Abstract
Strains of Bacillus sphaericus exhibit varying levels of virulence against mosquito larvae. The most potent strain, B. sphaericus 2362, which is the active ingredient in the commercial product VectoLex®, together with another well-known larvicide Bacillus thuringiensis subsp. israelensis, are used to control vector and nuisance mosquito larvae in many regions of the world. Although not all strains of B. sphaericus are mosquitocidal, lethal strains produce one or two combinations of three different types of toxins. These are (1) the binary toxin (Bin) composed of two proteins of 42 kDa (BinA) and 51 kDa (BinB), which are synthesized during sporulation and co-crystallize, (2) the soluble mosquitocidal toxins (Mtx1, Mtx2 and Mtx3) produced during vegetative growth, and (3) the two-component crystal toxin (Cry48Aa1/Cry49Aa1). Non-mosquitocidal toxins are also produced by certain strains of B. sphaericus, for examples sphaericolysin, a novel insecticidal protein toxic to cockroaches. Larvicides based on B. sphaericus-based have the advantage of longer persistence in treated habitats compared to B. thuringiensis subsp. israelensis. However, resistance is a much greater threat, and has already emerged at significant levels in field populations in China and Thailand treated with B. sphaericus. This likely occurred because toxicity depends principally on Bin rather than various combinations of crystal (Cry) and cytolytic (Cyt) toxins present in B. thuringiensis subsp. israelensis. Here we review both the general characteristics of B. sphaericus, particularly as they relate to larvicidal isolates, and strategies or considerations for engineering more potent strains of this bacterium that contain built-in mechanisms that delay or overcome resistance to Bin in natural mosquito populations.
Keywords: Bacillus sphaericus, mosquitocidal, Bin, resistance, recombinant
Introduction
Bacillus sphaericus is a mesophilic, aerobic Gram-positive bacterium that occurs worldwide in soil and aquatic habitats. It produces a characteristic spherical spore located at one end of the swollen sporangium (Fig. 1), a phenotype that makes it readily distinguishable from other Bacillus species, including the best characterized bacterial entomopathogen, Bacillus thuringiensis, which produces an ovoidal spore (Federici et al., 2007; Park and Federici, 2009). During the past 25 years, much interest has been focused on isolating strains of B. sphaericus primarily because of their potential for use as mosquito larvicides. Many of these isolates have been partially characterized, and antigenic studies indicate that they can be grouped in 49 serotypes based on epitope differences in flagellar (H) antigens. Five major groups (I-V) are known based on DNA homologies (Krych et al., 1980). Larvicidal strains of B. sphaericus comprise DNA subgroup IIA and nine serotypes (H1, H2, H3, H5, H6, H9, H25, H26, and H48). As a group, little is known about their genomics, and presently the genome of only one mosquitocidal strain (C3-41; H5a5b, Hue et al., 2008a) has been sequenced.
Fig. 1.
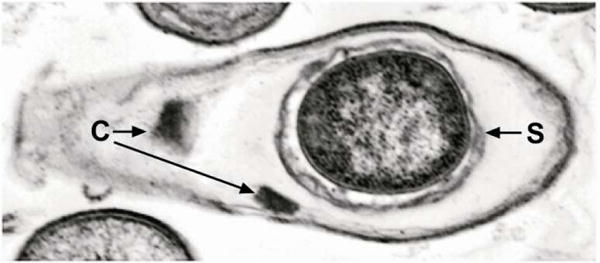
Transmission electron micrograph of a fully sporulated Bacillus sphaericus 2362 cell. C, BinAB crystal; S, spore.
Unlike strains of B. thuringiensis, for which virulence against insects has long been established, B. sphaericus was not considered insecticidal until a strain active against mosquitoes was isolated from dead larvae of Culiseta incidens (Kellen et al., 1965). The larvicidal activity of this isolate, however, was comparatively low and its use in mosquito control was not considered feasible. Later, identification of the SSII-1 strain in India renewed interest in the search for more active strains of B. sphaericus (Singer, 1973). However, it was not until the discovery of more potent strains lethal to larvae of Culex species, strains such as 1593 and 2297 (Wickremesinghe and Mendis, 1980) and 2362 (Weiser, 1984) isolated in, respectively, Indonesia, Sri Lanka and Nigeria, that the potential of B. sphaericus as a cost-effective bacterial larvicide was taken seriously (Singer, 1974). In fact, strain 2362 is the most widely used commercial larvicide based on B. sphaericus, the active ingredient in VectoLex® (Valent BioSciences, Libertyville, IL). Independently, strain C3-41 that has comparable toxicity to strain 2362 has also been commercialized (Xingtai Biotechnology Co., Ltd., Wuhan, China) and used more widely in China and Southeast Asia.
Since the development and commercial implementation of B. sphaericus 2362, efforts to isolate more virulent strains of this bacterium have been unproductive (Brownbridge and Margalit, 1987; Guerineau et al., 1991; Orduz-Peralta et al., 1992; Khyami-Horani et al., 1999; Cavados et al., 2001). Recent studies however, suggest that more potent strains of B. sphaericus could exist in nature. For example, one such isolate, WBM 1-1-13, closely related to 2362, was cultured from a salt marsh habitat in Indian River County, Florida. Comparative analysis of 2362 and WBM 1-1-13 showed that latter produced more binary toxin (Bin, see below) per unit medium and was more toxic to Culex quinquefasciatus larvae (Park et al., 2007b).
Mosquitocidal proteins of Bacillus sphaericus
Bin toxins
The primary mosquitocidal activity of B. sphaericus is due to the binary toxin (Bin). Bin is composed of two proteins of molecular masses 42 kDa (BinA) and 51 kDa (BinB) that co-crystallize to form a single parasporal inclusion that is encapsulated with the spore during late stage of bacterial growth (Charles et al., 1996; Federici et al., 2007; Park and Federici, 2009). BinB, the binding domain, functions as a specificity determinant, whereas BinA, the toxic domain, binds to BinB to amplify its toxicity. Generally, Bin is highly toxic to all known Culex species, and many important anopheline species, such as the key malaria vector in Africa, Anopheles gambiae. In addition, many Aedes species are sensitive to Bin, but not important species such as Aedes aegypti. To be sensitive to the Bin toxin, the α-glucosidase receptor much be present on midgut microvilli (see Receptors below). Although BinA alone is active against target mosquito species, the presence of both proteins is required for optimal larvicidal activity (Limpanawat et al., 2009). Regarding their primary structures, the Bin toxins are highly conserved among strains of different serotypes and phage groups. However, based on differences in their amino acid sequence and relative virulence, Bin toxins are classified in four different groups. The type strains for Types 1, 2, 3 and 4 are IAB59, 2362, 2297 and LP-1G, respectively (Table 1) (Priest et al., 1997; Humphreys and Berry, 1998).
Table 1.
