One of the great challenges facing developmental neuroscience is to understand how complex social interactions can shape brain development during ontogeny to affect adult functioning in social and behavioral contexts (1). Some of the most vexing behavioral problems, such as autism spectrum disorder that involves impaired social interactions, including verbal and nonverbal communication capabilities, are thought to be related to a complex interplay of endogenous and exogenous risk factors (2). How can one possibly sort out these various factors and develop coherent hypotheses about candidate genes in a causal context? Animal models are the obvious preferred approach to address such challenges, but many animal models selected for their potential to exploit powerful cellular/genetic methods do not capture the richness of the social and behavioral processes that underlie a phenomenon such as autism spectrum disorder (2). Oscine songbirds (i.e., members of the suborder passeres) constitute nearly 50% of the over 10,000 extant species of birds (3), and they exhibit a pattern of vocal development that has many features in common with language learning in humans (4). In zebra finches, a species derived from Australia, developing nestlings require social interactions often with their father but also with other conspecific males to form auditory memories that guide song development (5). Deprivation of these particular social interactions can result in enduing anomalies in vocal communication, such that the nestlings are never able to attain normal communicative functioning as adults (5). In a study in PNAS by Ahmadiantehrani and London (6), the authors implicate the mechanistic target of rapamycin (mTOR) signaling cascade as being a key player in the molecular basis of social interactions in zebra finches that result in auditory memory formation essential for functional vocal communication as an adult. These studies broaden our understanding of mTOR signaling and suggest that its implication in autism spectrum disorder may be related to a general role in the cellular basis of early experience effects on later adult behavior.
Birdsong, Autism, and mTOR
Why are songbirds a valuable model system for Ahmadiantehrani and London’s (6) study? In humans, social development is intimately tied to language development. The way we develop language capabilities and learn how to communicate in social contexts is firmly embedded in a process of complex social interactions. Few nonhuman species exhibit such a reliance on social factors to develop a species-typical vocal repertoire and use it in an appropriate social context. In oscine songbird species (members of the suborder passeres), it has been established that for the learning of song, and in some cases for calls as well, there is a profound dependence on auditory input usually from the father or other adult conspecifics (7). There is great variance among the ≈4,500 extant living oscine songbirds (8) but zebra finches are commonly studied and illustrate the general principle. Zebra finches, natives of the Australian outback, develop relatively rapidly and male nestlings start forming memories from tutors as early as day 30 posthatch (9). This sensory phase is when they form an auditory memory. Relatively rapidly after the onset of the sensory phase, they start a process known as the sensorimotor phase that involves matching their vocal output to the auditory memory they have previously formed. In some species, this learning can occur solely in response to the playback of auditory tapes (7). However, in zebra finches the presence of a conspecific is essential, indicating that the social interaction is fundamental to the learning occurring (5). This process has been modeled in a way that results in successful song learning in zebra finches by using experimental designs that require juvenile zebra finches to actively press keys that play vocalizations and thus mimic social interactions (10). Social interactions, therefore are fundamental to the development of successful vocal behavior in male zebra finches.
The mTOR protein is a serine/threonine kinase involved in a wide range of cellular processes, including protein synthesis and mRNA translation (11). mTOR forms at least two distinct multiprotein complexes, the mTOR complex 1(mTORC1) and mTOR complex 2 (mTORC2). This signaling pathway is known to respond to both intracellular and extracellular signals in an integrative fashion (11). In recent years, mutations in the mTOR signaling pathway have been implicated in select cases of autism spectrum disorders (12). Manipulations of rodent models of select aspects of autism spectrum disorders suggest that hyperactivity of the mTOR pathway may be involved in that inhibitors of mTOR mitigate these symptoms (12). Could mTOR play a wide role in coding social signals that have enduring consequences for adult behavior? For these reasons Ahmadiantehrani and London (6) hypothesize that mTOR might play an important role in the effects of interactions with a song tutor on song development in zebra finches.
