Abstract
Introduction
Renal sodium (Na+) retention and extracellular fluid volume expansion are hallmarks of nephrotic syndrome, which occurs even in the absence of activation of hormones that stimulate renal Na+ transporters. Plasmin-dependent activation of the epithelial Na+ channel has been proposed to have a role in renal Na+ retention in the setting of nephrotic syndrome. We hypothesized that the epithelial Na+ channel inhibitor amiloride would be an effective therapeutic agent in inducing a natriuresis and lowering blood pressure in individuals with macroscopic proteinuria.
Methods
We conducted a pilot double-blind randomized cross-over study comparing the effects of daily administration of either oral amiloride or hydrochlorothiazide to patients with type 2 diabetes and macroscopic proteinuria. Safety and efficacy were assessed by monitoring systolic blood pressure, kidney function, adherence, weight, urinary Na+ excretion, and serum electrolytes. Nine subjects were enrolled in the trial.
Results
No significant difference in systolic blood pressure or weight was seen between subjects receiving hydrochlorothiazide and those receiving amiloride (P ≥ 0.15). Amiloride induced differences in serum potassium (P < 0.001), with a 0.88 ± 0.30 mmol/l greater acute increase observed. Two subjects developed acute kidney injury and hyperkalemia when treated with amiloride. Four subjects had readily detectable levels of urinary plasminogen plus plasmin, and 5 did not. Changes in systolic blood pressure in response to amiloride did not differ between individuals with versus those without detectable urinary plasminogen plus plasmin.
Discussion
In summary, among patients with type 2 diabetes, normal renal function, and proteinuria, there were reductions in systolic blood pressure in groups treated with hydrochlorothiazide or amiloride. Acute kidney injury and severe hyperkalemia were safety concerns with amiloride.
Keywords: amiloride, hyperkalemia, nephrotic syndrome, plasmin, plasminogen, proteinuria
Proteinuria is a reflection of glomerular damage, but it also is a risk factor for cardiovascular disease, stroke, and end-stage kidney disease.1, 2, 3 It has also been associated with extracellular volume expansion and high blood pressure (BP) in various human populations.4, 5, 6 Multiple studies have examined the role of proteinuria as a risk factor for the development of elevated BP. A study involving normotensive adult men and women from Okinawa revealed the annual frequency for development of hypertension to be 2.4-fold higher in individuals with non-nephrotic range proteinuria at baseline.7 Examination of 9 potential biomarkers for hypertension risk in the normotensive, healthy male and female offspring of the Framingham Heart Study participants indicated that urinary albumin/creatinine (Cr), a marker of proteinuria, determined from a single-void morning urine sample predicted the development of hypertension with an odds ratio of 1.21.8 Another study revealed that higher levels of urinary albumin, despite being considered within the normal range, predicted incident hypertension in a population of healthy nondiabetic female nurses.9
The relationship between proteinuria and BP is complicated because hypertension can cause renal damage resulting in increased proteinuria, and the development of essential hypertension does not require preexisting proteinuria.10, 11, 12 Studies involving patients with type 2 diabetes reflect this complicated relationship between proteinuria and hypertension. In the natural course of type 2 diabetes, microalbuminuria and elevations in BP are thought to occur at around the same time. BP in patients with diabetes and microalbuminuria is more sensitive to dietary salt intake than BP in patients with diabetes and normal urinary albumin excretion despite both groups having similar aldosterone and plasma renin activity levels.13 However, proteinuria is not consistently identified as a risk factor for incipient elevation in BP, and in some studies, elevated BP predicts the advent of microalbuminuria.14, 15, 16 Analyses of normotensive subjects with normal urinary albumin excretion in the Diabetes Control and Complications Trial study showed that higher urinary albumin levels, though still in the normal range, predicted incident hypertension.17 A similar finding was seen in a cohort of subjects without diabetes.18 These disparate results regarding hypertension predicting microalbuminuria versus microalbuminuria predicting hypertension may be related to the cut-off value that was used to define microalbuminuria.
Studies have suggested that activation of sodium (Na+) transporters in the distal nephron is responsible for the enhanced renal Na+ retention that is seen in proteinuric states and that Na+ retention in this setting does not require activation of the renin-angiotensin-aldosterone system.19, 20, 21, 22 In rats with experimentally induced nephrotic syndrome, proteinuria-associated Na+ retention is attenuated by the epithelial Na+ channel (ENaC) blocker, amiloride, suggesting a role for ENaC in this process.22, 23 Recent work has suggested that enhanced ENaC activation by filtered proteases may contribute to renal Na+ retention in nephrotic syndrome.24, 25, 26, 27 Proteases activate ENaC by cleaving 2 of the channel subunits (α and γ) at multiple sites flanking embedded inhibitory tracts, releasing these tracts and transitioning the channel to higher activity states (for review, see Kleyman et al.28). The protease furin, constitutively expressed in the trans-Golgi network, has an important role in this process. Furin cleaves the α subunit twice, releasing an inhibitory tract and transitioning channels from a low to a moderate activity state.29, 30 Furin cleaves the γ subunit at a site preceding its inhibitory tract. Numerous other proteases, including plasmin, have been shown to cleave the γ subunit distal to its inhibitory tract, thus releasing this tract and transitioning ENaC to a high activity state.25, 31
Plasminogen is filtered by damaged glomeruli and can be converted to its active form, plasmin, by urokinase in kidney tubules.24, 32, 33, 34 It has been suggested that tubular plasmin may be an important factor contributing to renal Na+ retention in proteinuric states by directly or indirectly (via activation of the tubular protease prostasin) cleaving and activating ENaC.26, 27, 35 Urine from patients with diabetes, preeclampsia, and nephrotic syndrome, often containing more than 100 μg of plasminogen plus plasmin (uPl) per gram of Cr (uPl/Cr), activates ENaC in vitro.36, 37, 38 If proteinuria leads to ENaC activation by plasmin and renal Na+ retention, ENaC inhibitors such as amiloride should serve as an effective tool to induce a natriuresis and improve BP in this setting. In addition to blocking ENaC,39 amiloride is also a urokinase inhibitor that will reduce the conversion of plasminogen to plasmin.40
Although previous studies have examined the role of amiloride in low-renin hypertension41, 42 and as an agent added to the conventional treatment of hypertension,43 there is limited clinical information regarding the impact of ENaC inhibitors on BP and volume status in the setting of proteinuria.44 In this setting, amiloride should block ENaC-dependent Na+ retention and subsequent volume expansion and hypertension. We performed a pilot study to determine the effect size and safety of amiloride as a therapeutic agent compared with hydrochlorothiazide (HCTZ) in individuals with type 2 diabetes, normal renal function, and proteinuria. The primary outcome for this pilot study was change in systolic blood pressure (SBP). Another goal was to confirm previous study findings that uPl is correlated with urinary albumin in this clinical setting.38
Methods
Participants
Inclusion criteria for this study were a history of type 2 diabetes, age 18 to 80 years, presence of systolic hypertension or prehypertension at time of screening (average SBP ≥ 120 mm Hg and <180 mm Hg), urinary protein/Cr ratio ≥ 100 mg/g or albumin/creatinine ≥ 100 mg/g at screening, and glycosylated hemoglobin ≤ 9% (as glucosuria would confound the endpoints related to natriuresis and diuresis). Exclusion criteria were as follows: serum potassium ( K+) level <3.5 mEq/l or >5.0 mEq/l at screening, history of hyperkalemia (serum K+ > 5.5 mEq/l) in the last 2 years, estimated glomerular filtration rate <60 ml/min per 1.73 m2 as determined by Modification of Diet in Renal Disease 4-variable equation, contraindication to use of HCTZ or amiloride, symptomatic heart failure, acute cardiac issues, cirrhosis, organ transplantation, dementia, evidence of poor adherence (as indicated by missed clinic visits), and large arm circumference.