Comparison of binary toxin sequences for B. sphaericus strain types
| Gene | Base position | Amino acid position | Nucleotide and amino acid in straina | |||
|---|---|---|---|---|---|---|
| IAB59 (Type 1) |
2362 (Type 2) |
2297 (Type 3) |
LP-1G (Type 4) |
|||
| binB | 700 | 69 | G Ala | T Ser | T Ser | T Ser |
| 705 | 70 | A Lys | C Asn | C Asn | C Asn | |
| 824 | 110 | T Ile | C Thr | C Thr | T Ile | |
| 1206 | 239 | G Ala | G Ala | G Ala | A Ala | |
| 1435 | 314 | C His | C Leu | T Tyr | C His | |
| 1436 | 314 | A His | T Leu | A Tyr | A His | |
| 1446 | 317 | G Leu | T Phe | G Leu | G Leu | |
| 1455 | 320 | C Ser | T Ser | C Ser | C Ser | |
| 1660 | 389 | T Leu | T Leu | A Met | A Met | |
| 1677 | 394 | G Ser | G Ser | G Ser | A Ser | |
| binA | 2139 | 42 | C Ile | C Ile | T Ile | T Ile |
| 2169 | 52 | T Asn | T Asn | C Asn | C Asn | |
| 2253 | 80 | C Ala | C Ala | T Ala | T Ala | |
| 2291 | 93 | T Leu | T Leu | T Leu | C Ser | |
| 2308 | 99 | G Val | G Val | T Phe | G Val | |
| 2323 | 104 | G Glu | G Ala | T Ser | T Ser | |
| 2324 | 104 | A Glu | C Ala | C Ser | C Ser | |
| 2386 | 125 | C His | C His | A Asn | A Asn | |
| 2412 | 133 | T Leu | T Leu | C Leu | C Leu | |
| 2417 | 135 | A Tyr | A Tyr | T Phe | T Phe | |
| 2490 | 159 | A Ser | A Ser | T Ser | T Ser | |
| 2643 | 210 | C Thr | C Thr | G Thr | G Thr | |
| 2745 | 243 | C Ile | C Ile | T Ile | T Ile | |
| 2813 | 267 | G Arg | G Arg | A Lys | A Lys | |
The nucleotide numbering is based on the sequence of Baumann et al. (1988). The sequences described have been submitted to the EMBL data base under Accession Nos. Y13311 to Y13320, Data modified from Humphreys and Berry (1998). Copyright 1998 Elsevier.
The binA and binB coding sequences are contained in an operon in which expression presumably allows for synthesis of near equimolar amounts of BinA and BinB. Unlike B. thuringiensis in which the insecticidal crystal (cry) and cytolytic (cyt) entomotoxin genes are located on large plasmids, it is known that the bin operon is located on chromosomal DNA (Poncet et al., 1997; Servant et al., 1999). However, analyses of the complete genome sequence of B. sphaericus strain C3-41 (Hu et al., 2008a), a strain closely related to 1593 and 2362, revealed that the bin operon is located in an ~35-kb duplicate fragment present both in the chromosome and in a large plasmid (pBsph) (Fig. 2). Based on this finding, it has been suggested that the bin operon and even the ~35-kb fragment could be the remnant of a transduction event mediated by a bacteriophage (Hu et al., 2008a), a plausible explanation for the absence of bin in several B. sphaericus (bin−) strains that conceivable escaped invasion by such a transducing phage.
Fig. 2.
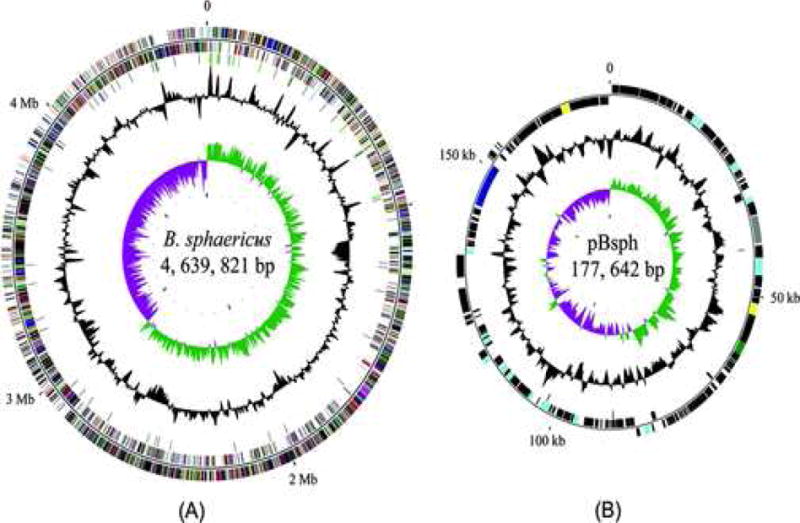
Circular representations of the genome of Bacillus sphaericus C3-41. (A) Chromosome. (B) Plasmid pBsph. From the inside: circles 1 and 2, GC skew and G+C content (20-kb window with 5-kb step); circle 3, blue and green bars show positions of tRNA and rRNA, respectively, and black bars show positions of repeats; circles 4 and 5, CDSs (coding sequences) on the − and + strands. Colors reflect functional categories of CDSs. Teal, chromatin structure and dynamics; blue, energy production and conversion; orange, cell cycle control, cell division, and chromosome partitioning; maroon, amino acid transport and metabolism; dark blue, nucleotide transport and metabolism; silver, carbohydrate transport and metabolism; dark green, coenzyme transport and metabolism; dark purple, lipid transport and metabolism; navy, translation, ribosomal structure, and biogenesis; light brown, transcription; aqua, replication, recombination, and repair; green, cell wall/membrane/envelope biogenesis; fuchsia, cell motility; gray, posttranslational modification, protein turnover, and chaperones; dark yellow, inorganic ion transport and metabolism; dark blue, secondary metabolite biosynthesis, transport, and catabolism; dark red, general function prediction only; dark gray, function unknown; lime, signal transduction mechanisms; yellow, intracellular trafficking, secretion, and vesicular transport; olive, defense mechanisms; black, not classified by COG. The “0” coordinates marked on the outmost circles correspond to the putative replication origins, and the putative replication termination site is located near 2.9 Mb. Hu et al. (2008). Copyright 2008 American Society for Microbiology.
Among mosquitocidal strains of B. sphaericus, strains 2297 and 2362 are the most widely studied (Poncet et al., 1997; Servant et al., 1999; Federici et al., 2007; Park and Federici, 2009). Between the two, strain 2362 is significantly more active against target mosquito species. It has been suggested that the amino acid replacements at around position 100 (position 99: valine in 2362 and phenylalanine in 2297; position 104: alanine in 2362 and serine in 2297) in BinA confer the difference in toxicity between the two strains (Berry et al., 1993). It is also interesting to note that although strain 2362 is more toxic than 2297, the latter produces larger Bin crystalline inclusions. The mechanisms involved with Bin crystal synthesis and the factors that influence crystal size have yet to be elucidated. However, based on recent studies (Park et al., 2009), it is unlikely that post-translational processing, including proteolysis, is an important determinant of Bin crystal size. More likely, crystal size is determined by unknown genes that regulate bin transcription. Two lines of evidence support this hypothesis. First, additional copies of the bin operon in strain 2362 correlated with increased Bin yield and crystal size (Park et al., unpublished data). More importantly, Park et al. (2009) have shown that strain 2362 contains a 1.6-kb insertion downstream from the bin operon, a sequence that is absent at the same position in 2297. The insertion harbors two potential open reading frames (ORFs) that putatively encode proteins of 6 kDa and 21 kDa (Fig. 3). When a 1.1-kb minimal fragment within the insertion containing the two ORFs was integrated downstream from the bin operon in strain 2297, crystal size and yield were decreased in the recombinant. In fact, the size of the inclusion and yields of BinA and BinB were similar to that observed in 2362. The influence of the two ORFs and potential epigenetic effects exerted by the insertion on expression of bin in 2362 remain to be resolved.
Fig. 3.
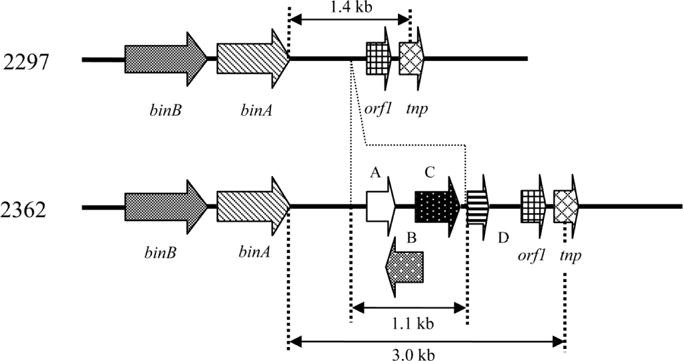
Schematic illustration of genomic DNA of Bacillus sphaericus strains 2362 and 2297 in the region of the bin operon. Strain 2362 contains a 1.6-kb fragment downstream from the bin operon not found in strain 2297. Park et al. (2009). Copyright 2009 American Society for Microbiology.
