Abstract
α0-thalassemia of SEA deletion (—SEA) is common among Southeast Asian and Chinese. Using haplotype and phylogenetic analyses, we examined the origin of this defect in Southeast Asian populations. Study was done on both normal and α0-thalassemia alleles in 3 ethnic groups including 96 Thai, 52 Laotian and 21 Cambodian. Five SNPs encompassing the (—SEA) including (rs3760053 T>G), (rs1211375 A>C), (rs3918352 A>G), (rs1203974 A>G) and (rs11248914 C>T) were examined using high-resolution melting assays. It was found that 94.0% of Thai, 100% of Laotian and 100% of Cambodian α0-thalassemia alleles were linked to the same haplotype: the haplotype H4 (AAGC), representing an Asian specific origin. An G allele of the (rs3760053) was found to be in strong linkage disequilibrium with the α0-thalassemia allele in these populations. A multiplex PCR assay was developed to detect simultaneously the (—SEA) allele and genotyping of a linked (rs3760053) to improve accuracy of prenatal diagnosis of α0-thalassemia. Application of this multiplex PCR assay for routine prenatal diagnosis of α0-thalassemia in 12 families revealed a 100% concordant result with conventional gap-PCR assay. Therefore, a single genetic origin is responsible for the spread and high prevalence of the (—SEA) in the region. The multiplex PCR assay developed should provide a double-check PCR system for more accurate diagnosis and allow the monitoring of possible maternal contamination at prenatal diagnosis of this important genetic disorder.
Introduction
The Southeast Asian deletion α0-thalassemia (—SEA) is the most common and severe form of α-thalassemia found in Southeast Asia and south China.1, 2 Association of this severe form with a milder form of α+-thalassemia leads to the Hb H disease (—SEA/-α), commonly encountered in the region. Homozygous α0-thalassemia (—SEA /—SEA) can cause severe thalassemia syndrome known as Hb Bart’s hydrops fetalis. Pregnancy with this Hb Bart’s hydrops fetalis has increased risk of severe maternal complications and infant with this syndrome usually die in utero, or soon after birth.3 Prenatal diagnosis of homozygous state of α0-thalassemia is therefore preferable in routine practice. Generally, this can be done by using gap-PCR analysis of fetal tissues collected during the first or second trimesters of pregnancy. Although this is simple, allele dropout or maternal contamination leading to a misdiagnosis can happen and diagnosis using two different methods is usually employed.4, 5, 6 Alternatively, analysis of fetal blood obtained by cordocentesis for Hb Bart’s quantification could also provide accurate diagnosis of the disease.7 However, cordocentasis is not practical and is relatively outdate, DNA analysis of fetal tissues is preferred method.
Qiu et al. reported an evidence of recent natural selection on the α0-thalassemia (SEA deletion) in south China triggered by malaria.8 A single origin of α0-thalassemia (SEA deletion) as characterized by haplotype analysis was found among southern Chinese, the haplotype which was likely constructed from an Asian specific haplotype. Unfortunately, no haplotype study has been documented for other Asian populations with high prevalence of this genetic disorder. Here we reported the results of studying genetic backgrounds of α0-thalassemia (SEA deletion) in Southeast Asian populations using haplotype and phylogenetic analysis. An accurate method for prenatal diagnosis of this α0-thalassemia (SEA deletion) based on simultaneous detection of the SEA deletion and analysis of a linked single-nucleotide polymorphism (SNP) to the deletion was developed.
Materials and methods
Samples
Ethical approval of the study protocol was obtained from the Ethical Committee of Khon Kaen University, Thailand (HE592190). Archival DNA samples with α0-thalassemia at the Centre for Research and Development of Medical Diagnostic Laboratories, Faculty of Associated Medical Sciences, Khon Kaen University, were obtained from the previous study.9 Identification of α0-thalassemia is routinely performed in our laboratory using gap-PCR described elsewhere.9, 10 A total of 169 DNA specimens obtained from 96 Thai, 52 Laotian and 21 Cambodian individuals were recruited. Among 96 Thai individuals, 59 were α0-thalassemia carriers (—SEA/αα), 7 were homozygotic fetuses with Hb Bart’s hydrops fetalis (—SEA /—SEA) and the remaining 30 had wild-type (αα/αα). Of the 52 Laotian individuals, 28 were heterozygous (—SEA/αα) and 24 had wild-type (αα/αα). Cambodian subjects included 7 heterozygous (—SEA/αα) and 14 wild-type (αα/αα).
SNPs genotyping
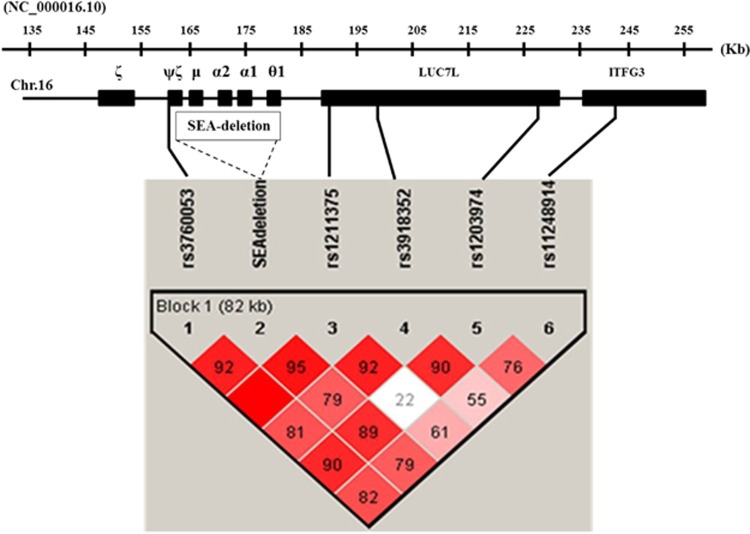
Five SNPs encompassing the SEA deletion α0-thalassemia breakpoints were examined. These SNPs, spanning about 82 kb in length, included a SNP located upstream of the SEA deletion breakpoint (rs3760053 T>G) and four other SNPs located downstream; [(rs1211375 A>C), (rs3918352 A>G), (rs1203974 A>G) and (rs11248914 C>T)] as shown in Figure 1.8 All of them were genotyped using the high-resolution melting (HRM) assays described8 and further confirmed by DNA sequencing. Oligonucleotide primers used in this study were listed in Supplementary Table S1. PCR was carried out using the KAPA HRM FAST PCR kit (KAPA Biosystems, Wimington, MI, USA). HRM analysis was performed on a Light Scanner System (Idaho Technology Inc., Salt Lake City, UT, USA) and the data was analyzed with the Light Scanner System 2.0 Software program (Idaho Technology Inc.).
