows: anti-CD8a-PacificBlue (53-6.7; BD Biosciences), anti-CD4-PerCP/Cy5.5 (RM4-5; BD Pharmingen), anti-CD68-APC (FA-11; BioLegend), anti-CD31-FITC (MEC13.3; BioLegend), PI (51-66211E; BD Biosciences), anti-BATF2 (l-24) (sc-130972; Santa Cruz Biotechnology), and Alexa Fluor 488 goat anti-rabbit IgG (A-11034; Life Technologies). Fluorescent images were obtained using a BZ-9000 (Keyence) or IN Cell Analyzer 6000 (GE Healthcare).
Quantitative PCR.
RNA was extracted from cells using a High Pure RNA Isolation Kit (Roche), and reverse transcription was performed with ReverTra Ace (Toyobo) according to the manufacturer’s instructions. For quantitative PCR, cDNA fragments were amplified using real-time PCR Master Mix (Toyobo). Fluorescence from the TaqMan probe for each cytokine was detected using a 7500 Real-time PCR System (Applied Biosystems). The mRNA expression level of each gene was normalized to the 18S rRNA expression level (18S rRNA; Applied Biosystems).
Stimulation by DOTAP Liposomal Formulations of RNA.
After the isolation of total RNA from B16-F1 cells using a High Pure RNA Isolation Kit (Roche), 15 μg of the resulting RNA was complexed with DOTAP Liposomal Transfection Reagent (Roche) as previously described (12). BMDMs were then stimulated for 4 h using DOTAP with the RNA, DOTAP alone, or the RNA alone.
ELISAs.
IL-12 p40, TNF-α, and IL-6 levels were determined by ELISAs performed according to the manufacturer’s instructions (DuoSet ELISA; R&D). The absorbance of each ELISA plate was measured using a microplate reader (iMark; Bio-Rad).
Luciferase Reporter Assays.
Luciferase reporter assays were performed according to the manufacturer’s instructions (Dual-Luciferase Reporter Assay System; Promega). For luciferase transfections, 1 × 106 RAW 264.7 cells were suspended in 100 μL of a solution (buffer R) containing 8 μg of Batf2 or empty vector control, 8 μg of Il12b 400-bp pro-Luc plasmid, and 1 μg of pRL-TK. Cells were electroporated using the Neon Transfection System (Thermo Fisher Scientific) at 1,680 V for 20 ms. Each transfection product was plated in a 24-well plate with 1 × 105 cells per well. Twenty-four hours after electroporation, cells were activated by LPS (100 ng/mL) or R848 (100 ng/mL). Following activation, cell extracts were prepared by using Passive Lysis Buffer (Promega). Luciferase activity was determined from a 20-μL cell extract and measured on the microplate reader Centro X3 LB 960 (Berthold Technologies). For deletion mutants, the mutant DNA fragments were produced by PCR and cloned into the KpnI-BglII sites of the restricted Il12b pro-Luc construct as previously described (13). For substitution mutants, the mutant constructs were produced using a PrimeSTAR Mutagenesis Basal Kit (Takara) according to the manufacturer’s recommendations. All plasmids were verified by restriction mapping and by sequencing using ABI 3130xl (Applied Biosystems).
Microwell p65 DNA-Binding Assay.
The p65 DNA-binding activity was measured using the TransAM NF-κB Flexi ELISA kit (Active Motif) according to the manufacturer’s recommendations (46, 47). A biotinylated probe containing positions −132 to −110 within the Il12b promoter was used as the DNA probe.
Immunoblot Analysis.
