Abstract
Background:
Sequential anthracyclines and taxanes are standard adjuvant chemotherapy for patients with high-risk axillary node-positive breast cancer. We compared a sequential to a concurrent regimen in high-risk node-negative early breast cancer.
Methods:
Patients were eligible if they had tumours >2 cm or T1c with two of the following characteristics: no oestrogen receptor (ER) and progesterone receptor (PR) expression, histological grade III, Ki67 >40% and vascular, lymphovascular or perineural invasion. They were randomised to receive four cycles of epirubicin 90 mg m−2 followed by four cycles of docetaxel 75 mg m−2 (sequential regimen) or six cycles of epirubicin 75 mg m−2 plus docetaxel 75 mg m−2 (concurrent regimen). All chemotherapy cycles were administered every 21 days with G-CSF prophylaxis only for the concurrent arm. The primary endpoint was disease-free survival (DFS).
Results:
Between 2001 and 2013, 658 women received the sequential (n=329) or the concurrent (n=329) regimen. The median age was 53 years, 43.9% of the patients were premenopausal and of the tumours 44.2% were ⩽2 cm, 52.7% histological grade 3 and 35.3% hormone receptor-negative. After a median follow-up of 70.5 months, there were 29 (8.8%) vs 42 (12.8%) disease relapses (P=0.102) and 11 (3.3%) vs 19 (5.8%) deaths (P=0.135), in the sequential and concurrent arm, respectively. The 5-year DFS rates were 92.6% vs 88.2% for sequential and concurrent arm, respectively (hazard ratio (HR): 1.591; 95% confidence interval (CI): 0.990–2.556; P=0.055). Toxicity included grade 2–4 neutropenia in 54% vs 41% (P=0.001), febrile neutropenia 2.7% vs 6.1% (P=0.06), nausea/vomiting 18.5% vs 12.4% (P=0.03) of patients in the sequential and concurrent arm. There were no toxic deaths.
Conclusions:
Sequential compared with the concurrent administration of anthracyclines and taxanes is associated with a non-significant but possibly clinically meaningful improvement in DFS. In the era of molecular selection of patients for adjuvant chemotherapy, this study offers valuable information for the optimal administration of anthracyclines and taxanes in patients with node-negative disease.
Keywords: taxanes, anhtracyclines, breast cancer, adjuvant chemotherapy, node-negative, high-risk
Adjuvant chemotherapy reduces significantly the risk of recurrence and death for women with early breast cancer (Early Breast Cancer Trialists' Collaborative Group (EBCTCG), 2005). The addition of a taxane to an anthracycline-containing regimen is associated with further reduction in the risk of disease relapse and death (Saloustros et al, 2008).
In phase 3 trials in metastatic breast cancer setting, the concurrent administration of doxorubicin and docetaxel has been proven to be superior to doxorubicin–cyclophosphamide and doxorubicin, cyclophosphamide, and docetaxel (also known as the TAC regimen) superior to fluorouracil, doxorubicin, and cyclophosphamide (Mackey et al, 2002; Nabholtz et al, 2003). In early breast cancer, the concurrent vs sequential administration of anthracyclines and taxanes has been investigated in at least four studies: the Breast Cancer International Group (BCIRG) 005 (Mackey et al, 2016), the Breast International Group (BIG) 02–98 (Oakman et al, 2013), the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 and B-38 trials (Swain et al, 2010, 2013). A significant benefit in both disease-free survival (DFS) and overall survival (OS) has been shown for sequential over concurrent regimens in a meta-analysis including three of these studies (Sha et al, 2012). In all these studies, only patients with node-positive disease have been included.
Owing to the relative lack of data for sequential and concurrent chemotherapy in node-negative early disease and given the fact that the benefit of taxanes in the adjuvant setting was independent of the nodal status (Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) et al, 2012), we compared the sequential vs the concurrent administration of anthracyclines and taxanes in patients with node-negative, high-risk disease. This question is crucial, especially in the era of precision medicine when molecular signatures are being used to select the patients for whom adjuvant chemotherpy is beneficial (Cardoso et al, 2016).
Patients and methods
This randomized study was conducted mainly at ten sites of the Hellenic Oncology Research Group (HORG). The institutional review boards and independent ethics committees have approved the protocol and related materials. All patients signed the informed consent before entering the study.
