Significance
When a pathogen emerges in a host population, will it evolve to do more or less harm to its host? A single strain of myxoma virus was released as a biocontrol agent against Australian rabbit populations in 1950. The subsequent coevolution has become a textbook classic, although there has been little experimental work on this topic since the early 1980s. Here, we show that the host–pathogen arms race continued with the evolution of highly lethal viruses that cause immune collapse. The possibility that pathogens can become highly immunosuppressive in response to increases in host resistance needs to be considered where genetic and immunologic manipulations are used to enhance host resistance, as, for instance, in agriculture.
Keywords: emergent virus, coevolution, virulence, immunosuppression, septic shock
Abstract
In host–pathogen arms races, increases in host resistance prompt counteradaptation by pathogens, but the nature of that counteradaptation is seldom directly observed outside of laboratory models. The best-documented field example is the coevolution of myxoma virus (MYXV) in European rabbits. To understand how MYXV in Australia has continued to evolve in wild rabbits under intense selection for genetic resistance to myxomatosis, we compared the phenotypes of the progenitor MYXV and viral isolates from the 1950s and the 1990s in laboratory rabbits with no resistance. Strikingly, and unlike their 1950s counterparts, most virus isolates from the 1990s induced a highly lethal immune collapse syndrome similar to septic shock. Thus, the next step in this canonical case of coevolution after a species jump has been further escalation by the virus in the face of widespread host resistance.
Emerging infectious disease is one of the great health challenges of the 21st century. A central question is whether an infectious agent becomes more or less virulent as it adapts after a successful host jump (1–5). In 1950, a single strain of myxoma virus of South American rabbits (Sylvilagus brasiliensis) was released in Australia as a biological control agent against European rabbits (Oryctolagus cuniculus). Frank Fenner, realizing this would be a grand experiment in virulence evolution, set in motion a series of experimental studies to monitor the subsequent evolution of viral virulence. These studies involved measuring the lethality of virus isolates taken from the field in standardized laboratory rabbits. The work showed that the original highly lethal strain, with a case fatality rate (CFR) of close to 100%, was rapidly replaced by strains with case fatality rates of 70–95% or lower, and sometimes even less than 50%. Fenner and colleagues then went on to show that this attenuation was favored by natural selection because, by killing hosts so rapidly, highly virulent viruses had shorter infectious periods than more attenuated strains, which did not kill so rapidly (6–10). This work became the bedrock of the mathematical theory of virulence evolution developed in the 1980s (11–19), and it remains so because the combination of temporal field sampling and controlled experimentation demonstrating the relevant trade-offs is unique for a disease of vertebrates.
However, the story did not end with virulence declines. Genetic resistance rapidly evolved in the rabbit population, demonstrated by testing field-caught rabbits with a standard virus. For instance, a viral strain that once killed 90% of rabbits caught at Lake Urana was killing only 26% of rabbits caught at the same location 7 y later (20, 21). This increase in host resistance apparently halted and then changed the direction of viral evolution because viral lethality began to climb, although with extensive regional variation and frequent changes in virulence grade across the MYXV phylogeny (22). Most notably, strains with case fatality rates of less than 50% became extremely rare. More virulent viruses likely have longer infectious periods in resistant rabbits because they are less readily controlled by innate and adaptive immune responses (23, 24). Highly virulent and immunosuppressive viruses can overcome genetic resistance, indicating this evolutionary pathway is open to the virus (25, 26).
Although the story of myxomatosis in rabbits has become a textbook example of a host–parasite arms race, an obvious question arises: If increases in host resistance are countered by increases in viral lethality, which in turn select for increases in host resistance, will there be indefinite escalation of viral virulence?
There has been no systematic phenotyping of viral virulence of Australian field isolates of MYXV since the early 1980s (9). We examined, in two experimental trials, the clinical phenotype of viruses isolated from the field in the 1990s and compared these with several of the viral strains phenotyped by Fenner, including the progenitor strain SLS released in 1950 (Table 1). This allowed us to test whether the viral phenotype continues to evolve as part of an ongoing virus–host arms race.
Table 1.