Figure 1.
Structure of linkage disequilibrium (LD) constructed from 5 SNPs and —SEA deletion α0-thalassemia allele in sample pooled of 338 chromosomes of Southeast Asian origin. Number in each square is the percentage of D′-value between the pair of loci. The SEA deletion α0-thalassemia allele showed strong LD with all single-nucleotide polymorphisms (SNPs) investigated. A full color version of this figure is available at the Journal of Human Genetics journal online.
Data analysis
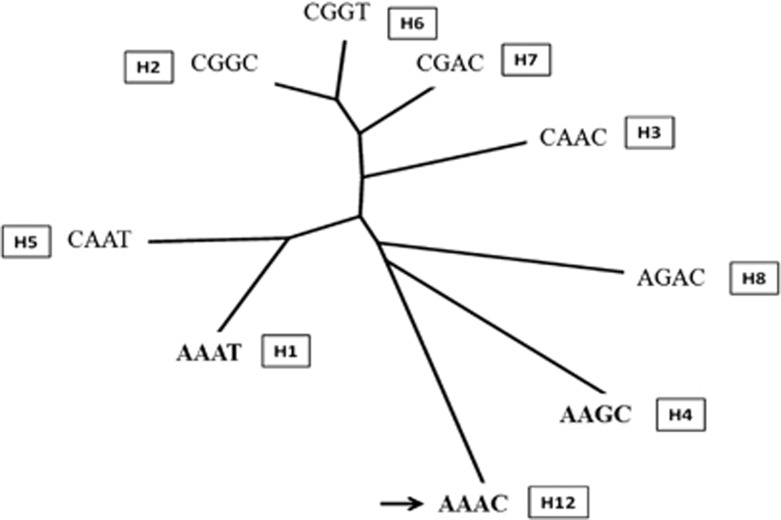
The basic statistics including allele frequency, genotype frequency, minor allele frequency and Hardy–Weinberg Equilibrium (P-value>0.05) were calculated using the PEAS V1.0: a package for elementary analysis of SNP data.11 Haplotype patterns, constructed using data from the four SNPs located downstream of the SEA deletion breakpoint, and pairwise linkage disequilibrium (LD) test were determined using the HAPLOVIEW 4.2 software, which examined haplotypes >1% observed in the study.12 The D′ value of the linkage disequilibrium test was calculated and shown in LD pattern. Using haplotype data, the phylogenetic tree (Figure 2) was generated using the online DendroUPGMA software (http://genomes.urv.cat/UPGMA) applying Jaccard (Tanimoto) coefficient with default settings.13
Figure 2.
Phylogenetics (cladogram) analysis of haplotypes found in Asian populations, constructed using DendroUPGMA software with Cophenetic Correlation Coefficient=0.7759. The H1, H4 and H12 are haplotypes associated with the SEA deletion α0-thalassemia found in Southeast Asian populations and H4 (AAGC) is the most common one. Arrow indicates an ancestral haplotype H12.
Development of multiplex PCR assay for simultaneous detection of α0-thalassemia and genotyping of SNP rs3760053
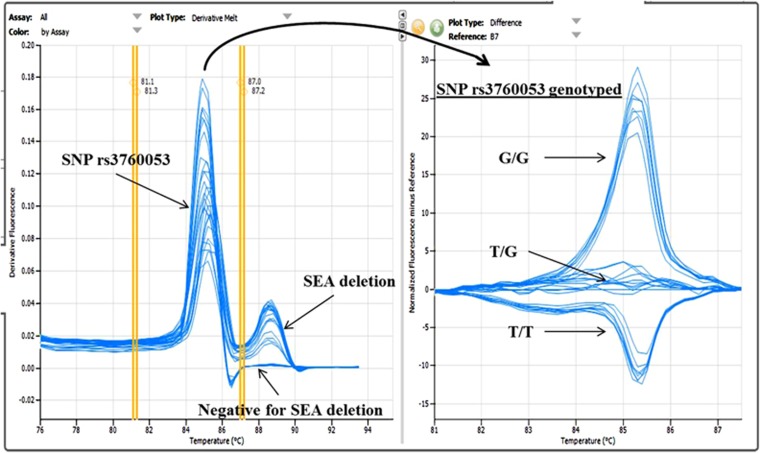
A novel method based on HRM analysis was developed. Two primer pairs with sequences shown in Supplementary Table S1 were designed and used to produce specific amplicons for detecting the SEA deletion α0-thalassemia and genotyping of the SNP rs3760053. Detection of the SEA deletion α0-thalassemia allele was by gap-PCR but genotyping of the (rs3760053) was done by HRM analysis on the Eco Real-Time PCR system (Illumina Co., Ltd., San Diego, CA, USA; Figure 3). The multiplex HRM reaction mixture (20 μl) contained 50–100 ng DNA in a buffer consisting of 60 mmol l−1 Tris HCl, pH 9.0, 40 mmol l−1 2 m KCl, 16 mmol l−1 (NH4)2SO4, 3 mmol l−1 MgCl2, 50 μmol l−1 each dNTP, 480 nmol l−1 of each specific primer for SEA deletion, 900 nmol l−1 of each primer for SNP (rs3760053), 1 μmol l−1 SYBR green and 0.02 units Taq DNA polymerase (Biolabs Co., Ltd., Ipswich, MI, USA). The PCR amplification started with heating at 95 oC (15 min) followed by 32 cycles of (95 °C, 1 min; 60 °C, 1 min; 72 °C, 1 min 20 s). After PCR, HRM analysis was carried out from 55 to 95 °C at 0.1 °C per second by the Eco Real-Time PCR system and data was analyzed using HRM analysis software. A total of 221 left-over DNA samples randomly collected at our routine service for thalassemia screening were examined in blinded trials with this new technique and the results were compared with conventional gap-PCR assay performed routinely.
