Abstract
A national crowdsourcing-based tick collection campaign was organized in 2015 with the objective of producing novel data on tick distribution and tick-borne pathogens in Finland. Nearly 20 000 Ixodes ticks were collected. The collected material revealed the nationwide distribution of I. persulcatus for the first time and a shift northwards in the distribution of I. ricinus in Finland. A subset of 2038 tick samples containing both species was screened for Borrelia burgdorferi sensu lato (the prevalence was 14.2% for I. ricinus and 19.8% for I. persulcatus), B. miyamotoi (0.2% and 0.4%, respectively) and tick-borne encephalitis virus (TBEV; 0.2% and 3.0%, respectively). We also report new risk areas for TBEV in Finland and, for the first time, the presence of B. miyamotoi in ticks from mainland Finland. Most importantly, our study demonstrates the overwhelming power of citizen science in accomplishing a collection effort that would have been impossible with the scientific community alone.
Keywords: Borrelia burgdorferi, Borrelia miyamotoi, crowdsourcing, distribution, Ixodes persulcatus, Ixodes ricinus, tick-borne encephalitis virus, tick-borne pathogens
INTRODUCTION
Ticks are the primary vectors for several zoonotic infections worldwide. Ticks and tick-transmitted pathogens are presently under active investigation due to the status of tick-borne diseases as emerging infections. The most important tick-borne diseases in Finland are Lyme borreliosis (LB; with ~1900 microbiologically confirmed cases yearly and estimated 6000–7000 total cases yearly; ~120 cases per 100 000 individuals) and tick-borne encephalitis (TBE; with ~40–60 microbiologically confirmed cases yearly; ~1 case per 100 000 individuals) according to the National Infectious Disease Register maintained by National Institute for Health and Welfare (https://www.thl.fi/ttr/gen/rpt/tilastot.html). Borrelia miyamotoi is a spirochete belonging to the relapsing fever group of Borrelia with an unknown prevalence and geographic distribution in Finland. The distribution of tick species in Finland is exceptional because it is the northernmost border of tick distribution in Europe, and the distribution borders of two important tick species (Ixodes ricinus and I. persulcatus) are both located within the country. The distribution of these tick species and the diversity of their associated pathogens have never been intensely studied in Finland. Surveys conducted in neighboring countries suggest a northward shift in the distribution of Ixodes ticks as well as an increase in abundance over the past few decades.1, 2, 3, 4 However, the current tick situation in Finland and elsewhere in northern Europe has not been fully characterized.
Tick collection using the traditional methods such as cloth dragging and flagging is both time-consuming and laborious, and covers a relatively small geographical area in a certain time in most research frames. Large-scale sample collection cannot be carried out with a limited number of researchers. Crowdsourcing is utilized relatively rarely but is an effective method for gathering data in health-related research.5 To construct a comprehensive, nationwide collection of ticks, we launched a national campaign using an innovative crowdsourcing approach in which citizens were asked to participate in tick collection. The national tick collection campaign was organized in 2015, advertised on the internet, television and newspapers, and was a success. Approximately 7000 shipments were received containing nearly 20 000 individual ticks from all over Finland. The samples gathered formed the so-called ‘Tickbank’ and constitute unique material for ecological, taxonomical, medical and veterinary medical studies. Here we present the first results from this vast material.
MATERIALS AND METHODS
Tick collection and metadata gathering
From April to November 2015, citizens were asked to send ticks (dead or alive) via postal mail to the Department of Biology at the University of Turku as a part of the tick collection campaign. Along with the ticks, they were asked to provide information on the collection site and date, and the species of the possible host. This collection resulted in a Tickbank of 19 923 individual ticks. Ticks lacking adequate date information or collected outside the campaign period (n=1788) were stored in the Tickbank but were not used in the further analyses. The species, life stage and sex of tick samples were identified based on morphological characteristics under a microscope, if possible. Almost all the received samples were recognized correctly as Ixodes ticks by citizens; those that represented other species (for example, deer keds, spiders and moss mites) were not stored in the Tickbank or analyzed in this study. After identification, ticks were stored at −80 °C. The geographical information of the ticks was stored as ETRS-TM35FIN coordinates with an accuracy of 100 m. In most cases, the collection site information provided by citizens was accurate enough. In a minority of the cases (∼300), the collection site information was inaccurate and therefore those tick samples were not used in the distribution analyses. Distribution maps were created using MapInfo Professional 12.0 software (Pitney Bowes Business Insight, Troy, NY, USA).
DNA and RNA extraction
A subset of 2038 ticks (1044 I. ricinus and 994 I. persulcatus) were selected for screening for B. burgdorferi s.l., B. miyamotoi and tick-borne encephalitis virus (TBEV). The samples were manually selected to represent the major collection areas, tick life stages and sex distribution of the whole Tickbank. However, we selected approximately the same number of I. ricinus and I. persulcatus samples to obtain a comprehensive picture of both species. DNA and RNA were extracted from the tick samples sequentially using NucleoSpin RNA kits and RNA/DNA buffer sets (Macherey-Nagel, Düren, Germany) following the kit protocols (RNA Kit: Rev. 16 May 2014 and RNA/DNA buffer set: Rev. 08 May 2014). RNA extracts were stored at −80 °C and DNA extracts were stored at −20 °C.