Recruitment of Patients
The study sample consisted of male and female subjects with type 2 diabetes who were recruited through the University of New Mexico Hospital clinics (Nephrology Clinic, Endocrinology Clinic, General Medicine Clinic, and Pharmacy Clinic) and the University of New Mexico Clinical and Translational Science Center Patient Recruitment Services. Participants with type 2 diabetes and macroscopic proteinuria who were seen in the University of New Mexico Hospital clinics of the research investigators or identified from Patient Recruitment Services were invited to participate in this pilot study. The study protocol 13-017 was approved by the University of New Mexico Institutional Review Board. The study was recorded in clincaltrials.gov NCT01804777.
Objectives and Study Design
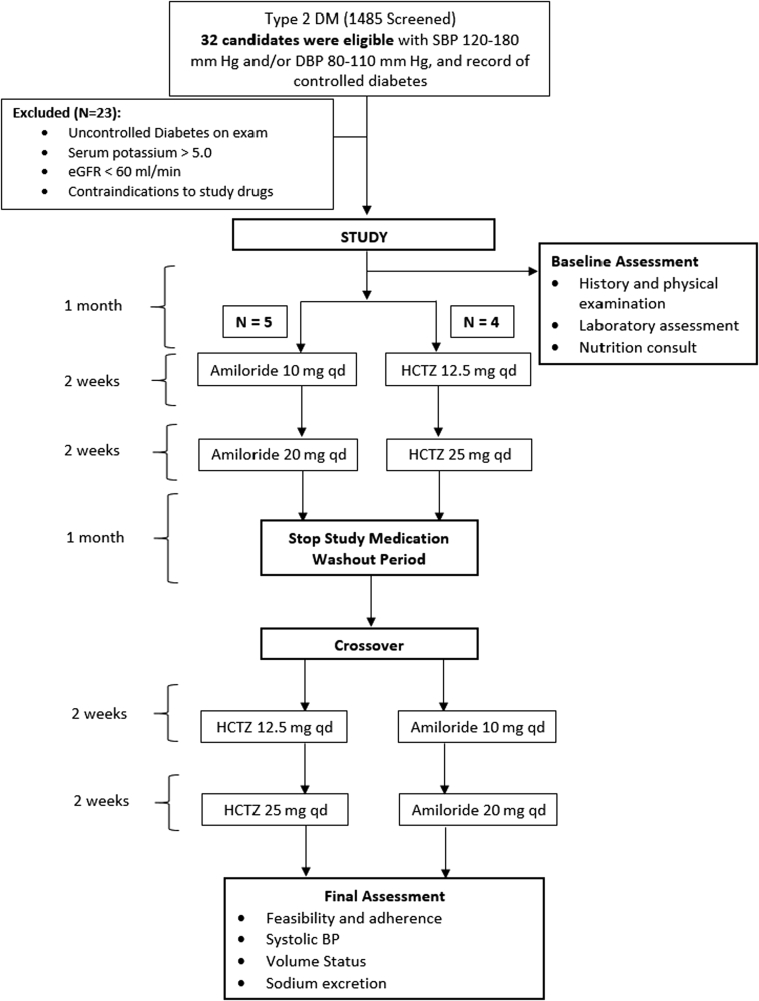
The study had a randomized, controlled, double-blind, single-center, crossover design. Orally administered amiloride in escalating doses of 10 to 20 mg daily was compared with HCTZ in doses of 12.5 mg to 25 mg daily for 2-week treatment periods (Figure 1). Responses to therapy were measured at the end of each treatment period with the assumption that any carryover effects from the previous treatment would be eliminated during a 4-week washout period. The University of New Mexico Hospital research pharmacist performed randomization and blinding and provided the study medications. Endpoint measurements were performed at the end of each 2-week active treatment period, with the exception of the 24-hour urine Na+ determinations, which occurred approximately 4 days after initiation of the study drug in order to measure natriuresis.
Figure 1.
Flowchart illustrating the design of the randomized trial. BP, blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; qd, every day; SBP, systolic blood pressure.
Outcomes
This pilot study examined feasibility and estimated preliminary effect sizes for the primary and secondary outcomes. Participants were closely monitored for development of hyperkalemia or hypokalemia, gastrointestinal intolerance, and acute kidney injury (increase in serum Cr >0.5 mg/dl45) to determine safety and feasibility of amiloride use for patients with type 2 diabetes and proteinuria. Adherence was measured by means of pill counts and self-report, and the target for adherence was use of >80% of pills and high levels of adherence by self-report. The primary outcome was a change in clinic SBP, as determined by the OMRON Digital Blood Pressure Monitor (Omron Healthcare, Lake Forest, IL), and calculated as the average of 3 serial BP measurements taken 1 minute apart after 5 minutes of sitting quietly. Changes in volume status were assessed by measurements of weight and percentage of total body water using a body composition analyzer to demonstrate effect sizes on clinically relevant hypertension outcomes, such as volume status and urinary Na+ excretion.46 Na+ excretion was assessed with the 24-hour urine excretion of Na+.