Figure 3.
A multiplex high-resolution melting (HRM) analysis for simultaneous detection of SEA deletion α0-thalassemia and genotyping of single-nucleotide polymorphism (SNP) rs3760053. On the left side, the specific amplicon of SNP rs3760053 was detected at Tm 85.09±0.12 oC whereas that of the SEA deletion α0-thalassemia was identified at Tm 88.64±0.09 oC. Further differentiation of the specific SNP rs3760053 amplicon for genotyping (T/T, T/G and G/G) was shown on the right hand side. A full color version of this figure is available at the Journal of Human Genetics journal online.
Development of multiplex PCR assay for simultaneous detection of α0-thalassemia and a T allele of SNP rs3760053
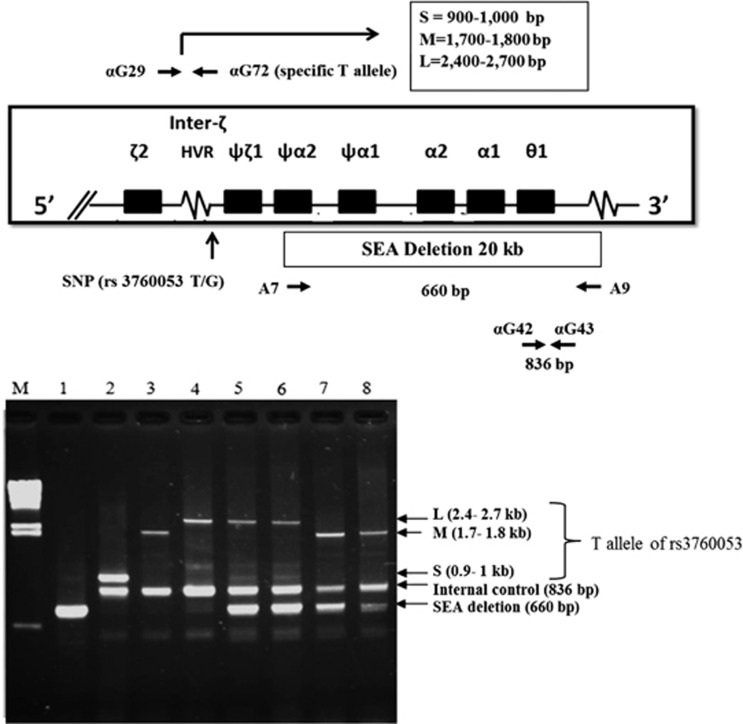
In this development, three primer pairs (listed in Supplementary Table S1) were used to generate specific fragments for normal allele (836 bp), the SEA deletion α0-thalassemia (660 bp) and a T allele of SNP rs3760053 with size ranging from 800 bp to 2.7 kb depending on the inter-ζ hypervariable region (HVR) that is, S=900–1000 bp, M=1.7–1.8 kb, L=2.4–2.7 kb, respectively. Again, identification of an α0-thalassemia allele was straightforward by using gap-PCR but detection of a T allele of the SNP rs3760053 was based on allele specific PCR (Figure 4). The multiplex PCR reaction mixture (50 μl) contained 50–100 ng DNA in a buffer containing 10 mmol l−1 Tris HCl, pH 8.3, 50 mmol l−1 KCl, 1.5 mmol l−1 MgCl2, 50 μmol l−1 each dNTP, 1.02 pmol of primer A7, 1.32 pmol of primer A9, 480 nmol of primer αG29, 240 nmol each of primers αG72, αG42 and αG43, and 0.03 units Taq DNA polymerase (Biolabs Co. Ltd.). The multiplex PCR was carried out on a Biometra T Personal Thermocycler (Biometra GmbH Co., Ltd., Göttingen, Germany) by initial heating at 95 oC (5 min), followed by a 35 cycles of (95 oC, 1 min - 58 oC, 1 min - 72 oC, 1 min) and a final step of 72 oC for 5 min. PCR product was then analyzed by agarose gel electrophoresis after ethidium bromide staining. A total of 155 left-over DNA samples randomly collected at our routine service were examined in blinded trials with this new technique and results were compared with conventional gap-PCR assay performed routinely.
Figure 4.
A multiplex PCR assay for simultaneous detection of the SEA deletion α0-thalassemia and a T allele of the single-nucleotide polymorphism (SNP) rs3760053 using combined gap-PCR (for SEA deletion) and allele specific PCR (for T allele). The locations and orientations of primers used were depicted. Gel electrophoresis represented results of the multiplex PCR amplification. ‘M’ is the λ/HindIII size markers, whereas 1=homozygote α0-thalassemia (Hb Bart’s hydrops fetalis); 2, 3, 4=non α0-thalassemia carriers carrying T alleles with S, M, L polymorphisms, respectively; 5 and 6, and 7 and 8=α0-thalassemia carriers with T allele of L and M polymorphisms, respectively.
Prenatal diagnosis of α0-thalassemia using a double-check system
Prenatal diagnosis of α0-thalassemia was done in 12 families at risk of having fetuses with Hb Bart’s hydrops fetalis. Fetal DNA was prepared from fetal tissues obtained routinely by chorionic villus sampling or amniocentesis.14 Prenatal diagnosis was carried out with the two newly developed systems (multiplex HRM analysis and multiplex PCR amplification) and results compared with conventional gap-PCR assay run routinely at our center.