Virulence phenotype of Australian isolates of myxoma virus
| Virus | Year of virus isolation | Survival times (no. surviving)* | AST (normalized) | Inferred virulence grade† | Clinical syndromes observed (no. of rabbits)‡ | Genes disrupted/duplicated compared with SLS (known virulence genes, in bold) |
| Trial 1 | ||||||
| SLS | 1950 | 10.6–14.8 | 12.6 | 1 | A (6) | |
| KM13 | 1952 | 26–S (5) | na | 5 | D (6) | ΔM014 |
| Ur | 1953 | All S (6) | na | 5 | D (6) | ΔM005; ΔM014; ΔM134 |
| BD23 | 1999 | 11.2–24.5 | 13.3 | 2¶ | B (5) C (1) | ΔM147; ΔM009; Duplication M156; M154 |
| BRK 12/2/93 | 1993 | 11.9–24.5 | 13.5 | 2¶ | B (5) C (1) | ΔM009 |
| SWH 8/2/93 | 1993 | 11.1–20.5 | 14.3 | 2 | B (4) C (2) | ΔM009 |
| WS6 346 | 1995 | 12.2–29 | 15.8 | 2 | B (3) C (3) | ΔM005; ΔM009 |
| SWH1209 | 1996 | 13.3–32 | 19.1 | 3 | B (2) C (4) | ΔM009; duplication M156, M154 |
| Trial 2 | ||||||
| BRK 4/93 | 1993 | 10.6–12 | 11.4 | 1 | B (6) | ΔM009; ΔM036 |
| BD23 | 1999 | 10.6–12.6 | 11.85 | 1¶ | B (6) | ΔM147; ΔM009; duplication M156; M154 |
| BRK 12/2/93 | 1993 | 11.6–13.6 | 12.03 | 1¶ | B (6) | ΔM009 |
| BD44 | 1999 | 11.3–14.6 | 12.1 | 1 | B (6) | ΔM00.05; ΔM008.1; ΔM009 ΔM153; duplication M156, M154 |
| SWH 9/92 | 1992 | 10–23 | 12.2 | 1 | B (4) A (2) | ΔM009 |
| SWH 805 | 1993 | 12.6–28 | 14.4 | 2 | B (5) C (1) | ΔM009 |
| BRK 897 | 1995 | 12.6–28 | 16.4 | 3 | B (4) C (2) | ΔM009 |
| WS6 1071 | 1995 | 12.2–22.5 | 18.2 | 3 | B (2) C (4) | ΔM009; ΔM153 |
| Meby | 1991 | 10.6–S (1) | 16.9 | 3 | A-B§ (5) D (1) | ΔM009; ΔM153 |
| OB3 Y317 | 1994 | All S (6) | na | 5 | D (6) | ΔM009; ΔM012 |
A total of 16 isolates were assayed in groups of six laboratory rabbits in two trials. Two virus strains, BD23 and BRK 12/2/93, were tested in both trials. na, not applicable.
Survival times, unnormalized range. S, survived, with number surviving shown in brackets.
Grade 1, AST ≤13 d, CFR 100%; Grade 2, AST 14–16 d, CFR 95–99%; Grade 3, AST 17–28 d, CFR 70–95%; Grade 4, AST 29–50 d, CFR 50–70%; Grade 5, indeterminate AST, CFR <50%.
Syndrome A, typical cutaneous myxomatosis; B, acute collapse with little overt signs of myxomatosis; C, progressive “amyxomatous” myxomatosis in rabbits infected with viruses causing syndrome B but that did not suffer acute collapse; D, attenuated cutaneous myxomatosis (see SI Appendix for definitions).
BD23 and BRK 12/2/93 were graded as 2 in the first trial and as 1 in the repeat trial; the combined results are grade 1 for both strains.
Intermediate phenotype. Four animals died from acute collapse, and a fifth with bacterial infection associated with acute inflammation. All animals developed primary lesions consistent with typical cutaneous myxomatosis, but only two developed secondary lesions (SI Appendix, Table S1). The Meby strain is from Tasmania and has been isolated from viruses on continental Australia for at least 20 y.
Results
Viruses were chosen on the basis of mutations in known virulence genes, including reading frame disruptions resulting from insertion/deletion events and duplications in virulence genes, and to be representative of the full MXYV phylogeny (SI Appendix, Fig. S1). In total, 15 viral strains isolated from the field in the 1990s were phenotyped (Table 1; Materials and Methods). Virulence phenotyping was conducted over two trials in groups of six male New Zealand White laboratory rabbits (Materials and Methods).
Fenner and Marshall classified the virulence of field isolates of MYXV into 5 virulence grades based on CFR, average survival time (AST), and clinical phenotype in four to six laboratory rabbits (6, 9, 27), with grade 1 the most virulent and grade 5 the least virulent. To allow direct comparison with earlier data, we have followed their basic approach; quantitative definitions of the grades are given in Table 1.
Most viruses we assayed were of grade 2 or 3 virulence, with a lower number of grade 1 and grade 5 viruses (Table 1 and SI Appendix, Fig. S2), consistent with the general trend observed after the progenitor release. The virus strains from the 1950s caused typical cutaneous (nodular) myxomatosis (Fig. 1, Table 1, SI Appendix, Table S1 and Fig. S3). In contrast, all the grade 1–3 isolates from the 1990s induced an acute collapse syndrome in which rabbits became moribund over a few hours and died between days 10–15, with only minor signs of typical myxomatosis (Fig. 1 and Table 1). Unlike typical cutaneous myxomatosis, the viral inoculation site was poorly differentiated from the surrounding skin (SI Appendix, Fig. S3A), and secondary cutaneous lesions were absent. Massive pulmonary edema (Fig. 1C), often with hemorrhage (SI Appendix, Fig. S3D) or severe swelling and hemorrhage of one hind leg, was typical (Fig. 1D), as well as pale and swollen liver (SI Appendix, Fig. S3E), subcutaneous edema (SI Appendix, Fig. S3B), and bleeding from minor injuries (SI Appendix, Fig. S3C) The few animals infected with the grade 1–3 viruses from the 1990s that survived longer than 14–16 d also failed to develop clear delineation of the primary lesion at the inoculation site and rarely developed secondary cutaneous lesions, and then only very late in the course of disease. The phenotype of these survivors resembled the amyxomatous syndrome described in some European MYXV isolates (28–30). The Meby strain, which was isolated from Tasmania and so has evolved separately for >20 y from the other viruses analyzed here, induced an intermediate disease phenotype with some acute collapse, but elements of the cutaneous form.