Sample Size
The pilot study was designed to enroll 20 participants. Assuming an SD of change in SBP = 5 mm Hg, alpha = 0.05, we estimated having approximately 22% power to detect a difference in SBP change between the 2 treatments if they differed by 2 mm Hg. Regardless of the significance of a difference, the targeted sample size would have enabled the estimation of the effects of the intervention on SBP with a precision of about ± 2.4 mm Hg, thus enabling the design of a future study with acceptable statistical power if the magnitude of the estimates appeared to be clinically meaningful in this pilot study.
Randomization
The randomization was performed centrally by the University of New Mexico Hospital Research Pharmacy. Patients were allocated to the different treatment sequences using random numbers. The Research Pharmacy was responsible for coordinating and managing the investigational drug inventory, storage, distribution, and record keeping for this clinical research study and conducted this clinical trial by following established standard operating procedures for drug preparation and delivery (blinding of study patients and investigators).
Visit Schedule and Study Measures
After the screening visit, a 1-month run-in period was included to assess changes in BP after withdrawal of diuretics (loop, thiazide, and K-sparing) and adherence to the study diet. All other antihypertensive medications and dosages were maintained during the study. During the run-in period, subjects met with the University of New Mexico Clinical and Translational Science Center study nutritionist to discuss eating habits and were educated on how to record their food intake, given examples of food choices, and established the study diet. Each participant was advised to adhere to an individualized diet including 1.1 g/kg protein per day, 70 mmol of Na+ (4 g), and 50 mmol of K+ (2 g) as prescribed by the dietician. Nutrition analysis of food diary records was performed using Nutrition Data System for Research 2012 (University of Minnesota, Minneapolis, MN). The nutritionist contacted subjects on day 14 and on day 30 to confirm compliance. Quality control reviews of dietary records were used to minimize missing nutrient values and errors. Participants were advised not to make any dietary changes during the course of the study except under guidance of the study dietician.
BP, weight, body composition, adherence, and adverse effects of therapy were recorded during clinic visits. The time of day for these visits was not standardized. Body composition was assessed by bioelectrical impedance analysis. Bioelectrical impedance analysis determines the electrical impedance, or opposition to the flow of an electric current through body tissues, which can then be used to calculate an estimate of total body water. Adherence to study medication was assessed by means of pill counts and self-report. At the end of the study period, the participants had a close-out visit with the study dietician and physician to return diet and BP regimen to their usual care in collaboration with each participant’s primary physician.
Immunoblotting for Urinary Plasminogen and Plasmin
Urine specimens collected at the initiation of the study were used to determine relative amounts of plasminogen and plasmin, normalized to Cr (uPl/Cr). Urinary proteins were precipitated and de-salted with chloroform/methanol.47 The volume of urine precipitated was adjusted to optimize detection of plasminogen and plasmin from each patient by immunoblotting. Precipitated proteins were suspended in Bio-Rad 2x Laemmli Sample Buffer (Bio-Rad, Hercules, CA), heated for 2 minutes at 90°C, and subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis on Bio-Rad Criterion TGX precast 10% polyacrylamide gels. For the purpose of separating plasminogen (relative molar mass = 88 kDa) and plasmin (relative molar mass = 75 kDa) bands on the gel, Bio-Rad Precision Plus Protein All Blue Standards were included in a gel lane, and proteins were electrophoresed until the 37-kDa standard reached the bottom of the gel. Proteins were electrophoretically transferred to nitrocellulose (Merck-Millipore, Billerica, MA) and incubated with a mouse anti-plasminogen antibody (MAB2596, R&D Systems, Inc, Minneapolis, MN) overnight and horseradish peroxidase–tagged secondary antibody (Jackson Labs, Bar Harbor, ME) for 90 minutes before incubation with PerkinElmer Western Lightning Plus ECL (PerkinElmer, Billerica, MA) and collection of the signal with a Bio-Rad Versadoc, as previously described.48 Bands for plasminogen or plasmin were quantified using Bio-Rad Quantity One software. Purified plasminogen (Sigma-Aldrich Corporation, St. Louis, MO) was run on each sodium dodecylsulfate gel to determine levels of plasminogen and plasmin in patient samples based on a standard curve. Immunoblot analyses were performed 3 to 6 times for each patient sample and used to calculate mean and SD.
Statistical Analysis
We summarized patient characteristics as mean ± SD for quantitative measures and as number (percentage) for qualitative features. We assessed the primary study outcomes of feasibility by estimating the proportions of patients with hyperkalemia and the rates of study adherence for participants who were receiving each of the study treatments. Change in SBP and other study outcomes, within and between the low- and high-dose 2-week treatment periods were estimated and compared using mixed model analysis of variance to account for the repeated per-subject assessments. To determine whether uPl/Cr has a role in amiloride treatment effects, we performed an additional series of mixed model analyses of variance, comparing the degree to which uPl/Cr status was associated with treatment outcomes. This was achieved by testing for an interaction between uPl/Cr and treatment group. We estimated the degree of change observed for each treatment within groups stratified on the basis of uPl/Cr above or below 100 μg/g. Estimated effects from the mixed models are presented as mean ± SE, and all reported P values reflect 2-sided tests of significance. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
One thousand four hundred eighty-five patients with diabetes were screened for study participation; patients were recruited from endocrine and nephrology clinics and a University of New Mexico Clinical and Translational Science Center registry. Most of these patients were excluded because of low levels of proteinuria or elevated glycosylated hemoglobin levels. Of those remaining after initial screening, 32 were eligible for study participation and agreed to an in-person screening visit. Of those, 23 were excluded because of elevated glycosylated hemoglobin levels and low levels of proteinuria. Table 1 shows the participant characteristics at baseline. Mean age (± SD) was 58.4 ± 10.0, and 5 (56%) were women. Mean body mass index (± SD) was 32.2 ± 6.2 kg/m2, and mean albumin/Cr (± SD) was 1120 ± 780 mg/g. The participants had a mean (± SD) estimated glomerular filtration rate of 87.4 ± 20.4 ml/min per 1.73m2, and a mean SBP (± SD) of 132 ± 13 mm Hg. Seven (78%) of the patients were receiving an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and the number of antihypertensive agents was 1.6 ± 1.3. Adherence to study protocols was medium to high for 7 of the 9 patients completing treatment with HCTZ. Adherence was similar for those completing the amiloride treatment protocol, with 5 of 7 patients reporting medium to high adherence.
Table 1.