Results
Genetic background of the SEA deletion α0-thalassemia among Southeast Asian populations
From a pool of 169 Southeast Asian subjects examined (including 96 Thai, 52 Laos and 21 Cambodian, that is, 338 chromosomes in total), DNA analysis for α0-thalassemia (SEA deletion) identified 7 cases with homozygous (—SEA/—SEA), 94 heterozygous (—SEA/αα) and 68 wild-type (αα/αα). This led to a total number of 230 wild type (αα) and 108 mutant alleles (—SEA). Analysis of the 5 SNPs surrounding the SEA deletion as shown in Figure 1 were performed on all these 169 subjects. The results of this analysis including allele frequencies, genotype frequencies and minor allele frequencies as well as the χ2-test for Hardy–Weinberg Equilibrium were summarized in Supplementary Table S2. As shown in the table, χ2-test indicated in all SNPs, the assumption of Hardy–Weinberg Equilibrium of the studied population (P>0.05) and minor allele frequency of all SNPs were found to be >0.1. Because of having the highest number of subjects, data from a group of Thai individuals including 59 SEA deletion α0-thalassemia carriers and 30 normal subjects were analyzed further. The derived allele frequency (DAF) of each SNP (G of rs3760053, C of rs1211375, G of rs3918352, G of rs1203974 and T of rs11248914) was calculated and compared between α0-thalassemia carriers (n=59) and wild type (n=30). For instance, a G allele of rs3760053 was found to be more common in α0-thalassemia carrier (with DAF of 0.4831) than that of a normal subject (with DAF of 0.0333). This is also the case for a G allele of the rs1203974 (with DAF of 0.6694). For the rs11248914, however, a T allele is less common in α0-thalassemia carrier (with DAF of 0.2712) than that of a normal subject (with DAF of 0.5000). Fisher's exact test revealed significant differences for the rs3760053 (P<0.001), rs1203974 (P=0.002) and rs11248914 (P=0.002) but not the two remaining SNPs.
As shown in Figure 1, pairwise analysis of linkage disequilibrium (LD) between these SNPs and the SEA deletion α0-thalassemia in a pooled of 338 subjects demonstrated strong LD of the SEA deletion allele with all 5 SNPs with the D′-values ranging from 0.79 to 0.95 (79–95%). In addition, this analysis also indicated the strongest LD between rs3760053 & rs1211375 and the lowest LD with D′-value of 0.22 (22%) between rs1211375 and rs1203974.
The four SNPs located downstream of the SEA deletion breakpoint including rs1211375, rs3918352, rs1203974 and rs11248914 were used to construct haplotype using the HAPLOVIEW 4.2 software. Frequencies of these haplotypes of Southeast Asian populations were compared to those of other populations described in the HapMap project including CHB (Han Chinese in Beijing, China), JPT (Japanese in Tokyo, Japan), GIH (Gujarat Indians in Houston, Texas), CEU (Utah residents with Northern and Western European ancestry), TSI (Toscans in Italy), YRI (Yoruba Ibadan, Nigeria) and MEX (Mexican ancestry in Los Angeles) as shown in Table 1. Haplotype H12 (AAAC), an ancestral haplotype, was rarely found in Thai, Cambodian and MEX populations. The three most common haplotypes for a wild-type allele in Southeast Asian populations were H1 (AAAT), H2 (CGGC) and H3 (CAAC) whereas H4 (AAGC) was found to be Asian specific haplotype. Interestingly, we found that most of the SEA deletion α0-thalassemia alleles identified in Southeast Asian populations were associated with haplotype H4 (AAGC; 94.05% in Thai and 100% in Laos & Cambodian), similar to that described previously in Chinese population (100%).8 This finding likely indicates the same origin of the SEA deletion α0-thalassemia in Asian populations and could partly explain why this common α0-thalassemia in Asian populations had never been found in other populations lacking the H4 (AAGC) haplotype. Further phylogenetic analysis using the DendroUPGMA software indicated that this haplotype H4 (AAGC) arose from the ancestral haplotype H12 (AAAC) by a mutational event (A>G) at the SNP rs1203974 before an introduction of the SEA deletion α0-thalassemia mutation onto this haplotype (Figure 2).
Table 1. Haplotypes generated using 4 SNPs located downstream of the SEA deletion α0-thalassemia in Southeast Asian populations investigated as compared to other populations described in the HapMap project.
| rs1211375 | rs3918352 | rs1203974 | rs11248914 |
(αα) Allele frequency, % |
(—SEA) Allele frequency, % |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Asia |
Europe |
Africa | America |
Asia |
||||||||||||||
| THAa | LAOa | CAMa | CHBb | JPTb | GIHb | CEUb | TSIb | YRIb | MEXb | THAa | LAOa | CAMa | CHNc | |||||
| Haplotype | A>C | A>G | A>G | C>T | (n=119) | (n=76) | (n=35) | — | — | — | — | — | — | — | (n=73) | (n=28) | (n=7) | (n=30) |
| H1 | A | A | A | T | 44.9 | 32.9 | 39.6 | 34.5 | 36.6 | 31.2 | 26.1 | 29 | 19.2 | 3.12 | ||||
| H2 | C | G | G | C | 24.4 | 25.3 | 14.2 | 35.7 | 30.8 | 11.4 | 9.8 | 11.4 | 3.9 | 26 | ||||
| H3 | C | A | A | C | 17.2 | 12.4 | 17.2 | 14.3 | 22.1 | 24.4 | 10.5 | 11.9 | 27.8 | 17.3 | ||||
| H4 | A | A | G | C | 6.7 | 11.9 | 14.3 | 10.7 | 5.8 | 1.1 | 94.05 | 100 | 100 | 100 | ||||
| H5 | C | A | A | T | 2.8 | 5.6 | 5.7 | 2.4 | 2.3 | 1.5 | 1.1 | 23 | ||||||
| H6 | C | G | G | T | 2.3 | 6.2 | 5.7 | 1.2 | 1.7 | 18.7 | 35.6 | 31.2 | 10 | 26.9 | ||||
| H7 | C | G | A | C | 5.2 | 1.2 | 6.8 | 7.1 | 6.8 | 4.8 | ||||||||
| H8 | A | G | A | C | 2.3 | 6.3 | 5.7 | |||||||||||
| H9 | A | A | G | T | 27 | |||||||||||||
| H10 | C | G | A | T | 3 | |||||||||||||
| H11 | A | G | G | T | 2.6 | |||||||||||||
| H12d | A | A | A | C | 1.8 | 3 | 1.9 | 2.83 | ||||||||||
Populations under study; THA (Thai), LAO (Laotian), CAM (Cambodian).