Fig. 1.
(A, 1–4) Typical clinical outcomes for clinical syndromes observed (Table 1 and SI Appendix). (1) Syndrome A (typical cutaneous myxomatosis). (2) Syndrome B (acute collapse with minimal signs of myxomatosis). (3) Syndrome C: progressive “amyxomatous” myxomatosis in a rabbit with prolonged survival. (4) Syndrome D (attenuated nodular cutaneous myxomatosis). (B) Kaplan-Meier plots for representative infections (SLS, 1950 progenitor virus, grade 1 virulence; BRK 4/93, 1993, grade 1 virulence; SWH 805, 1993, grade 2 virulence; OB3 Y317, 1994, grade 5 virulence). All rabbits recovered from the attenuated OB3 Y317 infection. (C) Pulmonary edema at autopsy. (D) Hemorrhage in hind leg at autopsy. (E) Histology of lymph node showing complete absence of lymphocytes and bacteria packed into subcapsular sinus (broad arrow) and other parts of the node (arrows). (Scale bar, 100 μm.) (F) Histology of lung showing edema and bacterial colonies (arrows). (Scale bar, 20 μm.)
The acute collapse phenotype is strikingly distinct from that seen with the progenitor SLS virus or those from the early radiation (6). Notably, death was associated with a form of septic or toxic shock characterized by profound immune system depression seen as a near or complete depletion of lymphocytes from lymph nodes and spleen, and frequently the presence of bacteria throughout tissues such as lung, liver, kidney, heart, spleen, and lymph nodes; necrosis of lymphocytes in gut lymphoid tissue; and massive bacterial invasion, often of Staphylococcus aureus (Fig. 1 E and F and SI Appendix, Fig. S4). Despite the high abundance and wide distribution of bacteria, there was no inflammatory cell response to the bacteria on histology. Peripheral blood taken 10 d after infection from rabbits with this syndrome showed lymphocytosis and neutropenia (SI Appendix, Fig. S5). Across all rabbits tested in trial 2 (Table 1), neutrophil levels in the blood at day 10 were positively correlated with survival time (SI Appendix, Fig. S6A; Spearman rank order correlation, 0.81; P < 0.001). Unlike classical myxomatosis, rectal temperatures in animals dying with acute collapse syndrome were commonly not elevated except for a spike to >40–42 °C in the 24 h before death, probably associated with bacterial invasion, and an initial temperature rise at day 4–5 around the time of virus generalization (SI Appendix, Fig. S6B).
Not all rabbits in groups infected with viruses that caused acute collapse syndrome succumbed to acute collapse. Those that did not developed high titers of virus at the inoculation site despite the lack of typical cutaneous pathology (SI Appendix, Fig. S7). Importantly, secondary bacterial infection in these animals caused a range of pathologies, including pericarditis, suggesting these animals were profoundly immunosuppressed. However, unlike the acute deaths, this pathology was accompanied by an inflammatory response in the tissues. These animals also had prolonged temperature elevation, similar to that seen with attenuated 1950s viruses, and consistent with ongoing inflammatory responses (SI Appendix, Fig. S6Biii). S. aureus was isolated from tissues of some rabbits, consistent with the clusters of coccoid bacteria seen in the acute cases (SI Appendix, Fig. S8).
Time course studies comparing the 1950 progenitor strain SLS with two strains from the 1990s, the hyperacute BRK 4/93 and the highly attenuated OB3 Y317, showed that BRK 4/93 caused a very different disease to the progenitor or the attenuated modern virus. BRK 4/93 infections were characterized by prominent subcutaneous edema (SI Appendix, Fig. S9) and profound neutropenia (SI Appendix, Fig. S10) and had markedly higher titers of virus in spleen, lung, and liver (SI Appendix, Fig. S11). However, titers in the primary lesion were comparable across all infection, despite the difference in lesion appearance (SI Appendix, Fig. S11). Bacteria were not detectable in tissues by histology at 10 d after infection, although the lymphoid tissue depletion in BRK 4/93 had already occurred. In contrast, animals infected with SLS often showed large influxes of neutrophils into tissues such as lymph nodes and testes (SI Appendix, Fig. S12). Taken together with titer data from the clinical phenotype experiments, these data show an alteration in tissue tropism, with much higher viral titers in liver and lung than occurred with the progenitor virus. Loss of lymphoid tissue and peripheral blood neutropenia indicate a collapse of immune system function that likely facilitates opportunistic bacterial infection.
