Significance
How heterochromatin affects RNA processing is unclear. The chromatin regulators ASI1 and EDM2 function in regulating alternative polyadenylation at genes with intronic heterochromatin. We found that ASI1 and EDM2 are associated in planta through interactions with a putative RNA-binding protein, AIPP1. Protein interaction assays suggest that the RNA Pol II C-terminal domain phosphatase CPL2 and two other proteins (AIPP2 and AIPP3) are associated with the ASI1-AIPP1-EDM2 complex. Like ASI1 and EDM2, AIPP1 also functions in promoting the expression of heterochromatin-containing genes. However, the function of CPL2, AIPP2, and AIPP3 is antagonistic to that of ASI1, EDM2, and AIPP1. Our discovery of the ASI1-AIPP1-EDM2 complex and associated proteins is important for understanding how heterochromatin regulates RNA processing.
Keywords: RNA processing, heterochromatin, DNA methylation, polyadenylation, transposable element
Abstract
In several eukaryotic organisms, heterochromatin (HC) in the introns of genes can regulate RNA processing, including polyadenylation, but the mechanism underlying this regulation is poorly understood. By promoting distal polyadenylation, the bromo-adjacent homology (BAH) domain-containing and RNA recognition motif-containing protein ASI1 and the H3K9me2-binding protein EDM2 are required for the expression of functional full-length transcripts of intronic HC-containing genes in Arabidopsis. Here we report that ASI1 and EDM2 form a protein complex in vivo via a bridge protein, ASI1-Immunoprecipitated Protein 1 (AIPP1), which is another RNA recognition motif-containing protein. The complex also may contain the Pol II CTD phosphatase CPL2, the plant homeodomain-containing protein AIPP2, and another BAH domain protein, AIPP3. As is the case with dysfunction of ASI1 and EDM2, dysfunction of AIPP1 impedes the use of distal polyadenylation sites at tested intronic HC-containing genes, such as the histone demethylase gene IBM1, resulting in a lack of functional full-length transcripts. A mutation in AIPP1 causes silencing of the 35S-SUC2 transgene and genome-wide CHG hypermethylation at gene body regions, consistent with the lack of full-length functional IBM1 transcripts in the mutant. Interestingly, compared with asi1, edm2, and aipp1 mutations, mutations in CPL2, AIPP2, and AIPP3 cause the opposite effects on the expression of intronic HC-containing genes and other genes, suggesting that CPL2, AIPP2, and AIPP3 may form a distinct subcomplex. These results advance our understanding of the interplay between heterochromatic epigenetic modifications and RNA processing in higher eukaryotes.
A substantial portion of eukaryotic genomes consists of transposable elements (TEs) and other repetitive elements (TREs). Because of their potential deleterious effects on genome integrity, TREs are generally repressed by epigenetic silencing mechanisms (1, 2). Therefore, epigenetic silencing marks, such as DNA methylation and repressive histone modifications like H3K9me2 are enriched, and heterochromatin (HC) is formed in these regions. Heterochromatic TREs (HC-TREs) can affect the expression of their associated genes in various ways. In both plant and animal genomes, HC exists not only in promoter regions, but also within transcribed regions, especially within introns, and most intronic HC is a result of TRE insertion (3–6). In the human genome, 60% of TEs are located within introns that compose only 24% of the genome. Approximately 10% of the genes in the maize genome have intronic TEs >1 kb in length, and ∼0.7% of the annotated genes contain intronic TEs in the genome of the model plant Arabidopsis (6). Thus, intronic TEs are widespread in human and plant genomes. Although the repressive effect of promoter HC-TREs on the expression of downstream genes has been well documented (7, 8), the effect of intronic HC-TREs on the expression of their associated genes remains largely unknown (9).
The polyadenylation of mRNAs, which involves 3′-end cleavage of pre-mRNAs and addition of poly (A) tails, is an important step in gene expression in eukaryotes that is achieved through a finely tuned biochemical process. This process involves numerous protein factors, including the CPSF (cleavage and polyadenylation specificity factor) and CstF (cleavage stimulation factor) complexes, as well as a number of cis elements, such as poly(A) signal elements (10). Previous studies have shown that >50% of human genes, >70% of Arabidopsis genes, and 50–82% of rice genes have multiple polyadenylation sites that generate various mRNA isoforms with different 3′ ends (10–12). This regulatory mechanism of gene expression is termed alternative polyadenylation.
The RNA Pol II carboxyl-terminal domain (CTD) is critical for coupling transcription with RNA processing (13). Studies in yeasts and animals have found that the Ssu72 RNA polymerase II Ser-5 CTD phosphatase regulates alternative polyadenylation (14, 15). Ssu72 binds CTD heptapeptides and forms a complex with Symplekin, a subunit of the CPSF complex (16).
Little is known about the interplay between RNA processing and heterochromatic histone modifications. Recent reports have indicated that histone modifications and nucleosome occupancy might influence polyadenylation site selection (17, 18). In humans, strong nucleosome depletion around polyadenylation sites and nucleosome enrichment downstream of polyadenylation sites have been observed (17). Moreover, the heterochromatic H3K9me2 and H3K27me1 modifications have been shown to be enriched in low-use polyadenylation sites, whereas the H3K27me2/3 marks are enriched in high-use polyadenylation sites (18). The mechanism underlying this polyadenylation site selection remains unclear, however.
Our previous study indicated that the Arabidopsis chromatin regulator and RNA-binding protein ASI1 (anti-silencing 1)/IBM2/SG1 functions in controlling polyadenylation site selection for genes with intronic HC-TREs (19–21). ASI1 is a BAH domain- and RNA recognition motif (RRM)-containing protein that represses the use of intronic proximal polyadenylation site near intronic TREs, thereby promoting the generation of full-length transcripts (19–21). Another chromatin regulator, EDM2 (enhanced downy mildew 2), functions in a similar manner as ASI1 in regulating alternative polyadenylation for intronic HC-TRE–containing genes (22, 23). EDM2 has three PHD (plant homeo domain) domains that bind H3K9me2 marks, but not H3K27me3 marks (22, 23). ASI1 and EDM2, as well as the heterochromatic markers H3K9me2 and H3K27me1, but not H3K27me3, are enriched in the intronic heterochromatic regions of the affected genes (19, 20, 22, 23). Although we identified ASI1 and EDM2 from the same genetic screen, whether these two proteins may function in the same protein complex in alternative polyadenylation regulation for genes with intronic HC is not known. In oil palm, the DNA methylation status of an intronic LINE-related transposon Karma also affects the RNA processing of the homeotic gene DEFICIENS (24), although whether homologs of ASI1 and EDM2 may be involved in this regulation is unclear. Loss of DNA methylation in somaclonal variants at the Karma transposon causes reduced accumulation of functional full-length DEFICIENS transcripts and increased accumulation of short transcripts, resulting in the unproductive mantled fruits (24).
In this study, we found that ASI1 is associated with EDM2 in vivo even though they do not directly interact with each other. The association between ASI1 and EDM2 is bridged by another RRM protein, ASI1 immunoprecipitated protein 1 (AIPP1). AIPP1 dysfunction causes increased use of proximal polyadenylation sites in tested ASI1 and EDM2 target genes. Like asi1 and edm2 mutations, AIPP1 mutations lead to defects in the expression of the intronic HC-TRE–containing gene IBM1, which encodes an H3K9me2 demethylase. These defects result in transgene silencing and genome-wide CHG hypermethylation at gene body regions. Our protein interaction results suggest that the ASI1-AIPP1-EDM2 complex is associated with three other proteins—AIPP2, AIPP3 and AIPP4/CPL2—and that these three associated proteins function antagonistically with ASI1, AIPP1, and EDM2 in regulating intronic HC-TRE–containing genes. Given that CPL2 is a plant RNA Pol II Ser-5 CTD phosphatase homologous to the yeast Ssu72 (25), and that Pol II Ser-5 CTD phosphatase is known to associate with CPSF (16) and regulate alternative polyadenylation in yeasts and animals (14, 15), our results suggest that the ASI1-AIPP1-EDM2 complex may regulate alternative polyadenylation by modulating the function of CPL2. Moreover, we have identified a nonintronic HC-TRE gene that is also regulated by the ASI1-AIPP1-EDM2 complex, suggesting that the complex may function beyond the regulation of alternative polyadenylation. Our findings uncover a protein complex critical for the regulation of genes with HC.
Results
AIPP1 Interacts with ASI1 and EDM2 to Form a Protein Complex in Vivo.
