Abstract
Background
Many patients with X-linked hypophosphataemic rickets (X-LHPR) demonstrate significant lower limb deformity despite optimal medical management. This study evaluates the use of guided growth by means of hemi-epiphysiodesis to address coronal plane deformity in the skeletally immature child.
Methods
Since 2005, 24 patients with X-LHPR have been referred to our orthopaedic unit for evaluation. All patients had standardised long leg radiographs that were analysed sequentially before and after surgery if any was performed. The rate of correction of deformity was calculated based on peri-articular angles and diaphyseal deformity angles measured at regular intervals using Traumacad software. Clinical records were reviewed to obtain relevant clinical and demographic details. Statistical analysis was performed using SPSS 23 (SPSS Inc., Chicago, IL, USA).
Results
The indication for surgical intervention was a mechanical axis progressing through Zone 2 or in Zone 3 despite one year of optimised medical treatment. The 15 patients underwent 16 episodes of guided growth (30 limbs, 38 segments) at a mean age of 10.3 years. In four limbs, surgery has only taken place recently; and in three limbs, correction is ongoing. Neutral mechanical axis was restored in 16/23 (70%) limbs: six improved and one limb (one segment) required osteotomy for residual deformity. The mean rate of angular correction per month was 0.3° for the proximal tibia and 0.7° for the distal femur. Patients with ≥ 3 years of growth remaining responded significantly better than older patients (p = 0.004). Guided growth was more successful in correcting valgus than varus deformity (p = 0.007). In younger patients, diaphyseal deformity corrected at a rate of 0.2° and 0.6° per month for the tibia and the femur, respectively. There has been one case of recurrent deformity. Patients with corrected coronal plane alignment did not complain of significant residual torsional malalignment. Serum phosphate and alkaline phosphatase levels did not affect response to surgery.
Conclusions
Guided growth is a successful, minimally invasive method of addressing coronal plane deformity in X-LHPR. If coronal plane deformity is corrected early in patients with good metabolic control, osteotomy can be avoided.
Keywords: Guided growth, hypophosphataemic rickets, lower limb deformity
Introduction
Following the initial reports of vitamin D resistant rickets by Albright et al1 in 1937, much progress has been made in understanding the genetic and pathological processes involved in this disorder, which affects approximately 1 in 20 000 births.2 Eighty percent of cases are inherited in an X-linked fashion with the remainder resulting from autosomal recessive inheritance or spontaneous mutations. The mutation leading to the clinical picture of hypophosphataemic rickets (X-LHPR) affects the PHEX (phosphate regulating gene homologous to endopeptidases on the X-chromosome) gene and leads to increased production of fibroblast growth factor-23 (FGF-23).3,4 Elevated levels of FGF-23 in turn lead to decreased phosphate reabsorption in the proximal renal tubule as well as decreased renal production of 1,25 (OH)2Vit-D.3,5
The biochemical picture of X-LHPR is that of low serum phosphate, elevated urinary phosphate, increased alkaline phosphatase (ALP) levels and low or low normal circulating 1,25 (OH)2Vit-D. Parathyroid hormone (PTH) levels are usually normal on presentation but need to be monitored throughout growth.5
Patients present with delayed linear growth and lower limb deformity. In cases of high clinical suspicion or if the genetic cause is known, the diagnosis can be made in infancy.3
The typical skeletal manifestations include genu varum or valgum and radiographic features of rickets including generalised osteopaenia, widened, irregular physes and cupped and flared metaphyses (Fig. 1).
Fig. 1.
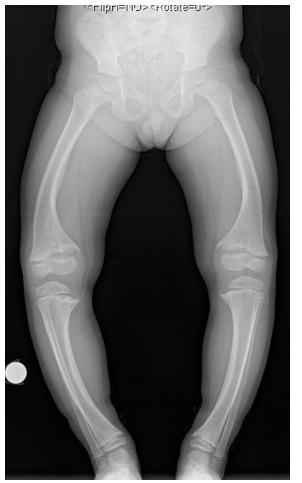
Anteroposterior standing leg alignment radiographs of a four-year-old girl with X-linked hypophosphataemic rickets after 2.2 years of medical treatment.