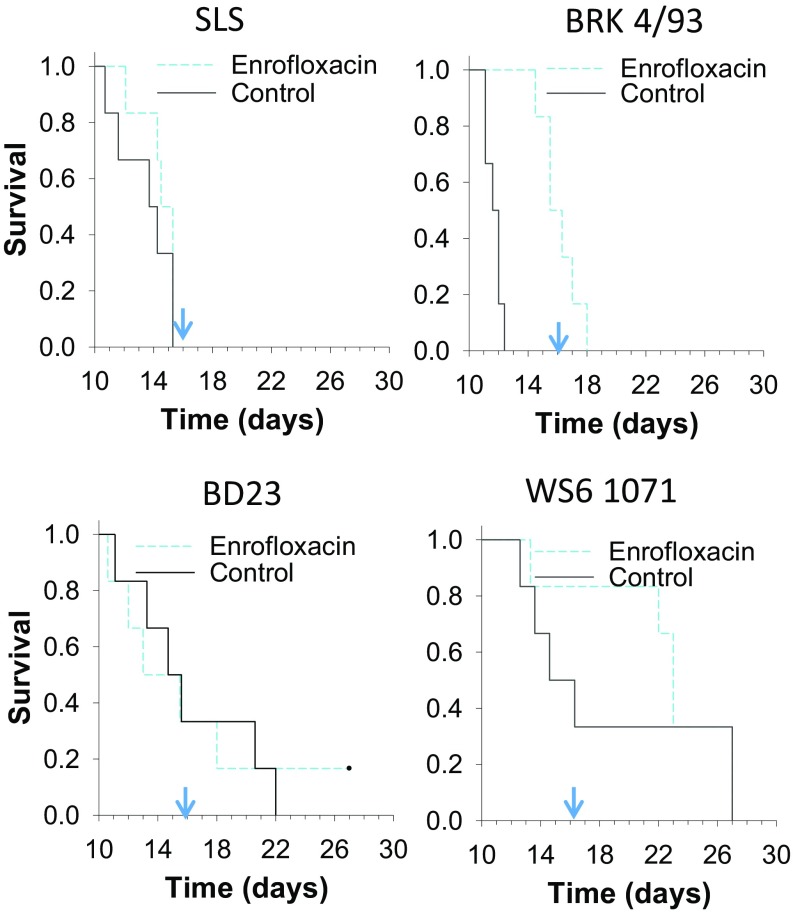
To examine the role of bacterial sepsis, rabbits treated with the broad-spectrum antibacterial enrofloxacin were compared with untreated controls infected with the same virus (SI Appendix). As predicted, there was no difference in survival times for the groups infected with SLS, since bacterial sepsis played no role in the disease from this virus. However, rabbits infected with the hyperacute BRK 4/93 survived significantly longer if they were treated with enrofloxacin (Fig. 2), and only 1/6 of the treated animals showed bacteria in the tissues histologically, and then only in the lymph nodes. In contrast, animals from the control group had widely disseminated bacteria. The importance of bacterial infection in disease and survival is also seen in the high temperatures from control animals infected with WS6 1071 or BD23, two strains from the 1990s that are less virulent than BRK 4/93 (SI Appendix, Fig. S13). Together, these results confirm that the progenitor virus SLS, although undoubtedly immunosuppressive, does not induce the same degree of immune system collapse. Even though antibacterial treatment significantly delayed time to death in rabbits infected with BRK 4/93, these cases subsequently succumbed to virus infection. Thus, the hypervirulent BRK 4/93 caused very acute immune suppression with overwhelming bacteremia. WS6 1071 and BD23 still induced pulmonary edema and acute deaths often with bacteremia, but could also cause death resulting from the virus alone.
Fig. 2.
(A) Kaplan-Meier plots for rabbits treated with enrofloxacin from days 5–16 after infection (blue line) or controls treated with PBS (black line) and infected with SLS, BRK 4/93, WS6 1071, or BD23. Arrow indicates day 16. ASTs (range) were as follows. SLS: control, 13.1 d (10.7–15.3 d), and enrofloxacin, 14.3 d (12.1–15.3 d); BRK 12/2/93: control, 11.7 d (11.1–12.4 d), and enrofloxacin, 16.1 d (14.5–18 d); BD23: control, 15.3 d (11.1–22 d), and enrofloxacin, 15.7 d (10.6 d—survived); WS6 1071: control, 16.9 d (12.6–27 d), and enrofloxacin, 21.4 d (13.3–27 d).
Discussion
Fenner and colleagues measured the virulence of hundreds of field isolates in the 1950s and 1960s, but they did not report the acute shock syndrome observed here (6–8). We also found no evidence of acute collapse in SLS or two other isolates from the 1950s (Table 1). Strikingly, however, all grade 1–3 viruses from the 1990s caused acute collapse, a phenotype that was markedly distinct from classical cutaneous myxomatosis seen with the progenitor SLS virus or those from the early virus radiation. Indeed, only two viral strains from the 1990s, the attenuated Meby strain from Tasmania and the highly attenuated OB3 Y317, caused a cutaneous form of disease in laboratory rabbits. The acute collapse phenotype is thus a novel syndrome that emerged sometime after Fenner’s work ended in the early 1960s. It is difficult to pinpoint the year of emergence with any precision, as virulence phenotyping in the period between Fenner’s work and ours focused on CFR and AST and did not report detailed pathology. However, given its widespread phylogenetic distribution and our previous molecular clock dating studies (31), it is possible that viruses causing this syndrome first emerged sometime between the mid-1970s and early 1980s.