Eligible patients have undergone either lumpectomy or modified radical mastectomy with tumour-free surgical margins, plus sentinel-node biopsy or axillary node dissection. The tumour had to be invasive adenocarcinoma without infiltrated axillary lymph nodes on pathologic examination. Patients with tumours larger than 2 cm in maximum diameter were eligible. Patients with T1c tumours according to TNM classification were eligible if two of the following criteria were fulfilled: no oestrogen receptor (ER) and progesterone receptor (PR) expression, histological grade III, Ki67 >40% and vascular, lymphovascular or perineural invasion. Determination of the Ki67 expression of the primary tumour was performed at local laboratories, with manual counting (∼1000 cells were counted). Patients with HER2-positive tumours (as determined by local institutional laboratories) were initially considered eligible for this study, but the protocol was amended in May 2008 and Her2-neu overexpression or FISH amplification was added to the exclusion criteria. Patients with a previous history of invasive breast cancer or ductal carcinoma in situ (in either breast), as well as patients who had received any prior radiation, chemotherapy or hormonal therapy were considered ineligible.
Chemotherapy
Eligible women were randomized (1:1) using a computer software to receive four cycles of epirubicin (90 mg m−2 of body-surface area, given by slow intravenous (iv) push during a period of 5–15 min) followed by four cycles of docetaxel (75 mg m−2 by iv infusion over 1 h) (sequential regimen) or six cycles of epirubicin 75 mg m−2 plus docetaxel 75 mg m−2 (concurrent regimen). All chemotherapy cycles were administered every 21 days with primary prophylactic G-CSF support on days 3–10 only for the concurrent arm. Secondary prophylaxis with G-CSF for patients on the sequential arm was at the discretion of the treating physician. Stratification parameters were the menopausal status (pre vs post), tumour maximum diameter (⩽2 cm vs >2 cm) and hormone receptor status (oestrogen and/or progesterone receptor positive vs both negative).
Hormonal and radiation therapy and follow-up
Patients who had breast-conserving surgery received adjuvant radiotherapy following chemotherapy completion. Women who had a modified radical mastectomy were also allowed to receive radiotherapy after completion of all chemotherapy, if they had large (>5 cm) primary tumours. Patients with hormone receptor-positive disease received 20 mg of tamoxifen daily or an aromatase inhibitor for 5 years. Ovarian function suppression for the first 2–3 years of hormonal treatment was optional for premenopausal patients receiving tamoxifen.
Surveillance was recommended for all patients every 3–4 months for the first 2 years, every 6 months for the subsequent 3 years and yearly thereafter. History and physical examination were performed at each visit, mammograms yearly and imaging studies as clinically indicated and according to the discretion of the treating physician.
Study endpoints
The primary endpoint of the study was to compare the DFS rates between the two regimens. A DFS event was defined as the time from randomisation to the date of breast cancer recurrence (either locoregional or distant), breast cancer in the contralateral breast, second non-breast primary cancer or death from any cause, whichever occurred first. Secondary endpoints were to compare OS, defined as the time from the date of randomisation to death from any cause and toxicity of the regimens. Toxicity was graded using the Common Terminology Criteria for Adverse Events of the National Cancer Institute version 3.0.
Statistical analysis
Despite the administration of adjuvant chemotherapy, it was estimated that 35% of high-risk patients with node-negative disease will relapse in 5 years (DFS=65%). On the basis of this assumption, a sample size of 329 patients per arm (658 in total) was required to detect a 5% absolute difference in 5-year DFS between the two arms. This sample size study a power of 80% at an overall two-sided significant level of 0.05. The absolute difference of 5% in 5-year DFS rate corresponds to a hazard ratio (HR) of 0.86, or an absolute relative risk reduction of 14%.
The final analysis was scheduled to perform 5 years after the last patient’s enrolment and the occurrence of 127 events, whereas an interim analysis was planned after the observation of 64 (50%) events. No subgroup analysis was planned before the start of the trial. Analysis was performed on an intent-to-treat basis and all patients who received at least one cycle of treatment were included. Categorical variables were summarised in frequency tables. Continuous variables were presented with descriptive statistics (median and range). Differences of rates between groups for qualitative factors were compared by Pearson’s χ2 contingency table analysis or Fisher’s exact test, whenever appropriate. Differences between groups, in terms of continuous variables, were compared by the non-parametric Mann–Whitney test. The Kaplan–Meier method was used to estimate the distribution of DFS and OS. Comparisons between treatment arms were assessed using the log-rank test. A univariate Cox regression analysis was performed to compute HRs and 95% confidence intervals (CIs) for treatment arms and other exploratory variables. The independent effect of treatment and these variables (age, histology, grade, tumour size, nodal and hormone receptor (HR) status, as well as type of surgery) on DFS and OS was examined in a multivariate analysis using the Cox model. All tests were conducted at a two-sided alpha level of 0.05, and all CIs were given at a two-sided 95% level. Clinical data were held centrally (Clinical Trial Office, Hellenic Oncology Research Group) and analysed using the SPSS (version 22.0) program. Data were current as of May 2015.