Historically, patients frequently presented late or were initially misdiagnosed, and treatment failed to achieve the goals of limiting or improving skeletal deformity. Osteotomies performed early in childhood were associated with a high rate of recurrent deformity.6,7 More recently, with earlier and better medical care, not all patients develop severe deformity. This, combined with the development of guided growth techniques, suggests that extensive surgery and the associated risks may be avoidable. At our institution, the use of eight-plate (Orthofix, Verona, Italy) hemi-epiphysiodesis for guided growth in these patients was introduced ten years ago as the preferred primary surgical treatment. This study evaluates the effect of this technique on coronal plane lower limb deformity in X-LHPR and determines which factors influence the patient’s response to surgical treatment.
Patients and methods
A search of the electronic database at our institution identified 24 patients suffering from X-LHPR who were referred to the orthopaedic clinic for evaluation of their lower limb deformity. The clinical records and radiographs of these patients were obtained and demographic data gathered, including clinical and laboratory findings, deformity change over time, type and timing of surgical interventions and response to surgery.
All patients completed at least 12 months of optimised medical treatment consisting of phosphate and 1-alfacalcidol supplementation before being considered for surgery (Fig. 2).6 Patients were reviewed every three months, more frequently if necessary. The biochemical treatment goals were normalisation of plasma PTH and ALP levels: subnormal plasma phosphate levels were accepted to avoid high dose phosphate supplementation. The clinical goal was an improvement in linear growth and deformity correction, concurrent with radiological healing.
Fig. 2.
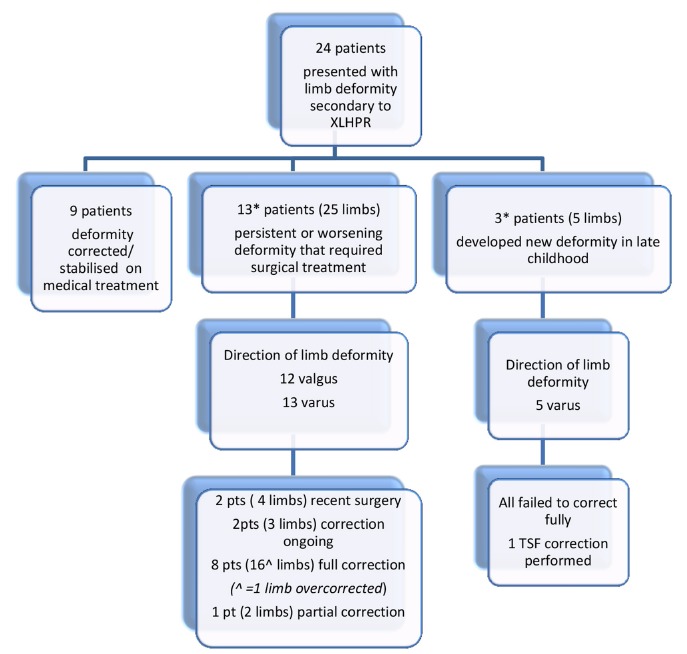
Flow diagram that details management of this patient cohort. *One patient underwent two episodes of treatment: left leg varus and subsequently right leg varus.
Our patients underwent regular standardised long leg alignment radiographs (Fig. 1) at ages 5, 8, 10 and 12 years, in addition to those performed for a specific clinical indication. On each occasion, for each limb, the mechanical axis was determined according to Stevens’ method;8 an axis outside Zone 1 at knee joint level (Fig. 3) was considered abnormal. A mechanical axis in Zone 3 or a progressively deteriorating Zone 2 alignment, despite 12 months of optimised medical management, was an indication for surgery.
Fig. 3.
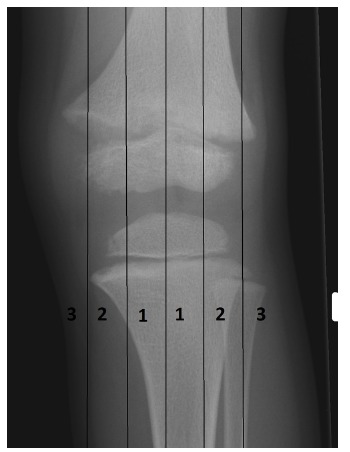
Diagram of the knee demonstrating that a mechanical axis may pass medial or lateral to the centre of the knee joint or indeed pass outside the knee joint. The degree of displacement of the mechanical axis can be defined in terms of Zones 1, 2 and 3 (medial or lateral). An axis within either medial or lateral Zone 1 is considered to be within normal limits: surgically induced guided growth defined central Zone 1 as fully corrected.