The syndrome is characterized by an absence of cellular inflammatory responses, lymphocyte depletion from lymphoid tissues, and particularly the loss of neutrophils in the most virulent infections. Neutrophils normally form a major barrier to bacterial infection, and their disappearance here was associated with bacteremia throughout the tissues and animal collapse with a “septic shock” type of condition. Arguably, this has generated viruses that are more virulent than the highly lethal SLS strain that was released in 1950. In our only head-to-head experiment comparing the virulence of the highly lethal ancestor and the most lethal of the strains from the 1990s, the more recent strain killed rabbits more quickly than did the ancestor (BRK 4/93 vs. SLS; two-tailed P = 0.059, comparing controls only from the enrofloxacin experiment; Fig. 2 A and B). Moreover, while SLS and BRK 4/93 both caused typical myxomatosis in wild rabbits in previous experiments, five of nine wild rabbits experimentally infected with BRK 4/93 died of severe disease (32), whereas SLS killed just one of five, and that one death was due to pneumonia when the remaining rabbits were recovering (25).
Similar to other poxviruses, myxoma virus encodes multiple proteins that manipulate and suppress the host immune response (33). The data we report here show that MXYV in Australia has evolved enhanced abilities to suppress the immune system of nonresistant laboratory rabbits. The evolution of enhanced immunosuppressive abilities might be expected here because host resistance depends on enhanced innate antiviral responses. In the field, MYXV imposes strong selection for resistance in rabbit populations because infection can cause substantial morbidity and mortality (6). Host resistance substantially affects MYXV transmission because immunity reduces viral titers at cutaneous sites and leads to more rapid viral clearance (23). This must strongly select for counter adaptation by the virus because high viral titers are essential for MYXV transmission to vectors (34), and rabbits that survive infection are poor sources of infectivity for vectors (35). We propose that viral genes encoding the acute collapse syndrome became favored by natural selection sometime between the mid-1970s and the early 1980s because they allowed the virus to overcome the newly evolved resistance of wild rabbits. A strong prediction is that the 1990s viral isolates we tested here would, in 1990s wild rabbits, cause more pronounced cutaneous lesions with higher titers of virus for longer than would progenitor viruses. Consistent with that, wild rabbits of that era infected with BRK 4/93 and other 1990s viruses had essentially no primary lesion at the inoculation site, but the virus successfully spread to distal cutaneous tissues such as eyelids and the base of ears (32). Moreover, field reports from that era and today continued to describe rabbits with classical cutaneous myxomatosis, implying that viruses with the genetic machinery to cause acute collapse in laboratory rabbits were able to overcome enhanced resistance in wild rabbits. These observations, together with our experimental data, provide no evidence that MYXV is evolving toward an innocuous fibroma type of disease, as it induces in its presumed natural host, the Brazilian tapeti (Sylvilagus brasiliensis). Instead, MYXV in Australia is evolving mechanisms to maintain high viral titers in the cutaneous tissues of resistant wild rabbits via generalized disease. Whether the long-term evolutionary trajectory continues toward even more aggressive immunosuppression remains to be determined.
There are three possible genetic explanations for the acute collapse syndrome we report here. The simplest is that it is the result of a single key mutation. All the 1990s viruses tested here have reading frame disruptions in M009L, a member of a three-gene family that encodes a putative E3 ubiquitin ligase (36), although this gene has not previously been reported to affect virulence (37). It is unclear how the loss of this gene could cause a gain of function (massive immunosuppression). Beyond M009, there are 17 nonsynonymous mutations shared by all the 13 virus strains that cause the acute collapse (i.e., that fall on the branch leading to all of the modern Australian isolates of MYXV) (22, 31) (SI Appendix, Fig. S1 and Table S2). The Meby strain caused the most diverse range of phenotypes in our study (Table 1) and had evolved in isolation on Tasmania for at least 20 y. Excluding that strain, there are just four nonsynonymous mutations shared by the remaining acute-collapse strains (SI Appendix, Table S2), any of which could be a key mutation.
The second possibility is that rather than being a single key mutation, the acute collapse syndrome is caused by independent mutations in different viral lineages, such that there is convergence at the phenotypic level, but not the genetic level. There being multiple genetic routes to the same virulence phenotypes is a consistent feature of the radiation of the Australian (22, 31, 37) and European (38) MYXV lineages. For instance, the hypervirulent BRK 4/93, the most lethal of the viruses we tested, has only 2 unique nonsynonymous mutations outside M009L: a large deletion with probable loss of function in M036L and A47V in M112, a Holliday junction resolvase. In contrast, strain BD44, which also causes acute collapse and was of grade 1 virulence (Table 1), has neither of these mutations, but instead has nonsynonymous changes in M005 E81A and M134 N741T, T851I, and disruptions to ORFs in M000.5L/R, M008.1L/R (with consequent loss of active site in the protein) and M153R. The latter two proteins are important virulence factors (39, 40). In addition, along with other viruses, BD44 possesses a mutation L71P in M156 (an inhibitor of the antiviral protein kinase R), which has been demonstrated to cause loss of function (41). Thus, the loss of major virulence functions is compatible with high virulence, suggesting compensatory mutations are playing an important role. Similarly, it is difficult to pinpoint a unique mutation that is responsible for the attenuation and nodular phenotype in OB3 Y317, as there are no reversions of earlier mutations that are unique to this virus (SI Appendix, Fig. S1 and Table S2).
A final possibility is that the genetic causes of acute collapse are not a result of amino acid changes in coding regions of the genome; previous examination of putative promoter sequences showed three of the phenotyped viruses had single-nucleotide indels that might have some effect on transcription, although there was no consistent pattern: WS6 346 (M008.1L/R, secreted serine proteinase inhibitor), SWH 8/2/93 (M138L, sialyl-transferase), and WS6 1071 (M153R, E3 Ub ligase; however, this ORF is disrupted in WS6 1071) (31). All three genes have reported virulence functions (39, 40, 42–44).