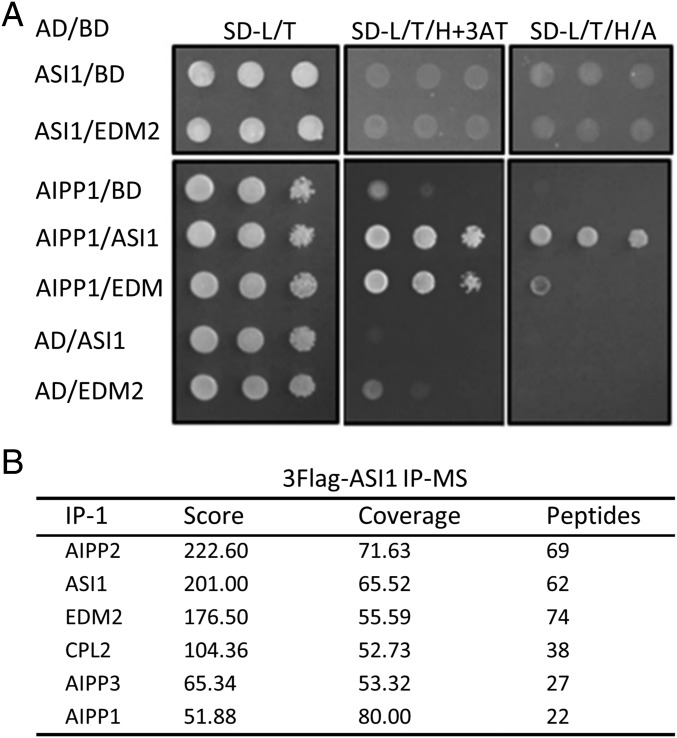
Previous findings have shown that both ASI1 and EDM2 are required to facilitate the distal polyadenylation of IBM1 and other genes with intronic HC-TREs (19, 20, 22, 23) and to prevent transcriptional silencing at the 35S-SUC2 reporter gene (19, 22). The shared functions of ASI1 and EDM2 prompted us to explore whether the two proteins may physically interact and thus function together in plants. In a yeast two-hybrid (Y2H) assay, direct interaction of ASI1 with EDM2 was not observed (Fig. 1A), consistent with the results of a split luciferase assay (Fig. S1A). However, immunoprecipitation coupled with mass spectrometry (IP-MS) revealed that EDM2 could be copurified with 3×Flag-tagged ASI1 in a previously created asi1 mutant complementation line (Fig. 1B and Fig. S2) (19). Based on these data, we suspected that one or more unknown components may mediate the physical interaction between ASI1 and EDM2 in vivo.
Fig. 1.
AIPP1 interacts with ASI1 and EDM2. (A) Y2H assay showing that ASI1 does not directly interact with EDM2, but both ASI1 and EDM2 directly interact with AIPP1. (B) IP-MS results showing that EDM2, AIPP1, AIPP2, AIPP3, and CPL2 copurified with 3×Flag-ASI1. Results from one of five independent IP-MS experiments are shown. The “score” was calculated as the sum of the negative algorithms of the posterior error probability values of the connected peptide-spectrum matches. “Coverage” indicates the percentage of amino acid residues covered by the identified peptides. “Peptide” refers to the total number of identified peptide sequences matching the protein.
Fig. S1.
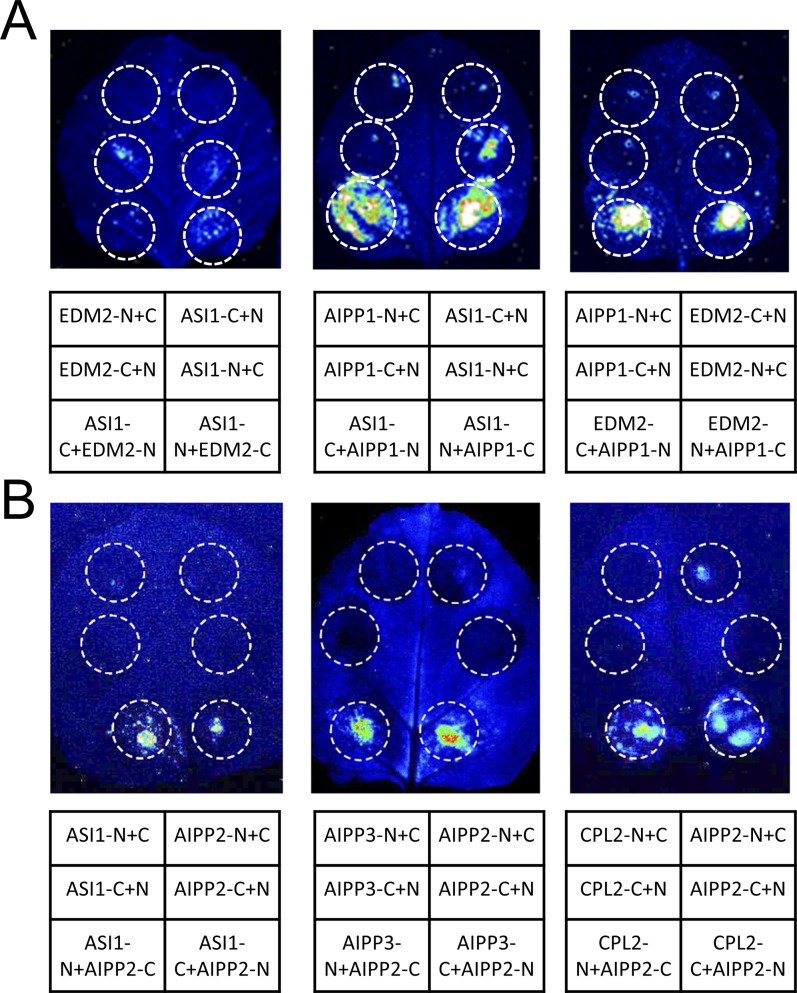
Split luciferase assays showing protein interactions among the ASI1-copurified proteins. Protein interactions among the indicated proteins were examined by split luciferase assays. (A) Split luciferase assays showing protein interactions between AIPP1 and ASI1, AIPP1 and EMD2 but not ASI1 and EDM2. (B) Split luciferase assays showing that AIPP2 can interact with ASI1, AIPP3, and CPL2. Luciferase activity was determined at 3 d after infiltration. N and C indicate the N-terminal and C-terminal fragments of luciferase, respectively.
Fig. S2.
ASI1 immunoprecipitated proteins identified by IP-MS assays. Results from four of five independent IP-MS experiments are shown.
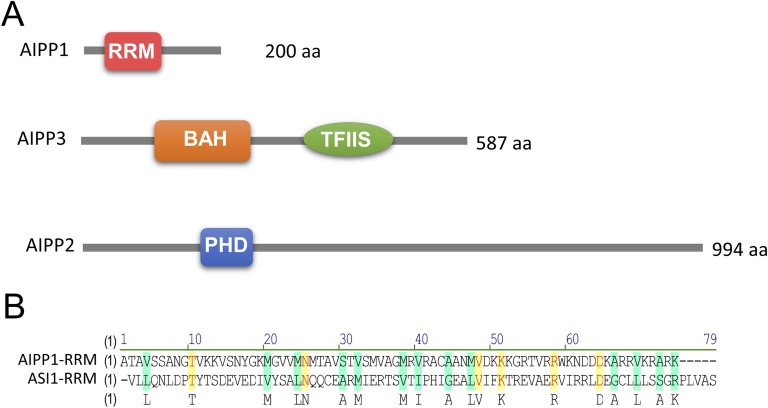
Further examination of the IP-MS data revealed other proteins copurified with 3×Flag-ASI1 besides EDM2, including AIPP1, AIPP2, AIPP3, and AIPP4/CPL2 (Fig. 1B and Fig. S2). Like ASI1 (19), AIPP1 contains an RRM domain (Fig. S3A), although the RRM domains of ASI1 and AIPP1 share only a very limited sequence homology (Fig. S3B). To test whether the two RRM proteins may interact, we performed Y2H assays and split luciferase assays in tobacco leaves. Both assays showed that ASI1 directly interacts with AIPP1, and that AIPP1 also directly interacts with EDM2 (Fig. 1A and Fig. S1A). These results suggest that AIPP1 may bridge the interaction between ASI1 and EDM2.
Fig. S3.
Domain structures of ASI1 immunoprecipitated proteins. (A) Domain structures of AIPP1, AIPP2, and AIPP3 proteins. Conserved domains are predicted by the National Center for Biotechnology Information’s online tool (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). (B) Sequence alignment between the RRM domains of AIPP1 and ASI1.
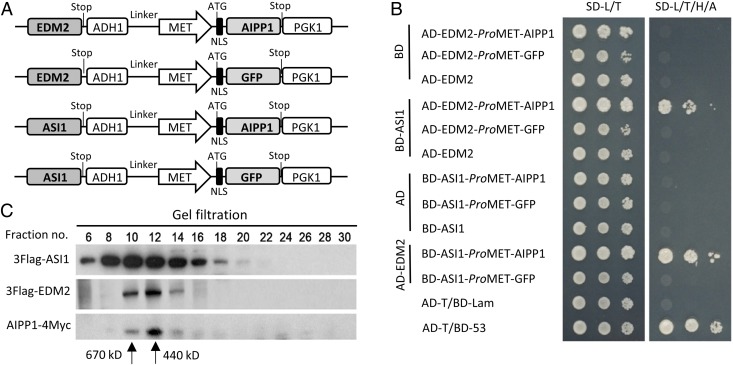
To test this hypothesis, we conducted a yeast three-hybrid (Y3H) assay. EDM2 and AIPP1 were coexpressed in the same prey vector under the control of ADH1 and MET promoters, respectively (Fig. 2A). This coexpression allowed yeast cells to grow in a high-stringency selection medium when the bait BD-ASI1 was present (Fig. 2B). However, the yeast cells failed to grow when AIPP1 was replaced with an irrelevant protein, GFP, or when AIPP1 was simply removed (Fig. 2 A and B). Similarly, the coexpression of ASI1 and AIPP1, but not of ASI1 and GFP, in the bait vector also enabled the survival of yeast cells in the selection medium in the presence of prey AD-EDM2 (Fig. 2B). These results strongly support our hypothesis that AIPP1 can function as a bridge protein between ASI1 and EDM2 in vivo.
Fig. 2.