Sagittal plane deformity was evaluated clinically and radiographs were only taken when the deformity was deemed to be contributing significantly to gait disturbance or cosmetic deformity. In our patient cohort, sagittal plane deformity was not a significant clinical concern in any patient at the time of initial surgery; therefore, only the coronal plane deformity was addressed and reported on.
The pre-operative radiograph defines the site(s) of deformity and orientation of the knee joint axis to allow selection of the ideal site(s) for eight-plate placement. Diaphyseal deformity was not addressed directly.
For each patient, the long leg radiographs taken at regular intervals during surveillance and treatment periods were analysed for the following parameters: mechanical axis deviation (MAD); the mechanical lateral proximal femoral angle (mLPFA); the mechanical lateral distal femoral angle (mLDFA); the mechanical medial proximal tibial angle (mMPTA); and the mechanical lateral distal tibial angle (mLDTA). The peri-articular angles were measured using Traumacad software (TraumaCAD, Voyant Health, Tel Aviv, Israel) (Fig. 4). Following guided growth surgery, alignment films were taken within one month of surgery and at intervals of four to six months thereafter as the deformity corrected. These radiographs determined the rate of correction. In order to quantify the diaphyseal deformity in the coronal plane, the centre of rotation of angulation (CORA) was determined and the deformity recorded (Fig. 5).
Fig. 4.
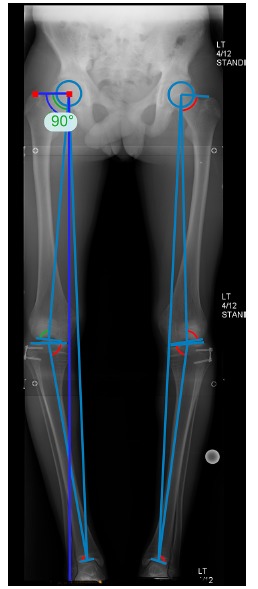
Anteroposterior standing long leg radiographs of a 12-year-old by showing the measurements made by the TraumaCad software (Voyant Health, Tel Aviv, Israel).
Fig. 5.
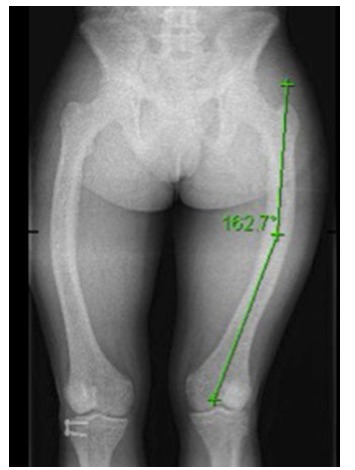
Anteroposterior view of the left femur of a skeletally mature girl with the diaphyseal deformity of measured at 18°.
In skeletally immature patients, eight plates were removed once the deformity corrected to central Zone 1 (i.e. Zone ‘0’). Clinical and radiographic follow-up was continued until skeletal maturity.
For analysis, patients were divided into two groups depending on their age at the time of surgery for guided growth. The young cohort were patients that were > 3 years from skeletal maturity (< 11 years in girls, < 13 years in boys). The older cohort were < 3 years from skeletal maturity at the time of surgery.
Statistical analysis was performed using SPSS 23 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used for analysis of continuous data and Fisher’s exact test for categorical data. Categorical variables are expressed as frequency (percent) and continuous variables are expressed as mean (range), unless otherwise stated. Statistical significance was considered at p < 0.05.
Results
The presenting features of this patient cohort are summarised in Tables 1 and 2. Fourteen of our patients started medical treatment before the age of 2 years, and none after the age of 3 years. Compliance with medical treatment was defined as attendance of three out of the four planned renal clinics per year.9
Table 1.
Clinical variables of the 24 patients included in the study.
| Patients (n) | 24 (48 limbs) |
|---|---|
Gender
|
|
| Mean age at initiation of medical therapy | 1 year 6 months (1 month to 3 years) |
| Known family history | 16 (66%) |
PHEX mutation
|
|
Direction of deformity (limbs)
|
|
Treatment
|
|
Table 2.