The ongoing evolution of MYXV in Australia has already contributed hugely to the understanding of pathogen adaptation and virulence evolution (1, 11–19). Critically, our data show that enhancements in host resistance have led to the evolution of strains of MYXV that are far more immunosuppressive than any of their ancestors. It will clearly be important to determine when and if the evolution of immunosuppressive disease phenotypes might evolve in other contexts. In agriculture and aquaculture, for example, there are major efforts to enhance the resistance of farm animals via traditional artificial selection (45, 46), genetic engineering (47) and immunization (48), all of which have the potential to affect virulence evolution unless they completely prevent transmission (49, 50). This could lead to arms races between agriculturalists and viruses if farm animals with artificially enhanced resistance prompt the evolution of pathogens with the capacity to immunosuppress their hosts. In the meantime, the natural arms race between MYXV and Australian rabbits continues to escalate. In the decades to come, it will be intriguing to learn which of the antagonists first fails to find a genetic solution to the selection imposed by the other.
Materials and Methods
Rabbits.
Rabbits were male, outbred New Zealand White laboratory rabbits (Oryctolagus cuniculus). For further details, see SI Appendix. All procedures were approved by The Pennsylvania State University Institutional Animal Care and Use Committee (permit numbers: 33615, 42748, 46919).
Viruses.
Viruses were isolated from wild rabbits from ad hoc samples submitted by field workers from New South Wales, Australian Capital Territory (Canberra district), Victoria, and Tasmania or sampled systematically at field study sites in the Australian Capital Territory or Queensland; the complete genome sequence was determined for all viruses tested (22, 31, 51–53). Virus stocks were prepared in RK13 cells; infections were with the same virus stock used for sequence analysis (31). Tissues were assayed as previously described (23). Titers are expressed as plaque-forming units per gram tissue.
Virulence Phenotyping.
Two separate trials were conducted to examine virus phenotypes (Table 1 and SI Appendix). A total of 100 plaque-forming units of virus in 100 µL PBS was injected intradermally into the rump on the right-hand side of each of 6 rabbits. Each rabbit was examined daily and, from d10 postinfection, monitored twice daily (SI Appendix).
Challenge Experiments.
To ensure the acute syndrome was not a result of an adventitious agent in the inocula, rabbits recovered from infection with the attenuated OB3 Y317 strain, and therefore considered immune to MYXV, were inoculated with viruses causing the acute death syndrome: three rabbits with BD23 and three with BRK. None of the rabbits developed any clinical disease other than a rapid swelling at the inoculation site typical of the response of an immune rabbit to challenge (54).
Time Course Experiments.
A sample of 12 rabbits was infected with each of SLS, BRK, and OB3Y317 in the dorsum of the right foot. Rabbits were monitored daily as described earlier. At 4, 8, and 10 d after infection, four rabbits in each group were killed and samples collected for virological and histological analysis.
Antibacterial Treatments.
Groups of 12 rabbits were each infected with SLS (1950 progenitor virus; grade 1) or three of the viral strains from the 1990s that caused the acute mortality syndrome: BRK 4/93 (1993; grade 1), BD23 (1999; grade 2), and WS6 1071 (1995; grade 3). In each group, six rabbits were treated with the broad-spectrum antibacterial enrofloxacin from day 5 to day 16 after infection at a dose of 26.25 mg twice daily. The remaining six rabbits were treated with PBS as a control. Bacteriological monitoring was done on nasal swabs at days −10, 0, and 10 (SI Appendix).
Inferring Virulence Grades.
Fenner’s original experiments used death as an endpoint. Because this is no longer ethically acceptable, we defined endpoints based on detailed clinical examination, as described in SI Appendix.
Supplementary Material
Acknowledgments
This work was funded by the National Institute of Allergy and Infectious Diseases (R01AI093804). E.C.H. is supported by an National Health and Medical Research Council Australia Fellowship (GNT1037231).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710336114/-/DCSupplemental.