The relationship between the primary and tertiary structures and toxicity of Bin has received little attention in comparison to similar studies on crystal (Cry) and cytolytic (Cyt) toxins produced by various strains of entomopathogenic B. thuringiensis (Pigott and Ellar, 2007). Although much remains to be learned, site-directed mutagenesis of BinA showed that replacements of a cysteine residue at position 195 with either serine or alanine significantly reduced mosquitocidal activity, whereas replacements of cysteines at positions 31 and 47 completely abolished toxicity against Cx. quinquefasciatus larvae (Promdonkoy et al., 2008). These mutations, however, did not affect the production of BinA, and all 3 cysteine residues were not involved in disulfide bridges within the BinA molecule. In another study, replacements of selected charged residues in BinA (arginines at positions 97 and 101, and glutamate at positions 98 and 114) with alanine did not affect toxin yield (Sanitt et al., 2008). However, the replacement at position 97 resulted in complete loss of mosquitocidal activity, and replacements at positions 101, 98 and 114 resulted in significant reduction in toxicity against Cx. quinquefasciatus larvae.
Mtx toxins
Several mosquitocidal strains of B. sphaericus are known to produce another class of mosquitocidal proteins called Mtx (mosquitocidal toxin). These proteins are unrelated to BinA and BinB (Charles et al., 1996; Federici et al., 2007; Park and Federici, 2009). At present, three categories of Mtx have been described, Mtx1 (~100 kDa), Mtx2 (~31 kDa) and Mtx3 (~36 kDa). The amino-terminal of these toxins contains short leader signal peptide sequences that are characteristic in many proteins encoded by Gram-positive bacteria (Thanabalu et al., 1991; Liu et al., 1996; Thanabalu and Porter, 1996). Mtx proteins are soluble toxins that are synthesized during vegetative growth, and unlike Cry and Cyt proteins of B. thuringiensis, do not form crystalline inclusions during sporulation. Once synthesized in wild type B. sphaericus, Mtx proteins are rapidly degraded by cellular proteases, and as a result, they contribute very little to mosquitocidal activity in wild type strains. Increased yields and improved toxicities of Mtx proteins against target mosquito have been achieved by heterologous expression of mtx genes and synthesis of the toxins in Escherichia coli or protease-deficient recombinant B. sphaericus (Thanabalu and Porter, 1995; Wirth et al., 2007). Bioassay results using Mtx1 and Bin produced in E. coli and B. thuringiensis, respectively, showed that against Cx. quinquefasciatus, Mtx1 incurs significantly higher pupal mortality and abnormal pupal emergence when compared with Bin (Wei et al., 2006).
X-Ray crystallographic studies on the catalytic domain of Mtx1 linked by 44 residues in its carboxyl-terminus to four ricin B-like domains revealed that the crystallized fragment has structural characteristics similar to ADP-ribosylating enzymes, and that the cleavage site for toxin activation is in a highly mobile loop that is exposed in the monomer (Reinert et al., 2006). More recently, the crystal structure of full-length Mtx1 toxin was determined by the molecular replacement method (Treiber et al., 2008). In this study, Mtx1 was produced as a single polypeptide chain of 870 residues consisting of the putative signal sequence (residues 1–29), a catalytic domain (residues 30–264), a linker (265–295) and the remaining 575 residues (Fig. 4) (Thanabalu et al., 1992; 1993; Reinert et al., 2006; Treiber et al., 2008). Structural analysis revealed that the complete chain consisted of four ricin B-type domains curling around the catalytic domain in a hedgehog-like assembly. In addition, the amino acid sequences of the 12 putative sugar-binding sites revealed a structural and functional relationship to piericin, a glycolipophilic cytotoxin (Carpusca et al., 2006). Therefore, it is possible that with the binding of Mtx to glycolipids, and/or following endocytosis and its subsequent exposure to low pH in late endosomes, the hedgehog structure could uncurl to form a string of domains where the seven amino-terminal segments then enter into the membrane and cause the translocation of the catalytic domain into the cytosol (Hazes and Read, 1997).
Fig. 4.
Stereo view of a ribbon plot of MTXholo. The catalytic domain (blue), the linker (residues 265–295, red) and the four ricin B-like (RBL) domains (cyan, green, yellow and orange) are color-coded and labeled. The missing residues at the ARTT loop (189–192) and the activation loop (262–270) are bridged by dotted lines. Loop 117–124 is labeled. Residues 30–70 preceding the common chain fold of ADP-ribosylating enzymes are purple. They are not tightly fastened to the catalytic domain. The depicted NAD+ molecule was modeled for MTXcali but also applies for MTXholo. Treiber et al. (2008). Copyright 2008 Elsevier.
Cry toxins
In a recent study, sequence analyses of B. sphaericus strains IAB59 and NHA15b identified a previously undescribed type of two-component toxin (Jones et al., 2007). One subunit, Cry48Aa1, is related to the three-domain Cry toxins of B. thuringiensis (Jones et al., 2007; Pigott and Ellar, 2007). Interestingly, the mosquitocidal activity of Cry48Aa1 is dependent on the presence of Cry49Aa1, a second accessory protein that is related to both the Bin toxin of B. sphaericus and Cry35 and Cry36 of B. thuringiensis. Heterologous expression of cry48Aa1 and cry49Aa1 in the acrystalliferous 4Q7 strain of B. thuringiensis subsp. israelensis using the robust cyt1A-p/STAB promoter system developed by Park et al. (1999) or its own promoter, respectively, resulted in the synthesis of small bipyramidal (Cry48Aa1) and amorphous (Cry49Aa1) crystalline inclusions (Fig. 5). Although purified Cry48Aa1/Cry49Aa1 proteins showed high mosquitocidal activity against Cx. quinquefasciatus, wild type B. sphaericus strains producing these toxins were less virulent, presumably due to a low level of Cry48Aa1 produced in native strains. Investigations on target specificities of this new binary toxin showed that it is not active against other species of mosquitoes, such as Aedes and Anopheles, and coleopterous and lepidopterous insects (Jones et al., 2008).
Fig. 5.
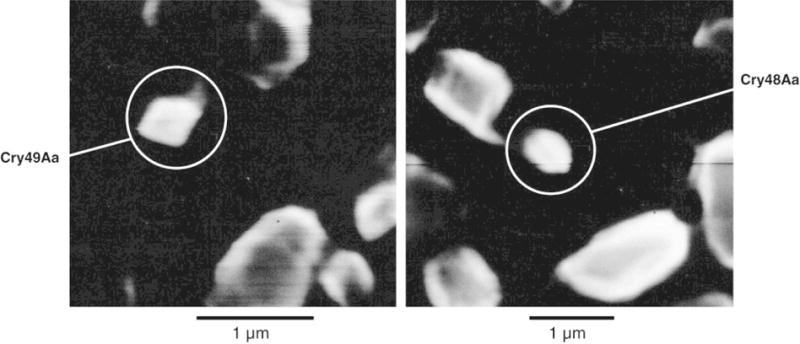
Electron micrographs of recombinant protein crystals. Left panel: Cry49Aa crystals; image taken at × 20,000. Right panel: Cry48Aa crystals; image taken at ×15,000. Jones et al. (2007). Copyright 2007 Federation of American Societies for Experimental Biology
Resistance and receptors
Interactions among the multiple toxins of B. thuringiensis subsp. israelensis and their respective receptors explain the high potency of this strain against target mosquito species, and it is likely that these multiple intermolecular interactions are the major reason for the absence of resistance to B. thuringiensis subsp. israelensis in field populations (Federici et al., 2007; Park and Federici, 2009). Unlike B. thuringiensis subsp. israelensis, B. sphaericus produces only the Bin crystalline inclusion, the primary active ingredient in commercial formulations. For this reason, resistance to B. sphaericus in field populations of Culex mosquitoes, including very high levels of resistance in China and Thailand, has been reported from several countries (Rao et al., 1995; Silva-Filha et al., 1995; Yuan et al., 2000; Nielsen-LeRoux et al., 2002; Su and Mulla, 2004).