Comparison between patients that underwent guided growth more or less than three years prior to skeletal maturity.
| Younger cohort | Older cohort | |
|---|---|---|
| Patients (n (limbs)) * | 8* (15) | 8* (15) |
Gender
|
4 4 |
4 4 |
| Mean age at initiation of medical therapy (mths) | 16 | 24 |
| Known family history | 3 (37.5%) | 6 (75%) |
PHEX mutation
|
4 2 2 |
5 2 1 |
Direction of deformity
|
9 6 |
9 6 |
| Mean age at surgery | 7 years 6 months | 13 years 0 months |
One patient had two separate episodes of surgery for guided growth, one at nine years and the other at 15 years
In all patients, serum phosphate levels were low (normal range 1.2 to 1.8 mmol/L) throughout their clinical course. Metabolic control and compliance to medical treatment was good in all our patients and we can therefore not comment on the effect of poor metabolic control on the response to guided growth. The presence of a mutation of the PHEX gene did not predict requirement for surgery (NS; Fisher’s exact test). Similarly, neither gender nor a positive family history were predictors for deformity requiring surgery.
In total, 15 patients (62.5%) had deformity severe enough to warrant surgical intervention. In total, 38 limb segments (21 distal femora and 17 proximal tibiae) were treated in 30 limbs; 12 limbs were in valgus and 18 in varus. The mean age at treatment was 10.3 years (4.8 to 14.75). One patient underwent two surgical procedures: initially a unilateral varus deformity was corrected, and then six years later he developed a contralateral deformity which required treatment. All other patients underwent bilateral treatment: in all limbs, the deformity was in the same direction although severity was sometimes asymmetrical.
Two patients (four limbs) underwent surgery within the last three months and therefore have been excluded from further analysis. In the remaining 13 patients (26 limbs), there have been no significant complications following surgery but one prominent screw required early revision.
In 3/26 limbs, deformity correction is ongoing. Fifteen of the remaining 23 limbs (65%) have achieved a neutral limb alignment with a mean correction time of 16 months (9 to 27) (Fig. 6). Seven limbs in four patients improved, but not sufficiently to restore a neutral axis at skeletal maturity. These patients all had varus deformities and all were in the older cohort. One limb (one segment) has required further surgery to address residual deformity and was successfully treated by means of gradual correction with a Taylor Spatial Frame (Smith & Nephew, TX, USA). The median follow-up for patients who have not yet reached skeletal maturity is 48 months (8 to 90).
Fig. 6.
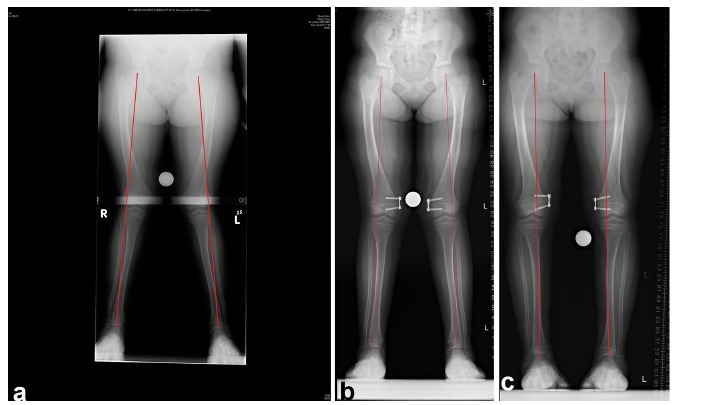
(a)Long leg standing films of a ten-year-old girl with bilateral genu valgum and a mechanical axis in Zone 2. (b) Four months after insertion of medial distal femoral eight plates. The mechanical axis is still in Zone 2, but improving. (c) Mechanical axis in Zone 1 ten months after insertion of eight plates.
One patient treated successfully for bilateral varus knees was overcorrected to Zone 2 on one side at plate removal: this has not improved at two-year follow-up. In the corrected group, there has been no case of recurrent deformity in the ten limbs that have reached skeletal maturity. One patient has developed bilateral recurrent varus deformity at the age of ten years, four years after plate removal (Fig. 2).
Table 3 contains a summary of the rate of correction (degrees/month) for all measured indices following application of eight plates for guided growth for the two age groups. Overall, femoral indices improved at a faster rate than tibial indices, in keeping with the growth rates of the respective physes.10 Use of this technique around the knee resulted in an improvement in abnormal peri-articular angles at the hip and ankle at a rate similar to that in the proximal tibia.