Cells were lysed with lysis buffer (20 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, and 1% Nonidet P-40) containing a Complete Protease Inhibitor Mixture (Roche). The cell lysates were separated by standard SDS/PAGE (e-PAGEL; ATTO) and analyzed by immunoblotting. Antibodies for Western blotting were purchased as follows: goat anti–NF-κB p50 (D-17) (sc-1192; Santa Cruz Biotechnology), rabbit anti–NF-κB p50 (H-119) (sc-7178; Santa Cruz Biotechnology), anti–NF-κB p65 (C-20) (sc-372; Santa Cruz Biotechnology), rabbit anti–c-Myc (A-14) (sc-789; Santa Cruz Biotechnology), mouse anti–c-Myc (9E10) (sc-40; Santa Cruz Biotechnology), anti–actin-HRP (C-11) (sc-1615 HRP; Santa Cruz Biotechnology), anti-rabbit IgG-HRP (NA934V; GE Healthcare), anti-mouse IgG-HRP (NA931V; GE Healthcare), and anti-goat IgG-HRP (sc-2056; Santa Cruz Biotechnology). The Luminata Forte Western HRP Substrate (Millipore) was used for the development of positive signals, and the chemiluminescence was detected using an ImageQuant LAS 4000 (GE Healthcare).
ChIP.
ChIP analysis was performed using ChIP-IT Express Enzymatic (Active Motif) according to the manufacturer’s recommendations with some modifications. First, 3 × 107 RAW 264.7 cells were transfected with 120 μg of Myc-tagged Batf2 using the Neon Transfection System (Thermo Fisher Scientific) at 1,680 V for 20 ms. Twelve hours later, the cells were fixed with 1% formaldehyde for 10 min followed by a wash with PBS, and the fixation reaction was stopped by adding glycine stop fix solution for 5 min. Cell extracts were prepared in lysis buffer [10 mM Hepes-KOH, pH 7.8, 10 mM KCL, 0.1 mM EDTA, pH 8.0, protease inhibitor mixture (Roche), and 0.1% Nonidet P-40] and were homogenized using a Dounce homogenizer (BioMasherII, Nippi) on ice. Enzymatic shearing was performed for 5 min, and DNA fragments were obtained (Input). Immunoprecipitation was performed using 4 μg of the following antibodies: normal rabbit IgG (sc-2027; Santa Cruz Biotechnology), anti–c-Myc (A-14) (sc-789; Santa Cruz Biotechnology), or rabbit anti–NF-κB p65 (C-20) (sc-372; Santa Cruz Biotechnology). After samples were incubated overnight with 25 μL of protein G magnetic beads, the beads were washed. DNA was eluted in elution buffer AM2, and 50 μL of reverse cross-linking buffer was added. The supernatants were then collected and incubated at 95 °C for 15 min. After samples were returned to room temperature, 1 μg of proteinase K was added, and the samples were incubated for 1 h at 37 °C. Input samples and precipitated DNA were analyzed by PCR using primers that detect sequences containing the NF-κB binding site of the Il12b promoter: 5′-GCAGTCTGTGGAATCGGGAG-3′ and 5′-CTGGTGCTTATATACTCTACTC-3′.
Coimmunoprecipitation.
For coimmunoprecipitations, 1 × 106 cells were suspended in 100 μL of a solution (buffer R) containing 8 μg of Myc-tagged Batf2 and 8 μg of Nfkb1 (truncated at the p105 ligation site) or Rela plasmid. Cells were electroporated using the Neon Transfection System (Thermo Fisher Scientific) at 1,680 V for 20 ms. Transfected cells were plated at 5 × 106 cells per dish. Twelve hours after electroporation, cell extracts were prepared using lysis buffer [10 mM Hepes-KOH, pH 7.8, 10 mM KCL, 0.1 mM EDTA, pH 8.0, protease inhibitor mixture (Roche), and 0.1% Nonidet P-40]. The anti-p50 or anti-p65 antibodies (4 μg) were added to the lysates (50 μg) in a total volume of 100 μL. Antibodies for immunoprecipitation were purchased as follows: normal rabbit IgG (sc-2027; Santa Cruz Biotechnology), normal goat IgG (sc-2028; Santa Cruz Biotechnology), goat anti–NF-κB p50 (d-17) (sc-1192; Santa Cruz Biotechnology), and rabbit anti–NF-κB p65 (C-20) (sc-372; Santa Cruz Biotechnology). The solution was incubated at 4 °C for 1 h on a tube rotator. Protein G-Sepharose beads (50 μL of 50% slurry; GE Healthcare) were added, and the mixture was agitated at 4 °C for 1 h. After centrifugation, the pellet was washed four times with TBS (50 mM Tris and 150 mM NaCl, pH 7.5). Samples were analyzed by standard SDS/PAGE immunoblotting using anti–c-Myc antibody from a different host species, as described above.