Real-time PCR assays
Tick species, if unknown after morphological identification (n=98), was determined in a species-specific duplex real-time PCR assay as previously described6 (detailed protocol in Supplementary Materials). IXO-I2-F4 and IXO-I2-R4 primers targeting a 94-bp fragment of Ixodes spp. internal transcribed spacer 2 (ITS2) gene were used to amplify genus-specific segments, and Ipe-I2-P4 and Iri-I2-P4 probes were used to match the ITS2 region for either tick species (I. persulcatus or I. ricinus, respectively; Table 1).7, 8, 9, 10 DNA samples from I. ricinus and I. persulcatus confirmed by sequencing in an earlier study9 were used as positive controls, and double-distilled water (ddH2O) was used as a negative control in each assay.
Table 1. Primers and probes used in tick species determination and pathogen screening.
| Primer/probe name | Target name | Nucleotide sequence (5′→3′) | Reference |
|---|---|---|---|
| Real-time PCR | |||
| Bbsl-ospA-F | B. burgdorferi ospA | AATATTTATTGGGAATAGGTCTAA | 7 |
| Bbsl-ospA-R | CACCAGGCAGCAAATCTACTGA | ||
| Bbsl-ospA-P | [6FAM]-TTAATAGCATGTAAGCAAAATGTTAGCA-[DDQ1] | ||
| Bm-fla-F | B. miyamotoi flagellin | AGAAGGTGCTCAAGCAG | 8 |
| Bm-fla-R | TCGATCTTTGAAAGTGACATAT | ||
| Bm-fla-P | [6FAM]-AGCACAACAGGAGGGAGTTCAAGC-[DDQ1] | ||
| IXO-I2-F4 | Ixodes spp. ITS2 | TCTCGTGGCGTTGATTTGC | 9 |
| IXO-I2-R4 | Ixodes spp. ITS3 | CTGACGGAAGGCTACGACG | |
| Ipe-I2-P4 | I. persulcatus ITS4 | [FAM]-TGCGTGGAAAGAAAACGAG-[BHQ1] | |
| Iri-I2-P4 | I. ricinus ITS5 | [VIC]-TGCTCGAAGGAGAGAACGA-[BHQ1] | |
| Real-time RT-PCR | |||
| F-TBEV1 | 3′-non-coding region of the TBEV genome | GGGCGGTTCTTGTTCTCC | 10 |
| R-TBEV1 | ACACATCACCTCCTTGTCAGACT | ||
| P-TBEV-WT | [FAM]-TGAGCCACCATCACCCAGACACA-[TAMRA] | ||
Abbreviations: internal transcribed spacer 2, ITS2; outer surface protein A, ospA; reverse transcription-PCR, RT-PCR; tick-borne encephalitis virus, TBEV.
Bbsl-ospA-F and Bbsl-ospA-R primers, and a Bbsl-ospA-P probe (Table 1) amplifying a 102-bp fragment of the outer surface protein A (ospA) gene as previously described 7 were used to detect B. burgdorferi s.l. DNA (Supplementary Methods). Positive and negative controls (B. burgdorferi sensu stricto strain B31 ATCC 35210 and ddH2O, respectively) were included in all runs. For B. miyamotoi, Bm-fla-F and Bm-fla-R primers, and a Bm-fla-P probe (Table 1) targeting the B. miyamotoi flagellin gene (156 bp) were used as previously described8 with minor modifications. DNA samples from B. miyamotoi confirmed by sequencing in earlier studies6, 11 were used as positive controls; B. burgdorferi sensu stricto strain B31 (ATCC 35210) and ddH2O were used as negative controls.
For TBEV screening, aliquots of the original RNA samples were first pooled (10 samples per pool, 5 μL of each sample) because a low prevalence was expected. Then, the pools were examined using real-time reverse transcription-PCR with F-TBEV1 and R-TBEV1 primers, and a P-TBEV-WT probe (Table 1) amplifying the 3′-non-coding region of the TBEV genome as previously described10, 12 (Supplementary Materials). Individual RNA samples were re-analyzed if a pooled sample tested positive. Positive (TBEV-Sib and TBEV-Eur)13, 14 and negative (ddH2O) controls were included in each run.
Statistical analyses
Data were managed using Microsoft Excel 2013 (Redmond, WA, USA). Because the independence of observations is an underlying assumption of most basic statistical tests, statistical analysis of citizen-collected data is a challenging task. On many occasions, we received many ticks in one letter, indicating that these ticks were dependent on each other, for example, similar collection times, locations, hosts, and often by tick species and developmental stage. Therefore, we refrained from formal statistical analyses apart from testing one specific hypothesis and controlling for dependent observations (see below).
Previous studies have suggested a higher prevalence of B. burgdorferi s.l. among samples of I. persulcatus compared to I. ricinus.15, 16, 17 We tested this hypothesis using a generalized linear mixed model (GLMM) for the screened adults of both tick species. Larvae and nymphs were ignored because of their low sample sizes (Supplementary Table S1). To separate the possible effect of tick species from that of dissimilar environments (for example, due to weather or distance to the southern coast; Figure 1), we restricted the analysis to I. persulcatus (n=885; 658 females and 227 males) and I. ricinus (n=527; 393 females and 134 males) samples collected from the area of their sympatric occurrence. In practice, this was done by simply filtering the data according to the N coordinate of the southernmost I. persulcatus and northernmost I. ricinus.