Table 3.
Rate of correction of all physes in patients who had surgery around the knee for guided growth.
| Angle | Mean rate of correction for all patients (degrees/mth) (range) | Mean rate of correction in young cohort (degrees/mth) | Mean rate of correction in older cohort (degrees/mth) |
|---|---|---|---|
| mLPFA | 0.3 (0 to 1.1) | 0.4 | 0.11 |
| mLDFA | 0.6 (0 to 1.7) | 0.8 | 0.32 |
| Operated * | 0.7 (0.1 to 1.7) | 0.8 | 0.35 |
| Non-operated | 0.3 (0 to 0.64) | n/a | 0.30 |
| mMPTA | 0.3 (0 to 1.4) | 0.46 | 0.19 |
| Operated * | 0.3 (0 to 1.4) | 0.6 | 0.2 |
| Non-operated | 0.31 (0 to 0.78) | 0.39 | 0.19 |
| mLDTA | 0.4 (0 to 1.6) | 0.48 | 0.21 |
| Femoral diaphyseal bow | 0.3 (0 to 1.4) | 0.62 | 0.07 |
| Tibial diaphyseal bow | 0.2 (0 to 0.8)] | 0.25 | 0.07 |
Operated physes refers to the physes (distal femoral or proximal tibial) to which the eight-plate was applied
Patients undergoing surgery for guided growth three or more years prior to skeletal maturity had a higher rate of successful mechanical axis restoration (10/11 (90%) vs 5/15 (33%); Fisher’s exact test, p = 0.004). Significantly greater rates of correction at the operated physes were seen in the younger age group in comparison to those undergoing surgery less than three years prior to skeletal maturity (Mann-Whitney U test, p = 0.007). Table 4 summarises the time to correction of deformity for the respective groups.
Table 4.
Time to correction in successfully treated patients (months).
| Young cohort | Old cohort | |
|---|---|---|
| Varus limbs | 14 | n/a: all failed to correct |
| Valgus limbs | 11.8 | 16.5 |
Of the 23 limbs that have completed correction, those with a varus deformity had less satisfactory outcome with guided growth than those with valgus deformity. Eight out of 15 (53%) varus limbs were considered fully corrected at the end of treatment compared with eight out of eight (100%) of valgus limbs (Fisher’s exact test p = 0.004). Overall, the age at time of surgery was similar for the varus (ten years) and the valgus (10.5 years) groups, but five of seven varus limbs in four patients that failed to correct had been straight until the sudden development of lower limb deformity at the time of the adolescent growth spurt.
Discussion
The literature suggests that 24% to 65% of patients will require surgical intervention for lower limb deformity resulting from X-LHPR, despite optimal medical treatment.11 This fact was confirmed by our study where 62.5% had a persistent or worsening coronal plane deformity that merited surgery. The presence of a defined mutation on the PHEX gene did not predict requirement for surgery, nor did gender or family history. Serum phosphate levels were below normal levels in all patients and therefore not predictive of requirement for surgery. Serum phosphate levels below 2.5 mg/dL (0.81 mmol/L) have been reported to be predictive of worse outcome after surgery;12 but this was not reflected in our cohort.
Historically, at our institution and elsewhere, surgery for rachitic bone consisted of osteotomy, preferably at skeletal maturity, using a variety of internal and external fixation devices. Published results of corrective osteotomies for these patients are varied and consist mostly of small case series reporting on a variety of surgical techniques.6,12-19 Reported recurrent deformity is in the range of 0% to 90%6,16-18 and appears to be related to age at time of surgery and the fixation method used. External fixation over an intramedullary rod appears to have reliably fair outcome,12,15,19 although recurrent deformity above and below the intramedullary device has been noted by some authors in a large proportion of patients.16 Patients who had surgery before skeletal maturity generally had a higher rate of recurrent deformity, and several authors also emphasised the importance of medical compliance, both for maintenance of correction and prevention of complications such as non-union.6,12-14,17
Little has been published on the use of guided growth for patients with hypophosphataemic rickets. Evans et al13 employed stapling of the medial femoral physes for correction of valgus deformity in one patient nearing skeletal maturity, but performed osteotomies for all varus deformities. Gigante et al20 used guided growth in seven patients with renal osteodystrophy and corrected all deformities, but three recurred and required further surgery. Novais and Stevens11 showed full or partial deformity correction in 7/9 X-LHPR patients treated by hemi-epiphysiodesis. Most complications were related to staple migration and the failures were seen in adolescent patients nearing the end of growth. We had similar results with this intervention, with 7/30 limbs not corrected fully, but only one that required further surgery. We agree with these authors that younger age at surgery predicts a better response to guided growth.11 We furthermore suggest that valgus deformity generally responds better to guided growth than varus deformity; particularly the adolescent onset varus deformity. Recurrent deformity has not been a significant concern in our patients but we would not consider it a failure of management as we still believe that simple treatment, even if repeated, may be more acceptable than an osteotomy. Our single overcorrected patient remained overcorrected at skeletal maturity, two years after plate removal. Due to this, and the relative lack of recurrence in the corrected patients, we do not recommend overcorrection with the guided growth technique.