ASI1, EDM2, and AIPP1 may form a protein complex in vivo. (A) Diagram of the construction of the entry vectors harboring two expression units. (B) The ASI1–EDM2 interaction mediated by AIPP1 was examined by yeast growth on a stringent medium deficient in Met, Leu, Trp, His, and adenine (SD-M/L/T/H/A). GFP, which was used as an unrelated donor to replace AIPP1, could not mediate the interaction between EDM2 and ASI1. The plates were photographed after incubation at 28 °C for 3 d. (C) Western blot analysis showing that the epitope-tagged ASI1, EDM2, and AIPP1 proteins are present in the same eluted fractions in gel filtration assays. The arrows indicate the molecular mass corresponding to the indicated fractions.
To test whether these three proteins may exist in the same protein complex in planta, we performed gel filtration assays with epitope-tagged ASI1, EDM2 (19, 22), and AIPP1 transgenic lines. For 4×Myc-tagged AIPP1 transgenic plants, AIPP1 genomic DNA, including a 2-kb promoter sequence, was transformed into Col-0 plants. The gel filtration results showed that ASI1, EDM2, and AIPP1 can be eluted in the same fractions, indicating that they exist in a complex with an estimated size of 440–670 kDa (Fig. 2C). Taken together, our results show that ASI1 and EDM2 form a protein complex in vivo via a bridge provided by AIPP1.
AIPP1 Is Required for the Accumulation of Full-Length Transcripts of Intronic HC-Containing Genes.
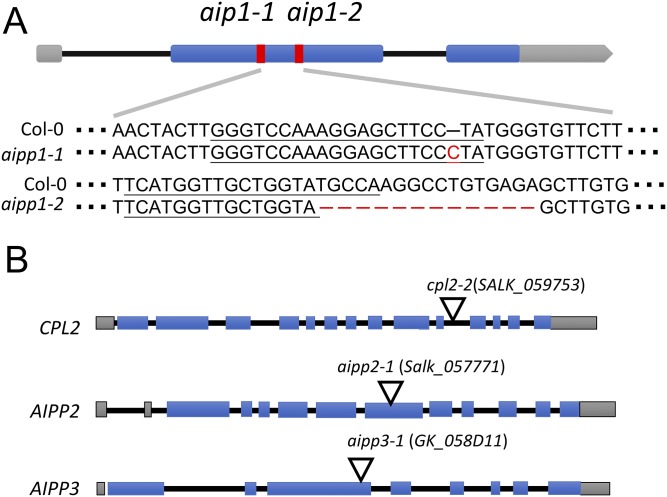
ASI1 and EDM2 function in controlling the polyadenylation site selection of intronic HC-containing genes by facilitating distal polyadenylation (19, 22). Therefore, we next determined whether AIPP1 also controls the proper expression of intronic HC-containing genes targeted by ASI1 and EDM2. Because we failed to identify a T-DNA insertion mutant of the AIPP1 gene, we used CRISPR/Cas9-mediated genome editing to generate AIPP1 mutants. We designed two Cas9 target sites in the second exon of the AIPP1 gene (Fig. S4A). By DNA sequencing, we identified two homozygous mutants from the CRISPR/Cas9-transformed plants. The first mutant has a single C insertion between the nucleotides 179 and 180 downstream of the ATG translation initiation, and the second mutant has a 17-nt deletion from nucleotides 269 to 285 downstream of the start codon (Fig. S4A). Both mutations would lead to a frame shift in the AIPP1 amino acid sequence. Hereinafter, we refer to these two mutants as aipp1-1 and aipp1-2, respectively (Fig. S4A).
Fig. S4.
Mutant alleles for ASI1 immunoprecipitated proteins. (A) The genomic structure of the AIPP1 gene and mutations detected by DNA sequencing in two aipp1 mutant alleles. The underscored bases represent single guide RNA sequences used for gene editing by CRISPR/Cas9. Gray and blue boxes represent untranslated regions and exons, respectively. Red columns indicate mutated regions. (B) Diagram of T-DNA insertion lines for the CPL2, AIPP2, and AIPP3 genes. Inverted triangles indicate the insertion sites of T-DNA.
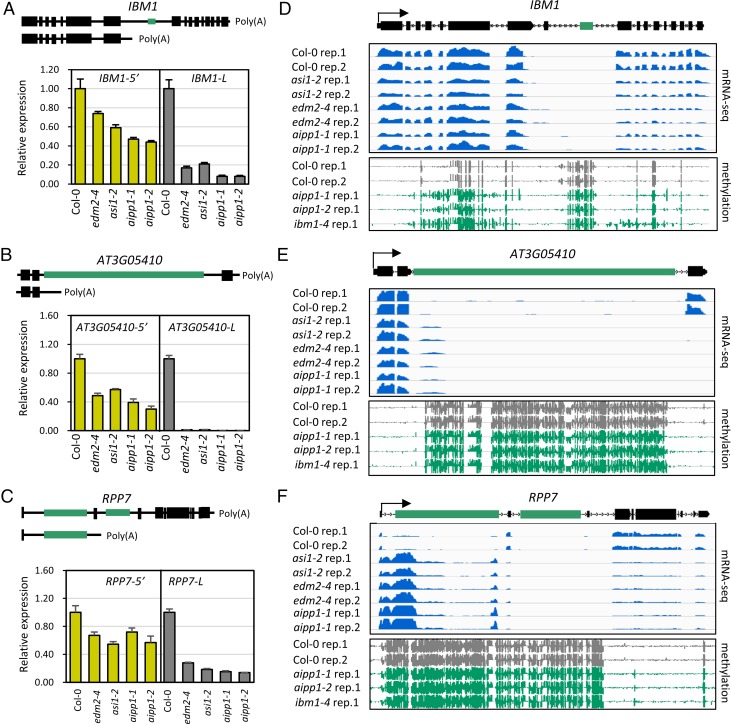
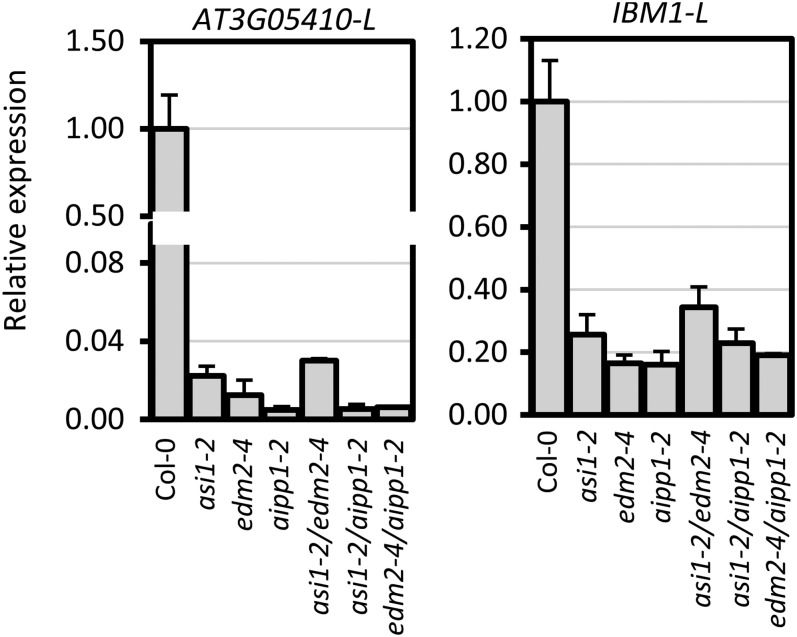
We used reverse-transcription quantitative PCR (RT-qPCR) to quantify the accumulation of full-length and short transcripts at three loci targeted by ASI1 and EDM2, including the histone H3K9me2 demethylase gene IBM1. We used 5′-terminal–specific and 3′-terminal–specific primer pairs to quantify total and full-length transcripts in aipp1-1 and aipp1-2 mutants. asi1-2/ibm2-3 (26) and edm2-4 (22) mutants, which are in the Col-0 background, served as parallel controls. The IBM1 gene produces two transcript forms, a large, full-length form (IBM1-L) and a short form (IBM1-S) (19). Our RT-qPCR results show that the IBM1-L transcript was substantially reduced in aipp1-1 and aipp1-2 mutant plants compared with Col-0 wild-type (WT) plants, similar to the reduction observed in asi1-2 and edm2-4 mutants (Fig. 3A). The amount of total IBM1 transcripts (IBM1-5′), including IBM1-L and IBM1-S together, was reduced to a lesser extent than the amount of IBM1-L transcript (Fig. 3A). Reduced expression of full-length transcripts was also observed in aipp1 as well as asi1 and edm2 mutants for two other genes with intronic HC, AT3G05410 (Fig. 3B) and AT1G58602 (RPP7) (Fig. 3C). These results show that AIPP1 functions with ASI1 and EDM2 in regulating the expression of intronic HC-containing genes.
Fig. 3.
AIPP1 is required for the expression of full-length transcripts of selected ASI1 and EDM2 target intronic HC-TRE genes. (A–C) Three ASI1 and EDM2 target genes that contain intronic HC, including the H3K9me2 demethylase gene IBM1 (A), AT3G05410 (B), and AT1G58602 (RPP7) (C) were selected for expression analysis in aipp1 mutants. Col-0 served as the WT control, and asi1-2 and edm2-4 served as positive controls. Two representative forms of pre-mRNA transcripts with different polyadenylation sites are shown in the diagram. Black and green boxes represent exons and intronic HC elements, respectively. 3′- and 5′-specific primer pairs were used for detection of long (L) and 5′ (sum of distal and proximal transcripts) transcripts by RT-qPCR. The relative expression of different transcripts was first normalized to ACTIN2 and then to Col-0 plants. Error bars indicate SD; n = 3. (D–F) Snapshots of mRNA-seq and DNA methylation profiles from the IGV showing the read coverage (Upper) and DNA methylation levels (Lower) of the IBM1 (D), AT3G05410 (E), and RPP7 (F) genes. The genomic structure of each gene is shown at the top. Green boxes represent TREs.