mTOR, Tutors and Song Memories
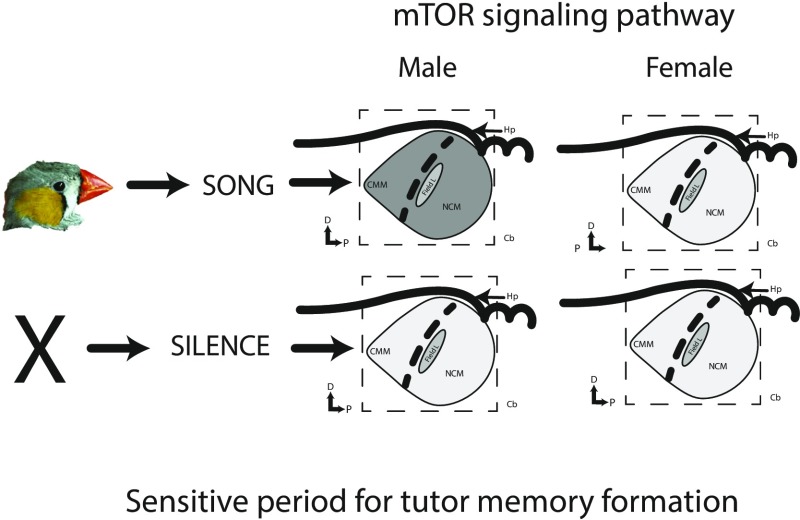
To investigate the hypothesized role played by mTOR in the regulation of song learning from a tutor, Ahmadiantehrani and London (6) took advantage of what is known about intraspecific variation in the song-learning process in zebra finches. First, unlike many other songbird species, in zebra finches only males learn the species-typical song, though males and females can produce calls (5). Second, during ontogeny there is a sensitive period in which exposure to conspecific tutors is especially effective in facilitating the formation of song memories (9). Therefore, Ahmadiantehrani and London (6) hypothesize that the mTOR signaling cascade, if it is related to the socially facilitated formation of song memories, would be induced preferentially in males during ontogeny and during a time period coincident with the known sensitive period for song memory formation. It was also thought that this induction would occur preferentially in specialized areas of the auditory telencephalon, namely the caudomedial nidopallium (NCM) and the caudomedial mesopallium (CMM), known to be related to song memory formation (13). In an initial study, Ahmadiantehrani and London (6) establish the presence of the mTORC1 signaling pathway in juvenile male and female finches by localizing ribosomal protein S6 kinase and the 40S subunit ribosomal protein S6. The authors then played song to male and female finches on posthatch day 23 before tutoring supports the formation of song memories, and on day 30 when it does. They found induction of mTOR signaling in NCM and CMM but only in juvenile males, not in females, and only in males who were 30 d of age, not 23 (Fig. 1). These correlational findings strongly implicated the mTOR signaling system in formation of tutor song memories.
Fig. 1.
Zebra finch juveniles hearing song during a known sensitive period for the formation of the memory of conspecific tutor song (posthatch day 30) exhibit a significant increase in the mTOR signaling pathway in auditory areas critical for song memory formation related to song development. This increase does not occur in an adjacent auditory area, Field L, which is involved in general auditory processing. The increase is noted by the dark shading in the CMM and NCM. Males hearing silence do not exhibit this increase. In addition, juvenile females who do not go on to develop species-typical song do not exhibit this increase during the same time of development. Neither males nor females hearing song at an earlier age (posthatch day 23) exhibit any induction of the mTOR signaling pathway in response to song at this time (result not illustrated in this figure). Ahmadiantehrani and London (6) in subsequent experiments manipulated the expression of mTOR and found significant effects to song tutor copying (see text for more detail). Cb, cerebellum; D, dorsal; Hp, hippocampus; P, posterior.
However, correlation does not indicate causation. Ahmadiantehrani and London (6) therefore designed an experimental paradigm that resulted in a bidirectional manipulation of the mTOR signaling pathway. The authors housed juvenile zebra finches with females only and then introduced male tutors for 90-min tutoring sessions. In this way, the authors could control tutoring access, and it is known that this type of exposure is sufficient to maintain normal song. Ahmadiantehrani and London found that either inhibiting (via administration of the selective mTOR inhibitor rapamycin) or constituently enhancing mTOR signaling (via administration of SC79) resulted in a significant disruption of crystallized (i.e., final adult) song compared with control juveniles treated with the vehicle.