EMSAs, in Vivo Migration Assays, Th1 Differentiation Assays, and Antigen-Presentation Assays.
Detailed methods are described in SI Materials and Methods.
SI Materials and Methods
Mice.
Batf2−/− mice were generated as previously described (35). The Batf2 genes were isolated from genomic DNA, and the targeting vectors were constructed by replacing the Batf2 ORF (exon 1,2) with a neomycin-resistance gene cassette (neo) and inserting herpes simplex virus thymidine kinase (HSV-TK) driven by the PGK promoter into the genomic fragment for negative selection. Batf2−/− mice were back-crossed for more than four generations on a C57BL/6J background. C57BL/6J mice were obtained from CLEA Japan. WT male littermates were used as controls. OT-I transgenic mice were provided by W. R. Heath (36). OT-II transgenic mice have been described previously (37, 48–50). RAG2-deficient mice on a C57BL/6 background have been described previously (38). All mice were bred and maintained in our specific pathogen-free animal facility according to the institutional guidelines. Experiments were generally performed at 6–12 wk of age. All animal experiments were performed with approval from the Animal Research Committee of the Research Institute for Microbial Diseases, Osaka University.
BM Transfer Experiments.
BM transfer experiments were performed as previously described (35). Recipient 6-wk-old C57BL/6J mice were lethally irradiated with 10 Gy and injected i.v. with 2 × 107 BM cells obtained from Batf2−/− or WT littermates. After 8 wk, tumor implantation experiments were performed.
Flow Cytometry.
All data were collected on a FACSCantoII instrument (BD Biosciences) or Attune NxT Flow Cytometer (Thermo Fisher Scientific) and analyzed using FlowJo analysis software (TreeStar). After being washed with MACS buffer (0.5% BSA and 2 mM EDTA in PBS, pH 7.2), the cells were incubated with antibodies for 15 min and washed twice. For intracellular cytokine staining, cells were generally incubated for 4 h in the presence of monensin and analyzed by using a BD Cytofix/Cytoperm Kit unless otherwise stated. Antibodies for flow cytometry were purchased from commercial sources as follows: anti–F4/80-FITC (BM8; BioLegend), anti–I-A/I-E (M5/114.15.2; BD Pharmingen), anti–CD68-APC (FA-11; BioLegend), anti–IFN-γ (XMG1.2; BioLegend), anti–CD4-PerCP/Cy5.5 (RM4-5; BD Pharmingen), anti–CD8a-PE (53-6.7; BD Pharmingen), anti–CD8a-APC (53-6.7; BioLegend), anti–CD8a-PerCP/Cy5.5 (53-6.7; Tonbo Biosciences), anti–IL-2 (JES6-5H4; BioLegend), anti–IL-4 (11B11; BD Pharmingen), anti–IL-12/IL-23 p40-PE (C15.6; BioLegend), anti–CD11b-PerCP/Cy5.5 (M1/70; BioLegend), anti–CD45.2-PerCP/Cy5.5 (104; BD Pharmingen), anti–CD45.2- Brilliant Violet 421 (104; BioLegend), anti–CD4- PerCP/Cy5.5 (RM4-5; BD Pharmingen), anti–Ki-67-APC (16A8; BioLegend), anti–CD11c-APC (N418; BioLegend), anti–NK-1.1-APC (PK136; BioLegend), anti–CD3ε-APC (145-2C11; BioLegend), anti–CD3ε-Pacific Blue (145-2C11; BioLegend), anti–CD3e-FITC (145-2C11; Tonbo Biosciences), anti–Ly6G-PE (1A8; BD Pharmingen), anti-CD45R/B220 (RA3-6B2; BD Pharmingen), anti–CD49b-PE (DX5; BD Pharmingen), anti–Ly6C-FITC (HK1.4; BioLegend), anti–CD80-PE (16-10A1; BD Pharmingen), anti–CD86-FITC (GL-1; BioLegend), and anti–H-2-FITC (M1/42; BioLegend). The flow cytometry gating was mostly performed as previously described with some modifications (51, 52). Leukocyte subpopulations were generally defined as follows: TAMs, CD45+ CD11b+ F4/80+ CD68+ MHC II+ cells; neutrophils, CD45+ CD11b+ Ly6Cint Ly6Ghigh cells; monocytes, CD45+ CD11b+ Ly6Chigh Ly6G− cells; DCs, CD45+ CD11b+ CD11c+ MHC II+ F4/80− cells; CD4+ T cells, CD45+ CD3+ CD4+ CD8− cells; CD8+ T cells, CD45+ CD3+ CD4− CD8+ cells; and natural killer cells, CD45+ CD3− NK1.1+ CD49b+ cells.