Results
Patients
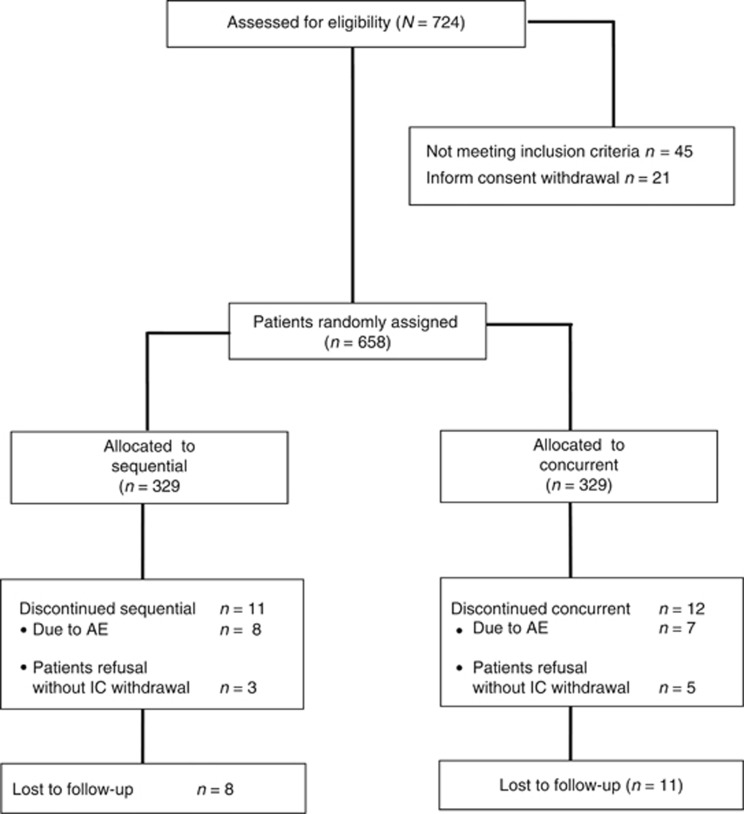
Between June 2001 and August 2013, 724 patients were assessed, of whom 658 (91%) were found to be eligible. Forty-five patients did not meet all the eligibility criteria and 21 patients withdrew their consent. Therefore, 658 patients were centrally randomized to the sequential (n=329 patients) or concurrent (n=329 patients) treatment arm (Figure 1). The treatment groups were well balanced regarding prognostic characteristics (Table 1). The median age was 52 years (range: 28–78). Forty-four percent of the patients were premenopausal at diagnosis. The tumour was positive for ER, PR, or both in 64% of the patients and negative for both hormonal receptors in 36%. Finally, 60% of the patients had undergone breast-conservation, and 40% mastectomy.
Figure 1.
CONSORT diagram.
Table 1. Characteristics of the patients.
|
Treatment groups |
|||||
|---|---|---|---|---|---|
|
Sequential (329) |
Concurrent (329) |
||||
| N | % | N | % | P-value | |
|
Age | |||||
| Median | 53 | 52 | 0.476 | ||
| Min–Max | 29–76 | 28–78 | |||
|
Menopausal status | |||||
| Pre-menopausal | 144 | 43.8 | 145 | 44.1 | 0.937 |
| Post-menopausal | 185 | 56.2 | 184 | 55.9 | |
|
Histology type | |||||
| Ductal | 293 | 89.1 | 288 | 87.5 | 0.544 |
| Lobular | 26 | 7.9 | 33 | 10 | |
| Mixed | 5 | 1.5 | 3 | 0.9 | |
| Others | 5 | 1.5 | 5 | 1.5 | |
|
Performance status (ECOG) | |||||
| 0 | 284 | 86.3 | 293 | 89.1 | 0.286 |
| 1 | 45 | 13.7 | 36 | 10.9 | |
|
HER2 status | |||||
| 3+ | 37 | 11.2 | 56 | 14 | 0.284 |
| Negative | 284 | 86.3 | 270 | 82.1 | |
| Unknown | 8 | 2.4 | 13 | 4 | |
|
T size | |||||
| ⩽2cm | 145 | 44.1 | 146 | 44.4 | 0.937 |
| >2cm | 184 | 55.9 | 183 | 55.6 | |
|
Hormone receptors | |||||
| At least one (+) | 220 | 66.9 | 206 | 62.6 | 0.253 |
| Both (−) | 109 | 33.1 | 123 | 37.4 | |
|
Histology grade | |||||
| 1 | 15 | 4.6 | 18 | 5.5 | 0.385 |
| 2 | 121 | 36.8 | 101 | 30.7 | |
| 3 | 171 | 52 | 176 | 53.5 | |
| Lobular | 16 | 4.9 | 16 | 4.9 | |
| Not applicable | 6 | 1.8 | 18 | 5.5 | |
|
Type of surgery | |||||
| Breast conserving surgery | 212 | 64.4 | 188 | 57.1 | 0.034 |
| Mastectomy | 113 | 34.3 | 141 | 42.9 | |
| Unknown | 4 | 1.2 | — | — | |
Bold values statistical significance of P<0.05.