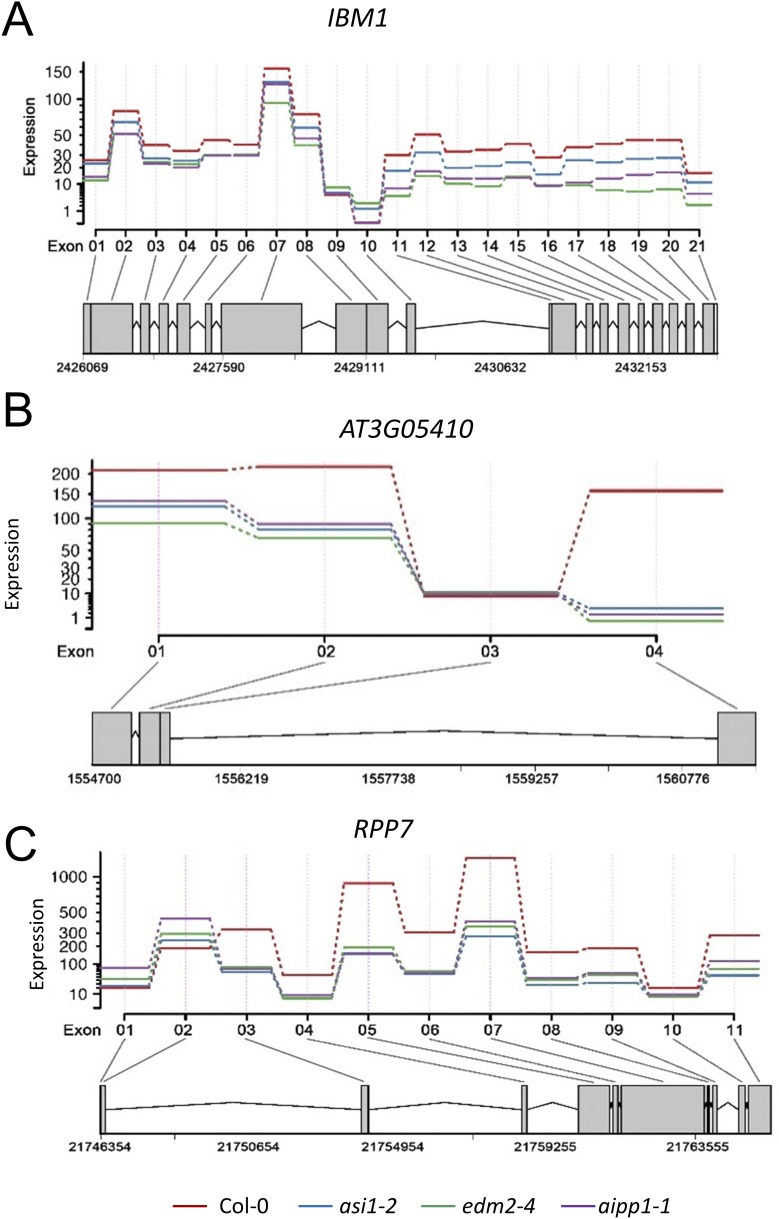
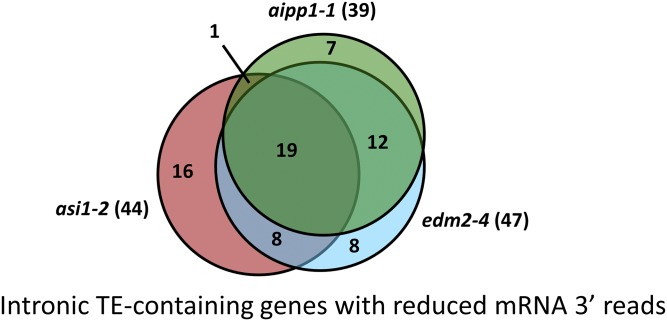
We further investigated the effect of aipp1, asi1, and edm2 mutations on the intronic HC-containing genes by examining the distribution of mRNA-seq reads. Fig. 3D shows the mRNA-seq reads mapped to the IBM1 locus, which contains a large intronic and methylated repetitive sequence. In the WT, abundant reads were mapped to sequences 3′ to the intronic HC; however, the 3′ reads were greatly decreased in all three mutants (Fig. 3D). Reductions in 3′ reads also were observed at two other genes, AT3G05410 (Fig. 3E) and RPP7 (Fig. 3F). We quantified the use of individual exons using the DEXSeq method (27). The DEXSeq results showed greatly decreased expression levels of exons 3′ to the HC-TREs in all three mutants, with expression levels of exons 5′ to the HC-TREs reduced to a lesser extent (Fig. S5 A and B) or even increased (Fig. S5C) in the three mutants. Previous work has shown that asi1 and edm2 mutations cause decreased 3′ mRNA-seq reads of intronic HC-TRE genes by reducing the use of distal polyadenylation sites (19, 20, 22, 23). The altered patterns of mapped mRNA-seq reads in aipp1 mutants are similar to those seen in asi1 and edm2 mutants, suggesting that, like ASI1 and EDM2, AIPP1 also affects the selection of polyadenylation sites. From the mRNA-seq data, we identified intronic TE-containing genes with reduced levels of RNA-seq reads downstream of the intronic TE relative to reads upstream of the intronic TE in aipp1-1 as well as asi1-2 and edm2-4 mutants, as described previously (19). We found 39, 44, and 47 intronic TE-containing genes with reduced 3′ reads in the aipp1-1, asi1-2, and edm2-4 mutants, respectively (Dataset S1). Substantial portions of these genes are shared by the three mutants, as shown in Fig. S6.
Fig. S5.
DEXSeq results showing exon expression at the IBM1 (A), AT3G05410 (B), and RPP7 (C) genes. The plot indicates the estimated expression of each exon based on mRNA-seq (two replicates for each genotype). The estimates are from counts of mRNA-seq reads using each exon as a unit. The plots were generated with the R package “DEXSeq” function “plotDEXSeq” (21).
Fig. S6.
Intronic TE-containing genes affected in aipp1-1, asi1-2, and edm2-4 mutants. The Venn diagram shows the overlap of intronic TE-containing genes with reduced mRNA-seq 3′ reads in aipp1-1, asi1-2, and edm2-4 mutants.
We generated asi1-2edm2-4, asi1-2aiipp1-1, and edm2-4aipp1-1 double mutants and compared the accumulation of full-length transcripts between the double mutants and single mutants. Our RT-qPCR results indicate a similar reduction of full-length transcripts in the double mutants and their corresponding single mutants (Fig. S7). These results support the idea that AIPP1, ASI1, and EDM2 function in the same genetic pathway, consistent with the three proteins functioning together in a complex. Our results suggest that the ASI1-AIPP1-EDM2 complex is required for promoting the accumulation of full-length transcripts of intronic HC-containing genes.
Fig. S7.
Comparison of the effects of indicated single and double mutants on the accumulation of full-length transcripts of the tested loci. The expression of full-length transcripts (L) of the intronic HC-containing genes AT3G05410 and IBM1 was examined in aipp1-1, asi1-2, and edm2-4 single mutants and in their double mutants using 3′-specific primer pairs. Error bars indicate SD; n = 3.
AIPP1 Is an Antisilencing Factor That Affects Gene Body CHG Methylation.