In a study in PNAS by Ahmadiantehrani and London, the authors implicate the mechanistic target of rapamycin (mTOR) signaling cascade as being a key player in the molecular basis of social interactions in zebra finches that result in auditory memory formation essential for functional vocal communication as an adult.
These data indicate that mTOR expression needs to be at a specific level of expression in the auditory telencephalon for normal vocal development to occur. The authors also discovered that social interactions were impaired (as measured by how much time the juveniles spent close to and facing their tutors, who were in adjacent cages) in finches that experienced an enhancement of mTOR via SC79 administration. This finding is consistent with other studies on constitutive increases in mTOR expression and social interactions (6).
In this study Ahmadiantehrani and London (6), using both correlational methods and pharmacological manipulations, have implicated the mTOR signaling pathway in the acquisition of tutor song memories. In particular, they show that song can induce mTOR expression in males but not females (only males learn and develop species-typical song) during a period in which the birds are sensitive to learning conspecific song (5). Bidirectional manipulations of the mTOR pathway resulted in similar deficits in the quality of song learning as measured, based on the match between the tutor song and the song produced by the subjects. Thus, there is a nonlinear relationship between mTOR expression and song learning, which as noted by Ahmadiantehrani and London (6) is not unprecedented. Although both compounds distorted song copying from the tutor, only SC79 disrupted the measure of social interactions (time spent facing the tutor in close proximity). These findings indicate that effects on the ability to form a song memory can be dissociated from effects on social interactions per se. Not surprisingly, suppressing the mTOR signaling pathway may well result in different effects than constituently increasing this pathway. These findings, like all good studies, provide us with novel insight into the mechanism of important processes but also raise additional questions as to how this intriguing signaling molecule can have such long-lasting and significant effects.
Acknowledgments
I thank Sarah Goff-Tlemsani for help with the figure. My research is supported by National Institute of Mental Health/NIH R01 MH 50388.
Footnotes
The author declares no conflict of interest.
See companion article on page 9463.
References
- 1.Bale TL, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez M, Mollindeo-Gajate I, Peñagarikano O. Neural circuits for social cognition: Implications for autism. Neuroscience. July 17, 2017 doi: 10.1016/j.neuroscience.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Ball GF, Hulse SH. Birdsong. Am Psychol. 1998;53:37–58. doi: 10.1037//0003-066x.53.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Doupe AJ, Kuhl PK. Birdsong and human speech: Common themes and mechanisms. Ann Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 5.Slater PJB, Eales LA, Clayton NS. Song learning in zebra finches (Taeniopygia guttata): Progress and prospect. Adv Stud Behav. 1988;18:1–34. [Google Scholar]
- 6.Ahmadiantehrani S, London SE. Bidirectional manipulation of mTOR signaling disrupts socially mediated vocal learning in juvenile songbirds. Proc Natl Acad Sci USA. 2017;114:9463–9468. doi: 10.1073/pnas.1701829114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marler P. Three models of song learning: Evidence from behavior. J Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- 8.Brenowitz EA, Beecher MD. Song learning in birds: Diversity and plasticity, opportunities and challenges. Trends Neurosci. 2005;28:127–132. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Roper A, Zann R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology. 2006;112:458–470. [Google Scholar]
- 10.Adret P. Operant conditioning, song learning and imprinting to taped song in the zebra finch. Anim Behav. 1993;46:149–159. [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato A. mTOR, a potential targe to treat autism spectrum disorder. CNS Neurol Disord Drug Targets. 2016;15:533–543. doi: 10.2174/1871527315666160413120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolhuis JJ, Moorman S. Birdsong memory and the brain: In search of the template. Neurosci Biobehav Rev. 2015;50:41–55. doi: 10.1016/j.neubiorev.2014.11.019. [DOI] [PubMed] [Google Scholar]