Treatment
Patients on the sequential arm received a median of 8 (range: 3–8) cycles of treatment, whereas on the concurrent arm, they received a median of 6 (range: 2–6) cycles. The proportion of women who received all eight cycles of the sequential regimen was 96.7% vs 96.4% for the concurrent arm who received all six cycles (P=0.122). The main reason for treatment discontinuation was adverse event probably associated with the treatment in 2.4% and 2.1% (P=0.794) of the patients for the sequential and the concurrent arm, respectively. In both arms treatment discontinuation was mainly due to non-haematologic toxicities. Treatment was administered on time without delay in 96.7% and 96.4% of cycles (P=0.557). Dose reduction for toxicity was required in 1.2% and 3% of administered cycles, in the sequential vs the concurrent group (P=0.001). A total of 8 patients in the sequential and 11 in the concurrent group were lost to follow-up (P=0.641; Figure 1).
Disease-free and overall survival
After a median follow-up of 70.5 months, 71 (10.8%) patients experienced disease recurrence (local n=16, distant n=44) or cancer in the contralateral breast (n=11), and 30 (4.5%) patients died. According to the protocol, the number of events for the interim analysis had been reached and due to the better outcome of the sequential arm, the steering committee decided to report the results.
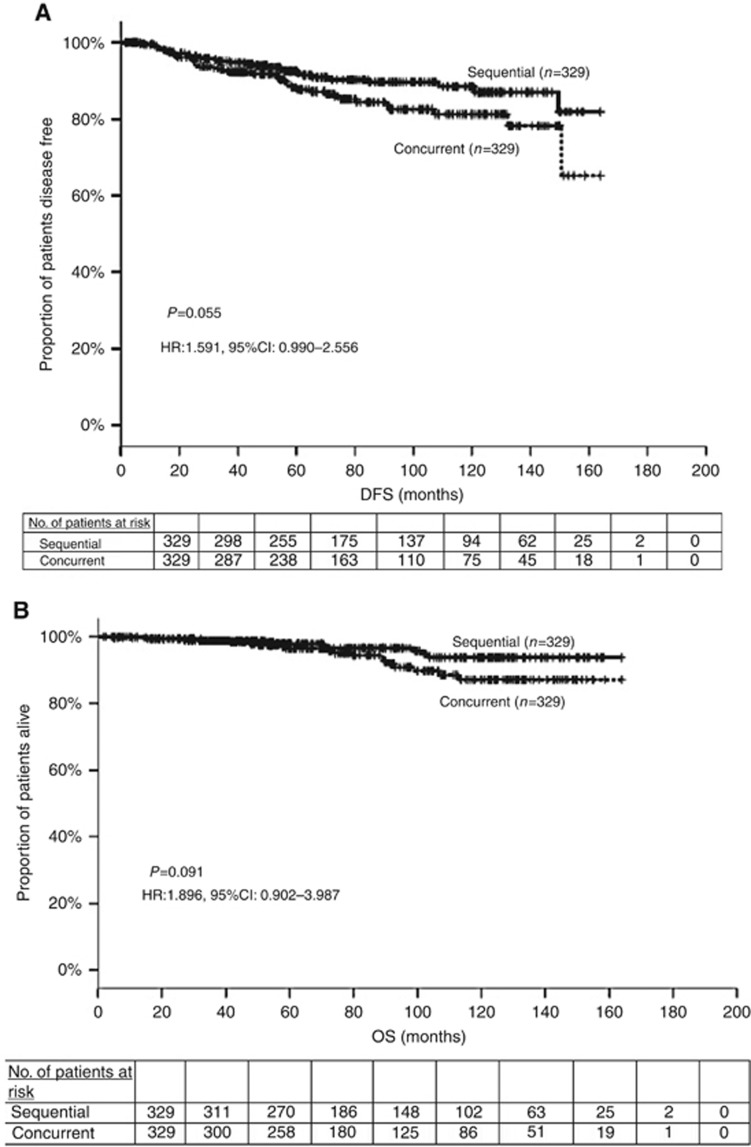
The DFS events were distant relapses in ∼66% of the cases in both groups (Table 2). Although the median DFS has not yet been reached, there was a trend favoring the sequential administration (HR: 1.591; 95% CI: 0.990–2.556; P=0.055). Figure 2A illustrates the Kaplan–Meier curves for DFS in the two treatment groups. The 5-year DFS rates were 88.2% and 92.6% for the groups receiving concurrent and sequential treatment, respectively. An unplanned subgroup analysis for the 188 triple-negative patients, revealed a similar trend; the 5-year DFS rates were 91.4% vs 82.2% in favour of the sequential arm (HR: 1.93; 95% CI: 0.886–4.205; P=0.098). However, for patients with hormone receptor positive, HER2-negative disease no difference was found; the 5-year DFS was 92.5% vs 92.2% for the sequential and the concurrent group (HR: 1.185; 95% CI: 0.592–2.371; P=0.631).