Characteristics of participants on enrollment in the study
| All subjects |
Amiloride–HCTZ |
HCTZ–Amiloride |
||||
|---|---|---|---|---|---|---|
| N | % | n | % | n | % | |
| Females | 5 | 55.6 | 4 | 80.0 | 1 | 25.0 |
| Caucasian | 4 | 44.4 | 2 | 40.0 | 2 | 50.0 |
| Asian | 1 | 11.1 | 0 | 0.0 | 1 | 25.0 |
| Hispanic | 5 | 55.6 | 4 | 80.0 | 1 | 25.0 |
| Mean | SD | Mean | SD | Mean | SD | |
|---|---|---|---|---|---|---|
| Age (yr) | 58.4 | 10.0 | 56.4 | 13.2 | 61.0 | 4.3 |
| BMI (kg/m2) | 32.2 | 6.2 | 28.3 | 2.5 | 37.1 | 6.1 |
| Albumin/Creatinine (mg/g) | 1120 | 780 | 1070 | 650 | 1170 | 1030 |
| Weight (kg) | 84.0 | 21.3 | 68.7 | 7.6 | 103.1 | 16.1 |
| Creatinine (mg/dl) | 0.8 | 0.3 | 0.7 | 0.2 | 1.0 | 0.2 |
| Potassium (mmol/l) | 4.4 | 0.4 | 4.2 | 0.4 | 4.6 | 0.2 |
| Mean SBP (mm Hg) | 132 | 13 | 123 | 20 | 126 | 19 |
| Mean DBP (mm Hg) | 80.3 | 8.1 | 75.1 | 3.9 | 75.0 | 11.3 |
| eGFR (ml/min) | 87.4 | 20.4 | 92.6 | 23.0 | 77.4 | 14.8 |
Of the 9 participants in the trial, 5 were randomized to amiloride followed by HCTZ (Amiloride – HCTZ) and 4 were randomized to HCTZ followed by amiloride (HCTZ – Amiloride).
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure.
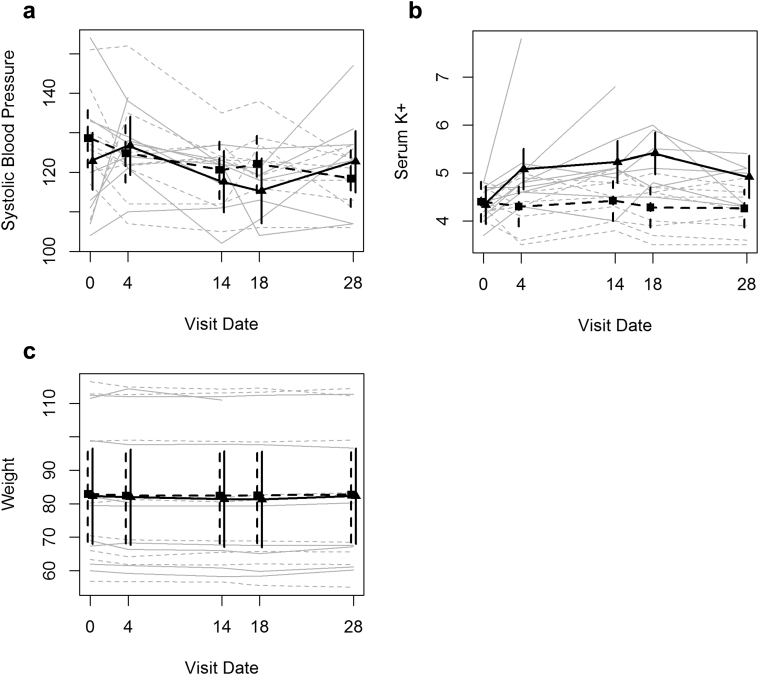
Table 2 and Figure 2 summarize the mean SBP estimates observed at treatment initiation and over the subsequent 4 weeks of treatment, during which patients received the lower amiloride or HCTZ dose for 2 weeks and subsequently received the higher amiloride or HCTZ dose. The within-person decline in SBP, estimated from the linear mixed-effects model over the first 2 weeks after treatment, was 8.0 ± 4.4 mm Hg in the HCTZ group and 5.1 ±4 .5 mm Hg in the amiloride group, with a resulting treatment difference between the amiloride and HCTZ treatment groups of 2.9 ± 6.3 mm Hg. Over the full 4 weeks of treatment, those in the HCTZ group experienced an estimated decline of 10.2 ± 4.4 mm Hg, and those in the amiloride group experienced an estimated decline of 0.5 ± 4.6 mm Hg. Those treated with HCTZ experienced a 10.2 ± 7.5 mm Hg greater decline in SBP over the 4 weeks of therapy than those treated with amiloride. However, these changes in SBP over time did not differ significantly by treatment (P = 0.15).
Table 2.
Summaries of outcomes over the course of the study
| Treatment |
Dose |
N |
SBP (mm Hg) |
Serum K+ (mmol/l) |
Weight (kg) |
|||
|---|---|---|---|---|---|---|---|---|
| All patients | Mean | SD (SE for Δ) | Mean | SD (SE for Δ) | Mean | SD (SE for Δ) | ||
| Amiloride | Baseline | 9 | 123 | 16 | 4.4 | 0.4 | 82.5 | 20.5 |
| ΔLow | 8 | -5.1 | 4.5 | 0.9 | 0.2 | -0.9 | 0.6 | |
| ΔHigh | 7 | -0.1 | 4.6 | 0.6 | 0.2 | -0.1 | 0.7 | |
| HCTZ | Baseline | 9 | 127 | 13 | 4.4 | 0.3 | 83.0 | 21.8 |
| ΔLow | 9 | -8.0 | 4.4 | 0.0 | 0.2 | -0.6 | 0.6 | |
| ΔHigh | 9 | -10.2 | 4.4 | -0.1 | 0.2 | -0.3 | 0.6 | |
| High plasminogen | Mean | SD / error (for Δ) | Mean | SD / error (for Δ) | Mean | SD / error (for Δ) | ||
|---|---|---|---|---|---|---|---|---|
| Amiloride | Baseline | 4 | 135 | 14 | 4.5 | 0.3 | 91.3 | 24.9 |
| ΔLow | 4 | -11.8 | 6.7 | 1.0 | 0.3 | -0.7 | 0.8 | |
| ΔHigh | 3 | -0.9 | 7.1 | 0.9 | 0.3 | 0.3 | 0.9 | |
| HCTZ | Base | 4 | 131 | 19 | 4.5 | 0.3 | 93.8 | 25.4 |
| ΔLow | 4 | -10.1 | 6.7 | 0.1 | 0.3 | -1.1 | 0.9 | |
| ΔHigh | 4 | -9.0 | 6.7 | -0.1 | 0.3 | -0.9 | 0.9 | |
| Low plasminogen | Mean | SD / error (for Δ) | Mean | SD / error (for Δ) | Mean | SD / error (for Δ) | ||
|---|---|---|---|---|---|---|---|---|
| Amiloride | Baseline | 5 | 113 | 10 | 4.2 | 0.4 | 75.5 | 15.3 |
| ΔLow | 4 | 1.4 | 6.4 | 0.8 | 0.3 | -1.0 | 0.8 | |
| ΔHigh | 4 | 1.1 | 6.3 | 0.4 | 0.3 | -0.5 | 0.8 | |
| HCTZ | Base | 5 | 123 | 5 | 4.3 | 0.2 | 74.4 | 16.0 |
| ΔLow | 5 | -6.7 | 6.0 | 0.0 | 0.3 | -0.2 | 0.8 | |
| ΔHigh | 5 | -11.8 | 6.0 | -0.2 | 0.3 | 0.1 | 0.8 | |
Baseline estimates are simple averages and SDs obtained across study participants at the appropriate period. ΔLow and ΔHigh estimates are estimates of change from baseline for the study participants at the end of the low-dose and high-dose periods, respectively. Summaries are presented overall and stratified by urinary plasminogen plus plasmin per gram of creatinine.