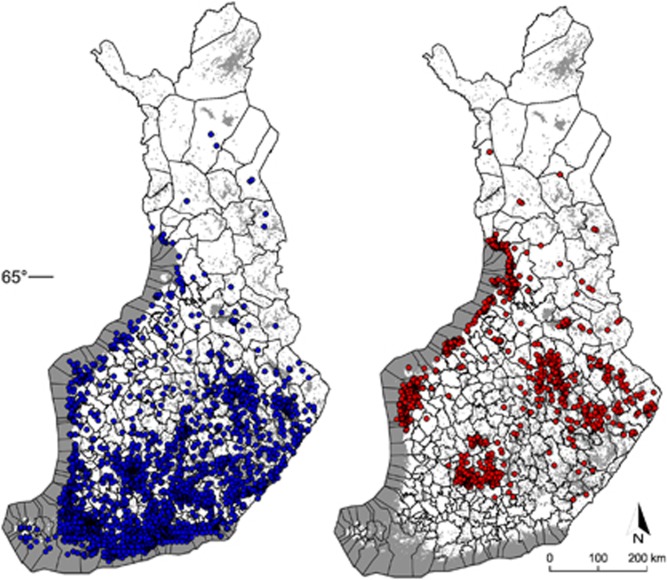
Figure 1.
Map illustrating the distribution of I. ricinus and I. persulcatus in Finland based on the coordinates of 17 603 tick samples collected in 2015 via the collection campaign. Blue dots indicate collection points for I. ricinus (n=13 847) and red dots indicate collection points for I. persulcatus (n=3756).
We modeled the probability of an adult tick testing positive for B. burgdorferi s.l. by running a generalized estimating equation, a specific type of GLMM for clustered observations, with a binomial error distribution and logit link function. The shipment ID was set as a clustering factor, whereas the species and sex of the tick were fixed explanatory factors. The model was run with the GENMOD procedure in SAS statistical software, v. 9.4. (Cary, NC, USA).18
RESULTS
Characteristics of the Tickbank
Our crowdsourcing-based tick collection was extremely successful, with nearly 7000 shipments from all over Finland. These resulted in the Tickbank of 19 923 individual ticks, of which nearly 80% were I. ricinus. After samples lacking adequate date or collection site information were excluded, the remaining 17 603 coordinates were used in distribution analyses. Most tick samples were received from central Finland (the so-called Finnish Lakeland) and coastal areas (Figure 1). A considerable number of ticks were received from the southern coast of Finland, all of which were I. ricinus. The northernmost collection sites were beyond the Arctic Circle, at latitudes of 67° N in Lapland. Both tick species were received from northern Finland (north of latitude 65° N), although almost all of these samples (97% 760/784) were I. persulcatus. Whereas I. ricinus was more evenly distributed over southern and eastern Finland, and the coastal areas, I. persulcatus seemed to have three distinct clusters in distribution: on the coast of the Gulf of Bothnia, in eastern Finland and in the middle of southern Finland (Figure 1).
Of the ticks collected in 2015 (n=18 135), 17 936 could be identified morphologically to species (14 133 I. ricinus and 3803 I. persulcatus). Of these, most were adults (Table 2). I. ricinus samples contained relatively more young developmental stages (larvae and nymphs) than I. persulcatus (5.5% vs. 1.1%, respectively). Adult samples were more often female (n=12 246) than male (n=4880), with similar proportions for both species. The most frequently reported host was dog (54.2% for I. ricinus and 62.2% for I. persulcatus). I. persulcatus was detected more often in humans (19.7% vs. 14.5%) whereas I. ricinus was found more often in cats (30.3% vs. 17.3% Table 2).
Table 2. Information for the samples collected in 2015 via the collection campaign.
| Number (%) of I. ricinus samples | Number (%) of I. persulcatus samples | Total | |
|---|---|---|---|
| Amount | 14 133 (78.8) | 3803 (21.2) | 17 936 (100.0) |
| Sex of adult ticks | |||
| Female | 9555 (71.5) | 2691 (71.6) | 12 246 (71.5) |
| Male | 3810 (28.5) | 1070 (28.4) | 4880 (28.5) |
| Total | 13 365 (100.0) | 3761 (100.0) | 17 126 (100.0) |
| Developmental stage | |||
| Adult | 13 365 (94.5) | 3761 (98.9) | 17 126 (95.5) |
| Nymph | 743 (5.3) | 41 (1.1) | 784 (4.4) |
| Larva | 25 (0.2) | 1 (0.0) | 26 (0.1) |
| Total | 14 133 (100.0) | 3803 (100.0) | 17 936 (100.0) |
| Collected from | |||
| Dog | 7289 (54.2) | 2195 (62.2) | 9484 (55.9) |
| Cat | 4075 (30.3) | 609 (17.3) | 4684 (27.6) |
| Human | 1945 (14.5) | 695 (19.7) | 2640 (15.6) |
| Other animal | 88 (0.7) | 2 (0.0) | 90 (0.5) |
| Nature | 46 (0.3) | 27 (0.8) | 73 (0.4) |
| Total | 13 443 (100.0) | 3528 (100.0) | 16 971 (100.0) |
*Of all these samples (n=18 135), 17 936 were identified as Ixodes ricinus or I. persulcatus; 199 samples could not be identified.