References
- 1.Bolker BM, Nanda A, Shah D. Transient virulence of emerging pathogens. J R Soc Interface. 2010;7:811–822. doi: 10.1098/rsif.2009.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepin KM, Lass S, Pulliam JRC, Read AF, Lloyd-Smith JO. Identifying genetic markers of adaptation for surveillance of viral host jumps. Nat Rev Microbiol. 2010;8:802–813. doi: 10.1038/nrmicro2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser C, et al. Virulence and pathogenesis of HIV-1 infection: An evolutionary perspective. Science. 2014;343:1243727. doi: 10.1126/science.1243727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diehl WE, et al. Ebola Virus glycoprotein with increased infectivity dominated the 2013–2016 epidemic. Cell. 2016;167:1088–1098.e6. doi: 10.1016/j.cell.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbanowicz RA, et al. Human adaptation of Ebola virus during the West African outbreak. Cell. 2016;167:1079–1087. doi: 10.1016/j.cell.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenner F, Marshall ID. A comparison of the virulence for European rabbits (Oryctolagus cuniculus) of strains of myxoma virus recovered in the field in Australia, Europe and America. J Hyg (Lond) 1957;55:149–191. doi: 10.1017/s0022172400037098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall ID, Fenner F. Studies in the epidemiology of infectious myxomatosis of rabbits. VII. The virulence of strains of myxoma virus recovered from Australian wild rabbits between 1951 and 1959. J Hyg (Lond) 1960;58:485–488. doi: 10.1017/s0022172400038614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenner F, Woodroofe GM. Changes in the virulence and antigenic structure of strains of myxoma virus recovered from Australian wild rabbits between 1950 and 1964. Aust J Exp Biol Med Sci. 1965;43:359–370. doi: 10.1038/icb.1965.69. [DOI] [PubMed] [Google Scholar]
- 9.Fenner F. The Florey lecture, 1983. Biological control, as exemplified by smallpox eradication and myxomatosis. Proc R Soc Lond B Biol Sci. 1983;218:259–285. doi: 10.1098/rspb.1983.0039. [DOI] [PubMed] [Google Scholar]
- 10.Fenner F, Fantini B. Biological control of vertebrate pests. The history of myxomatosis: An experiment in evolution. CAB International; New York: 1999. [Google Scholar]
- 11.Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RM, May RM. Infectious diseases of humans. Oxford University Press; New York: 1991. [Google Scholar]
- 13.Dwyer G, Levin S, Buttel L. A simulation model of the population dynamics and evolution of myxomatosis. Ecol Monogr. 1990;60:423–447. [Google Scholar]
- 14.Bull JJ. Virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 15.Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- 16.Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet. 2002;32:569–577. doi: 10.1038/ng1202-569. [DOI] [PubMed] [Google Scholar]
- 17.Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol Lett. 2013;16:556–567. doi: 10.1111/ele.12076. [DOI] [PubMed] [Google Scholar]
- 18.Alizon S, Hurford A, Mideo N, Van Baalen M. Virulence evolution and the trade-off hypothesis: History, current state of affairs and the future. J Evol Biol. 2009;22:245–259. doi: 10.1111/j.1420-9101.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- 19.Cressler CE, McLeod DV, Rozins C, Van Den Hoogen J, Day T. The adaptive evolution of virulence: A review of theoretical predictions and empirical tests. Parasitology. 2016;143:915–930. doi: 10.1017/S003118201500092X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall ID, Fenner F. Studies in the epidemiology of infectious myxomatosis of rabbits. V. Changes in the innate resistance of Australian wild rabbits exposed to myxomatosis. J Hyg (Lond) 1958;56:288–302. doi: 10.1017/s0022172400037773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall ID, Douglas GW. Studies in the epidemiology of infectious myxomatosis of rabbits. VIII. Further observations on changes in the innate resistance of Australian wild rabbits exposed to myxomatosis. J Hyg (Lond) 1961;59:117–122. doi: 10.1017/s0022172400038766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr PJ, et al. Evolutionary history and attenuation of myxoma virus on two continents. PLoS Pathog. 2012;8:e1002950. doi: 10.1371/journal.ppat.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Best SM, Kerr PJ. Coevolution of host and virus: The pathogenesis of virulent and attenuated strains of myxoma virus in resistant and susceptible European rabbits. Virology. 2000;267:36–48. doi: 10.1006/viro.1999.0104. [DOI] [PubMed] [Google Scholar]
- 24.Best SM, Collins SV, Kerr PJ. Coevolution of host and virus: Cellular localization of virus in myxoma virus infection of resistant and susceptible European rabbits. Virology. 2000;277:76–91. doi: 10.1006/viro.2000.0505. [DOI] [PubMed] [Google Scholar]
- 25.Kerr PJ, et al. Expression of rabbit IL-4 by recombinant myxoma viruses enhances virulence and overcomes genetic resistance to myxomatosis. Virology. 2004;324:117–128. doi: 10.1016/j.virol.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Silvers L, et al. Virulence and pathogenesis of the MSW and MSD strains of Californian myxoma virus in European rabbits with genetic resistance to myxomatosis compared to rabbits with no genetic resistance. Virology. 2006;348:72–83. doi: 10.1016/j.virol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Fenner F, Ross J. Myxomatosis. In: Thompson HV, King CM, editors. The European rabbit. The history and biology of a successful colonizer. Oxford University Press; Oxford: 1994. pp. 205–240. [Google Scholar]
- 28.Joubert L, Duclos P, Toaillen P. La myxomatose des garennes dans le sud-est. La myxomatose amyxomateuse. Rev Méd Vét. 1982;133:739–753. [Google Scholar]
- 29.Marlier D, et al. Study of the virulence of five strains of amyxomatous myxoma virus in crossbred New Zealand White/Californian conventional rabbits, with evidence of long-term testicular infection in recovered animals. J Comp Pathol. 2000;122:101–113. doi: 10.1053/jcpa.1999.0345. [DOI] [PubMed] [Google Scholar]