Quantitative PCR.
RNA was extracted from cells using a High Pure RNA Isolation Kit (Roche), and reverse transcription was performed with ReverTra Ace (Toyobo) according to the manufacturer’s instructions. For quantitative PCR, cDNA fragments were amplified using real-time PCR Master Mix (Toyobo). Fluorescence from the TaqMan probe for each cytokine was detected using a 7500 Real-time PCR System (Applied Biosystems). The mRNA expression level of each gene was normalized to the 18S rRNA expression level (18S rRNA; Applied Biosystems). TaqMan probes for quantitative PCR were purchased from Life Technologies with the following assay ID numbers: Il12b (Mm00434174_m1), Batf2 (Mm01231591_m1), Il6 (Mm00446190_m1), Ifnb1 (Mm00439552_s1), Tnf (Mm00443258_m1), Nos2 (Mm00440502_m1), Vegfa (Mm01281449_m1), and Tbx21 (Mm00450960_m1).
EMSAs.
EMSAs were performed as previously described (13, 41, 53). Probes were made by annealing single-stranded oligonucleotides (FASMAC) that contained positions −110 to −69 of the Il12b promoter as the C/EBP binding site or −132 to −110, which includes the NF-κB half-site within the Il12b promoter. Nuclear extracts were prepared from 1 × 106 RAW 264.7 cells transfected with 8 μg of Batf2 or empty constructs. After the cells were activated with LPS (100 ng/mL) or R848 (100 ng/mL) for 1 h, cell lysates were prepared in lysis buffer [10 mM Hepes-KOH, pH 7.8, 10 mM KCL, 0.1 mM EDTA, pH 8.0, protease inhibitor mixture (Roche), and 0.1% Nonidet P-40], and nuclear extracts were obtained in buffer [50 mM Hepes-KOH, pH 7.8, 420 mM KCL, 0.1 mM EDTA, pH 8.0, 5 mM MgCl2, protease inhibitor mixture (Roche), and 20% glycerol] as previously described (54, 55). Then, 105 cpm of [α-32P]dCTP- (PerkinElmer) radiolabeled probes were incubated with 10 μg of nuclear extract and 4 μg of poly(dI-dC) (Sigma-Aldrich) at room temperature for 30 min. The EMSA products were separated on a 5% acrylamide-1×Tris-glycine-EDTA gel run at 4 °C for 3 h at 150 V. The radioactivities were measured by laser scanner FLA-7000 (FUJIFILM).
Topical Application of Imiquimod.
WT and Batf2−/− mice received a daily topical dose of 62.5 mg of 5% imiquimod cream (Beselna Cream 5%; Mochida) on a shaved patch of back for six consecutive days (corresponding to a daily dose of 3.125 mg of the active compound), as previously described (56). Control mice were treated similarly with a control vehicle cream (Vaseline; Unilever). Based on the clinical Psoriasis Area and Severity Index, erythema was scored on a scale from 0 to 4: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked (56).
In Vivo Migration Assay.
The in vivo migration assays were performed as previously described (57). CD8+ T cells isolated from WT splenocytes (n = 14) were labeled with CFSE (BioLegend), and 2 × 107 CD8+ T cells were injected i.v. in Batf2−/− or WT littermates. After 20 h, lymphocytes were obtained from the spleen, lymph nodes, and tumor tissues and analyzed by flow cytometry.
Naïve CD8+ T-Cell Analysis.