The mechanisms of resistance to Bin have been elucidated over the past decade since the receptor for the toxin was identified. In Culex pipiens, the receptor (Cpm1) for Bin is a 60-kDa midgut brush border membrane protein (Silva-Filha et al., 1999). Cpm1 is anchored in the mosquito midgut membrane by its linkage to glycosyl-phosphatidylinositol (GPI), and is partially released by phosphatidylinositol specific-phospholipase (PI-PLC). Cloning and sequencing of cpm1 indicated that the open reading frame encodes a predicted peptide of 580 residues with a putative signal peptide in the amino-terminus and a putative GPI-anchoring signal in the carboxyl-terminus (Darboux et al., 2001). The deduced amino acid sequence of the cloned cpm1 gene showed 39–43% identities with insect maltases (α-glucosidases and α-amylases). Recombinant Cpm1 produced in E. coli specifically bound the Bin toxin and had α-glucosidase activity but no α-amylase activity. In addition, in separate studies, it was demonstrated that Types 1 and 2 Bin toxins produced by B. sphaericus IAM59 and 2362, respectively, share a binding site in the midgut brush border membrane of Cx. pipiens larvae (Silva-Filha et al., 2004). More recently, gene orthologues encoding the Bin toxin receptor were cloned from a malaria-vector, Anopheles gambiae (Agm3) and the Bin-resistant species, Aedes aegypti (Opota et al., 2008). Comparison of these two genes with cpm1 showed that all three share a very similar organization and are strongly conserved at the amino acid level, in particular in the amino-terminus, a region believed to contain the ligand binding site. The structural similarities of the Cpm1 orthologues (Fig. 6) suggest that relatively few amino acids residues are critical for high affinity binding of the toxin.
Fig. 6.
Alignment of the deduced amino acid sequences of Cpm1 genes in Culex, Anopheles and Aedes mosquitoes. Identical residues in the alignment are colored in black boxes. Grey boxes indicate conservative replacements. Dots indicate alignment gaps. GenBank accession no: Anopheles, EU166335; Culex, AF222024; Aedes, AAEL010537-PA. Opota et al. (2008). Copyright 2008 Elsevier.
The cpm1 gene from the Bin-resistant Cx. pipiens (cpm1GEO) was also cloned and compared with cpm1. Sequence alignment revealed that cpm1GEO differed from cmp1 by seven mutations. Six of these mutations were mis-sense mutations that led to the following amino acid replacements: alanine at position 95 to aspartate, lysine at position 115 to methionine, glutamate at position 178 to threonine, aspartate at position 230 to histidine, asparagine at position 265 to aspartate, and leucine at position 486 to methionine. The seventh mutation at position 569 converted the codon for leucine to a stop codon resulting in the shortening of the hydrophobic tail of Cpm1 and preventing the processing of this protein and its subsequent attachment to the midgut membrane. The toxin binding site was unaffected by the mutation (Darboux et al., 2002). In an independent study, the gene encoding the Cx. quinquefasciatus Bin toxin receptor, cqm1 was cloned (Romão et al., 2006), and the deduced amino acid sequence confirmed its identity as an α-glucosidase, similar to cpm1. However, unlike cpm1GEO, analysis of the corresponding gene sequence from resistant larvae (cqm1REC) implicated a 19-nucleotide deletion as the basis for resistance. Furthermore, another novel mechanism of resistance to the Bin toxin in a natural population of Cx. pipiens was elucidated by Darboux et al. (2007). In this study, it was shown that the insertion of a transposable-like DNA element into the coding sequence of the midgut toxin receptor induces a new mRNA splicing event, unmasking cryptic donor and acceptor sites located in the host gene. The creation of the new intron causes the expression of an altered membrane protein, which is incapable of interacting with the toxin, thus providing the host with an advantageous phenotype. In another study, resistance in non-treated and B. sphaericus-treated field populations of Cx. quinquefasciatus was assessed through bioassays as well as a specific PCR assay designed to detect the cqm1REC allele in individual larvae (Chalegre et al., 2009). The results indicated that the cqm1REC allele was present at a detectable frequency in non-treated populations, while the higher frequency in samples from the treated area may be correlated with exposure to B. sphaericus.
Midgut cells from Cx. quinquefasciatus larvae resistant to the Bin and Cry48Aa1/Cry49Aa1 toxins were also observed using transmission electron microscopy (de Melo et al., 2008; 2009). These columnar epithelial cells were characterized by pronounced production of lipid inclusions throughout the 4th instar. At the end of this stage, resistant larvae were larger in size and had an increased number of inclusions in the midgut cells when compared to those observed in cells from susceptible larvae. The morphological differences in midgut cells of resistant larvae suggested that the lack of the Cqm1 receptor, also an α-glucosidase, could be correlated to changes in cellular metabolism. The cytopathological alterations observed in cells of susceptible larvae treated with a lethal concentration of toxin included breakdown of the endoplasmic reticulum, mitochondrial swelling, and microvillar disruption and vacuolization. Some of these effects were observed in cells from resistant larvae, although those alterations did not lead to larval death, indicating that Cqm1 is essential to mediate the larvicidal action of the toxin.
A series of mosquito bioassays using recombinant Mtx1 and Bin produced in E. coli and B. thuringiensis, respectively, showed that Mtx1 has moderate toxicity against Aedes albopictus and high toxicity against both susceptible and Bin-resistant Cx. quinquefasciatus, suggesting that Mtx1 has a different mode of action than Bin and lacks cross-resistance to B. sphaericus-resistant Cx. quinquefasciatus (Wei et al., 2007).
Synergism and resistance management
As resistance in field population of mosquitoes is more likely to develop against Bin when compared to the Cry and Cyt multi-toxin components in B. thuringiensis subsp. israelensis, much interest has been focused on developing strategies to prevent or delay the onset of resistance to B. sphaericus in these insects and/or to confer Bin toxicity on more naturally resistant species, including Aedes species. To this end, Wirth et al. (2000a) have reported that Cyt1Aa, a detergent-like lipophilic membrane-binding protein (Manceva et al., 2005) from B. thuringiensis subsp. israelensis, synergizes the activity of B. sphaericus against Ae. aegypti, a mosquito species highly refractive to B. sphaericus (Wirth et al., 2000a). In this study, a ratio of 10:1 of B. sphaericus/Cyt1Aa was 3,600-fold more toxic to Ae. aegypti than when B. sphaericus was used alone. Similarly, two other Cyt toxins, Cyt1Ab from B. thuringiensis subsp. medellin and Cyt2Ba from B. thuringiensis subsp. israelensis also synergized B. sphaericus activity against Ae. aegypti (Wirth et al., 2001). More importantly, when used in combination with B. sphaericus, Cyt1Aa was shown to reestablish activity of Bin against B. sphaericus-resistant Cx. quinquefasciatus (Wirth et al., 2000b). A combination of B. sphaericus 2362 in a 10:1 ratio with a strain of B. thuringiensis subsp. israelensis that only produces Cyt1Aa reduced resistance in Cx. quinquefasciatus by more than 30,000 fold, indicating a synergistic interaction between Cyt1A and Bin. Significant levels (from 883-fold to 59,000-fold) of reduction in resistance using different Cry combinations and/or crystal-spore preparations of B. thuringiensis subsp. israelensis and B. sphaericus against B. sphaericus-resistant Cx. quinquefasciatus and Ae. aegypti have also been observed (Wirth et al., 2004).