Data taken from the HapMap project; CHB (Han Chinese in Beijing, China), JPT (Japanese in Tokyo, Japan), GIH (Gujarat Indians in Houston, TX, USA), CEU (Utah residents with Northern and Western European ancestry), TSI (Toscans in Italy), YRI (Yoruba Ibadan, Nigeria) and MEX (Mexican ancestry in Los Angeles).
From Qiu QW, et al., BMC Evolutionary Biology 2013; 13: 63. CHN (Chinese).
Ancestral haplotype.
Validation of multiplex PCR assays for simultaneous detection of α0-thalassemia and SNP rs3760053
As shown in Table 2, a highly significant difference (P<0.001) in DAF between α0-thalassemia carrier and normal subject for the rs3760053 (T>G) was observed and G allele was strongly linked to the SEA deletion α0-thalassemia in this population. In order to improve accuracy for prenatal diagnosis of α0-thalassemia (SEA deletion) in routine practice, we have developed two different multiplex assays to detect the SEA deletion α0-thalassemia and the SNP rs3760053, simultaneously.
Table 2. Derived allele frequencies of five SNPs surrounding the SEA deletion α0-thalassemia in normal and α0-thalassemia carrier in Thai population.
|
Derived allele frequencies |
|||||
|---|---|---|---|---|---|
| SNP ID | Polymorphisma | Positionb | SEA carrier (n=59) | Normal (n=30) | P-valuec |
| rs3760053 | T>G | 161244 | 0.4831 | 0.0333 | < 0.001 |
| rs1211375 | A>C | 190281 | 0.2881 | 0.4667 | 0.100 |
| rs3918352 | A>G | 197889 | 0.2034 | 0.2667 | 0.511 |
| rs1203974 | A>G | 227459 | 0.6694 | 0.3333 | 0.002 |
| rs11248914 | C>T | 243563 | 0.2712 | 0.5000 | 0.002 |
Abbreviation: SNP, single-nucleotide polymorphism.
Allele on the positive strand and the first character represents an ancestral allele.
NCBI position in chromosome 16.
By Fisher’s exact test.
The first assay based on HRM analysis was shown in Figure 3. As shown in the figure, a specific peak of the SEA deletion α0-thalassemia was detected at Tm; 88.64±0.09 oC. A peak corresponding to the SNP rs3760053 was found separately at Tm; 85.09±0.12 oC from which with further differentiation could demonstrate the GG, TG and TT genotypes of this SNP. The method was applied to 211 blind specimens and the results shown in Table 3. As shown in the table, a 100% concordant result for detection of the SEA deletion α0-thalassemia as compared to the conventional gap-PCR was obtained for all 134 normal, 71 heterozygous and 6 homozygous subjects. In addition, genotypes of the SNP rs3760053 of all subjects were readily identified. Among 134 normal subjects, 126 had T/T and 8 had T/G genotypes. In heterozygotes, 66 of 71 had T/G and 5 had G/G genotypes. Interestingly, all the 6 homozygotes carried G/G genotype; the data confirmed that most of the SEA deletion α0-thalassemia in Southeast Asian population was linked to a G allele of the SNP rs3760053.
Table 3. Validation of a multiplex HRM analysis for simultaneous identification of the SEA deletion α0-thalassemia and genotyping of the SNP rs3760053 in 211 individuals as compared to the conventional gap-PCR assay.
|
Multiplex HRM analysis |
||||
|---|---|---|---|---|
| SEA deletion α0-thalassemia | SNP rs3760053 genotyping | No | Gap-PCR assay for SEA deletion α0-thalassemia | No |
| Negative | T/T | 126 | αα/αα | 134 |
| Negative | T/G | 8 | ||
| Positive | T/G | 66 | αα/—SEA | 71 |
| Positive | G/G | 5 | ||
| Positive | G/G | 6 | —SEA/—SEA | 6 |
| Total | 211 | Total | 211 | |
Abbreviations: HRM, high-resolution melting; SNP, single-nucleotide polymorphism.
We also used another method to detect the SEA deletion α0-thalassemia by gap-PCR and identification of a T allele of the SNP rs3760053 by allele specific PCR. As shown in Figure 4, the amplified DNA fragments specific for SEA deletion (660 bp), normal allele (836 bp) and a T allele of the SNP rs3760053 with size polymorphism of the inter-ζ HVR (S, M or L) could be demonstrated on the gel electrophoresis. Since the SEA deletion α0-thalassemia was linked to a G allele of the SNP rs3760053, homozygous α0-thalassemia would generate only one fragment at 660 bp, but not 836 bp and T allele specific fragments. This should provide an effective double-check system for the diagnosis of a homozygotic condition of this most severe thalassemia disease. Validation of this method was done in blinded trials on 155 subjects. A 100% concordant result with routine gap-PCR assay was obtained by this validation. As shown in Table 4, wild-type subjects (n=41) had no SEA-deletion-specific fragment (660 bp) but had internal control fragment (836 bp) and T allele with inter-ζ HVR (S=10, M=26, and L=5). Heterozygotes (n=104) had specific SEA-deletion fragment (660 bp), and either T allele of the SNP rs3760053 with inter-ζ HVR (S=14, M=58, L=31) or G allele of the SNP rs3760053 (n=1) as well as internal control fragment (836 bp). In contrast, all 10 homozygotes (—SEA/—SEA) presented, as expected, only one specific fragment at 660 bp without fragments specific for a T allele of SNP rs3760053 and normal control.