Table 2. Sites of disease relapse.
|
Sequential (329) |
Concurrent (329) |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Local | 6 | 1.8 | 10 | 3 |
| Distant | 19 | 5.8 | 25 | 7.6 |
| 2nd primary | 4 | 1.2 | 7 | 2.1 |
| Death (from any cause) | — | — | — | — |
Figure 2.
Kaplan Meier estimate of (A) disease-free and (B) overall survival for the two treatment arms. The P-value is from the log-rank test. The hazard ratio (HR) and its 95% confidence interval are obtained from the Cox proportional hazards model.
Figure 2B shows the Kaplan–Meier curves for OS. The estimated 5-year OS rates were excellent in both groups; 96.3% for the concurrent group and 98.7% for the sequential one. A trend for prolonged median OS was observed in favour of the sequential treatment group (HR: 1.896; 95% CI: 0.902–3.987, P=0.091). Interestingly, this trend towards better OS was more pronounced for patients with triple-negative tumours (HR: 3.369; 95% CI: 0.940–12.081, P=0.062).
From the exploratory variables included in the univariate analysis, only hormone receptors had a significant influence on DFS (HR: 1.683, 95% CI: 1.056–2.683, P=0.029), whereas the type of treatment had a marked trend but not statistically significant (HR: 1.591, 95% CI: 0.990–2.556, P=0.055). The interaction between treatment arms and hormone receptors was also examined and a statistically significant association was revealed (HR: 2.157, 95% CI: 1.174–3.963, P=0.013). The multivariate analysis confirmed this association (HR: 2.125, 95% CI: 1.152–3.921, P=0.016).
Toxic effects
Fifty eight percent of patients receiving epirubicin and docetaxel concurrently, developed grade 2–4 adverse events as compared with 65% of those receiving sequential treatment (P=0.078). The Table 3 summarises the most commonly reported adverse events. Patients in the sequential group despite the lower cumulative dose of chemotherapy received (sequential: 360 mg m−2 epirubicin and 300 mg m−2 docetaxel vs concurrent: 450 mg m−2 epirubicin and 450 mg m−2 docetaxel) were at higher risk for grade 2–4 neutropenia (54% vs 41%), presumably due to primary G-CSF prophylaxis in the concurrent arm. However, grade 2–4 febrile neutropenia was more common on the concurrent arm (6% vs 2.7%). The incidence of grade 2–4 neuropathy in the two groups was relatively low (0.3% vs 0.6%). Finally, grade 2–4 non-haematologic toxicities like chemotherapy-induced nausea and vomiting, constipation and nail toxicity were more common in the sequential arm, whereas patients in the concurrent group were at higher risk for oedema. There were no significant cardiac toxicity or toxic deaths.