Each category (sex, developmental stage and collected from) contains missing data, such that the total amount differs from the total number of collected ticks. ‘Other animal’ (n=90) includes animals such as horse, sheep, raccoon dog, European roe deer and white-tailed deer.
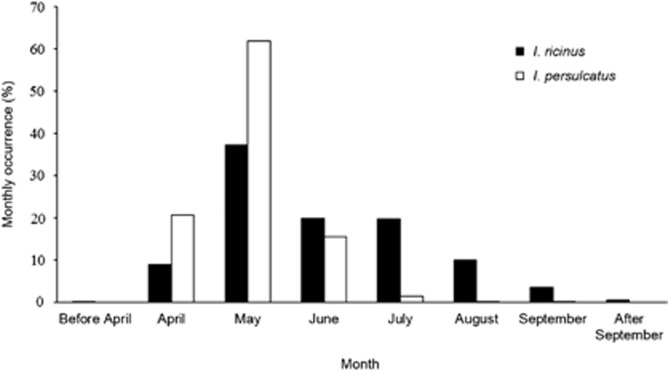
Most of the ticks were collected in May (Figure 2). I. persulcatus was collected mainly from April to June (98.1%), whereas the collection period for I. ricinus was more evenly distributed throughout the summer and early autumn. I. persulcatus was apparently no longer active in October and November, whereas almost 100 I. ricinus individuals were collected during the same period.
Figure 2.
A diagram showing the monthly occurrence of I. ricinus and I. persulcatus samples collected via the collection campaign.
The subset of 2038 ticks—characteristics and pathogen screening results
A total of 1044 I. ricinus and 994 I. persulcatus were selected for screening of B. burgdorferi s.l., B. miyamotoi and TBEV. These ticks represented the whole Tickbank in terms of collection site (Figures 1 and 3A), tick life stage and sex distribution, and reported host (Table 2; Supplementary Table S1). Of ticks that could not be identified morphologically by microscope, 57 were identified as I. ricinus and 41 as I. persulcatus by duplex real-time PCR.
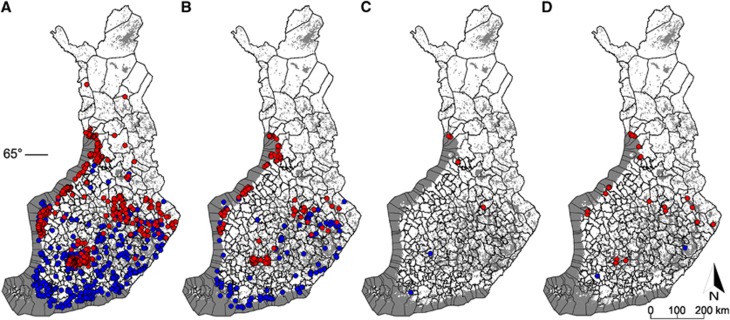
Figure 3.
(A) Distribution of the samples that were screened for pathogens (n=2038). Blue dots indicate collection points for I. ricinus samples (n=1044) and red dots indicate collection points for I. persulcatus samples (n=994). (B) Distribution of the samples that were positive for Borrelia burgdorferi s.l. (n=345). (C) Distribution of the samples that were positive for B. miyamotoi (n=6). (D) Distribution of the samples that were positive for TBEV (n=32).
In total, B. burgdorferi s.l. was detected in 16.9% (345/2038) of the screened DNA samples (Table 3). The prevalence was 14.2% (148/1044) for I. ricinus and 19.8% (197/994) for I. persulcatus. Divided by stages, the prevalence of B. burgdorferi s.l. was 17.1% (332/1945) for adult ticks and 14.4% (13/91) for nymphs. No larvae were found to be infected. The GLMM conducted for the adults in the sympatric region indicated a significantly higher probability of a positive finding for I. persulcatus (the estimated marginal mean (with 95% confidence interval) was 0.196 (0.166–0.232)) compared with that of I. ricinus (0.137 (0.106–0.174); Wald statistics, species: χ2=5.67, DF=1, P=0.017). No differences in the prevalence of B. burgdorferi s.l. were observed between females and males of either species (sex: χ2=1.03, DF=1, P=0.311; species × sex: χ2=0.03, DF=1, P=0.872). The distribution map drawn from the positive B. burgdorferi s.l. samples corresponded to the distribution of the whole subset of ticks (Figure 3B). B. miyamotoi was detected in six DNA samples, of which two were I. ricinus (0.2% 2/1044) and four I. persulcatus (0.4% 4/994). All of the B. miyamotoi-positive ticks were adults collected from southwestern Finland, central Finland and the coast of the Bothnian Bay (Figure 3C). Two ticks, both I. persulcatus, were co-infected with B. burgdorferi s.l. and B. miyamotoi.