Table 4. Validation of a multiplex PCR assay for simultaneous detection of the SEA deletion α0-thalassemia and a T allele of SNP rs3760053 with size polymorphism of inter-ζ HVR in 155 individuals as compared to the conventional gap-PCR assay.
| Inter-ζ HVR with T allele of rs3760053 | SEA α0-thal (660 bp) | Internal control (836 bp) | No | Diagnosis by multiplex PCR | Gap-PCR | No |
|---|---|---|---|---|---|---|
| S | − | + | 10 | Normal | αα/αα | 41 |
| M | − | + | 26 | |||
| L | − | + | 5 | |||
| S | + | + | 14 | Heterozygote (αα / —SEA) | αα/—SEA | 104 |
| M | + | + | 58 | |||
| L | + | + | 31 | |||
| — | + | + | 1 | |||
| — | + | − | 10 | Homozygote (—SEA / —SEA) | —SEA/—SEA | 10 |
| Total | 155 | 155 | ||||
Abbreviations: HVR, hypervariable region; SNP, single-nucleotide polymorphism.
The two new methods developed have been applied to prenatal diagnosis of Hb Bart’s hydrops fetalis in routine practice on 12 at-risk families. Again the concordant results of diagnosis using conventional gap-PCR method was obtained as shown in Supplementary Table S3. The two developed methods for simultaneous detection of α0-thalassemia, genotyping of SNP rs3760053 and identification of a T allele could improve the accuracy of diagnosis provided by gap-PCR alone. The method can also help in detecting maternal contamination of fetal specimen by looking at the pattern of size polymorphism associated with the T allele of the fetus. Analysis of the 12 families revealed 3 fetuses with normal (αα/αα), 5 fetuses with heterozygous α0-thalassemia (—SEA/αα) and 4 fetuses with homozygous α0-thalassemia (—SEA/—SEA) leading to the Hb Bart’s hydrops fetalis syndrome.
Discussion
The SEA deletion α0-thalassemia represents one of the most common α-thalassemia found among Southeast Asian and Chinese populations. It results from a DNA deletion on chromosome 16 spanning approximately 20.5 kb which removes the Ψα2-, Ψα1-, α2-, α1- and θ1 globin genes.15 Parents who are both carriers of this α0-thalassemia would have a 25% risk of having fetus with homozygous α0-thalassemia causing Hb Bart’s hydrops fetalis syndrome. Although some survivors with Hb Bart’s hydrops fetalis syndrome have been documented, the disease can lead to serious maternal complications during pregnancy such as hypertension, pre-eclampsia, polyhydramnios and severe postpartum hemorrhage.16, 17 Therefore, it is necessary to provide accurate diagnostic test for carrier screening and prenatal diagnosis. This SEA deletion α0-thalassemia has limited geographical distribution and has only been found among Chinese and Southeast Asian populations. High prevalence of this mutation has been reported among northeast Thai, Laos and Cambodian populations.18, 19 Although the evidence of malaria selection in α0-thalassemia at the cellular level is still unclear, this mutation is primarily found in malarious regions. In the study of Chinese population, Qiu et al.8 have hypothesized that this SEA deletion α0-thalassemia has been subjected to recent balancing selection, triggered by malaria and suggested a single origin of this mutation in Chinese population.
We reported for the first time the genetic background of this SEA deletion α0-thalassemia among three Southeast Asian populations. Five SNPs surrounding the SEA deletion α0-thalassemia spanning about 82 kb in length were examined in Thai, Laos and Cambodian subjects with and without the mutation. As shown in Figure 1, all these five SNPs were found to have linkage disequilibrium with the SEA deletion α0-thalassemia allele in these three Southeast Asian populations. When the data of these SNPs were compared between the α0-thalassemia carriers and normal controls, we found that the SNP rs3760053 T>G located upstream of SEA deletion breakpoint was uncommon in the normal population (with a DAF of 0.033) but was predominant in carriers (with DAF of 0.4831) (Table 2). The G allele of this SNP was mostly linked to a chromosome with the SEA deletion α0-thalassemia in the three populations. We observed no hematological difference between α0-thalassemia carriers with this most common G-linked and a rarer T-linked alleles. The difference in DAF between normal controls and carriers allowed us to develop the more reliable assays for screening of the SEA deletion α0-thalassemia as shown in Figures 3 and 4. In Figure 3, the SEA deletion α0-thalassemia was detected along with SNP rs3760053 genotyping on the HRM format. Analysis of 211 individuals confirmed that most of the normal subjects (αα/αα) had T/T genotype while heterozygotes (αα/—SEA) had T/G or G/G genotypes. In addition, only G/G genotype was observed for the 6 cases with homozygous α0-thalassemia (—SEA /—SEA; Table 3). Therefore, a T allele was not detected on a chromosome with SEA deletion α0-thalassemia in these Southeast Asian populations. This was also the case for the southern Chinese population in whose the DAF for SNP rs3760053 T>G were found to be 0.520 and 0.098 for α0-thalassemia carriers and normal population, respectively.8 Undetectable T allele at the SNP rs3760053 T>G in case with Hb Bart’s hydrops fetalis caused by homozygosity of the SEA deletion α0-thalassemia should provide additional confirmation for diagnosis of this important thalassemia syndrome as shown in Figure 4 and Table 4. This is very important in a routine prenatal diagnosis practice because misdiagnosis of Hb Bart’s hydrops fetalis can occur with maternal contamination and allelic dropout (a mechanism in which one of the alleles fails to be amplified) on a small amount of fetal DNA. In our laboratory, maternal contamination is usually monitored by PCR analysis of VNTR.14, 20 When a wild-type allele fails to be amplified in heterozygote, PCR will show homozygosity for the mutant allele.21 It is therefore necessary to perform at least two different PCR methods for accurate prenatal diagnosis of the case.4, 22 Although the SNP genotyping is not less prone to allelic dropout than gap-PCR method, diagnosis using a double-check system is preferred. The methods described in Figure 4 for simultaneous detection of the SEA deletion α0-thalassemia and a T allele at SNP rs3760053 should provide an effective system for this.