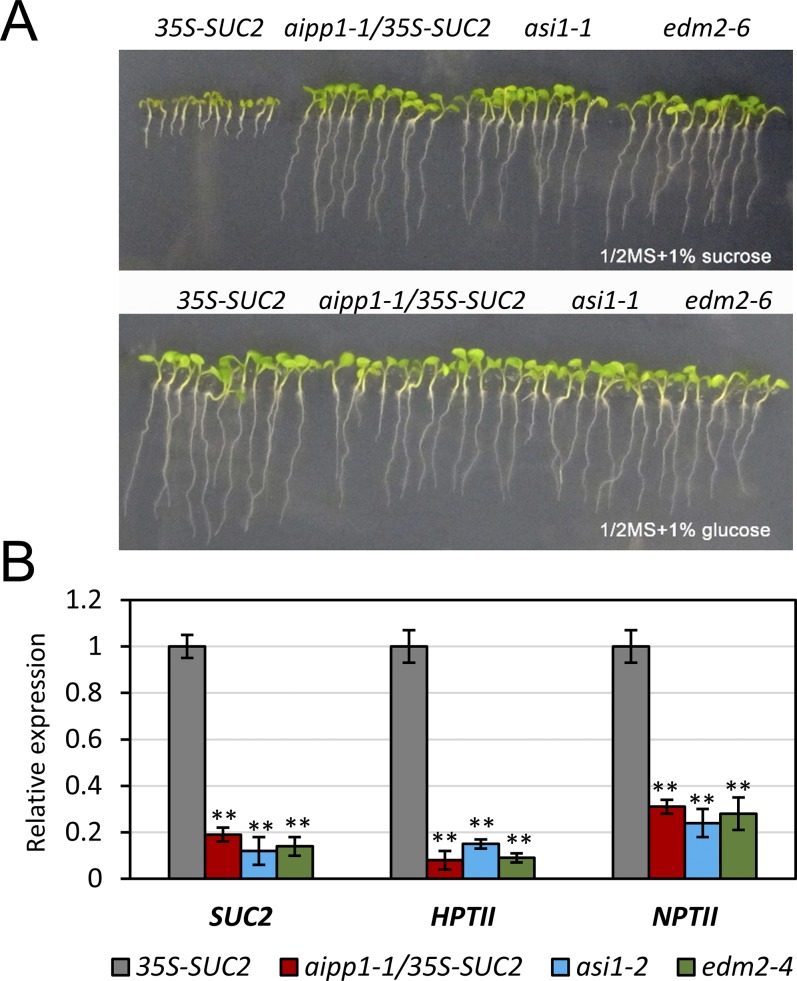
Because ASI1 and EDM2 were identified as antisilencing factors (19, 22), we determined whether AIPP1 may have a role in preventing transgene silencing as well. To explore the effect of AIPP1 dysfunction on transgene silencing, we introduced the aipp1-1 mutation into the 35S-SUC2 transgene background by crossing aipp1-1 mutant and 35S-SUC2 plants. Homozygous aipp1-1 mutant plants containing the 35S-SUC2 transgene, as well as asi1-1 (19) and edm2-6 (22) plants (already in the 35S-SUC2 background), were grown on sucrose- and glucose-containing half-strength Murashige and Skoog medium. All plants grew normally and had long roots on the glucose-containing medium (Fig. S8A). Like the asi1-1 and edm2-6 plants, the 35S-SUC2/aipp1-1 plants grew normally and had long roots in the sucrose-containing medium, compared with the inhibited growth and short roots of the WT parental 35S-SUC2 plants (Fig. S8A), suggesting AIPP1 dysfunction as the cause of 35S-SUC2 transgene silencing. Consistent with the root growth phenotype, our RT-qPCR results show that SUC2, NPTII, and HPTII transgenes were silenced in aipp1-1 mutant plants compared with WT 35S-SUC2 plants (Fig. S8B). These results indicate that AIPP1 is an antisilencing factor.
Fig. S8.
AIPP1 prevents silencing of the 35S-SUC2 transgene locus. (A) The root growth phenotype of the aipp1-1 mutant in the 35S-SUC2 transgene background in half-strength Murashige and Skoog MS medium supplemented with 1% sucrose or 1% glucose. asi1-1 and edm2-6, which were already in the 35S-SUC2 transgene background, were grown as parallel controls. (B) RT-qPCR results showing that expression of the SUC2, HPTII, and NPTII transgenes was significantly reduced in aipp1-1, asi1-1, and edm2-6 mutant plants in comparison with the WT parental 35S-SUC2 plants. All plants were of the 35S-SUC2 background. Relative expression of the transgenes was first normalized to ACTIN2 plants and then to 35S-SUC2 plants. Error bars indicate SD; n = 3. **P < 0.01, Student’s t test.
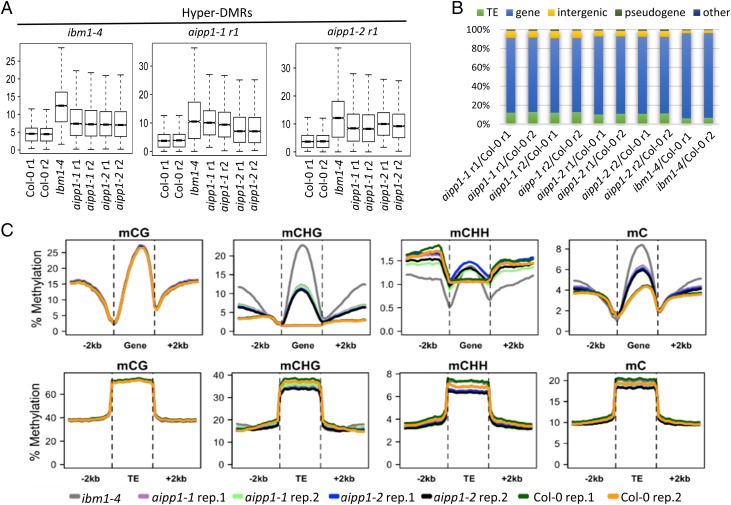
IBM1 suppresses gene body CHG methylation by demethylating histone H3K9me2 (28-30). Because AIPP1 is required for the appropriate expression of functional IBM1-L transcripts (Fig. 3 A and D), we next asked whether AIPP1 dysfunction may affect gene body CHG methylation. To explore this question, we subjected two aipp1 mutant alleles to whole-genome bisulfite sequencing (WGBS) with two biological replicates for each allele. Col-0 served as the WT control. Our WGBS analysis identified thousands of differentially methylated regions with increased methylation (hyper-DMRs) from the four methylomes of the aipp1 mutants. We used boxplot analysis to compare the hyper-DMRs of aipp1 with those of ibm1-4, which were identified from published DNA methylome data (19). The results show that at ibm1-4 hyper-DMRs, DNA methylation was greater in aipp1 mutants compared with Col-0 WT. Similarly, at aipp1 hyper-DMRs, DNA methylation levels were higher in the ibm1 mutant than in the Col-0 WT (Fig. 4A). These results suggest that AIPP1 and IBM1 suppress cytosine methylation of similar genomic regions. We next compared the genomic features of hyper-DMRs between aipp1 and ibm1-4 mutants, and found that ≈80% of aipp1 hyper-DMRs were mapped to gene regions, similar to the percentage in the ibm1-4 mutant (Fig. 4B).
Fig. 4.
AIPP1 controls gene body CHG methylation. (A) Boxplot diagram showing DNA methylation levels of each genotype at ibm1-4, aipp1-1, and aipp-2 hyper-DMRs. r1, replicate 1; r2, replicate 2. (B) Composition of the hyper-DMRs in aipp1-1, aipp1-2, and ibm1-4 mutants. (C) Distribution of DNA methylation along gene (Upper)/TE (Lower) bodies and their upstream/downstream 2-kb flanking sequences in different cytosine contexts.
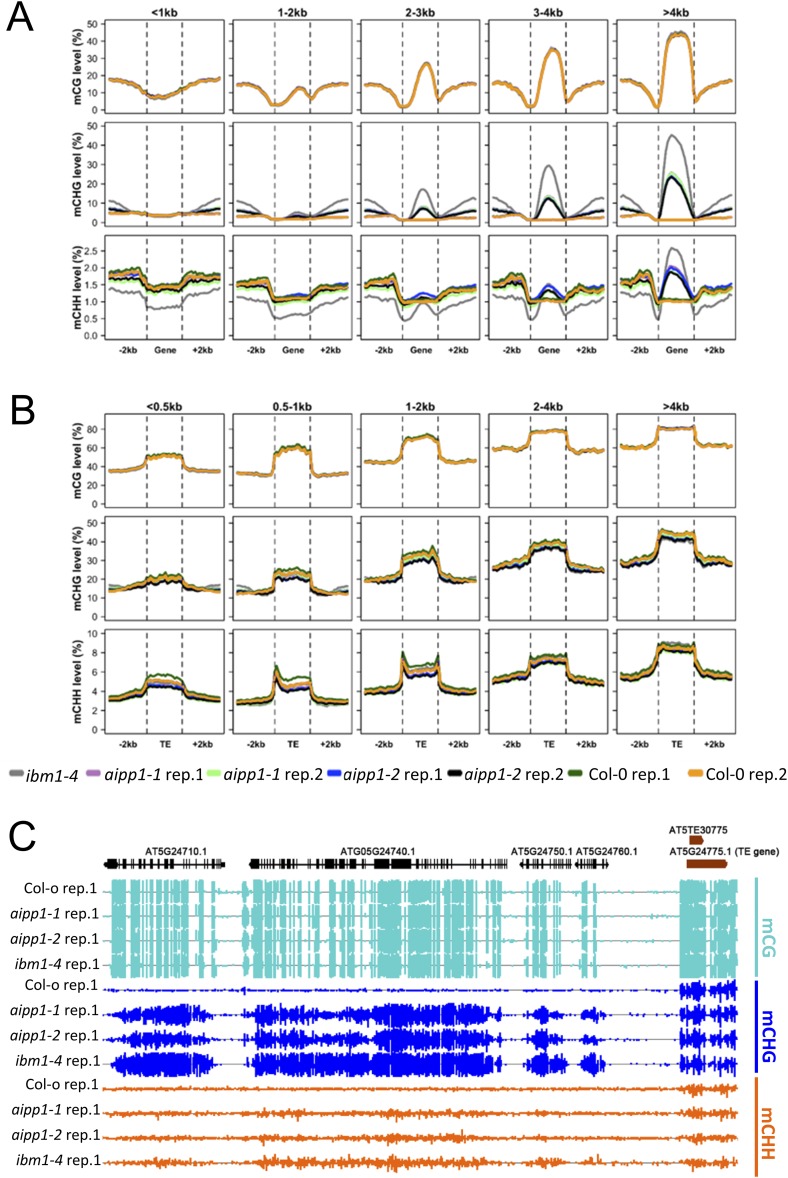
To further characterize the DNA methylation pattern in the aipp1 mutants, we examined the distribution of DNA methylation along the gene bodies, TEs, and their 2-kb upstream and downstream flanking sequences. The results indicate that CHG methylation, but not CG or CHH methylation, in the aipp1 mutants was significantly increased in gene body regions compared with the WT (Fig. 4C and Fig. S9), with greater increases in CHG methylation observed in longer genes (Fig. S9A). No change was evident in TEs or their flanking regions, however (Fig. 4C and Fig. S9B). This change in DNA methylation pattern of the aipp1 mutants is very similar to that seen in the ibm1-4 mutant. Considering the down-regulation of functional IBM1-L transcript in aipp1 mutant plants, these results suggest that AIPP1 suppresses gene body CHG methylation by facilitating expression of the functional IBM1-L transcript.