Naïve CD8+ T cells were obtained from the spleens of Batf2−/− mice and WT littermates without tumors by using MACS (CD8+ T Cell Isolation Kit; Miltenyi Biotec). Cells were stimulated with PMA (50 ng/mL) and ionomycin (1 μM) for 4 h, in the presence of monensin for the last 2 h. The intracellular IFN-γ of CD8+ T cells was stained, and its expression was analyzed by flow cytometry.
Adoptive Transfer of CD8+ T Cells into Rag2−/− Mice.
Splenic CD8+ T cells were negatively selected from Batf2−/− mice and WT littermates by MACS according to the manufacturer’s recommendations (CD8+ T Cell Isolation Kit; Miltenyi Biotec). Then, 2.5 × 106 CD8+ T cells from Batf2−/− or WT littermates were transferred i.v. into Rag2−/− recipients. After 24 h, 1 × 105 B16-F1 tumor cells were transplanted into each mouse.
Th1 Differentiation Assay.
The Th1 differentiation assays were performed as previously described (14). Naïve CD4+ CD62Lhi CD44dull CD25− T cells from Batf2−/− mice and WT littermates were purified by cell sorting on a flow cytometer (BD FACSAria II). After 96-well plates had been coated with anti-CD3 antibody (1 μg/mL, 145-2C11; BD Pharmingen) for 2 h at 37 °C, the cells were cultured at a concentration of 2 × 105 cells per well in the anti–CD3-coated 96-well plates in the presence of recombinant mIL-12 (10 ng/mL; R&D), anti–IL-4 (10 μg/mL, AF-404-NA; R&D), recombinant mIL-2 (20 ng/mL; R&D), and anti-CD28 (10 μg/mL, 37.51; BD Pharmingen). Medium was changed to fresh medium containing the same compounds at day 3. After 7 d, the cells were restimulated with PMA (50 ng/mL) and ionomycin (1 μM) for 4 h in the presence of monensin for an intracellular cytokine analysis by flow cytometry.
Antigen-Presentation Assays.
Antigen-presentation assays were performed as previously described (14, 58). Ovalbumin-specific CD8+ OT-I and CD4+ OT-II T cells were obtained from mouse spleens and lymph nodes by using a CD8+ T Cell Isolation Kit and CD4+ T Cell Isolation Kit, respectively (Miltenyi Biotec). T cells were labeled with CFSE (BioLegend) according to the manufacturer’s instructions. For OT-I T-cell proliferation, CD8+ T cells from the splenocytes and lymph nodes of OT-I transgenic mice were labeled with CFSE. BMDMs from Batf2−/− or WT littermates were pulsed with Ova 257-264 peptide (SIINFEKL, 1 μM for 3 h), except for the cells in control wells, and then all cells were washed twice with PBS and cocultured with 5 × 104 CFSE-labeled naïve OT-I transgenic CD8+ T cells at decreasing dilutions (1:1–1:1/8, OT-I:BMDMs) together with 100 ng/mL R848 (InvivoGen) or recombinant mIL-12. Proliferation of OT-I cells was measured after 60 h by flow cytometry. For MHC II-restricted antigen-presentation experiments, spleens from Batf2−/− mice and WT littermates were obtained, and splenic CD11c+ DCs were purified by MACS (CD11c MicroBeads; Miltenyi Biotec) according to the manufacturer’s recommendations. For all cultures, 5 × 104 CFSE-labeled OT-II transgenic CD4+ T cells were incubated with 1 × 104 splenic CD11c+ DCs, in the continuous presence of 1 mg/mL or 0.1 mg/mL chicken ovalbumin (Sigma-Aldrich) or with 1 μM Ova peptide (323-339) (InvivoGen) together with 1 µg/mL CpG1826 (InvivoGen). After 36–72 h, the percentages of CD4+ CFSElow cells were assessed by flow cytometry.
Adoptive Transfer of BMDMs.
The adoptive transfer of BMDMs was performed by a previously described protocol with some modifications (14, 52). BMDMs (1 × 106) induced by M-CSF from Batf2−/− or WT littermate donor mice were s.c. transferred into the backs of Batf2−/− recipient mice on days 0, 4, and 8. Tumors were measured with a dial caliper every 3 d, and their volumes were determined as described above.
In Vivo IL-12 Treatment.