Wirth et al. (2007) have also established that synergism occurs between B. sphaericus and Mtx1 or Mtx2 produced in E. coli and that Mtx toxins, like Cyt1A which also enhances Mtx1 activity (Zhang et al., 2006), could mask resistance in B. sphaericus-resistant mosquito larvae. Therefore the combined results reported by Wirth et al. (2000a; 2004; 2007) and Zhang et al. (2006) provide foundational principles for the applied use of Cyt1A, Cry, Cyt, and Mtx toxins in managing resistance to B. sphaericus in field population of mosquitoes.
Recombinant strains
The development of recombinant strains of B. sphaericus with improved efficacy and built-in mechanisms to prevent resistance in the field is of critical importance for continued success and applied use of this bacterium. The main strategy for this purpose is to introduce other genes including mtx, cry and cyt into wild-type strains of B. sphaericus that could synergize bin. For example, when synthesis of Mtx1 from B. sphaericus was redirected to the sporulation phase of growth by replacing its weak native promoter with the strong sporulation promoter of the bin operon (Yang et al., 2007), the recombinant strain displayed toxicity during early sporulation, but this declined rapidly in later stages. Protease inhibition studies showed that the decline in Mtx1 was due to the serine proteinase, sphericase (Yang et al., 2007). Mutant Mtx1 protein lacking the susceptible site was expressed in a recombinant strain in an attempt to overcome destructive cleavage while remaining capable of proteolytic activation. However, the apparently broad specificity of sphericase seems to make this impossible (Yang et al., 2007). The instability of Mtx2 when exposed to sphericase or culture supernatants was also observed (Yang et al., 2007). Thus, the exploitation of Mtx toxins may, therefore, be greatly limited by their susceptibility to proteases produced by the host bacterium and their inability to from stable crystalline inclusions.
The co-synthesis of Cry and Bin proteins has also been explored for creating more virulent strains of B. sphaericus, but with little success (Yang et al., 2007). Random mutations of the receptor binding loops of the Cry1Aa toxin, in contrast, allowed production of significant levels of spore-associated protein in the form of parasporal inclusions (Yang et al., 2007). Finally, as Cyt1A is known to synergize Bin and restore sensitivity to this toxin in Bin-resistant mosquito larvae (Wirth et al., 2000a; 2000b), we have attempted to produce Cyt1A in B. sphaericus strains 2362 and 2297 with the intent of developing strains that are less susceptible to resistance in the field, but have been unsuccessful in this endeavor (Park et al., unpublished data).
Finally, expression of the chiAC chitinase gene from B. thuringiensis in B. sphaericus 2297 using the bin operon promoter yielded a recombinant strain that was 4,297-fold more toxic than strain 2297 against resistant Cx. quinquefasciatus (Cai et al., 2007). These results show that chitinase can synergize the toxicity of Bin and thus may be useful in managing larval resistance to B. sphaericus.
Sphaericolysin, a non-mosquitocidal toxin produced by Bacullus sphaericus
Although B. sphaericus is an established pathogen of mosquitoes, a recent report has demonstrated that a strain isolated from ant lion (Myrmeleon borfe) larvae produces a novel insecticidal toxin unrelated to Bin and Mtx (Nishiwaki et al., 2007). This novel hemolytic toxin, called sphaericolysin, has a molecular mass of 53 kDa and its activity results from the protein’s pore-forming action (Fig. 7). When injected, sphaericolysin is highly active against the German cockroach (Blattella germanica). Interestingly, when co-injected with cholesterol, the toxicity of sphaericolysin was abolished, suggesting that cholesterol binding plays an important role in moderating the insecticidal activity of the hemolysin (Nishiwaki et al., 2007).
Fig. 7.
Nucleotide sequence of the gene encoding the 53-kDa sphaericolysin toxin and its amino acid sequence deduced from the nucleotide sequence. The underlined sequences were determined by Edman degradation. The putative Shine-Dalgarno, −10, and −35 sequences are indicated by the dotted lines. The bold line indicates the putative start codon of the precursor protein. An arrowhead indicates a possible cleavage point for the signal peptide that was predicted by employing the neural networks and hidden Markov models trained on sequences in the gram-positive-bacterium database. The NdeI site is indicated as italic characters (positions 467 to 472). A point mutation generated at position 159 is enclosed by a square. Nishiwaki et al. (2007). Copyright 2007 American Society for Microbiology.
Other characterized non-lethal genes in Bacillus sphaericus
Until recently, few genes in B. sphaericus were characterized. As such, very little is known about the major genetic differences that distinguish the 5 known DNA groups and 49 known serotypes of B. sphaericus. However, the recent report of the complete genome sequence of strain C3-41 (Hu et al., 2008a) that like strains 2362 and 1593 belongs to the flagellar serotype H5a5b (Yuan et al., 2001) could provide a foundation for understanding the molecular basis for their high virulence against mosquito larvae. Moreover, the sequence data will undoubtedly expose strategies to genetically manipulate strains to generate even more highly efficacious larvicides for applied use. For example, the genome sequence of C3-41 has revealed that this asaccharolytic strain lacks genes that code for enzymes involved in sugar transport systems, thus relying on alternative pathways, such as metabolism of amino acids and other organics beside sugars, for its propagation. Whether engineering genes involved in sugar transport or other pathways lacking in B. sphaericus could affect increases in yield of Bin and other virulence factors remains to be resolved, but nevertheless, this prospect is intriguing. Moreover, the identification of sporulation-dependent promoters is of special interest as these cis elements could prove useful for the efficient expression of heterologous Cry and Cyt crystal toxin genes.
Regardless, the few genes other than bin that have been well characterized include a genes that encode a thermostable DNA polymerase I (polI) (Han et al., 2006) and a glucokinase-encoding (glcK) (Han et al., 2007) that was cloned as a part of an effort to elucidate metabolic pathways of asaccharolytic B. sphaericus. Biochemical analysis of glcK revealed that it encodes a protein with a molecular mass of 33 kDa and that the purified recombinant glucokinase has Km values of 0.52 and 0.31 mM for ATP and glucose, respectively. It has been shown that this ATP-dependent glucokinase can also phosphorylate fructose and mannose. Sequence alignment of GlcK indicated that it belongs to Group B of the hexokinase family of proteins.
The surface layer (S-layer) protein genes of several B. sphaericus strains have also been characterized (Bowditch et al., 1989; Ilk et al., 2002; Pollmann et al., 2005; Hu et al., 2008b). Alignments of S-layer proteins, which are part of the cell envelope commonly found in bacteria and archaea and constitute from 10–15% of cellular proteins (Engelhardt, 2007), indicate that mosquitocidal B. sphaericus C3-41 and 2362 strains form a clade (Group 1) distinct from those of non-mosquitocidal strains (strains CCM2177, P1, NCTC9602 and JG-A12) (Group 2) (Ilk et al., 2002; Pollmann et al., 2005). As the toxicity of B. sphaericus is optimal with both the spore and Bin crystal combination, it has been suggested that components in the spore, including the S-layer protein, could contribute to its larvicidal activity. However, it is unlikely that the S-layer protein is involved in toxicity as it has been shown that both the truncated amino-terminal peptide and native S-layer protein, synthesized in E. coli, were not toxic to mosquito larvae (Hu et al., 2008b).