Table 3. Prevalence (%) of the studied pathogens in I. ricinus and I. persulcatus samples.
|
Number (%) of samples positive for
B. burgdorferi
s.l. |
Number (%) of samples positive for
B. miyamotoi |
Number (%) of samples positive for TBEV |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R | P | Total | R | P | Total | R | P | Total | |
| Total | 148 (14.2) | 197 (19.8) | 345 (16.9) | 2 (0.2) | 4 (0.4) | 6 (0.3) | 2 (0.2) | 30 (3.0) | 32 (1.6) |
| Sex | |||||||||
| Female | 99 (13.1) | 138 (19.0) | 237 (16.0) | 2 (0.3) | 1 (0.1) | 3 (0.2) | 2 (0.3) | 23 (2.3) | 25 (1.7) |
| Male | 37 (17.3) | 57 (22.9) | 94 (20.3) | 0 | 3 (1.2) | 3 (0.6) | 0 | 7 (2.8) | 7 (1.5) |
| Stage | |||||||||
| Adult | 137 (14.1) | 195 (20.0) | 332 (17.1) | 2 (0.2) | 4 (0.4) | 6 (0.3) | 2 (0.2) | 29 (3.0) | 32 (1.6) |
| Nymph | 11 (15.1) | 2 (11.8) | 13 (14.3) | 0 | 0 | 0 | 0 | 1 (5.6) | 1 (1.1) |
| Larva | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: I. persulcatus, P; I. ricinus, R; tick-borne encephalitis virus, TBEV.
Of 2038 screened RNA samples, 32 (1.6%) were TBEV positive (Table 3). The prevalence of TBEV was higher for I. persulcatus (3.0% 30/994) than for I. ricinus (0.2% 2/1044). One of the positive I. persulcatus samples was a nymph, but all others were adult ticks. TBEV-positive samples were collected from coastal areas in the Bothnian Bay, eastern Finland and south-central Finland (Figure 3D). Eight ticks (two males and six females), all I. persulcatus, were co-infected with TBEV and B. burgdorferi s.l.
DISCUSSION
Crowdsourcing is utilized relatively infrequently to solve scientific issues and gather data in health-related research.5 Using this novel method of collecting citizen-contributed samples, we succeeded in constructing a large and geographically comprehensive collection of ticks, the Tickbank. Using the collected material, we investigated the distribution of two tick species, I. ricinus and I. persulcatus, and the prevalence of tick-associated pathogens in Finland. Compared with the previous nationwide distribution map drawn according to a survey in Finland almost 60 years ago,19 the extent of spatial distribution for ticks has shifted 200–300 km northwards and populations have become established in new locations, mainly in coastal areas of the Bothnian Bay and in the eastern part of central Finland. Most of the ticks received were from the coastlines and around Finnish Lakeland, perhaps because of the dry continental climate elsewhere that is suboptimal for ticks. The northernmost tick samples were from latitudes of 67° N. However, only a few ticks were received from this latitude, thus one may speculate whether they came from stable populations or may be stragglers that were transported there by migratory birds, cervids or pet animals.
The observed extension in tick distribution in our study is in accordance with other studies conducted in Europe. Climate change is thought to be a major factor driving changes in tick distribution and abundance, through milder winters and extended growing seasons in the northern hemisphere, faster tick developmental rates and changes in the abundance of host animals.2, 3, 20, 21, 22, 23, 24, 25, 26 In Finland, the increase in the temperature has been remarkably rapid since the late 1960s,27 and at the same time, the ticks’ host animals have become more abundant.28, 29, 30, 31, 32, 33 However, this study and the survey conducted in 1956–195819 are not entirely comparable, due to different extents and methods used to determine the tick distribution (unselected vs. selected sampling).
The majority of received ticks were I. ricinus collected from urbanized areas in southern Finland, likely due to a higher human population density. However, I. persulcatus is now also widely established in Finland and is even more abundant than I. ricinus in certain areas. For instance, in northern Finland, I. persulcatus is clearly the dominant tick species. Previous studies suggest that I. persulcatus is more cold-resistant than I. ricinus,34 and hence could potentially survive better in the north. In contrast, all the samples from the southern coast of Finland were I. ricinus. However, I. persulcatus can be found in corresponding latitudes in Russian Karelia35 and even further south in Estonia and Latvia.36 This observation may be related to tick reproduction. In principle, I. ricinus and I. persulcatus can interbreed, but the offspring are sterile.37 Thus, it may be difficult for one species to gain ground in a new area where the other species is already established. This could partly explain why there are no established populations of I. persulcatus in the southern coast of Finland, where I. ricinus has long been abundant. In addition, possible species-specific landscape and biotopic preferences, different seasonal activity patterns, and other biological characteristics may have an influence. The exact reasons for the dominance of I. ricinus, and lack of I. persulcatus, in southern Finland remain unknown.