Figure 2.
Trends in study outcomes over the course of the randomized trial. Plots of observed trends in outcomes for each study participant (gray lines) and of estimated trends in means (heavy black lines). The 95% confidence intervals are shown for the time-specific estimates (vertical lines). Solid lines represent results observed during treatment with amiloride, and dashed lines represent results observed during treatment for hydorchlorothiazide. (a) SBP results, (b) serum potassium (K+) results, and (c) weight results.
Serum K+ increased with amiloride treatment (Table 2 and Figure 2). The estimated mean within-person increase in serum K+ was 0.9 ± 0.2 mmol/l in the amiloride group over the first 2 weeks of treatment, whereas it was unchanged (0.0 ± 0.2 mmol/l) in the HCTZ group, with a difference between the amiloride and HCTZ treatment groups of 0.9 ± 0.3 mmol/l. For those treated over the full 4 weeks of the study, levels of serum K+ were 0.6 ± 0.2 mmol/l higher in the amiloride group than at baseline, whereas levels decreased by 0.1 ± 0.2 mmol/l from baseline in the HCTZ group, with serum K+ levels increasing over the 4-week treatment period in the amiloride group by 0.7 ± 0.3 mmol/l more than those in the HCTZ group. Changes in serum K+ levels during treatment were significantly different between the amiloride and HCTZ groups (P < 0.001).
Table 2 and Figure 2 also summarize weights observed in the 2 study groups at baseline and at 2-week intervals during treatment. The estimated mean within-person decline in weight was 0.9 ± 0.6 kg over the first 14 days with low-dose amiloride, and for those treated with HCTZ, the estimated decline was 0.6 ± 0.6 kg. Average weight losses of 0.1 ± 0.7 kg and 0.3 ± 0.6 kg were observed during high-dose treatment with amiloride and HCTZ, respectively. Differences in weight changes between treatment groups were not statistically significant (P = 0.46). Our analyses excluded the 2 participants who dropped out of the study because of hyperkalemia and acute kidney injury.
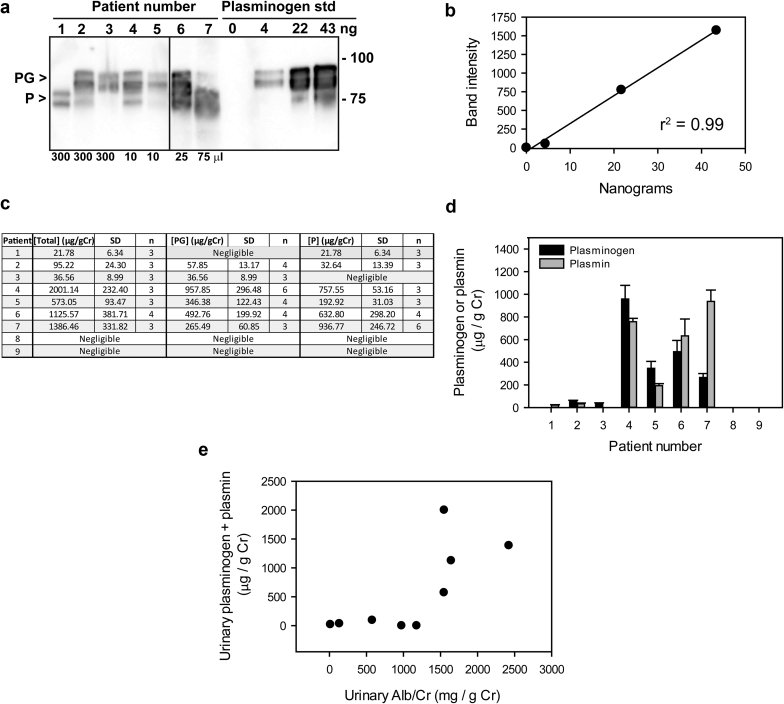
Immunoblotting of urine samples was performed to assess levels of plasminogen and plasmin, which were combined and normalized to urinary Cr (uPl/Cr, Figure 3). We defined high uPl/Cr as ≥100 μg/g and low uPl/Cr as < 100μg/g. Of the 9 study participants, 4 had high uPl/Cr at the start of the study, as assessed by immunoblotting (Figure 3). Surprisingly, 5 subjects had low or negligible levels. The individual measurements of either plasminogen or plasmin would have arrived at identical groupings of high versus low uPl/Cr.
Figure 3.
Quantitation of urinary plasminogen plus plasmin. Aliquots of urine from 9 patients were subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis and immunoblotting with anti-plasminogen/plasmin antibodies (n = 3–6). Urine volumes analyzed were optimized for detection of plasminogen and plasmin by immunoblotting (10–300 μl) and are listed at the bottom of the figure. Varying amounts of pure plasminogen (4–43 ng) were included on the same blot to create a standard curve and establish levels of plasminogen and plasmin for each patient. A representative immunoblot (a) and corresponding standard curve (b) are shown for 7 patients. (Two patients consistently lacked a signal.) Line between samples 5 and 6 indicates where the blot was cut. Arrowheads indicate plasminogen (PG) and plasmin (P). Data from 3 to 6 analyses were normalized to urine creatinine (Cr), and values are presented in table format (c) and as a bar graph (d). (e) Correlation between urinary plasminogen plus plasmin per gram of creatinine and urinary albumin (Alb)/Cr. std, standard.