Fig. S9.
AIPP1 dysfunction causes increased CHG methylation in gene body regions of long genes. (A and B) CHG methylation levels of body regions and their upstream and downstream 2-kb flanking sequences of different-sized genes (A) and TEs (B). (C) IGV snapshot showing increased CHG methylation at the body regions of genes in a representative genomic region in aipp1 and ibm1 mutants. Orange boxes represent TEs or TE genes.
Potential Roles of AIPP2, AIPP3, and AIPP4/CPL2.
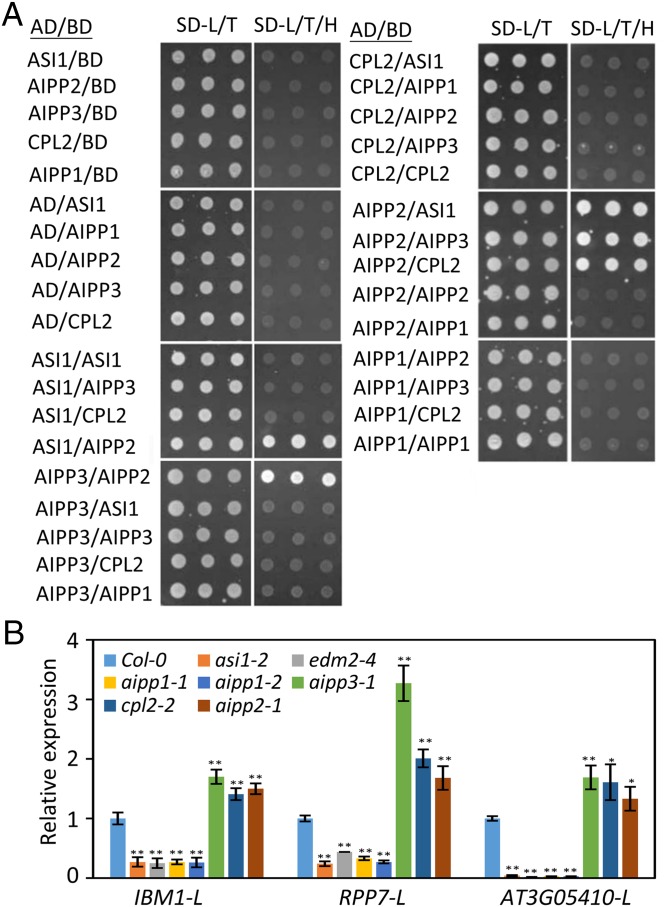
To better understand the functions of the ASI1-AIPP1-EDM2 complex, we investigated three other proteins that copurify with ASI1: AIPP2, AIPP3, and APIPP4/CPL2 (Fig. 1B and Fig. S2). AIPP2 is encoded by AT3G02890 and bears a PHD domain, whereas AIPP3 is encoded by AT4G11560 and bears a BAH domain and a TFIIS domain known to be involved in transcriptional regulation. AIPP4/CPL2 is an RNA Pol II CTD phosphatase that dephosphorylates the Ser-5 of RNA Pol II CTD (25). Using Y2H and split luciferase assays, we found that AIPP2 can interact directly with ASI1, AIPP3, and CPL2 (Fig. 5A and Fig. S1B). These results suggest that ASI1, EDM2, AIPP1, AIPP2, AIPP3, and CPL2 may form a large complex in plant cells.
Fig. 5.
AIPP2 can directly interact with ASI1, AIPP3, and CPL2 and regulate intronic HC-TRE genes. (A) Y2H assay results showing interaction of AIPP2 with AIPP3, ASI1, and CPL2. (B) Effect of the different mutations on the accumulation of full-length transcripts from the indicated intronic HC-TRE genes. 3′-specific primer pairs were used for detection of full-length transcripts (L) of IBM1, AT3G05410, and RPP7 by RT-qPCR. Relative expression of different transcripts was first normalized to ACTIN2 and then to Col-0 plants. Error bars indicate SD; n = 3. **P < 0.01; *P < 0.05, Student’s t test.
To probe the genetic function of AIPP2, AIPP3, and CPL2, we identified the T-DNA insertion lines salk_057771 and GK_058D11 for AIPP2 and AIPP3 genes, referred to as aipp2-1 and aipp3-1, respectively (Fig. S4B). For the CPL2 gene, a previously described mutant allele, cpl2-2 (31), was used. RT-qPCR was performed to examine the expression of full-length transcripts at three intronic HC-TRE genes: IBM1, AT3G05410, and RPP7. Surprisingly, in contrast to the reduction in asi1-2, edm2-4, aipp1-1, and aipp1-2 mutants, full-length transcripts for all three genes were increased in aipp2-1, aipp3-1, and cpl2-2 mutants compared with the WT Col-0 (Fig. 5B).
The ASI1-AIPP1-EDM2 Complex Is Required for Expression of a Nonintronic HC-TRE Gene.
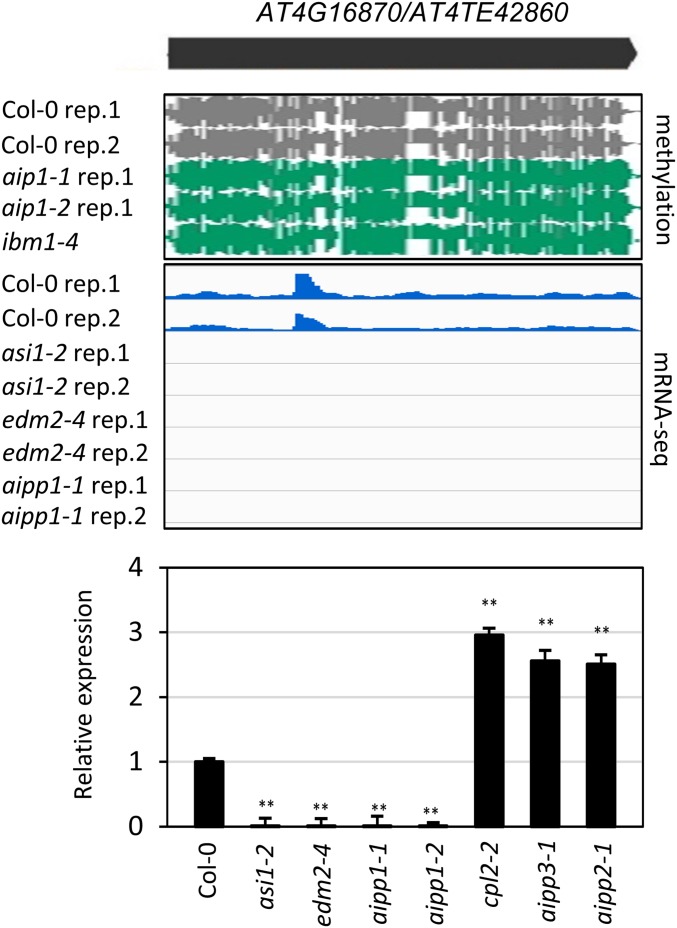
Our examination of mRNA-seq reads at HC-containing genes revealed that the transcript level of AT4G16870, a TE gene of Copia-like retrotransposon origin, was dramatically affected by asi1, edm2, and aipp1 mutations (Fig. 6). The AT4G16870 transcript was abolished in asi1-2, edm2-4, aipp1-1, and aipp1-2 mutants, but was significantly increased in aipp2, aipp3, and cpl2 mutants (Fig. 6). The AT4G16870 locus was highly methylated in Col-0 WT, as well as in aipp1 and ibm1 mutants (Fig. 6). Given that the HC at AT4G16870 does not reside in an intron, our results suggest that the ASI1-AIPP1-EDM2 complex has functions beyond the regulation of intronic HC-TRE genes.
Fig. 6.
Dysfunction of ASI1 and its associated proteins affects the expression of a nonintroinc HC gene. RT-qPCR shows that the ASI1-AIPP1-EDM2 subcomplex is required for the expression of AT4G16870, a TE gene with heavy DNA methylation. A snapshot of DNA methylation in this locus is shown. Relative expression in different mutants was first normalized to ACTIN2 and then to Col-0 plants. Error bars indicate SD; n = 3. **P < 0.01, Student’s t test.
Discussion
TRE insertions within transcribed regions, especially introns, are commonly observed in eukaryotic cells (3–6). The intronic HC-TREs do not appear to disturb gene transcription, given that chromatin immunoprecipitation assays have shown no effect on RNA Pol II occupancy (19, 22); however, intronic HC-TREs can cause alternative polyadenylation of the transcripts (19, 20, 22, 23). In previous studies, we identified two chromatin regulators, ASI1 and EMD2, that are required to promote distal polyadenylation so that full-length transcripts can accumulate (19, 22). It has been hypothesized that ASI1 and EDM2 may function together and interact through an unknown factor (32). In this study, we found that EDM2 can be copurified with ASI1, although the two proteins do not interact directly (Fig. 1A). AIPP1 interacts with both ASI1 and EDM2 and thus is the hypothesized unknown factor that serves as a bridge protein to mediate the in vivo association of ASI1 and EDM2 (Fig. 2). Consistent with AIPP1 being in the same protein complex with ASI1 and EDM2, AIPP1 also functions to promote the accumulation of full-length transcripts of intronic HC-TRE–containing genes. In addition, we found that AIPP1, as well as ASI1 and EDM2, are required for expression of the heterochromatinized TE gene AT4G16870.