In vivo IL-12 treatment assays were performed as previously described (15). Batf2−/− mice and WT littermates were injected intraperitoneally with 0.5 μg of recombinant murine IL-12 (Peprotech) in 200 μL of PBS on days 4, 3, and 1 before tumor implantation and again 24 h after tumor inoculation. Control mice were injected intraperitoneally with 200 μL of PBS in the same manner. After 1 × 105 B16-F1 cells were injected, the tumors were measured with a dial caliper every 3 d, and their volumes were determined as described above.
In Vivo Anti–IL-12 p40 Antibody Treatment.
In vivo treatment with anti–IL-12 p40 neutralizing antibody was performed as previously described (59). Batf2−/− mice and WT littermates were injected intraperitoneally with 1 mg/mouse of anti–IL-12 p40 antibody (C17.8; BioXCell), with follow-up doses of 0.5 mg every 5 d. Control mice were injected intraperitoneally with control IgG in the same protocols. After 1 × 105 B16-F1 cells per mouse were injected, the tumors were measured with a dial caliper every 3 d, and their volumes were determined as described above.
Statistical Analysis.
A two-tailed Student’s t test or Welch’s t test was used to compare two independent normally distributed groups. A Mann–Whitney U test was carried out for the comparison of means between two sets of nonnormally distributed data. For all tests, a P value of less than 0.05 was considered statistically significant.
Acknowledgments
We thank K. Nakagawa, G. Mao, L. Zhao, K. Koyama, H. Nabeshima, N. Miyamoto, R. Takenaka, T. Kidani, S. Saeki, X. Sun, M. Shimoda, S. K. Singh, Y. Nagahama, Y. Kozakai, Y. Kishi, H. Nakamura, T. Suzuki, and T. Sugita for discussions; K. Asakawa, C. Funamoto, E. Sugisawa, A. Wataki, R. Kawaguchi, C. Kadowaki, and K. Shinno for technical assistance; J. Hatazawa, H. Ikeda, Y. Kanai, and K. Isohashi for PET–CT experiments; and E. Kamada for secretarial assistance. We thank Katie Oakley from Edanz Group for editing a draft of this manuscript. This study was supported in part by Yuri Terao and Center for Medical Research and Education, Graduate School of Medicine, Osaka University. This work was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology, the Japan Society for the Promotion of Science through funding for Specially Promoted Research (Grant 15H05704), a grant from Project MEET, Osaka University Graduate School of Medicine, and Mitsubishi Tanabe Pharma Corporation.
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray dataset has been deposited in the NCBI Gene Expression Omnibus (GEO) database (accession no. GSE101781).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708598114/-/DCSupplemental.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 3.Senovilla L, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. OncoImmunology. 2012;1:1323–1343. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Domizio J, et al. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114:1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 8.Su ZZ, et al. Cloning and characterization of SARI (suppressor of AP-1, regulated by IFN) Proc Natl Acad Sci USA. 2008;105:20906–20911. doi: 10.1073/pnas.0807975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, et al. Additive melanoma suppression with intralesional phospholipid-conjugated TLR7 agonists and systemic IL-2. Melanoma Res. 2011;21:66–75. doi: 10.1097/CMR.0b013e328340ce6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-κ B half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tussiwand R, et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012;490:502–507. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matatall KA, et al. Chronic infection depletes hematopoietic stem cells through stress-induced terminal differentiation. Cell Rep. 2016;17:2584–2595. doi: 10.1016/j.celrep.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorsey MJ, et al. B-ATF: A novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene. 1995;11:2255–2265. [PubMed] [Google Scholar]
- 18.Echlin DR, Tae HJ, Mitin N, Taparowsky EJ. B-ATF functions as a negative regulator of AP-1 mediated transcription and blocks cellular transformation by Ras and Fos. Oncogene. 2000;19:1752–1763. doi: 10.1038/sj.onc.1203491. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, et al. Batf2/Irf1 induces inflammatory responses in classically activated macrophages, lipopolysaccharides, and mycobacterial infection. J Immunol. 2015;194:6035–6044. doi: 10.4049/jimmunol.1402521. [DOI] [PubMed] [Google Scholar]