During the low-dose phase of the study, the SBP of those with high urinary Pl/Cr fell an estimated 11.8 ± 6.7 mm Hg with amiloride and 10.1 ± 6.7 mm Hg with HCTZ, and the SBP of those with low Pl/Cr rose by 1.4 ± 6.4 mmHg with amiloride and fell by 6.7 ± 6.0 mm Hg with HCTZ. During high-dose therapy, the SBP of those with high Pl/Cr fell by 0.9 ± 7.1 mm Hg with amiloride and by 9.0 ± 6.7 mm Hg with HCTZ, and the SBP of those with low Pl/Cr levels rose by 1.1 ± 6.3 mm Hg with amiloride and fell by 11.8 ± 6.0 mm Hg with HCTZ. Although these differences are intriguing, we are not able to conclude that uPl/Cr correlates with treatment effects on SBP (P = 0.23).
Differences in treatment effect on serum K+ in those with different Pl/Cr levels were smaller than those observed for SBP. For instance, among subjects treated with amiloride, serum K+ rose by 0.8 ± 0.3 mmol/l in the low-dose study phase for those with low uPl/Cr and rose by 1.0 ± 0.3 mmol/l for those with high uPl/Cr. As with SBP, we are not able to conclude that uPl/Cr correlates with the effect of amiloride on serum K+ (P = 0.70).
In the low-dose study phase, the weight of subjects with high uPl/Cr levels fell by 0.7 ± 0.8 kg with amiloride and by 1.1 ± 0.9 kg with HCTZ, and the weight of subjects with low uPl/Cr fell by 1.0 ± 0.8 kg with amiloride and by 0.2 ± 0.8 kg with HCTZ. In the high-dose study phase, the weight of subjects with high levels of uPl/Cr levels rose by 0.3 ± 0.9 kg with amiloride and fell by 0.9 ± 0.9 kg with HCTZ, and the weight of those with low levels of plasminogen fell by 0.5 ± 0.8 kg with amiloride and rose by 0.1 ± 0.8 kg with HCTZ. These differences were not statistically significant (P = 0.60).
In the amiloride treatment arm, urinary albumin/Cr decreased by 10% from baseline during the low-dose phase of the trial and by 15% during the high-dose phase of the trial. The estimated effects of HCTZ were 7% and 14% reductions in albumin/Cr over the low- and high-dose phases, respectively. These treatment effects on albumin/Cr were not statistically different from pretreatment albumin/Cr values. Amounts of Na+ excreted in urine over 24 hours measured before initiation of treatment with a diuretic, 4 days after initiation of the diuretic (HCTZ or amiloride), and at the end of the 2-week period of treatment with the diuretic did not differ significantly.
Our assessment of treatment feasibility suggests that there are risks associated with amiloride treatment in this patient population. Of the 9 subjects who received amiloride, 2 experienced a serious adverse event (hyperkalemia and acute kidney injury). While taking the higher dose of amiloride, 1 participant was found to have both elevated serum K+ (6.8 mEq/l) and an acute rise in serum Cr (1.57 mg/dl, up from a baseline of 1.26) in a protocol blood collection, with no physical complaints. The subject experienced decreases in BP of up to 14 mm Hg and decreases in weight of up to 3.4 kg over the course of amiloride therapy. He was promptly directed to the University of New Mexico Hospital emergency department where he was admitted overnight for treatment and monitoring. In addition to discontinuing the amiloride, he received i.v. fluids and standard hyperkalemia therapy. This participant experienced rapid correction of serum K+ and Cr with i.v. volume resuscitation, consistent with prerenal azotemia. The second participant experienced hyperkalemia (serum K+ of 7.8 mEq/l) and acute kidney injury (serum Cr was 1.51 mg/dl, up from a baseline of 0.76) with the lower dose of amiloride. This individual was also hospitalized, and amiloride was discontinued. She received i.v. fluids and medical therapy for hyperkalemia and experienced rapid recovery.
Discussion
A growing body of literature suggests that ENaC activation occurs in patients with proteinuria because of proteases within the urinary space that cleave and activate ENaC, and that this process contributes to extracellular volume expansion and increased BP.24, 25, 26, 27, 35, 49 In this setting, filtered plasminogen (inactive precursor) is converted to plasmin (active protease) by urokinase that is expressed in the lumen of tubular epithelia.24, 32, 33, 34, 50
We expected that amiloride would substantially reduce the SBP and volume status by blocking both ENaC and urokinase,39, 40 when compared with HCTZ. We anticipated enrolling 20 subjects, but the trial was stopped early because of safety concerns related to 2 episodes of acute kidney injury and hyperkalemia. It was our expectation that the selection of patients with normal kidney function, with a serum K+ in a range from 3.5 to 5.0 and the use of a standardized diet would mitigate hyperkalemia. We observed a significant increase in serum K+ levels among those being treated with amiloride. This is in contrast to our primary outcomes: there were no significant reductions in weight or in SBP with amiloride when compared with HCTZ (Table 2). Our inability to detect significant effects on BP and weight may, in part, be due to the small sample size that resulted from stopping the trial early. It is interesting to note that a recent study in which patients with type 1 diabetes with and without nephropathy were compared, significant reductions in SBP were observed in both groups with short-term (2-day) amiloride (20 or 40 mg/d) administration, whereas mean arterial pressure was significantly reduced only in the group with nephropathy.44 Another study of 80 individuals with type 2 diabetes and resistant hypertension demonstrated a beneficial effect with amiloride (5 or 10 mg/d), with a 6 mm Hg reduction in SBP.51 The study included individuals with and without proteinuria, and a 9% incidence of hyperkalemia (K+ > 5.5 mEq/l) was reported.
The severity of hyperkalemia in our study was striking, given the multiple steps taken to mitigate it. The study protocol excluded patients with a history of hyperkalemia and an estimated glomerular filtration rate < 60. The study also included a moderate Na+ and low K+ diet that was instituted before randomization. There was evidence for adherence to the low-K+ diet as demonstrated by self-reported dietary records, where among the 49 instances of self-reported diet, the average ± SD K+ intake was 1.6 ± 0.6 g, and only 5 instances of intake above 2.5 g were reported. It is not surprising that the addition of a K+-sparing diuretic to an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker in individuals with some impairment in renal function increases the risk of significant hyperkalemia.52, 53 It remains unclear whether the risk of hyperkalemia would be adequately attenuated by novel agents to enhance intestinal K+ excretion when amiloride is administered to patients with diabetes also receiving an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.54 The combination of a loop or thiazide diuretic and/or a lower dose of amiloride could also reduce the risk of hyperkalemia in this setting.55
Although we expected to readily detect plasminogen and plasmin in the urine of patients with diabetes and proteinuria, we were surprised to find that 5 of 9 subjects had low or negligible levels according to immunoblot analyses. However, there was a correlation between the albumin/Cr and uPl/Cr, in agreement with findings from other groups.36, 37, 38 The differences in urinary Pl/Cr levels allowed us to examine whether high uPl/Cr is associated with a response to amiloride. We found that individuals with high uPl/Cr responded to low-dose amiloride with a fall in SBP by 11.8 ± 6.7 mm Hg. This is in contrast to an estimated increase in SBP by 1.4 ± 6.4 mm Hg with low-dose amiloride among those with low or negligible levels of urinary Pl/Cr. Lack of statistical significance (P = 0.16) may be attributable to low sample size. These findings suggest that, with a larger study population, we might observe significant reductions in SBP and weight with amiloride in individuals excreting readily detectable uPl/Cr in their urine.