RRM is an RNA-binding domain common in many proteins involved in RNA processing (33, 34). Both ASI1 (19) and AIPP1 contain an RRM domain, consistent with their function in regulating RNA processing. Because their RRM domains are quite divergent in sequence (Fig. S3), the two proteins may recognize different features of the pre-mRNAs during regulation of alternative polyadenylation of intronic HC-TRE–containing genes. Along with the RRM domain, ASI1 also contains a BAH domain that may interact with the chromatin (19–21). EDM2 contains three copies of the PHD and has been shown to bind to chromatin marks, including the heterochromatic mark H3K9me2 (22, 23). The ASI1-AIPP1-EDM2 complex is capable of interacting with both the chromatin and RNA, and thus is well suited for its function in connecting the epigenetic regulation of HC-TREs and RNA processing. EDM2 also has a putative N6-adenine methyltransferase domain (22). Methylated adenine is involved in 3′-end formation of mRNAs (35). It would be interesting to determine whether EDM2 may indeed methylate RNA, and how this RNA methylation may alter the use of proximal vs. distal polyadenylation sites.
Mutations in AIPP1, ASI1, and EDM2 have a significant impact on the DNA methylome. This can be explained by their role in regulating IBM1 expression. As a histone H3K9me2 demethylase, IBM1 is required for preventing gene body CHG methylation, because the H3K9me2 mark accumulates in the gene body regions of thousands of genes and the H3K9me2 mark attracts CMT3 to cause CHG methylation (27, 28, 36). IBM1 is an intronic HC-TRE–containing gene, and the accumulation of functional, full-length IBM1 transcript requires the ASI1-AIPP1-EDM2 complex (Fig. 3 A and D) (19, 22). IBM1 is also an antisilencing factor important for the prevention of promoter DNA hypermethylation and transcriptional silencing of the 35S-SUC2 transgene (19, 22). Because of their role in ensuring the accumulation of functional full-length IBM1 transcript, AIPP1 (Fig. 3 A and D), as well as ASI1 and EDM2 (19, 22), are antisilencing factors necessary for expression of the 35S-SUC2 transgene.
The estimated size of the ASI1-AIPP1-EDM2 protein complex is between 440 and 670 kDa. It is likely that this large complex contains other components as well. Our results suggest that CPL2, AIPP2, and AIPP3 are associated with this complex. Consistent with this notion, dysfunction in CPL2, AIPP2, and AIPP3 also affects the accumulation of full-length transcripts of intronic HC-TRE genes and modulates the expression of the TE gene AT4G16870; however, the role of these proteins appears to be opposite to that of ASI1, EDM2, and AIPP1. It is possible that AIPP2, AIPP3, and CPL2 may be in a subcomplex that differs from the ASI1-AIPP1-EDM2 subcomplex. In the putative AIPP3-AIPP2-CPL2 subcomplex, AIPP2 with a PHD domain and AIPP3 with a BAH domain may bind the HC, whereas CPL2, like the yeast and animal RNA Pol II Ser-5 CTD phosphatase SSU72, may be more directly involved in regulating alternative polyadenylation (14–16). Further work is needed to determine whether there are indeed two such subcomplexes and, if so, how they may function antagonistically in the regulation of intronic and nonintronic HC-TRE genes.
Materials and Methods
Plant Materials and Growth Conditions.
All plants were grown under a long-day photoperiod (16-h light/8-h dark). The aipp1-1 and aipp1-2 mutants were generated using CRISPR/Cas9 (37). For determination of root length phenotype, seeds were sown on half-strength Murashige and Skoog medium containing 1% sucrose or 1% glucose. Plants were photographed at 10 d after germination. 4×Myc-tagged AIPP1 transgenic expression driven by its native promoter was achieved by introducing the AIPP1 genomic DNA into Col-0 plants, and T3 generation plants were used for gel filtration analysis.
Protein Interaction Analysis.
Y2H and split luciferase assays were performed as described previously (36). For yeast three-hybrid (Y3H) assays, the entry vectors were constructed to harbor a second cassette expressing AIPP1 or GFP. The primers used are listed in Table S1. The corresponding entry vectors were recombined into pGBKT7-GW and pGADT7-GW vectors using the LR reaction (Invitrogen). Sets of constructs were cotransformed into the Y2H Gold yeast strain (Clontech). The AD-T and BD-53 combination served as a positive control, and the AD-T and BD-Lam combination served as a negative control. Yeast transformants were selected on synthetic minimal dropout medium deficient in Met, Trp, and Leu (SD-M/L/T). Protein interactions were assessed on the stringent dropout medium deficient in Met, Leu, Trp, His, and adenine (SD-M/L/T/H/A).
Table S1.
Primers used in this study
| Primer | Sequence (5′-3′) | Purpose |
| AIPP1-geF | caccTGTTAATCGGTAGAGATGGACATGTTG | Gateway cloning of AIP genomic DNA |
| AIPP-geR | CCGATAAGGAAAACCTTTCAGGTCGT | |
| AIPP1-CDS-NdeIF | ctgCATATGATGGAAGAATCTGTAGCATCTGAAG | ASI1 Y2H constructs |
| ASI1-CDS-EcoRIR | agtGAATTC aggaagtttcagcttgaagttaatc | |
| EDM2-CDS-XmaIF | attCCCGGGATGACGTTCGTTGACGATGATGAAG | EDM2 Y2H constructs |
| EDM2-CDS-PstIR | gacCTGCAGGTCATTAATCCAACCGCCAGATCGA | |
| EDM2-CDS-XhoIR | gagCTCGAGGTCATTAATCCAACCGCCAGATCGA | |
| AIP1-CDS-EcoRIF | agtGAATTCATGGCGACTCCGGAAGAAGTAGC | AIP1 Y2H constructs |
| AIP1-CDS-BamHIR | acgGGATCCCCGATAAGGAAAACCTTTCAGGTCG | |
| Y3HAarI-EDM2 F | aggattattCACCTGCagagAACCATGACGTTCGTTGACGATGATG | Y3H constructs |
| Y3HAarI-EDM2 R | aggattattCACCTGCagaggctgTTATCAGTCATTAATCCAACCGCCAG | |
| Y3HAarI-ASI1 F | aggattattCACCTGCagagAACCATGGAAGAATCTGTAGCATCTG | |
| Y3HAarI-ASI1 R | aggattattCACCTGCagaggctgTTATCAAGGAAGTTTCAGCTTGAAGTTAATCC | |
| Y3HAarI-ADH Terminator F | aggattattCACCTGCagagCAGCCAAGCTAATTCCGGGCG | |
| Y3HAarI-ADH Terminator R | aggattattCACCTGCagagGAACCCGGTAGAGGTGTGGTCA | |
| Y3HAarI-Met promoter F | aggattattCACCTGCagagGTTCGAATCCCTTAGCTCTCA | |
| Y3HAarI-Met promoter R | aggattattCACCTGCagagCCATTGCGGCCGCCACCTTTCTC | |
| Y3HAarI-AIPP1 F | aggattattCACCTGCagagATGGCGACTCCGGAAGAAGTG | |
| Y3HAarI-AIPP1 R | aggattattCACCTGCagagTACCTCACTACCGATAAGGAAAACCTTTCAGG | |
| Y3HAarI-GFP F | aggattattCACCTGCagagATGGtagatctgactAGTaaaggagaag | |
| Y3HAarI-GFP R | aggattattCACCTGCagagTACCTCACTAtttgtatagttcatccatgcc | |
| Y3HAarI-PGK Terminator F | aggattattCACCTGCagagGGTAAGGAATTGCCAGGTG | |
| Y3HAarI-PGK Terminator R | aggattattCACCTGCagagCTAGGCTTTAACGAACGCAGAATTTTCG | |
| Y3HAarI-ASI1 alone R | aggattattCACCTGCagagCTAGTTATCAAGGAAGTTTCAGCTTGAAGTTAATCC | |
| Y3HAarI-EDM2 alone R | aggattattCACCTGCagagCTAGTTACTAGTCATTAATCCAACCGCCAG | |
| AIP1-gwF | CACCATGGCGACTCCGGAAGAAGTAGC | Split luciferase constructs |
| AIP1-gwR | CCGATAAGGAAAACCTTTCAGGTCG | |
| RPP7-5′qF | ATGTGTGATTCTTCCCTAAGAGG | RPP7 RT-qPCR |
| RPP7-5′qR | CTTGATCGCGCCAAGGA | |
| RPP7-3′qF | GATGGGTTGGAAGAGTGGATAG | |
| RPP7-3′qR | TGCAATTGTGGAAACCCATTAG | |
| 305410–3′qF | GGTCAACGAGCAGATTTGAAAG | AT3G05410 RT-qPCR |
| 305410–3′qR | CGCAACAAACGGCTACATTAT | |
| 305410–5′qF | ATTCAACCGACGGCTTCTT | |
| 305410–5′qR | CCTGGCGATAAACCGAGTATAA | |
| IBM1-3′qF | TGAAAAGACCAAATATTGTGAGGAC | IBM1 RT-qPCR |
| IBM1-3′qR | caaaagatgatgttaattgaaaactactac | |
| IBM1-5′qF | AGGGAATTAGGATTGGGAAAAGG | |
| IBM1-5′qR | CGCTCTTTTGACATTGATGGC |
Immunoprecipitation and MS.