- 30.Marlier D, Cassart D, Boucraut-Baralon C, Coignoul F, Vindevogel H. Experimental infection of specific pathogen-free New Zealand White rabbits with five strains of amyxomatous myxoma virus. J Comp Pathol. 1999;121:369–384. doi: 10.1053/jcpa.1999.0335. [DOI] [PubMed] [Google Scholar]
- 31.Kerr PJ, et al. Genome scale evolution of myxoma virus reveals host-pathogen adaptation and rapid geographic spread. J Virol. 2013;87:12900–12915. doi: 10.1128/JVI.02060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr PJ, Merchant JC, Silvers L, Hood GM, Robinson AJ. Monitoring the spread of myxoma virus in rabbit Oryctolagus cuniculus populations on the southern tablelands of New South Wales, Australia. II. Selection of a strain of virus for release. Epidemiol Infect. 2003;130:123–133. doi: 10.1017/s0950268802007860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerr PJ. Myxomatosis in Australia and Europe: A model for emerging infectious diseases. Antiviral Res. 2012;93:387–415. doi: 10.1016/j.antiviral.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Fenner F, Day MF, Woodroofe GM. Epidemiological consequences of the mechanical transmission of myxomatosis by mosquitoes. J Hyg (Lond) 1956;54:284–303. doi: 10.1017/s0022172400044521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mead-Briggs AR, Vaughan JA. The differential transmissibility of Myxoma virus strains of differing virulence grades by the rabbit flea Spilopsyllus cuniculi (Dale) J Hyg (Lond) 1975;75:237–247. doi: 10.1017/s0022172400047276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Villa NY, McFadden G. Interplay between poxviruses and the cellular ubiquitin/ubiquitin-like pathways. FEBS Lett. 2009;583:607–614. doi: 10.1016/j.febslet.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Kerr PJ, et al. Myxoma virus and the Leporipoxviruses: An evolutionary paradigm. Viruses. 2015;7:1020–1061. doi: 10.3390/v7031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr PJ, et al. Genomic and phenotypic characterization of myxoma virus from Great Britain reveals multiple evolutionary pathways distinct from those in Australia. PLoS Pathog. 2017;13:e1006252. doi: 10.1371/journal.ppat.1006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macen JL, Upton C, Nation N, McFadden G. SERP1, a serine proteinase inhibitor encoded by myxoma virus, is a secreted glycoprotein that interferes with inflammation. Virology. 1993;195:348–363. doi: 10.1006/viro.1993.1385. [DOI] [PubMed] [Google Scholar]
- 40.Guérin JL, et al. Myxoma virus leukemia-associated protein is responsible for major histocompatibility complex class I and Fas-CD95 down-regulation and defines scrapins, a new group of surface cellular receptor abductor proteins. J Virol. 2002;76:2912–2923. doi: 10.1128/JVI.76.6.2912-2923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng C, Haller SL, Rahman MM, McFadden G, Rothenburg S. Myxoma virus M156 is a specific inhibitor of rabbit PKR but contains a loss-of-function mutation in Australian virus isolates. Proc Natl Acad Sci USA. 2016;113:3855–3860. doi: 10.1073/pnas.1515613113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upton C, Macen JL, Wishart DS, McFadden G. Myxoma virus and malignant rabbit fibroma virus encode a serpin-like protein important for virus virulence. Virology. 1990;179:618–631. doi: 10.1016/0042-6822(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 43.Jackson RJ, Hall DF, Kerr PJ. Myxoma virus encodes an α2,3-sialyltransferase that enhances virulence. J Virol. 1999;73:2376–2384. doi: 10.1128/jvi.73.3.2376-2384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutard B, et al. The α2,3-sialyltransferase encoded by myxoma virus is a virulence factor that contributes to immunosuppression. PLoS One. 2015;10:e0118806. doi: 10.1371/journal.pone.0118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stear MJ, Bishop SC, Mallard BA, Raadsma H. The sustainability, feasibility and desirability of breeding livestock for disease resistance. Res Vet Sci. 2001;71:1–7. doi: 10.1053/rvsc.2001.0496. [DOI] [PubMed] [Google Scholar]
- 46.Davies G, Genini S, Bishop SC, Giuffra E. An assessment of opportunities to dissect host genetic variation in resistance to infectious diseases in livestock. Animal. 2009;3:415–436. doi: 10.1017/S1751731108003522. [DOI] [PubMed] [Google Scholar]
- 47.Lyall J, et al. Suppression of avian influenza transmission in genetically modified chickens. Science. 2011;331:223–226. doi: 10.1126/science.1198020. [DOI] [PubMed] [Google Scholar]
- 48.Meeusen ENT, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20:489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- 50.Read AF, et al. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biol. 2015;13:e1002198. doi: 10.1371/journal.pbio.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saint KM, French N, Kerr P. Genetic variation in Australian isolates of myxoma virus: An evolutionary and epidemiological study. Arch Virol. 2001;146:1105–1123. doi: 10.1007/s007050170109. [DOI] [PubMed] [Google Scholar]
- 52.Kerr PJ, Hone J, Perrin L, French N, Williams CK. Molecular and serological analysis of the epidemiology of myxoma virus in rabbits. Vet Microbiol. 2010;143:167–178. doi: 10.1016/j.vetmic.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Berman D, Kerr PJ, Stagg R, van Leeuwen BH, Gonzalez T. Should the 40-year-old practice of releasing virulent myxoma virus to control rabbits (Oryctolagus cuniculus) be continued? Wildl Res. 2006;33:549–556. [Google Scholar]
- 54.Kerr PJ. An ELISA for epidemiological studies of myxomatosis: Persistence of antibodies to myxoma virus in European rabbits (Oryctolagus cuniculus) Wildl Res. 1997;24:53–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.