Table 3. Toxic effects of sequential and concurrent arm.
|
Sequential (329) |
Concurrent (329) |
Sequential (329) |
Concurrent (329) |
Sequential (329) |
Concurrent (329) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Gr II |
Gr II |
Gr III |
Gr III |
Gr IV |
Gr IV |
||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | P-value | |
| Neutropenia | 61 | 18.5 | 26 | 7.9 | 76 | 23.1 | 43 | 13.1 | 41 | 12.5 | 66 | 20.1 | 0.511* 0.001** |
| Anaemia | 36 | 10.9 | 56 | 17 | 4 | 1.2 | — | — | — | — | — | — | 0.124* 0.077** |
| Thrombocytopenia | 1 | 0.3 | 3 | 0.9 | — | — | — | — | 1 | 0.3 | 1 | 0.3 | 1.000* 0.682** |
| Febrile neutropenia | 5 | 1.5 | 1 | 0.3 | 2 | 0.6 | 4 | 1.2 | 2 | 0.6 | 15 | 4.6 | 0.003* 0.058** |
| Nausea | 51 | 15.5 | 32 | 9.7 | 9 | 2.7 | 9 | 2.7 | 1 | 0.3 | — | — | 1.000* 0.031** |
| Diarrhoea | 5 | 1.5 | 14 | 4.3 | 6 | 1.8 | 2 | 0.6 | — | — | — | — | — 0.432** |
| Mucositis | 11 | 3.3 | 6 | 1.8 | 3 | 0.9 | 3 | 0.9 | — | — | 1 | 0.3 | 1.000* 0.533** |
| Neurotoxicity | 1 | 0.3 | 1 | 0.3 | 1 | 0.3 | — | — | — | — | — | — | — 1.000** |
| Allergy | 5 | 1.5 | 2 | 0.6 | 1 | 0.3 | 3 | 0.9 | 1 | 0.3 | 1 | 0.3 | 0.682* 1.000** |
*P-value for comparison of grade III–IV toxicities between the two treatment groups.
**P-value for comparison of grade II/III/IV toxicities between the two treatment groups. Bold values statistical significance of P<0.05.
Discussion
We present the first study, to the best our knowledge, comparing the sequential vs concurrent administration of anthracyclines and taxanes as adjuvant therapy in patients with node-negative but high-risk early breast cancer. After a median follow-up of ∼6 years and 10.7% of the patients experiencing disease relapse, a preplanned interim analysis showed a non-statistically significant but possibly clinically meaningful trend towards longer DFS and OS in favour of the sequential administration of epirubicin and docetaxel. Moreover, the observed increased haematologic toxicity of the sequential regimen was not clinically harmful. The higher incidence of neutropenia did not result in more febrile neutropenia events, whereas there were no significant differences in the non-haematologic toxicities.
Anthracyclines and taxanes are recommended for the adjuvant treatment of women with operable breast cancer. Several regimens are being used by clinicians, including standard dose sequential, concurrent and dose-dense sequential (Saloustros et al, 2014). However, due to the relative paucity of data from head-to-head comparisons between these regimens, there is no consensus for the optimal chemotherapy regimen.
The sequential vs concurrent administration has been tested so far only in the population of node-positive early breast cancer. The concurrent TAC (docetaxel, doxorubicin and cyclophosphamide) was not superior in DFS and OS to the sequential AC→T regimen, according to the 10-year analysis of the BCIRG-005 study. At 10-years, the DFS rates were 66.5% in the AC→T arm and 66.3% in the TAC arm (P=0.749), whereas OS was 79.9% and 78.9% (P=0.506), respectively (Mackey et al, 2016). The efficacy was comparable across all stratification subgroups. Likewise, due to primary G-CSF support, the toxicity was more acceptable in the TAC than AC→T therapy. The BIG 02-98 trial compared the sequential vs the concurrent docetaxel arm (A 75 mg m−2 × 3 cycles→T 100 mg m−2 × 3 cycles→CMF × 3 cycles vs AT 50/75 mg m−2 × 4 cycles→CMF × 3 cycles) in patients with infiltrated lymph nodes. Sequential docetaxel significantly improved DFS (HR: 0.84, 95% CI: 0.72–0.99, P=0.035) and OS (HR: 0.79, 95% CI: 0.65–0.98, P=0.028) compared to the concurrent doxorubicin–docetaxel, after 8 years of median follow-up (Oakman et al, 2013). In the context of NSABP B-30 trial, 5351 patients were randomly assigned to four cycles of doxorubicin and cyclophosphamide followed by four cycles of docetaxel (AC→T) vs four cycles of doxorubicin and docetaxel and vs four cycles of doxorubicin, cyclophosphamide, and docetaxel (Swain et al, 2010). After a median follow-up of 73 months, OS was improved in the AC→T group (8-year OS, 83%) compared with the doxorubicin–docetaxel group (OS 79% P=0.03) or the concurrent-TAC group (OS 79% P=0.09). According to a meta-analysis of these studies, the sequential taxane- and anthracycline-based regimen resulted in a significant 12% reduction in mortality over the concurrent administration in patients with early-stage, node-positive breast cancer (Swain et al, 2013).