Conclusions
Since the discovery of the first mosquitocidal strain of B. sphaericus and the subsequent successful implementation of VectoLex®, there have been significant advances in understanding the molecular basis for Bin’s toxicity and the mechanisms of resistance to this toxin. The potential for genetically engineering more potent strains that synthesize combinations of Bin, Mtx, Cry, and Cyt proteins is promising. In this regard, the report of the first complete genome sequence of B. sphaericus (C3-41) (Hu et al., 2008a) could provide insights into molecular strategies that could be exploited to promote stable synthesis and perhaps crystallization of foreign proteins, similar to strategies employed in engineering highly efficacious B. thuringiensis subsp. israelensis recombinants (Park et al., 2005; 2007a; 2009). Indeed, the acquisition of a superior larvidical B. sphaericus strain with an expanded range of targets and built-in mechanisms that prevent or delay resistance in field populations of mosquitoes is desirable. The development and applied use of such a strain could circumvent current resistance management strategies such as rotating treatments between B. thuringiensis subsp. israelensis and B. sphaericus (Zahiri et al., 2002), or using formulations of mixture of the two bacteria, including the most recent product VectoMax® (Valent BioSciences, Libertyville, IL) developed for commercial use.
Acknowledgments
This work was partially supported by a grant to H.-W. P. from the U. S. Department of Agriculture (2007-38814-18497) and by grants to B. A. F. from the U. S. National Institutes of Health (AI145817 and AI054778).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berry C, Hindley J, Ehrhardt AF, Grounds T, de Souza I, Davidson EW. Genetic determinants of host ranges of Bacillus sphaericus mosquito larvicidal toxins. J Bacteriol. 1993;175:510–518. doi: 10.1128/jb.175.2.510-518.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch RD, Baumann P, Yousten AA. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989;171:4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbridge M, Margalit J. Mosquito active strains of Bacillus sphaericus isolated from soil and mud samples collected in Israel. J Invertebr Pathol. 1987;50:106–112. doi: 10.1016/0022-2011(87)90109-1. [DOI] [PubMed] [Google Scholar]
- Cai Y, Yan J, Hu X, Han B, Yuan Z. Improving the insecticidal activity against resistant Culex quinquefasciatus mosquitoes by expression of chitinase gene chiAC in Bacillus sphaericus. Appl Environ Microbiol. 2007;73:7744–7746. doi: 10.1128/AEM.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpusca I, Jank T, Aktories K. Bacillus sphaericus mosquitocidal toxin (MTX) and piericin: the enigmatic offspring from the family of ADP-ribosyltransferases. Mol Microbiol. 2006;62:621–630. doi: 10.1111/j.1365-2958.2006.05401.x. [DOI] [PubMed] [Google Scholar]
- Cavados CF, Fonseca RN, Chaves JQ, Rabinovitch L, Araújo-Coutinho CJ. Identification of entomopathogenic Bacillus isolated from Simulium (Diptera: Simuliidae) larvae and adults. Mem Inst Oswaldo Cruz. 2001;96:1017–1021. doi: 10.1590/s0074-02762001000700023. [DOI] [PubMed] [Google Scholar]
- Chalegre KD, Romão TP, Amorim LB, Anastacio DB, de Barros RA, de Oliveira CM, Regis L, de-Melo-Neto OP, Silva-Filha MH. Detection of an allele conferring resistance to Bacillus sphaericus binary toxin in Culex quinquefasciatus populations by molecular screening. Appl Environ Microbiol. 2009;75:1044–1049. doi: 10.1128/AEM.02032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles JF, Nielsen-LeRoux C, Delécluse A. Bacillus sphaericus toxins: molecular biology and mode of action. Annu Rev Entomol. 1996;41:451–472. doi: 10.1146/annurev.en.41.010196.002315. [DOI] [PubMed] [Google Scholar]
- Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D. Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae) Cell Microbiol. 2007;9:2022–2029. doi: 10.1111/j.1462-5822.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- Darboux I, Nielsen-LeRoux C, Charles JF, Pauron D. The receptor of Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae) midgut: molecular cloning and expression. Insect Biochem Mol Biol. 2001;31:981–990. doi: 10.1016/s0965-1748(01)00046-7. [DOI] [PubMed] [Google Scholar]
- Darboux I, Pauchet Y, Castella C, Silva-Filha MH, Nielsen-LeRoux C, Charles JF, Pauron D. Loss of the membrane anchor of the target receptor is a mechanism of bioinsecticide resistance. Proc Natl Acad Sci USA. 2002;99:5830–5835. doi: 10.1073/pnas.092615399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo JV, Jones GW, Berry C, Vasconcelos RH, de Oliveira CM, Furtado AF, Peixota CA, Silva-Filha MH. Cytopathological effects of Bacillus sphaericus Cry48Aa/Cry49Aa toxin on binary toxin-susceptible and —resistant Culex quinquefasciatus larvae. Appl Environ Microbiol. 2009;75:4782–4789. doi: 10.1128/AEM.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo JV, Vasconcelos RHT, Furtado AF, Peixoto CA, Silva-Filha MHNL. Ultrastructural analysis of midgut cells from Culex quinquefasciatus (Diptera: Culicidae) larvae resistant to Bacillus sphaericus. Micron. 2008;39:1342–1350. doi: 10.1016/j.micron.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Engelhardt H. Are S-layers exoskeletons? The basic function of protein surface layers revisited. J Struct Biol. 2007;160:115–125. doi: 10.1016/j.jsb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Federici BA, Park HW, Bideshi DK, Wirth MC, Johnson JJ, Sakano Y, Tang M. Developing recombinant bacteria for control of mosquito larvae. In: Floore TG, editor. Biorational Control of Mosquitoes. American Mosquito Control Association; Mount Laurel, NJ: 2007. pp. 164–175. [DOI] [PubMed] [Google Scholar]
- Guerineau M, Alexander B, Priest FG. Isolation and identification of Bacillus sphaericus strains pathogenic for mosquito larvae. J Invertebr Pathol. 1991;57:325–333. doi: 10.1016/0022-2011(91)90136-e. [DOI] [PubMed] [Google Scholar]
- Han B, Liu H, Hu X, Yuan Z. Preliminary characterization of a thermostable DNA polymerase I from a mesophilic Bacillus sphaericus strain C3-41. Arch Microbiol. 2006;186:203–209. doi: 10.1007/s00203-006-0135-3. [DOI] [PubMed] [Google Scholar]
- Han B, Liu H, Hu X, Cai Y, Zheng D, Yuan Z. Molecular characterization of a glucokinase with broad hexose specificity from Bacillus sphaericus strain C3-41. Appl Environ Microbiol. 2007;73:3581–3586. doi: 10.1128/AEM.02863-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazes B, Read RJ. A mosquitocidal toxin with a ricin-like cell-binding domain. Nat Struct Biol. 1997;2:358–359. doi: 10.1038/nsb0595-358. [DOI] [PubMed] [Google Scholar]
- Hu X, Fan W, Han B, Liu H, Zheng D, Li Q, Dong W, Yan J, Gao M, Berry C, Yuan Z. Complete genome sequence of the mosquitocidal bacterium Bacillus sphaericus C3-41 and comparison with those of closely related Bacillus species. J Bacteriol. 2008a;190:2892–2902. doi: 10.1128/JB.01652-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li J, Hansen BM, Yuan Z. Phylogenetic analysis and heterologous expression of surface layer protein SlpC of Bacillus sphaericus C3-41. Biosci Biotechnol Biochem. 2008b;72:1257–1263. doi: 10.1271/bbb.70747. [DOI] [PubMed] [Google Scholar]