- 20.Guler R, Roy S, Suzuki H, Brombacher F. Targeting Batf2 for infectious diseases and cancer. Oncotarget. 2015;6:26575–26582. doi: 10.18632/oncotarget.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, et al. Toward an understanding of the protein interaction network of the human liver. Mol Syst Biol. 2011;7:536. doi: 10.1038/msb.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciofani M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res. 2010;16:784–789. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biswas SK, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, et al. Decreased expression of BATF2 is associated with a poor prognosis in hepatocellular carcinoma. Int J Cancer. 2011;128:771–777. doi: 10.1002/ijc.25407. [DOI] [PubMed] [Google Scholar]
- 27.Wen H, et al. Decreased expression of BATF2 is significantly associated with poor prognosis in oral tongue squamous cell carcinoma. Oncol Rep. 2014;31:169–174. doi: 10.3892/or.2013.2863. [DOI] [PubMed] [Google Scholar]
- 28.Han T, et al. The tumor-suppressive role of BATF2 in esophageal squamous cell carcinoma. Oncol Rep. 2015;34:1353–1360. doi: 10.3892/or.2015.4090. [DOI] [PubMed] [Google Scholar]
- 29.Andrade AF, et al. The DNA methyltransferase inhibitor zebularine exerts antitumor effects and reveals BATF2 as a poor prognostic marker for childhood medulloblastoma. Invest New Drugs. 2017;35:26–36. doi: 10.1007/s10637-016-0401-4. [DOI] [PubMed] [Google Scholar]
- 30.Dash R, et al. Inhibition of AP-1 by SARI negatively regulates transformation progression mediated by CCN1. Oncogene. 2010;29:4412–4423. doi: 10.1038/onc.2010.194. [DOI] [PubMed] [Google Scholar]
- 31.Dash R, et al. Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction. Cancer Res. 2014;74:563–574. doi: 10.1158/0008-5472.CAN-13-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, et al. The function of SARI in modulating epithelial-mesenchymal transition and lung adenocarcinoma metastasis. PLoS One. 2012;7:e38046. doi: 10.1371/journal.pone.0038046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, et al. BATF2 deficiency promotes progression in human colorectal cancer via activation of HGF/MET signaling: A potential rationale for combining MET inhibitors with IFNs. Clin Cancer Res. 2015;21:1752–1763. doi: 10.1158/1078-0432.CCR-14-1564. [DOI] [PubMed] [Google Scholar]
- 34.Dai L, et al. SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat Commun. 2016;7:11996. doi: 10.1038/ncomms11996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Satoh T, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 36.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 37.Uehata T, et al. Malt1-induced cleavage of regnase-1 in CD4(+) helper T cells regulates immune activation. Cell. 2013;153:1036–1049. doi: 10.1016/j.cell.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi M, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto M, et al. Enhanced TLR-mediated NF-IL6 dependent gene expression by Trib1 deficiency. J Exp Med. 2007;204:2233–2239. doi: 10.1084/jem.20070183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 41.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: Evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–4588. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanjabi S, et al. A c-Rel subdomain responsible for enhanced DNA-binding affinity and selective gene activation. Genes Dev. 2005;19:2138–2151. doi: 10.1101/gad.1329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baccam M, Woo SY, Vinson C, Bishop GA. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: Involvement of NF-κ B, AP-1, and C/EBP. J Immunol. 2003;170:3099–3108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 44.O’Reilly MS, et al. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 45.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renard P, et al. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maruyama K, et al. The transcription factor Jdp2 controls bone homeostasis and antibacterial immunity by regulating osteoclast and neutrophil differentiation. Immunity. 2012;37:1024–1036. doi: 10.1016/j.immuni.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 49.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 50.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 51.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 52.Carretero R, et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 53.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97:12705–12710. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo K, Landau NR, Smale ST. LyF-1, a transcriptional regulator that interacts with a novel class of promoters for lymphocyte-specific genes. Mol Cell Biol. 1991;11:5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Fits L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 57.Iezzi G, Scheidegger D, Lanzavecchia A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J Exp Med. 2001;193:987–993. doi: 10.1084/jem.193.8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thevenot PT, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]