The more recently reported NSABP B38 trial compared the TAC regimen vs the dose-dense AC × 4 followed by paclitaxel × 4. No significant differences in efficacy were shown (Swain et al, 2013). Febrile neutropenia and diarrhoea were more common with TAC, and neuropathy, anaemia, transfusions and erythropoietin use with dose dense AC→paclitaxel. In both of these studies (Swain et al, 2013; Mackey et al, 2016), cyclophosphamide was included in the concurrent regimen. This observation raises the question whether the omission of cyclophosphamide in the concurrent arm of the other two studies, the BIG 02-98 and NSABP B-30, favored the sequential regimens.
Cyclophosphamide administration was not a ‘cofounding factor’ in our study, as it was not administered in either arm. However, in contrary to the NSABP B30 trial (Swain et al, 2010), which suggested that both a longer course (sequential regimen) and a higher dose of docetaxel are important for maximum efficacy, our study showed that for node-negative patients, less chemotherapy administered over a longer course is actually more beneficial. Given the results from recently presented trials, showing that in terms of invasive DFS, docetaxel plus cyclophosphamide × 6 was inferior to taxane plus anthracycline (Blum et al, 2017; Mavroudis et al, 2016), we conclude that for high-risk node-negative patients anthracyclines and taxanes are crucial for maximising clinical benefit. However, our study raises the question whether maximum benefit can be achieved with less cumulative chemotherapy dose.
An interesting issue in the interpretation of our data is the observation, based on an unplanned analysis, that the sequential regimen may be more active in patients with triple-negative tumours; 5-year DFS rates 91.4% vs 82.2% in favour of the sequential arm. Subset analyses of the other sequential vs concurrent taxane trials have shown contradictory results. Although no subtype specificity was observed in the BIG 02-98 and BCIRG trials, NSABP B-32 and B-38 trials suggested that the sequential regimen may be superior for patients with ER-negative tumours. Our results for triple-negative tumours should be interpreted with caution as this analysis has not been preplanned, we stratified patients according to hormonal status but not HER2 status (before 2008 amendment) and no central confirmation of ER, PR and HER2 expression was performed.
A major limitation of this report is that the results are based on an interim analysis. Both the slow accrual (12 years to enrol the required number of patients) and the better outcome observed in both groups than was initially anticipated, hampered the study. According to the study design, 65 more events will be needed for the final analysis. Given that the annual hazard rate of breast cancer relapse varies over time, with a peak near 3% per year between the second and third years after primary surgery and then declines to 1 to 2% per year thereafter (Jatoi et al, 2011), seven additional years of follow-up are needed for the 65 missing events to be observed for the final analysis. Therefore, the study committee decided to present the results of this preplanned interim analysis, based on the clear trend in favour of the sequential arm. This effect was more evident for the hormone receptor-negative patients. Therefore, our results should be interpreted with caution keeping in mind the importance of the question for the clinicians, and the possibility that with a longer follow-up our results may change. In addition, the relatively small sample size, the borderline significance of the primary outcome, the omission of cyclophosphamide on the anthracycline arm, as well as the non uniform assessment of HER2 status and the initial inclusion of a small number of HER2 positive patients are some other potential weaknesses of our study.
Over the last two decades, several trials have been conducted in an effort to define the optimal regimen for early-stage breast cancer. It seems highly unlikely that changes in dosing schedules will result in any substantial clinical benefit. Moreover, attempts to improve outcomes with combination regimens by adding more chemotherapeutic agents to anthracyclines and taxanes, have not been successful. Studies that will help us determine optimal treatments based on tumour biology seem to be more promising. The recently reported MINDACT (Microarray in Node-Negative and 1–3 Node-Positive Disease May Avoid Chemotherapy) trial showed that molecular analysis might improve the selection of patients with node-negative disease who derive benefit from adjuvant chemotherapy (Cardoso et al, 2016). The ongoing TAILORx (Trial Assigning Individualised Options for Treatment) study will address the question whether the addition of chemotherapy to hormonal therapy for women with node-negative, ER-positive breast cancer and intermediate Oncotype DX (Genomic Health, Redwood City, CA, USA) recurrence score improves outcome. In the era of molecular selection of patients with node-negative disease for whom adjuvant chemotherapy is indicated, we believe that our study provides valuable information regarding the optimal use of the most active drugs to achieve a better clinical outcome.