- Humphreys MJ, Berry C. Variants of the Bacillus sphaericus binary toxins: implications for differential toxicity of strains. J Invertebr Pathol. 1998;71:184–185. doi: 10.1006/jipa.1997.4711. [DOI] [PubMed] [Google Scholar]
- Ilk N, Vollenkle C, Egelseer EM, Breitwieser A, Sleytr UB, Sára M. Molecular characterization of the S-layer gene, sbpA, of Bacillus sphaericus CCM 2177 and production of a functional S-layer fusion protein with the ability to recrystallize in a defined orientation while presenting the fused allergen. Appl Environ Microbiol. 2002;68:3251–3260. doi: 10.1128/AEM.68.7.3251-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GW, Nielsen-LeRoux C, Yang Y, Yuan Z, Dumas VF, Monnerat RG, Berry C. A new Cry toxin with a unique two-component dependency from Bacillus sphaericus. FASEB J. 2007;21:4112–4120. doi: 10.1096/fj.07-8913com. [DOI] [PubMed] [Google Scholar]
- Jones GW, Wirth MC, Monnerat RG, Berry C. The Cry48Aa-Cry49Aa binary toxin from Bacillus sphaericus exhibits highly restricted target specificity. Environ Microbiol. 2008;10:2418–2424. doi: 10.1111/j.1462-2920.2008.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellen WR, Clark TB, Lindegren JE, Ho BC, Rogoff MH, Singer S. Bacillus sphaericus Neide as a pathogen of mosquitoes. J Invertebr Pathol. 1965;7:442–448. doi: 10.1016/0022-2011(65)90120-5. [DOI] [PubMed] [Google Scholar]
- Khyami-Horani H, Katbeh-Bader A, Mohsen ZH. Isolation of endospore-forming bacilli toxic to Culiseta longiareolata (Diptera; Culicidae) in Jordan. Lett Appl Microbiol. 1999;28:57–60. doi: 10.1046/j.1365-2672.1999.00469.x. [DOI] [PubMed] [Google Scholar]
- Krych VK, Johnson JL, Yousten AA. Deoxyribonucleic acid homologies among strains of Bacillus sphaericus. Int J Sys Bacteriol. 1980;30:476–484. [Google Scholar]
- Limpanawat S, Promdonkoy B, Boonserm P. The C-terminal domain of BinA is responsible for Bacillus sphaericus binary toxin BinA-BinB interaction. Curr Microbiol. 2009;59:509–513. doi: 10.1007/s00284-009-9468-x. [DOI] [PubMed] [Google Scholar]
- Liu JW, Porter AG, Wei BY, Thanabalu T. New gene from nine Bacillus sphaericus strains encoding highly conserved 35.8-kilodalton mosquitocidal toxins. Appl Environ Microbiol. 1996;62:2174–2176. doi: 10.1128/aem.62.6.2174-2176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceva SA, Pusztai-Carey M, Russo PS, Butko P. A detergent-like mechanism of action of the cytolytic toxin Cyt1A from Bacillus thuringiensis var. israelensis Biochemistry. 2005;44:589–597. doi: 10.1021/bi048493y. [DOI] [PubMed] [Google Scholar]
- Nielsen-LeRoux C, Pasteur N, Prètre J, Charles JF, Sheikh HB, Chevillon C. High resistance to Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae): the complex situation of west Mediterranean countries. J Med Entomol. 2002;39:729–735. doi: 10.1603/0022-2585-39.5.729. [DOI] [PubMed] [Google Scholar]
- Nishiwaki H, Nakashima K, Ishida C, Kawamura T, Matsuda K. Cloning, functional characterization, and mode of action of a novel insecticidal pore-forming toxin, sphaericolysin, produced by Bacillus sphaericus. Appl Environ Microbiol. 2007;73:3404–3411. doi: 10.1128/AEM.00021-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opota O, Charles JF, Warot S, Pauron D, Darboux I. Identification and characterization of the receptor for the Bacillus sphaericus binary toxin in the malaria vector mosquito, Anopheles gambiae. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:419–427. doi: 10.1016/j.cbpb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Orduz-Peralta S, Diaz T, Restrepo N, Rojas W, Yousten AA. Isolation and characterization of four new strains of Bacillus sphaericus from central Nigeria highly toxic to mosquito larvae. J Invertebr Pathol. 1992;60:107–108. doi: 10.1016/0022-2011(92)90162-w. [DOI] [PubMed] [Google Scholar]
- Park HW, Bideshi DK, Federici BA. The 20-kDa protein of Bacillus thuringiensis subsp. israelensis enhances Bacillus sphaericus 2362 Bin toxin synthesis. Curr Microbiol. 2007a;55:119–124. doi: 10.1007/s00284-006-0359-0. [DOI] [PubMed] [Google Scholar]
- Park HW, Bideshi DK, Johnson JJ, Federici BA. Differential enhancement of Cry2A versus Cry11A yields in Bacillus thuringiensis by the use of the cry3A STAB mRNA sequence. FEMS Microbiol Lett. 1999;181:319–327. doi: 10.1111/j.1574-6968.1999.tb08862.x. [DOI] [PubMed] [Google Scholar]
- Park HW, Bideshi DK, Wirth MC, Johnson JJ, Walton WE, Federici BA. Recombinant larvicidal bacteria with markedly improved efficacy against Culex vectors of West Nile virus. Am J Trop Med Hyg. 2005;72:732–738. [PubMed] [Google Scholar]
- Park HW, Federici BA. Genetic engineering of bacteria to improve efficacy using the insecticidal proteins of Bacillus species. In: Stock SP, Vandenberg J, Glazer I, Boemare N, editors. Insect Pathogens: Molecular Approaches and Techniques. CABI International; Oxfordshire, UK: 2009. pp. 275–305. [Google Scholar]
- Park HW, Mangum CM, Zhong H, Hayes SR. Isolation of Bacillus sphaericus with improved efficacy against Culex quinquefasciatus. J Am Mosq Control Assoc. 2007b;23:478–480. doi: 10.2987/5663.1. [DOI] [PubMed] [Google Scholar]
- Park HW, Tang M, Sakano Y, Federici BA. A 1.1-kilobase region downstream of the bin operon in Bacillus sphaericus strain 2362 decreases Bin yield and crystal size in strain 2297. Appl Environ Microbiol. 2009;75:878–881. doi: 10.1128/AEM.01444-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott CR, Ellar DJ. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 2007;71:255–281. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann K, Raff J, Schnorpfeil M, Radeva G, Selenska-Pobell S. Novel surface layer protein genes in Bacillus sphaericus associated with unusual insertion elements. Microbiology. 2005;151:2961–2973. doi: 10.1099/mic.0.28201-0. [DOI] [PubMed] [Google Scholar]
- Poncet S, Bernard C, Dervyn E, Cayley J, Klier A, Rapoport G. Improvement of Bacillus sphaericus toxicity against dipteran larvae by integration, via homologous recombination, of the Cry11A toxin gene from Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 1997;63:4413–4420. doi: 10.1128/aem.63.11.4413-4420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest FG, Ebdrup L, Zahner V, Carter PE. Distribution and characterization of mosquitocidal toxin genes in some strains of Bacillus sphaericus. Appl Environ Microbiol. 1997;63:1195–1198. doi: 10.1128/aem.63.4.1195-1198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promdonkoy B, Promdonkoy P, Wongtawan B, Boonserm P, Panyim S. Cys31, Cys47, and Cys195 in BinA are essential for toxicity of a binary toxin from Bacillus sphaericus. Curr Microbiol. 2008;56:334–338. doi: 10.1007/s00284-007-9065-9. [DOI] [PubMed] [Google Scholar]
- Rao DR, Mani TR, Rajendran R, Joseph AS, Gajanana A, Reuben R. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J Am Mosq Control Assoc. 1995;11:1–5. [PubMed] [Google Scholar]
- Reinert DJ, Carpusca I, Aktories K, Schulz GE. Structure of the mosquitocidal toxin from Bacillus sphaericus. J Mol Biol. 2006;357:1226–1236. doi: 10.1016/j.jmb.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Româo TT, de Melo-Chalegre KD, Key S, Ayres CFJ, de Oliveira CMF, de Melo-Neto OP, Silva-Filha MHNL. A second independent resistance mechanism to Bacillus sphaericus binary toxin targets its α-glucosidase receptor in Culex quinquefasciatus. FEBS J. 2006;273:1556–1568. doi: 10.1111/j.1742-4658.2006.05177.x. [DOI] [PubMed] [Google Scholar]