The findings from this study should be interpreted in light of several limitations. First, the use of other antihypertensive medications such as an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker may have attenuated the effects of amiloride by reducing levels of hormones that are known to activate ENaC.56, 57 These antihypertensive agents may also have contributed to the episodes of hyperkalemia observed in this study.52, 53 Second, the BP of our study sample may have been too low at randomization to demonstrate a significant effect of amiloride. The BP of the study sample was lower at randomization than at baseline, perhaps owing to regression to the mean or to our moderate Na+ diet and dietary monitoring. Third, the initial dosing of amiloride was based on that used for patients with Liddle’s syndrome. It may be that a lower dose of amiloride would have a better safety profile. Lastly, in this study, we explored whether amiloride had a differential effect among those with measurable uPl/Cr. A larger study prospectively measuring uPl/Cr would be needed to assess whether amiloride should be used in the subpopulation of patients with diabetes and proteinuria who have higher levels.
In summary, among individuals with normal renal function and proteinuria, we did not find evidence that amiloride was superior to HCTZ with regard to reductions in BP or weight. Furthermore, acute kidney injury and severe hyperkalemia were safety concerns with amiloride at a dose of 10 or 20 mg in patients with diabetes and proteinuria who were taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. Our study was stopped early because adverse effects occurred, and the evaluations of the primary study outcomes, BP and weight, were inconclusive. Data collected from this study provide important preliminary data for future studies of amiloride in the context of proteinuria and plasminuria. uPl/Cr varies among individuals with proteinuria. Further studies are needed to determine whether uPl/Cr provides a more robust biomarker than albuminuria for those individuals with proteinuria who develop Na+ retention and increases in BP.38
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by a grant from Dialysis Clinics, Inc. and by grants from the National Institutes of Health (T32 DK061296, T35 DK065521, P30 DK079307, K08 DK110332) and University of New Mexico Clinical and Translational Science Center (UL1TR00449). The study was recorded in clincaltrials.gov NCT01804777.
References
- 1.Hsu C.Y., Iribarren C., McCulloch C.E. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambers Heerspink H.J., Brantsma A.H., de Zeeuw D. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol. 2008;168:897–905. doi: 10.1093/aje/kwn209. [DOI] [PubMed] [Google Scholar]
- 3.Ninomiya T., Perkovic V., Verdon C. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53:417–425. doi: 10.1053/j.ajkd.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R., Andersen M.J. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–520. doi: 10.1161/01.HYP.0000178102.85718.66. [DOI] [PubMed] [Google Scholar]
- 5.Kim B.J., Lee H.J., Sung K.C. Comparison of microalbuminuria in 2 blood pressure categories of prehypertensive subjects. Circ J. 2007;71:1283–1287. doi: 10.1253/circj.71.1283. [DOI] [PubMed] [Google Scholar]
- 6.Schork A., Woern M., Kalbacher H. Association of plasminuria with overhydration in patients with CKD. Clin J Am Soc Nephrol. 2016;11:761–769. doi: 10.2215/CJN.12261115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue T., Iseki K., Higashiuesato Y. Proteinuria as a significant determinant of hypertension in a normotensive screened cohort in Okinawa, Japan. Hypertens Res. 2006;29:687–693. doi: 10.1291/hypres.29.687. [DOI] [PubMed] [Google Scholar]
- 8.Wang T.J., Gona P., Larson M.G. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–438. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 9.Forman J.P., Fisher N.D., Schopick E.L. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–1988. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feld L.G., Brentjens J.R., Van Liew J.B. Renal injury and proteinuria in female spontaneously hypertensive rats. Ren Physiol. 1981;4:46–56. doi: 10.1159/000172803. [DOI] [PubMed] [Google Scholar]
- 11.Rossi G.P., Bernini G., Desideri G. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 12.Wang G., Lai F.M., Kwan B.C. Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens. 2009;22:300–306. doi: 10.1038/ajh.2008.360. [DOI] [PubMed] [Google Scholar]
- 13.Trevisan R., Bruttomesso D., Vedovato M. Enhanced responsiveness of blood pressure to sodium intake and to angiotensin II is associated with insulin resistance in IDDM patients with microalbuminuria. Diabetes. 1998;47:1347–1353. doi: 10.2337/diab.47.8.1347. [DOI] [PubMed] [Google Scholar]
- 14.Coonrod B.A., Ellis D., Becker D.J. Predictors of microalbuminuria in individuals with IDDM. Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 1993;16:1376–1383. doi: 10.2337/diacare.16.10.1376. [DOI] [PubMed] [Google Scholar]
- 15.Mathiesen E.R., Ronn B., Storm B. The natural course of microalbuminuria in insulin-dependent diabetes: a 10-year prospective study. Diabet Med. 1995;12:482–487. doi: 10.1111/j.1464-5491.1995.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 16.Pambianco G., Costacou T., Ellis D. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 17.de Boer I.H., Kestenbaum B., Rue T.C. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008;168:1867–1873. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschenes G., Feraille E., Doucet A. Mechanisms of oedema in nephrotic syndrome: old theories and new ideas. Nephrol Dial Transplant. 2003;18:454–456. doi: 10.1093/ndt/18.3.454. [DOI] [PubMed] [Google Scholar]
- 19.Meltzer J.I., Keim H.J., Laragh J.H. Nephrotic syndrome: vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Ann Intern Med. 1979;91:688–696. doi: 10.7326/0003-4819-91-5-688. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa I., Rennke H.G., Hoyer J.R. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Seigneux S., Kim S.W., Hemmingsen S.C. Increased expression but not targeting of ENaC in adrenalectomized rats with PAN-induced nephrotic syndrome. Am J Physiol Renal Physiol. 2006;291:F208–F217. doi: 10.1152/ajprenal.00399.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lourdel S., Loffing J., Favre G. Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J Am Soc Nephrol. 2005;16:3642–3650. doi: 10.1681/ASN.2005040363. [DOI] [PubMed] [Google Scholar]