Acknowledgments
This study was funded by the Hellenic Oncology Research Group.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
References
- Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE, Jacobs SA, Robert NJ, Atkins JN, O'Shaughnessy J, Dang CT, Gomez HL, Fehrenbacher L, Vukelja SJ, Lyss AP, Devchand Paul D, Brufsky AM, Swain SM, Mamounas EP, Jones SE, Wolmark N (2017) Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP-B49 (NRG Oncology). J Clin Oncol doi:10.1200/JCO.2016.71.4147.
- Cardoso F, van’t Veer L, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M MINDACT Investigators (2016) 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375: 717–729. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 379: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatoi I, Anderson WF, Jeong JH, Redmont CK (2011) Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol 29: 2301–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey JR, Patrson A, Dirix LY, Dewar J, Chap L, Martin M, Chan S, Tang SC, Dugan W, Gil M, Zaluski J, Russel C, Vogel C, Efremedis A, Appia F, Brunel E, Hatteville L, Azli N, Nabholtz JM (2002) Final results of the phase III randomized trial comparing docetaxel (T), doxorubicin (A) and cyclophosphamide (C) to FAC as first line chemotherapy (CT) for patients (pts) with metastatic breast cancer (MBC). Proc Am Soc Clin Oncol 21: 35a. [Google Scholar]
- Mackey JR, Pieńkowski T, Crown J, Sadeghi S, Martin M, Chan A, Saleh M, Sehdev S, Provencher L, Semiglazov V, Press MF, Sauter G, Lindsay M, Houé V, Buyse M, Drevot P, Hitier S, Bensfia S, Eiermann W (2016) Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol 27: 1041–1047. [DOI] [PubMed] [Google Scholar]
- Mavroudis D, Matikas A, Malamos N, Papakotoulas P, Kakolyris S, Boukovinas I, Athanasiadis A, Kentepozidis N, Ziras N, Katsaounis P, Saloustros E, Georgoulias V (2016) Dose-dense FEC followed by docetaxel versus docetaxel plus cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 27: 1873–1878. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Falkson C, Campos D, Szanto J, Martin M, Chan S, Pienkowski T, Zaluski J, Pinter T, Krzakowski M, Vorobiof D, Leonard R, Kennedy I, Azil N, Murawsky M, Riva A, Pouillart P (2003) Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 21: 968–975. [DOI] [PubMed] [Google Scholar]
- Oakman C, Francis PA, Crown A, Quinaux E, Buyse M, De Azambuja E, Margeli Vila M, Andersson M, Nordenskjöld B, Jakesz R, Thürlimann B, Gutiérrez J, Harvey V, Punzalan L, Dell'orto P, Larsimont D, Steinberg I, Gelber RD, Piccart-Gebhart M, Viale G, Di Leo A (2013) Overall survival benefit for sequential doxorubicin-docetaxel compared with concurrent doxorubicin and docetaxel in node-positive breast cancer-8-year results of Breast International Group 02-98 phase III trial. Ann Oncol 24: 1203–1211. [DOI] [PubMed] [Google Scholar]
- Saloustros E, Malamos N, Boukovinas I, Kakolyris S, Kouroussis C, Athanasiadis A, Ziras N, Kentepozidis N, Makrantonakis P, Polyzos A, Christophyllakis C, Georgoulias V, Mavroudis D (2014) Dose-dense paclitaxel versus docetaxel following FEC as adjuvant chemotherapy in axillary node-positive early breast cancer: a multicenter randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Res Treat 148: 591–597. [DOI] [PubMed] [Google Scholar]
- Saloustros E, Mavroudis D, Georgoulias V (2008) Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother 9: 2603–2616. [DOI] [PubMed] [Google Scholar]
- Sha N, Wang S, Yao C, Xu Z, Zhang Y, Zhang Y, Lin Y (2012) Sequential versus concurrent anthracyclines and taxanes as adjuvant chemotherapy of early breast cancer: A meta-analysis of phase III randomized control trials. Breast 21: 389–393. [DOI] [PubMed] [Google Scholar]
- Swain SM, Jeong JH, Geyer CE, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J, Vogel VG, Erban JK, Rastogi P, Livingston RB, Perez EA, Mamounas EP, Land SR, Ganz PA, Wolmark N. (2010) Longer therapy, iatrogenic amenorhea, and survival in early breast cancer. N Engl J Med 362: 2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Tang G, Geyer CE, Rastogi P, Atkins JN, Donnellan PP, Fehrenbacher L, Azar CA, Robidoux A, Polikoff JA, Brufsky AM, Biggs DD, Levine EA, Zapas JL, Provencher L, Northfelt DW, Paik S, Costantino JP, Mamounas EP, Wolmark N (2013) Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 Trial. J Clin Oncol 31: 3197–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]