Over twice as many females as males were collected, with similar proportions for both species. Most of the collected samples were adults, probably due to the better visibility of adults and longer questing periods of adult females compared to nymphs and larvae. I. ricinus samples contained relatively more young developmental stages (nymphs and larvae) than I. persulcatus samples. According to previous observations, I. ricinus commonly attaches to people at the nymphal stage, whereas I. persulcatus prefers to do so at the adult stage.15, 38 Furthermore, in our study, I. persulcatus was collected from humans five percentage points more often. However, the most commonly reported host for both tick species was dog. I. persulcatus was detected from dogs eight percentage points more often, whereas I. ricinus was detected from cats over ten percentage points more often. This observation may be related to the different outdoor activity habits of cats compared to dogs and humans. However, samples collected from the reported individual host could include largely varying numbers of ticks, both attached and unattached, which could cause a bias in the frequencies of the reported host animals. Further studies of possible differences related to host animal preferences of I. ricinus and I. persulcatus are needed.
I. ricinus were collected throughout the summer months, whereas I. persulcatus were collected mostly during early summer, especially in May. Previous studies have shown that the seasonal activity of I. ricinus adults and nymphs is mainly two-peaked, whereas I. persulcatus adults have only one spring activity peak and are found to be questing only until July.34, 39
The subset of 2038 ticks selected for the pathogen screening represented the whole tick collection in terms of collection site, sex and developmental stage distribution, and reported hosts. However, due to our sampling method, a higher proportion of I. ricinus samples collected in May and June was analyzed for pathogens (84.1%) compared to their proportion in the whole Tickbank (60.5%).
Of the 1044 analyzed I. ricinus and 994 I. persulcatus ticks, 148 (14.2%) and 197 (19.8%) were positive for B. burgdorferi s.l., respectively. The results of the previous studies of Borrelia prevalence conducted in Europe vary among years and according to the methods used. In a meta-analysis from Europe, the prevalence of B burgdorferi s.l. in I. ricinus adults was 18.6%.40 Furthermore, there are great variations in tick infection rates within the country: in a study conducted in southwestern Finland in 2015, B. burgdorferi s.l. was detected in 23.5% of adult I. ricinus ticks,6 but a prevalence of up to 55% was reported in a study conducted in recreational parks in Helsinki in 1999.41 When investigating the prevalence of B. burgdorferi s.l. in the sympatric region only (excluding samples from the north and the southern coast of Finland), a lower prevalence was still observed for I. ricinus than for I. persulcatus adults. Shipment ID as a clustering factor was also found to influence prevalence, meaning that positive samples were correlated with the same sender. Our finding of a higher prevalence of B. burgdorferi s.l. in I. persulcatus than in I. ricinus ticks has also been observed in previous studies conducted in sympatric regions.15, 16, 17 As expected,40 the prevalence of B. burgdorferi s.l. appears to be higher in adults (17.1%) than in nymphs (14.3%) in the current study. This is the first report of B. miyamotoi in ticks from mainland Finland. B. miyamotoi was found in 6 out of 2038 (0.3%) ticks, which is approximately in accordance with the results of studies conducted in neighboring countries.11, 42, 43
The overall prevalence of TBEV was 1.6%. A TBEV prevalence of 0.2–2.0% has been reported in questing ticks in TBE-endemic areas in Europe.44 However, the annual prevalence of TBEV in ticks even in one site can vary remarkably.39 As the transmission cycle of TBEV is fragile, microclimatic conditions may affect its survival in nature45 and thus TBEV might not be distributed equally. This was observed also in our study, with positive samples aggregating in clear clusters. Moreover, some of the positive samples were correlated to the same collectors and collection sites. All but one of the TBEV-positive ticks were adults, probably due to the relatively small number of nymphs. As in the case of B. burgdorferi s.l., a higher TBEV prevalence was observed in I. persulcatus (1.6%) than in I. ricinus (0.2%), which has also been found in previous studies.39
In addition to previously known tick endemic areas, we received TBEV-positive ticks from areas where only sporadic TBE cases have been reported. Four samples were obtained from the Tampere region in the middle of southern Finland, suggesting a new TBEV endemic focus. This new focus area is inhabited by over 350 000 citizens and thus could pose an emerging threat to the local human population. Interestingly, none of the ticks from the southern and southwestern coasts of Finland were positive for TBEV, where the majority of human TBE cases in Finland are reported. This may be related to the clustered nature of TBEV distribution, as well as the low expected prevalence of the virus in nature; in other words, coincidence.
Although crowdsourcing is an effective method for gathering data, it inevitably has some limitations that can affect the generalization and reliability of the results. Because the samples are gathered by citizens instead of professional scientists, we cannot be sure of the reliability of all the collection information, such as the exact collection site or date. Moreover, it is possible that volunteer citizens are especially interested in ticks, which could cause a bias in the amount of ticks collected in certain areas. However, with nearly 7000 shipments received from all over the country, we expect that not only those especially interested in ticks participated, even when some of the shipments were received from the same sender. For ecological research, the collection is definitely biased by the proportion of different tick life stages. It is also obvious that most of the samples were received from the highly populated areas of Finland. However, a substantial proportion of the tick samples was also received from the sparsely inhabited areas, such as eastern Finland. Thus, we can conclude that the areas from where we received B. burgdorferi s.l.-infected ticks are obvious risk areas to obtain LB, but we cannot exclude the possibility that there are infected ticks in those areas that appear ‘white’ on our map. Interestingly, the map drawn from the incidence of microbiologically confirmed LB infections in Finland in 201546 substantially overlaps our map of tick distribution, further strengthening the idea that the main tick distribution areas presented in this study are indeed the areas of high risk for LB.