With a relatively high prevalence and spread of the SEA deletion α0-thalassemia among Southeast Asian and Chinese populations, information regarding the origin of this common thalassemic allele has been limited. Study on the inter-ζ HVR and IVS1 of Ψζ gene in a small number of patients has pointed to a single origin in Thailand. No haplotype data was reported.23 A more detailed study using pattern of linkage disequilibrium and a long-range haplotype based on 28 SNPs encompassing the SEA deletion, covering around 410 kb region on chromosome 16 has recently been conducted in southern Chinese population. The study has also suggested a single origin of the SEA deletion α0-thalassemia allele in Chinese.8
Data on the 5 SNPs flanking the deletion breakpoints and haplotypes reported herein are the first time this α0-thalassemia allele has been addressed for Southeast Asian populations. The results shown in the Table 1 for Thai, Laos and Cambodian demonstrated that while normal allele (αα) could be presented on diverse haplotypes; H1-H7 and H12, most of the SEA deletion α0-thalassemia (—SEA) were associated with haplotype H4 (AAGC), the data presented for the first time among Southeast Asian populations. Interestingly, as shown in the table, this haplotype H4 was found only in Asian populations including Thai, Laos, Cambodian, Chinese, Japanese and Asian Indians but not in European, African and American. In addition, phylogenetic analysis indicated that this haplotype H4 (AAGC) arose from the ancestral haplotype H12 (AAAC) before introduction of the SEA deletion α0-thalassemia mutation (Figure 2). All these data should partly explain why the SEA deletion α0-thalassemia has never been found among the non-Asian populations. This haplotype analysis pointed to a single origin of the SEA deletion α0-thalassemia in human population and further indicates that a single origin was responsible for the high prevalence and spread of SEA deletion α0-thalassemia in the regions. As for Chinese population, this should support the potential effect of natural selection on the SEA deletion α0-thalassemia in Southeast Asia especially the susceptibility to malaria infection.8, 24 A single origin of the Filipino β0-thalassemia in Asian population has been documented.25 However, these are in contrast with other hemoglobinopathies found in Southeast Asia like Hb E, Hb Constant Spring and Hb Q-Thailand in which multiple origins existed.26, 27, 28, 29
Acknowledgments
This work was supported by a grant to SF from the National Research University (NRU) program of Khon Kaen University and the Office of the Higher Education Commission, Ministry of Education, Thailand (NRU592015). WJ is supported by the Royal Golden Jubilee PhD program (PHD/0210/2553) of the Thailand Research Fund (TRF), Thailand. We thank Ms Kah Wei Yoong for helpful comments on the manuscript.
Author contributions
Performed the experiments: WJ; PC; and JM. Analyzed the data: WJ; GF; and SF. Contributed samples/reagents/materials/analysis tools: KS; XX; and SF. Initial preparation of the manuscript: WJ. SF is responsible for the design of the study, analysis of data, writing and editing the final manuscript, as well as acquisition of the grant. All authors have approved the final article.
Footnotes
Supplementary Information accompanies the paper on Journal of Human Genetics website (http://www.nature.com/jhg)
The authors declare no conflict of interest.
Supplementary Material
References
- Fucharoen, S. & Winichagoon, P. Thalassemia in Southeast Asia: problem and strategy for prevention and control. Southeast Asian J. Trop. Med. 23, 647–655 (1992). [PubMed] [Google Scholar]
- Xu, X. M., Zhou, Y. Q., Luo, G. X., Liao, C., Zhou, M., Chen, P. Y. et al. The prevalence and spectrum of alpha and beta thalassaemia in Guangdong province: implications for the future health burden and population screening. J. Clin. Pathol. 57, 517–522 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui, D. H. K. Thalassemia: Hb H disease and Hb Bart’s hydrops fetalis. Ann. N. Y. Acad. Sci. 1054, 25–32 (2005). [DOI] [PubMed] [Google Scholar]
- Karnpean, R., Fucharoen, G., Fucharoen, S., Sae-ung, N. & Sanchaisuriya, K. Accurate prenatal diagnosis of Hb Bart's hydrops fetalis in daily practice with a double-check PCR system. Acta. Haematol. 121, 27–33 (2009). [DOI] [PubMed] [Google Scholar]
- Ko, T. M., Tseng, L. H., Hwa, H. L., Hsu, P. M., Li, S. F., Chu, J. Y. et al. Misdiagnosis of homozygous alpha-thalassaemia 1 may occur if polymerase chain reaction alone is used in prenatal diagnosis. Prenat. Diagn. 17, 505–509 (1997). [PubMed] [Google Scholar]
- Kleanthous, M., Kyriacou, K., Kyrri, A., Kalogerou, E., Vassiliades, P., Drousiotou, A. et al. Alpha-thalassaemia prenatal diagnosis by two PCR based methods. Prenat. Diagn. 21, 413–417 (2001). [DOI] [PubMed] [Google Scholar]
- Srivorakun, H., Fucharoen, G., Sae-ung, N., Sanchaisuriya, K., Ratanasiri, T. & Fucharoen, S. Analysis of fetal blood using capillary electrophoresis system: a simple method for prenatal diagnosis of severe thalassemia diseases. Eur. J. Haematol. 83, 57–65 (2009). [DOI] [PubMed] [Google Scholar]