- 23.Deschenes G., Wittner M., Stefano A. Collecting duct is a site of sodium retention in PAN nephrosis: a rationale for amiloride therapy. J Am Soc Nephrol. 2001;12:598–601. doi: 10.1681/ASN.V123598. [DOI] [PubMed] [Google Scholar]
- 24.Svenningsen P., Bistrup C., Friis U.G. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299–310. doi: 10.1681/ASN.2008040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passero C.J., Mueller G.M., Rondon-Berrios H. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–36591. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray E.C., Rondon-Berrios H., Boyd C.R. Sodium retention and volume expansion in nephrotic syndrome: implications for hypertension. Adv Chronic Kidney Dis. 2015;22:179–184. doi: 10.1053/j.ackd.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svenningsen P., Andersen H., Nielsen L.H. Urinary serine proteases and activation of ENaC in kidney–implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch. 2015;467:531–542. doi: 10.1007/s00424-014-1661-5. [DOI] [PubMed] [Google Scholar]
- 28.Kleyman T.R., Carattino M.D., Hughey R.P. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughey R.P., Bruns J.B., Kinlough C.L. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 30.Carattino M.D., Sheng S., Bruns J.B. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem. 2006;281:18901–18907. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- 31.Bruns J.B., Carattino M.D., Sheng S. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 32.Lau S.O., Tkachuck J.Y., Hasegawa D.K. Plasminogen and antithrombin III deficiencies in the childhood nephrotic syndrome associated with plasminogenuria and antithrombinuria. J Pediatr. 1980;96:390–392. doi: 10.1016/s0022-3476(80)80678-0. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen P., Eriksen J., Dano K. Localization of urokinase-type plasminogen activator messenger RNA in the normal mouse by in situ hybridization. J Histochem Cytochem. 1991;39:341–349. doi: 10.1177/39.3.1899685. [DOI] [PubMed] [Google Scholar]
- 34.Wagner S.N., Atkinson M.J., Wagner C. Sites of urokinase-type plasminogen activator expression and distribution of its receptor in the normal human kidney. Histochem Cell Biol. 1996;105:53–60. doi: 10.1007/BF01450878. [DOI] [PubMed] [Google Scholar]
- 35.Passero C.J., Hughey R.P., Kleyman T.R. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens. 2010;19:13–19. doi: 10.1097/MNH.0b013e3283330fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen H., Friis U.G., Hansen P.B. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol Dial Transplant. 2015;30:781–789. doi: 10.1093/ndt/gfu402. [DOI] [PubMed] [Google Scholar]
- 37.Buhl K.B., Friis U.G., Svenningsen P. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension. 2012;60:1346–1351. doi: 10.1161/HYPERTENSIONAHA.112.198879. [DOI] [PubMed] [Google Scholar]
- 38.Buhl K.B., Oxlund C.S., Friis U.G. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J Hypertens. 2014;32:1672–1677. doi: 10.1097/HJH.0000000000000216. discussion 1677. [DOI] [PubMed] [Google Scholar]
- 39.Kleyman T.R., Cragoe E.J., Jr. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- 40.Vassalli J.D., Belin D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987;214:187–191. doi: 10.1016/0014-5793(87)80039-x. [DOI] [PubMed] [Google Scholar]
- 41.Hood S.J., Taylor K.P., Ashby M.J. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–275. doi: 10.1161/CIRCULATIONAHA.107.690396. [DOI] [PubMed] [Google Scholar]
- 42.Eide I.K., Torjesen P.A., Drolsum A. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Saha C., Eckert G.J., Ambrosius W.T. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–487. doi: 10.1161/01.HYP.0000179582.42830.1d. [DOI] [PubMed] [Google Scholar]
- 44.Andersen H., Hansen P.B., Bistrup C. Significant natriuretic and antihypertensive action of the epithelial sodium channel blocker amiloride in diabetic patients with and without nephropathy. J Hypertens. 2016;34:1621–1629. doi: 10.1097/HJH.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 45.Weisbord S.D., Mor M.K., Resnick A.L. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168:1325–1332. doi: 10.1001/archinte.168.12.1325. [DOI] [PubMed] [Google Scholar]
- 46.Jebb S.A., Cole T.J., Doman D. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br J Nutr. 2000;83:115–122. doi: 10.1017/s0007114500000155. [DOI] [PubMed] [Google Scholar]
- 47.Wessel D., Flugge U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 48.Hughey R.P., Mueller G.M., Bruns J.B. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 49.Kastner C., Pohl M., Sendeski M. Effects of receptor-mediated endocytosis and tubular protein composition on volume retention in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2009;296:F902–F911. doi: 10.1152/ajprenal.90451.2008. [DOI] [PubMed] [Google Scholar]
- 50.Piedagnel R., Tiger Y., Lelongt B. Urokinase (u-PA) is produced by collecting duct principal cells and is post-transcriptionally regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Cell Physiol. 2006;206:394–401. doi: 10.1002/jcp.20485. [DOI] [PubMed] [Google Scholar]
- 51.Oxlund C.S., Buhl K.B., Jacobsen I.A. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J Am Soc Hypertens. 2014;8:872–881. doi: 10.1016/j.jash.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Turgutalp K., Bardak S., Helvaci I. Community-acquired hyperkalemia in elderly patients: risk factors and clinical outcomes. Ren Fail. 2016;38:1405–1412. doi: 10.1080/0886022X.2016.1216714. [DOI] [PubMed] [Google Scholar]
- 53.Mavrakanas T.A., Gariani K., Martin P.Y. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med. 2014;25:173–176. doi: 10.1016/j.ejim.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN Randomized Clinical Trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 55.Brown M.J., Williams B., Morant S.V. Effect of amiloride, or amiloride plus hydrochlorothiazide, versus hydrochlorothiazide on glucose tolerance and blood pressure (PATHWAY-3): a parallel-group, double-blind randomised phase 4 trial. Lancet Diabetes Endocrinol. 2016;4:136–147. doi: 10.1016/S2213-8587(15)00377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce D., Soundararajan R., Trimpert C. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol. 2015;10:135–146. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossier B.C., Baker M.E., Studer R.A. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev. 2015;95:297–340. doi: 10.1152/physrev.00011.2014. [DOI] [PubMed] [Google Scholar]