In the present study, we report the first results from this unique national tick collection. Ten percent of the tick samples in the Tickbank were analyzed in this study, and this subset of 2038 samples gives us a reliable overview of B. burgdorferi s.l. and TBEV prevalence in Finnish ticks. Ongoing global climate change is expected to cause more changes in tick abundance and distribution patterns in future years, along with changes in tick-borne pathogen diversity and prevalence. To investigate temporal changes in tick distribution and pathogen diversity, a new collection of tick samples will be necessary in the future. However, for now, the Tickbank offers an exceptionally comprehensive overview of ticks and tick-borne pathogens in Finland. Finally, our tick assemblage offers a significant perspective on the emergence of rare and new potentially dangerous pathogens that would go undetected in a smaller collection effort.
Acknowledgments
We thank Jorma Nurmi (Department of Biology, University of Turku) for providing coordinates for tick distribution maps, Heidi Isokääntä (Department of Medical Microbiology and Immunology, University of Turku) for technical assistance with DNA/RNA extractions, Otto Glader (Department of Medical Microbiology and Immunology, University of Turku) for helping with the pathogen PCR runs, Julia Geller (Department of Virology, National Institute of Health Development, Tallinn, Estonia) for providing us with a positive B. miyamotoi control, and Elina Tonteri (Department of Virology, University of Helsinki, Helsinki, Finland) and Anu Jääskeläinen (Department of Virology, University of Helsinki, Helsinki, Finland) for providing TBEV strains for positive controls. This work was supported by the University of Turku, Sakari Alhopuro, Pfizer Inc. (Finland), Maj and Tor Nessling Foundation, Jane and Aatos Erkko Foundation, The Varsinais-Suomi Regional Fund of the Finnish Cultural Foundation, and the Academy of Finland.
DISCLAIMER
The funders of the study had no role in the study design, analysis or writing of the report.
Footnotes
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Supplementary Material
References
- Bugmyrin SV, Bespyatova LA, Korotkov YS et al. Distribution of Ixodes ricinus and I. persulcatus ticks in southern Karelia (Russia). Ticks Tick Borne Dis 2013; 4: 57–62. [DOI] [PubMed] [Google Scholar]
- Jaenson TG, Jaenson DG, Eisen L et al. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors 2012; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren E, Talleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ Health Perspect 2000; 108: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talleklint L, Jaenson TG. Increasing geographical distribution and density of Ixodes ricinus (Acari: Ixodidae in central and northern Sweden. J Med Entomol 1998; 35: 521–526. [DOI] [PubMed] [Google Scholar]
- Ranard BL, Ha YP, Meisel ZF et al. Crowdsourcing—harnessing the masses to advance health and medicine, a systematic review. J Gen Intern Med 2014; 29: 187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormunen JJ, Penttinen R, Klemola T et al. Tick-borne bacterial pathogens in southwestern Finland. Parasites Vectors 2016; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivacic L, Reed KD, Mitchell PD et al. A LightCycler TaqMan assay for detection of Borrelia burgdorferi sensu lato in clinical samples. Diagn Microbiol Infect Dis 2007; 57: 137–143. [DOI] [PubMed] [Google Scholar]
- Hovius JWR, de Wever B, Sohne M et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013; 382: 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormunen JJ, Klemola T, Vesterinen EJ et al. Assessing the abundance, seasonal questing activity, and Borrelia and tick-borne encephalitis virus (TBEV) prevalence of Ixodes ricinus ticks in a Lyme borreliosis endemic area in Southwest Finland. Ticks Tick Borne Dis 2016; 7: 208–215. [DOI] [PubMed] [Google Scholar]
- Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol 2003; 27: 136–145. [DOI] [PubMed] [Google Scholar]
- Geller J, Nazarova L, Katargina O et al. Detection and genetic characterization of relapsing fever spirochete Borrelia miyamotoi in Estonian ticks. PLoS One 2012; 7: e51914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonteri E, Jääskeläinen AE, Tikkakoski T et al. Tick-borne encephalitis virus in wild rodents in winter, Finland, 2008–2009. Emerg Infect Dis 2011; 17: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen AE, Tikkakoski T, Uzcátegui NY et al. Siberian subtype tickborne encephalitis virus, Finland. Emerg Infect Dis 2006; 12: 1568–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonteri E, Kipar A, Voutilainen L et al. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the bank vole (Myodes glareolus. PloS One 2013; 8: e81214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevskii YV, Korenberg EI. Differences in Borrelia infections in adult Ixodes persulcatus and Ixodes ricinus ticks (Acari: Ixodidae in populations of north-western Russia. Exp Appl Acarol 1995; 19: 19–29. [DOI] [PubMed] [Google Scholar]
- Alekseev AN, Dubinina HV, Antykova LP et al. Tick-borne borrelioses pathogen identification in Ixodes ticks (Acarina, Ixodidae collected in St. Petersburg and Kaliningrad Baltic regions of Russia. J Med Entomol 1998; 35: 136–142. [DOI] [PubMed] [Google Scholar]