- Sanitt P, Promdonkoy B, Boonserm P. Targeted mutagenesis at charged residues in Bacillus sphaericus BinA toxin affects mosquito-larvicidal activity. Curr Microbiol. 2008;57:230–234. doi: 10.1007/s00284-008-9180-2. [DOI] [PubMed] [Google Scholar]
- Servant P, Rosso ML, Hammon S, Poncet S, Delécluse A, Rapoport G. Production of Cry11A and Cry11Ba toxins in Bacillus sphaericus confers toxicity towards Aedes aegypti and resistant Culex populations. Appl Environ Microbiol. 1999;65:3021–3026. doi: 10.1128/aem.65.7.3021-3026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Filha MH, Oliveira CM, Regis L, Yuan Z, Rico CM, Nielsen-LeRoux C. Two Bacillus sphaericus binary toxins share the midgut receptor binding site: implication for resistance of Culex pipiens complex (Diptera: Culicidae) larvae. FEMS Microbiol Lett. 2004;241:185–191. doi: 10.1016/j.femsle.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Silva-Filha MH, Nielsen-LeRoux C, Charles JF. Identification of the receptor for Bacillus sphaericus crystal toxin in the brush border membrane of the mosquito Culex pipiens (Diptera: Culicidae) Insect Biochem Mol Biol. 1999;29:711–721. doi: 10.1016/s0965-1748(99)00047-8. [DOI] [PubMed] [Google Scholar]
- Silva-Filha MH, Regis L, Nielsen-LeRoux C, Charles JF. Low-level resistance to Bacillus sphaericus in a field-treated population of Culex quinquefasciatus (Diptera: Culicidae) J Econ Entomol. 1995;88:525–530. [Google Scholar]
- Singer S. Insecticidal activity of recent bacterial isolates and their toxins against mosquito larvae. Nature. 1973;244:110–111. doi: 10.1038/244110a0. [DOI] [PubMed] [Google Scholar]
- Singer S. Entomogenous bacilli against mosquito larvae. Dev Ind Microbiol. 1974;15:187–194. [Google Scholar]
- Su T, Mulla MS. Documentation of high-level Bacillus sphaericus 2362 resistance in field populations of Culex quinquefasciatus breeding in polluted water in Thailand. J Am Mosq Control Assoc. 2004;20:405–411. [PubMed] [Google Scholar]
- Thanabalu T, Berry C, Hindley J. Cytotoxicity and ADP-ribosylating activity of the mosquitocidal toxin from Bacillus sphaericus SSII-1: possible roles of the 27- and 70-kilodalton peptides. J Bacteriol. 1993;175:2314–2320. doi: 10.1128/jb.175.8.2314-2320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanabalu T, Hindley J, Berry C. Proteolytic processing of the mosquitocidal toxin from Bacillus sphaericus SSII-1. J Bacteriol. 1992;174:5051–5056. doi: 10.1128/jb.174.15.5051-5056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanabalu T, Porter AG. Efficient expression of a 100-kilodalton mosquitocidal toxin in protease-deficient recombinant Bacillus sphaericus. Appl Environ Microbiol. 1995;61:4031–4036. doi: 10.1128/aem.61.11.4031-4036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanabalu T, Porter AG. A Bacillus sphaericus gene encoding a novel type of mosquitocidal toxin of 31.8 kDa. Gene. 1996;170:85–89. doi: 10.1016/0378-1119(95)00836-5. [DOI] [PubMed] [Google Scholar]
- Thanabalu T, Hindley J, Jackson-Yap J, Berry C. Cloning, sequencing, and expression of a gene encoding a 100-kilodalton mosquitocidal toxin from Bacillus sphaericus SSII-1. J Bacteriol. 1991;173:2776–2785. doi: 10.1128/jb.173.9.2776-2785.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber N, Reinert DJ, Carpusca I, Aktories K, Schulz GE. Structure and mode of action of a mosquitocidal holotoxin. J Mol Biol. 2008;381:150–159. doi: 10.1016/j.jmb.2008.05.067. [DOI] [PubMed] [Google Scholar]
- Wei S, Cai Q, Cai Y, Yuan Z. Lack of cross-resistance to Mtx1 from Bacillus sphaericus in B. sphaericus-resistant Culex quinquefasciatus (Diptera: Culicidae) Pest Manag Sci. 2007;63:190–193. doi: 10.1002/ps.1319. [DOI] [PubMed] [Google Scholar]
- Wei S, Cai Q, Yuan Z. Mosquitocidal toxin from Bacillus sphaericus induces stronger delayed effects than binary toxin on Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2006;43:726–730. doi: 10.1603/0022-2585(2006)43[726:mtfbsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Weiser J. A mosquito-virulent Bacillus sphaericus in adult Simulium damnosum from northern Nigeria. Zentralbl Mikrobiol. 1984;139:57–60. [PubMed] [Google Scholar]
- Wickremesinghe RSB, Mendis CL. Bacillus sphaericus spore from Sri Lanka demonstrating rapid larvicidal activity on Culex quinquefasciatus. Mosq News. 1980;40:387–389. [Google Scholar]
- Wirth MC, Delécluse A, Walton WE. Cyt1Ab1 and Cyt2Ba1 from Bacillus thuringiensis subsp. medellin and B thuringiensis subsp. israelensis synergize Bacillus sphaericus against Aedes aegypti and resistant Culex quinquefasciatus (Diptera: Culicidae) Appl Environ Microbiol. 2001;67:3280–3284. doi: 10.1128/AEM.67.7.3280-3284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Jiannino JA, Federici BA, Walton WE. Synergy between toxins of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus. J Med Entomol. 2004;41:935–941. doi: 10.1603/0022-2585-41.5.935. [DOI] [PubMed] [Google Scholar]
- Wirth MC, Federici BA, Walton WE. Cyt1A from Bacillus thuringiensis synergizes activity of Bacillus sphaericus against Aedes aegypti (Diptera: Culicidae) Appl Environ Microbiol. 2000a;66:1093–1097. doi: 10.1128/aem.66.3.1093-1097.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Walton WE, Federici BA. Cyt1A from Bacillus thuringiensis restores toxicity of Bacillus sphaericus against resistant Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2000b;37:401–407. [PubMed] [Google Scholar]
- Wirth MC, Yang Y, Walton WE, Federici BA, Berry C. Mtx toxins synergize Bacillus sphaericus and Cry11Aa against susceptible and insecticide-resistant Culex quinquefasciatus larvae. Appl Environ Microbiol. 2007;73:6066–6071. doi: 10.1128/AEM.00654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang L, Gaviria A, Yuan Z, Berry C. Proteolytic stability of insecticidal toxins expressed in recombinant bacilli. Appl Environ Microbiol. 2007;73:218–225. doi: 10.1128/AEM.01100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Rang C, Maroun RC, Victor JP, Frutos R, Pasteur N, Vandrely C, Charles JF, Nielsen-LeRoux C. Identification and molecular structural prediction analysis of a toxicity determinant in Bacillus sphaericus crystal larvicidal toxin. Eur J Biochem. 2001;268:2751–2760. doi: 10.1046/j.1432-1327.2001.02176.x. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Zhang Y, Cia Q, Liu EY. High-level field resistance to Bacillus sphaericus C3-41 in Culex quinquefasciatus from southern China. Biocontr Sci Technol. 2000;10:41–49. [Google Scholar]
- Zahiri NS, Su T, Mulla MS. Strategies for the management of resistance in mosquitoes to the microbial control agent Bacillus sphaericus. J Med Entomol. 2002;39:513–520. doi: 10.1603/0022-2585-39.3.513. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu M, Yang Y, Yuan Z. Cytolytic toxin Cyt1Aa of Bacillus thuringiensis synergizes the mosquitocidal toxin Mtx1 of Bacillus sphaericus. Biosci Biotechnol Biochem. 2006;70:2199–2204. doi: 10.1271/bbb.60140. [DOI] [PubMed] [Google Scholar]