- Qiu, Q. W., Wu, D. D., Yu, L. H., Yan, T. Z., Zhang, W., Li, Z. T. et al. Evidence of recent natural selection on the Southeast Asian deletion (—SEA causing alpha-thalassemia in south China. BMC. Evol. Biol. 13, 63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaibunruang, A., Prommetta, S., Yamsri, S., Fucharoen, G., Sae-Ung, N., Sanchaisuriya, K. et al. Molecular and hematological studies in a large cohort of α0-thalassemia in northeast Thailand: data from a single referral center. Blood Cells Mol. Dis. 51, 89–93 (2013). [DOI] [PubMed] [Google Scholar]
- Sae-ung, N., Fucharoen, G., Sanchaisuriya, K. & Fucharoen, S. α0-Thalassemia and related disorders in northeast Thailand: a molecular and hematological characterization. Acta. Haematol. 117, 78–82 (2007). [DOI] [PubMed] [Google Scholar]
- Xu, S., Gupta, S. & Jin, L. PEAS V1.0: a package for elementary analysis of SNP data. Mol. Ecol. Resour. 10, 1085–1088 (2010). [DOI] [PubMed] [Google Scholar]
- Barrett, J. C., Fry, B., Maller, J. & Daly, M. J. Haploview analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005). [DOI] [PubMed] [Google Scholar]
- Bergman, I. M., Sandholm, K., Ekdahl, K. N., Okumura, N., Uenishi, H., Guldbrandtsen, B. et al. MBL1 genotypes in wild boar populations from Sweden, Austria, the Czech Republic, and Japan. Int. J. Immunogenet. 40, 131–139 (2013). [DOI] [PubMed] [Google Scholar]
- Yamsri, S., Sanchaisuriya, K., Fucharoen, G., Sae-ung, N., Ratanasiri, T. & Fucharoen, S. Prevention of severe thalassemia in northeast Thailand: 16 years of experience at a single university center. Prenat. Diagn. 30, 540–546 (2010). [DOI] [PubMed] [Google Scholar]
- Higgs, D. R. The molecular basis of α-thalassemia. Cold Spring Harb. Perspect. Med 3, a011718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songdej, D., Babbs, C. & Higgs, D. R. in collaboration with the BHFS International Consortium. An international registry of survivors with Hb Bart’s hydrops fetalis syndrome. Blood 129, 1251–1259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui, D. H. K. & Waye, J. S. Hydrops fetalis caused by α-thalassemia: an emerging health care problem. Blood 91, 2213–2222 (1998). [PubMed] [Google Scholar]
- Tritipsombut, J., Sanchaisuriya, K., Phollarp, P., Bouakhasith, D., Sanchaisuriya, P., Fucharoen, G. et al. Micromapping of thalassemia and hemoglobinopathies in different regions of northeast Thailand and Vientaine, Laos People's Democratic Republic. Hemoglobin 36, 47–56 (2012). [DOI] [PubMed] [Google Scholar]
- Carnley, B. P., Prior, J. F., Gilbert, A., Lim, E., Devenish, R., Sing, H. et al. The prevalence and molecular basis of hemoglobinopathies in Cambodia. Hemoglobin 30, 463–470 (2006). [DOI] [PubMed] [Google Scholar]
- Budowle, B., Chakraborty, R., Giusti, A. M., Eisenberg, A. J. & Allen, R. C. Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am. J. Hum. Genet. 48, 137–144 (1991). [PMC free article] [PubMed] [Google Scholar]
- Lam, C. W. & Mak, C. M. Allele dropout in PCR based diagnosis of Wilson disease: mechanisms and solutions. Clin. Chem. 52, 517–520 (2006). [DOI] [PubMed] [Google Scholar]
- Ho, S. S., Chong, S. S., Koay, E. S., Chan, Y. H., Sukumar, P., Chiu, L. L. et al. Microsatellite markers within —SEA breakpoints for prenatal diagnosis of Hb Bart’s hydrops fetalis. Clin. Chem. 53, 173–179 (2007). [DOI] [PubMed] [Google Scholar]
- Winichagoon, P., Higgs, D. R., Goodbourn, S. E., Clegg, J. B., Weatherall, D. J., Wasi, P. et al. The molecular basis of alpha-thalassaemia in Thailand. EMBO. J. 3, 1813–1818 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall, D. J. Genetic variation and susceptibility to infection: the red cell and malaria. Br. J. Haematol. 141, 276–286 (2008). [DOI] [PubMed] [Google Scholar]
- Yamsri, S., Sanchaisuriya, K., Fucharoen, G. & Fucharoen, S. Genetic origin and interaction of the Filipino β0-thalassemia with Hb E and α-thalassemia in a Thai family. Transl. Res. 159, 473–476 (2012). [DOI] [PubMed] [Google Scholar]
- Fucharoen, G., Fucharoen, S., Sanchaisuriya, K., Sae-Ung, N., Suyasunanond, U., Sriwilai, P. et al. Frequency distribution and haplotypic heterogeneity of βE-globin gene among eight minority groups of northeast Thailand. Hum. Hered. 53, 18–22 (2002). [DOI] [PubMed] [Google Scholar]
- Singsanan, S., Fucharoen, G., Savongsy, O., Sanchaisuriya, K. & Fucharoen, S. Molecular characterization and origins of Hb Constant Spring and Hb Paksé in Southeast Asian populations. Ann. Hematol. 86, 665–669 (2007). [DOI] [PubMed] [Google Scholar]
- Jomoui, W., Fucharoen, G., Sanchaisuriya, K., Nguyen, V. H. & Fucharoen, S. Hemoglobin Constant Spring among Southeast Asian populations: haplotypic heterogeneities and phylogenetic analysis. PLoS ONE 10, e0145230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsanan, S., Karnpean, R., Fucharoen, G., Sanchaisuriya, K., Sae-ung, N. & Fucharoen, S. Hemoglobin Q-Thailand related disorders: origin, molecular, hematological and diagnostic aspects. Blood Cells Mol. Dis. 45, 210–214 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.