- Korenberg E, Kryuchechnikov V, Kovalevsky YV. Advances in investigations of Lyme borreliosis in the territory of the former USSR. Eur J Epidemiol 1993; 9: 86–91. [DOI] [PubMed] [Google Scholar]
- Stroup WW. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications. Boca Raton, Florida: CRC Press, 2012. [Google Scholar]
- Öhman C. The geographical and topographical distribution of Ixodes ricinus in Finland. Acta Soc Fauna Flora Fennica 1961; 74: 1–38. [Google Scholar]
- Lindgren E, Jaenson TGT. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. In: Menne B, Ebi KL (eds). Climate change and adaptation strategies for human health. Steinkopf Verlag, Darmstadt & World Health Organization, Geneva. 2006. [Google Scholar]
- Dobson AD, Randolph SE. Modelling the effects of recent changes in climate, host density and acaricide treatments on population dynamics of Ixodes ricinus in the UK. J Appl Ecol 2011; 48: 1029–1037. [Google Scholar]
- Jaenson TG, Lindgren E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis 2011; 2: 44–49. [DOI] [PubMed] [Google Scholar]
- Rizzoli A, Hauffe HC, Tagliapietra V et al. Forest structure and roe deer abundance predict tick-borne encephalitis risk in Italy. Plos One 2009; 4: e4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Dautel H, Estrada-Pena A et al. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis 2009; 2009: 593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Bormane A et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013; 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine D, Roe R. Biology of Ticks. 2nd ed. New York, USA: Oxford University Press, 2014. [Google Scholar]
- Mikkonen S, Laine M, Mäkelä H et al. Trends in the average temperature in Finland, 1847–2013. Stoch Environ Res Risk Assess 2015; 29: 1521–1529. [Google Scholar]
- Burbaitė L, Csanyi S. Roe deer population and harvest changes in Europe. Estonian J Ecol 2009; 58: 169–180. [Google Scholar]
- Gripenberg U, Nygren T. Methods in Chromosome Studies in the Scandinavian moose (Alces alces). Sweden: Swedish Wildlife Research. 1987. [Google Scholar]
- Helle E, Kauhala K. Distribution history and present status of the raccoon dog in Finland. Ecography 1991; 14: 278–286. [Google Scholar]
- Kekkonen J, Wikström M, Brommer JE. Heterozygosity in an isolated population of a large mammal founded by four individuals is predicted by an individual-based genetic model. PLoS One 2012; 7: e43482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson R, Jaenson TGT, Gardulf A et al. Prevalence of Borrelia burgdorferi sensu lato infection in Ixodes ricinus in Sweden. Scand J Infect Dis 1995; 27: 597–601. [DOI] [PubMed] [Google Scholar]
- Lavsund S, Nygrén T, Solberg EJ. Status of moose populations and challenges to moose management in Fennoscandia. Alces 2003; 39: 109–130. [Google Scholar]
- Tokarevich N, Tronin A, Blinova O et al. The impact of climate change on the expansion of Ixodespersulcatus habitat and the incidence of tickborne encephalitis in the north of European Russia. Global Health Action 2011; 4: 8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen AE, Sironen T, Murueva GB et al. Tick-borne encephalitis virus in ticks in Finland, Russian Karelia and Buryatia. J Gen Virol 2010; 91: 2706–2712. [DOI] [PubMed] [Google Scholar]
- Katargina O, Russakova S, Geller J et al. Detection and characterization of tick-borne encephalitis virus in Baltic countries and eastern Poland. PloS One 2013; 8: e61374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov YS, Grigor'eva LA, Oliver J. Reproductive isolation and interspecific hybridization in ixodid ticks of the Ixodes ricinus-Ipersulcatus group (Acarina, Ixodidae). Entomol Rev 1998; 77: 713. [Google Scholar]
- Süss J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine 2003; 21: 19–35. [DOI] [PubMed] [Google Scholar]
- Bormane A, Lucenko I, Duks A et al. Vectors of tick-borne diseases and epidemiological situation in Latvia in 1993–2002. Int J Med Microbiol Suppl 2004; 293: 36–47. [DOI] [PubMed] [Google Scholar]
- Rauter C, Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodesricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol 2005; 71: 7203–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila J, Peltomaa M, Soini H et al. Prevalence of Borreliaburgdorferi in Ixodesricinus ticks in urban recreational areas of Helsinki. J Clin Microbiol 1999; 37: 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgoiakov VI, Fomenko NV, Panov VV et al. Study on the infection of taiga ticks with Borrelia in the territory of Novosibirsk Scientific Center SB PAS. Parazitologiia 2010; 44: 543–556. [PubMed] [Google Scholar]
- Wilhelmsson P, Fryland L, Borjesson S et al. Prevalence and diversity of Borrelia species in ticks that have bitten humans in Sweden. J Clin Microbiol 2010; 48: 4169–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss J, Schrader C, Abel U et al. Characterization of tick-borne encephalitis (TBE) foci in Germany and Latvia (1997-2000). Int J Med Microbiol 2002; 291: 34–42. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Rogers DJ. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc Biol Sci 2000; 267: 1741–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Welfare. Borrelian esiintyvyys 2015. Helsinki, Finland: THL. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.