One of the theoretical benefits of guided growth for deformity correction is that the deformity is addressed at its site of origin, the pathological physes. The peri-articular deformity corrected at a rate of 0.3° and 0.7° per month for the mMPTA and mLDFA, respectively. Interestingly, femoral and tibial diaphyseal bowing also improved, particularly in the younger children. Presumably, normalisation of loads across the physes would eliminate the ongoing imbalance in relative growth, according to the Heuter-Volkmann Principle, and thereby lead to gradual correction of the meta-diaphyseal deformity. This phenomenon was also observed by Novais and Stevens.11 Stevens and Klatt,7 in their series of 14 patients with pathological physes, observed that the appearance of the physes at the hip and the ankle normalised once the mechanical axis was restored by means of guided growth. We did not specifically evaluate the appearance of the physes, but we did note that that the mLPFA and the mLDTA improved or normalised with the application of eight plates around the knee. By restoring joint orientation around the hip, theoretically hip abductor function should improve with resolution of the waddling gait typically seen in these patients. Restoration of normal joint alignment at the knee diminishes the abnormal strain placed on the collateral ligaments in a varus or a valgus knee, and may decrease the incidence of early arthrosis associated with gross lower limb malalignment.21 In our cohort, although the diaphyseal bowing was not always corrected fully, no patient with a neutral mechanical axis in the coronal plane has requested surgical correction of a torsional deformity. The need for osteotomy with all its concomitant morbidity has been minimised.
The main limitation of this study is the fact that only coronal plane deformity was evaluated and addressed. Deformity in the sagittal and axial plane was not a significant problem in our patients, likely due to the early initiation of medical therapy and good metabolic control. The study is retrospective and does not compare different treatment modalities and the numbers are small.
All analyses were performed using computer software that has previously been verified.22 There are limitations in using a statistical analysis ‘per knee’ as each knee is not a fully independent variable. However, in a rare condition such as this, where the number of data points is limited, a multivariant analysis is not feasible. The analysis performed is the most appropriate option available, although care must be taken not to overestimate significance.
Medical management remains the mainstay of treatment for lower limb deformity in X-LHPR. For those patients that require surgical intervention for resistant or progressive deformity despite optimal medical treatment, guided growth by means of hemi-epiphysiodesis using eight plates is an attractive option. Successful deformity correction is more likely if guided growth is initiated three years or more before skeletal maturity: the adolescent onset tibial varus deformity does not respond well to treatment. Early treatment may facilitate improvement in the diaphyseal bowing.
Acknowledgement
The authors thank Paul Bassett, Statistical Consultant, who provided additional statistical advice.
Compliance with Ethical Standards
Funding Statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical statement
No funding was received for this study.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
As this was a retrospective cohort study, no informed consent was sought. Approval was granted by the Institutional review board.
ICMJE Conflict of interest statement
WvH has received travel and accommodation reimbursement from Ultragenyx and is receiving research funding, paid to his institution. DME receives royalties from the Oxford Textbook of Orthopaedics and Trauma. All other authors declare that they have no conflicts of interest.