Immunoprecipitation coupled with LC-MS/MS (IP-MS) assays were performed as described previously (30). The inflorescence tissues from 3×Flag-tagged ASI1 plants (16) were collected for total protein extraction. Total proteins were then precipitated by agarose-conjugated anti-Flag antibody (Sigma-Aldrich) for 3 h at 4 °C. After five washes with protein extraction buffer and five washes with PBS buffer, the precipitated proteins were subjected to MS analysis.
WGBS and DNA Methylation Analysis.
For whole-genome bisulfite sequencing analysis, genomic DNA was extracted from 2-wk-old seedlings using the DNeasy Plant Mini Kit (Qiagen). Differentially methylated regions (DMRs) and DNA methylation patterns in different genotypes were identified as described previously (38).
mRNA-seq Analysis.
For mRNA-seq assays, total RNAs were extracted from 2-wk-old seedlings and subjected to RNA deep sequencing. mRNA-seq libraries were prepared using the TruSeq Stranded mRNA LT Kit (Illumina) following the manufacturer's protocol. Paired-end sequencing of the library was performed with an Illumina HiSeq 2500 system using the Illumina v4 reagents at the Genomics Core Facility of the Shanghai Center for Plant Stress Biology. Clean reads were mapped to the Arabidopsis reference genome using TopHat. The mapped reads were visualized with the genome browser Integrative Genomics Viewer (IGV) in tdf file format. The tdf files were generated with igvtools using mapping results obtained from TopHat. The list of genes with reduced reads downstream of their intronic TEs in the mutants was generated according to a previously described method (19) with a threshold of a 1.5-fold reduction.
Supplementary Material
Acknowledgments
We thank Wei Jia and Aojun Chen for help with mRNA-seq library construction and sequencing. This work was supported by the Chinese Academy of Sciences and National Institutes of Health Grant R01 GM070795 (to J.-K. Z.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The aipp1-1 and aipp1-2 WGBS and mRNA-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE98655).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710683114/-/DCSupplemental.
References
- 1.Rebollo R, Romanish MT, Mager DL. Transposable elements: An abundant and natural source of regulatory sequences for host genes. Annu Rev Genet. 2012;46:21–42. doi: 10.1146/annurev-genet-110711-155621. [DOI] [PubMed] [Google Scholar]
- 2.Bennetzen JL, Wang H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu Rev Plant Biol. 2014;65:505–530. doi: 10.1146/annurev-arplant-050213-035811. [DOI] [PubMed] [Google Scholar]
- 3.van de Lagemaat LN, Medstrand P, Mager DL. Multiple effects govern endogenous retrovirus survival patterns in human gene introns. Genome Biol. 2006;7:R86. doi: 10.1186/gb-2006-7-9-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sela N, et al. Comparative analysis of transposed element insertion within human and mouse genomes reveals Alu’s unique role in shaping the human transcriptome. Genome Biol. 2007;8:R127. doi: 10.1186/gb-2007-8-6-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328:916–919. doi: 10.1126/science.1186366. [DOI] [PubMed] [Google Scholar]
- 6.West PT, et al. Genomic distribution of H3K9me2 and DNA methylation in a maize genome. PLoS One. 2014;9:e105267. doi: 10.1371/journal.pone.0105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollister JD, Gaut BS. Epigenetic silencing of transposable elements: A trade-off between reduced transposition and deleterious effects on neighboring gene expression. Genome Res. 2009;19:1419–1428. doi: 10.1101/gr.091678.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Weigel D, Smith LM. Transposon variants and their effects on gene expression in Arabidopsis. PLoS Genet. 2013;9:e1003255. doi: 10.1371/journal.pgen.1003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To TK, Saze H, Kakutani T. DNA methylation within transcribed regions. Plant Physiol. 2015;168:1219–1225. doi: 10.1104/pp.15.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian B, Manley JL. Alternative polyadenylation of mRNA precursors. Nat Rev Mol Cell Biol. 2017;18:18–30. doi: 10.1038/nrm.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, et al. Genome-wide landscape of polyadenylation in Arabidopsis provides evidence for extensive alternative polyadenylation. Proc Natl Acad Sci USA. 2011;108:12533–12538. doi: 10.1073/pnas.1019732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu H, et al. Genome-wide dynamics of alternative polyadenylation in rice. Genome Res. 2016;26:1753–1760. doi: 10.1101/gr.210757.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinhart A, Cramer P. Recognition of RNA polymerase II carboxy-terminal domain by 3′-RNA-processing factors. Nature. 2004;430:223–226. doi: 10.1038/nature02679. [DOI] [PubMed] [Google Scholar]
- 14.Lamas-Maceiras M, Singh BN, Hampsey M, Freire-Picos MA. Promoter-terminator gene loops affect alternative 3′-end processing in yeast. J Biol Chem. 2016;291:8960–8968. doi: 10.1074/jbc.M115.687491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, et al. Context-dependent modulation of Pol II CTD phosphatase SSUP-72 regulates alternative polyadenylation in neuronal development. Genes Dev. 2015;29:2377–2390. doi: 10.1101/gad.266650.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang K, et al. Crystal structure of the human symplekin-Ssu72-CTD phosphopeptide complex. Nature. 2010;467:729–733. doi: 10.1038/nature09391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spies N, Nielsen CB, Padgett RA, Burge CB. Biased chromatin signatures around polyadenylation sites and exons. Mol Cell. 2009;36:245–254. doi: 10.1016/j.molcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaladkar M, Smyda M, Hannenhalli S. Epigenomic and RNA structural correlates of polyadenylation. RNA Biol. 2011;8:529–537. doi: 10.4161/rna.8.3.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. RNA-binding protein regulates plant DNA methylation by controlling mRNA processing at the intronic heterochromatin-containing gene IBM1. Proc Natl Acad Sci USA. 2013;110:15467–15472. doi: 10.1073/pnas.1315399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saze H, et al. Mechanism for full-length RNA processing of Arabidopsis genes containing intragenic heterochromatin. Nat Commun. 2013;4:2301. doi: 10.1038/ncomms3301. [DOI] [PubMed] [Google Scholar]
- 21.Coustham V, et al. SHOOT GROWTH1 maintains Arabidopsis epigenomes by regulating IBM1. PLoS One. 2014;9:e84687. doi: 10.1371/journal.pone.0084687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei M, et al. Arabidopsis EDM2 promotes IBM1 distal polyadenylation and regulates genome DNA methylation patterns. Proc Natl Acad Sci USA. 2014;111:527–532. doi: 10.1073/pnas.1320106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya T, Eulgem T. An alternative polyadenylation mechanism coopted to the Arabidopsis RPP7 gene through intronic retrotransposon domestication. Proc Natl Acad Sci USA. 2013;110:E3535–E3543. doi: 10.1073/pnas.1312545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong-Abdullah M, et al. Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature. 2015;525:533–537. doi: 10.1038/nature15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koiwa H, et al. Arabidopsis C-terminal domain phosphatase-like 1 and 2 are essential Ser-5-specific C-terminal domain phosphatases. Proc Natl Acad Sci USA. 2004;101:14539–14544. doi: 10.1073/pnas.0403174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Rosso MG, Viehoever P, Weisshaar B. GABI-Kat SimpleSearch: An Arabidopsis thaliana T-DNA mutant database with detailed information for confirmed insertions. Nucleic Acids Res. 2007;35:D874–D878. doi: 10.1093/nar/gkl753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012;22:2008–2017. doi: 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saze H, Shiraishi A, Miura A, Kakutani T. Control of genic DNA methylation by a jmjC domain-containing protein in Arabidopsis thaliana. Science. 2008;319:462–465. doi: 10.1126/science.1150987. [DOI] [PubMed] [Google Scholar]
- 29.Miura A, et al. An Arabidopsis jmjC domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J. 2009;28:1078–1086. doi: 10.1038/emboj.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigal M, Kevei Z, Pélissier T, Mathieu O. DNA methylation in an intron of the IBM1 histone demethylase gene stabilizes chromatin modification patterns. EMBO J. 2012;31:2981–2993. doi: 10.1038/emboj.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda A, et al. The Arabidopsis thaliana carboxyl-terminal domain phosphatase-like 2 regulates plant growth, stress, and auxin responses. Plant Mol Biol. 2008;67:683–697. doi: 10.1007/s11103-008-9348-y. [DOI] [PubMed] [Google Scholar]
- 32.Ma L, Guo C, Li QQ. Role of alternative polyadenylation in epigenetic silencing and antisilencing. Proc Natl Acad Sci USA. 2014;111:9–10. doi: 10.1073/pnas.1321025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorković ZJ, Barta A. Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 2002;30:623–635. doi: 10.1093/nar/30.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maris C, Dominguez C, Allain FH. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005;272:2118–2131. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 35.Bodi Z, et al. Adenosine methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du J, et al. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell. 2012;151:167–180. doi: 10.1016/j.cell.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Z, et al. Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc Natl Acad Sci USA. 2014;111:4632–4637. doi: 10.1073/pnas.1400822111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan CG, et al. A pair of transposon-derived proteins function in a histone acetyltransferase complex for active DNA demethylation. Cell Res. 2017;27:226–240. doi: 10.1038/cr.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.