References
- 1.Albright F, Butler MA, Bloomberg E. Rickets resistant t vitamin D therapy. Am J Dis Child 1937;54:529-547. [Google Scholar]
- 2.Petersen DJ, Bonface AM, Schranck FW, Rupich RC, Whyte MP. X-linked hypophosphataemic rickets: A study (with literature review) of linear growth response to calcitriol and phosphate therapy. J Bone Miner Res 1992;7:583-597. [DOI] [PubMed] [Google Scholar]
- 3.Pavone V, Testa G, Gioitta Iachino S, et al. Hypophosphatemic rickets: etiology, clinical features and treatment. Eur J Orthop Surg Traumatol 2015;25:221-226. [DOI] [PubMed] [Google Scholar]
- 4.Sharkey MS, Grunseich K, Carpenter TO. Contemporary medical and surgical management of X-linked Hypophosphatemic rickets. J Am Acad Orthop Surg 2015;23:433-442. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 2011;26:1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petje G, Meizer R, Radler C, Aigner N, Grill F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin Orthop Relat Res 2008;466:3078-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens PM, Klatt JB. Guided growth for pathological physes: radiographic improvement during realignment. J Pediatr Orthop 2008;28:632-639. [DOI] [PubMed] [Google Scholar]
- 8.Stevens PM, Maguire M, Dales M, Robins AJ. Physeal stapling for idiopathic genu valgum. J Pediatr Orthop 1999;19:645-649. [PubMed] [Google Scholar]
- 9.Quinlan C, Guegan K, Offiah A, et al. Growth in PHEX-associated X-linked hypophosphatemic rickets: the importance of early treatment. Pediatr Nephrol 2012;27:581-588. [DOI] [PubMed] [Google Scholar]
- 10.Menelaus MB. Correction of leg length discrepancy by epiphyseal arrest. J Bone Joint Surg [Br] 1966;48-B:336-339. [PubMed] [Google Scholar]
- 11.Evans GA, Arulanantham K, Gage JR. Primary hypophosphataemic rickets. Effect of oral phosphate and vitamin D on growth and surgical treatment. J Bone Joint Surg [Am] 1980;62-A:1130-1138. [PubMed] [Google Scholar]
- 12.Novais E, Stevens PM. Hypophosphataemic rickets: the role of epiphysiodesis. J Pediatr Orthop 2006;26:238-244. [DOI] [PubMed] [Google Scholar]
- 13.Choi IH, Kim JK, Chung CY, et al. Deformity correction of knee and leg lengthening by Ilizarov method in hypophosphataemic rickets: outcomes and significance of serum phosphate level. J Pediatr Orthop 2002;22:626-631. [PubMed] [Google Scholar]
- 14.Ferris B, Walker C, Jackson A, Kirwan E. The orthopaedic management of hyphosphataemic rickets. J Pediatr Orthop 1991;11:367-373. [PubMed] [Google Scholar]
- 15.Fucentese SF, Neuhaus TJ, Ramseier LE, Ulrich Exner G. Metabolic and orthopedic management of X-linked vitamin D-resistant hypophosphatemic rickets. J Child Orthop 2008;2:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popkov A, Aranovich A, Popkov D. Results of deformity correction in children with X-linked hereditary hypophosphatemic rickets by external fixation or combined technique. Int Orthop 2015;39:2423-2431. [DOI] [PubMed] [Google Scholar]
- 17.Rubinovitch M, Said S, Glorieux F, Cruess RL, Rogala E. Principles and reuslts of corrective lower limb osteotomies for patients with vitamin-D resistant hypophosphataemic rickets. Clin Orthop Relat Res 1988;237:264-270. [PubMed] [Google Scholar]
- 18.Song HR, Soma Raju VV, Kumar S, et al. Deformity correction by external fixation and/or intramedullary nailing in hypophosphatemic rickets. Acta Orthop 2006;77:307-314. [DOI] [PubMed] [Google Scholar]
- 19.Stanitiski DF. Treatment of deformity secondary to metabolic bone disease using the Ilizarov technique. Clin Orthop Relat Res 1994;301:38-41. [PubMed] [Google Scholar]
- 20.Gigante C, Borgo A, Corradin M. Correction of lower limb deformities in children with renal osteodystrophy by guided growth technique. J Child Orthop 2017;11: 79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan FA, Koff MF, Noiseux NO, et al. Effect of local alignment on compartmental patterns of knee osteoarthritis. J Bone Joint Surg [Am] 2008;90-A:1961-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segev E, Hemo Y, Wientroub S, et al. Intra- and interobserver reliability analysis of digital radiographic measurements for pediatric orthopedic parameters using a novel PACS integrated computer software program. J Child Orthop 2010;4:331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]


