Abstract
Background
Gestational diabetes mellitus (GDM) is associated with increased availability of glucose and macronutrients in fetal circulation and macrosomia. Therefore, the role of GDM in the association between metabolism-disrupting chemicals and birth size deserves attention.
Objective
We examined whether GDM may mediate or modify the associations between maternal environmental pollutant exposures and offspring birth size measures.
Methods
We analysed 604 Faroese pregnant women and their offsprings born in 1997-2000. Maternal pregnancy serum concentrations of organochlorine compounds (OCs: polychlorinated biphenyl (PCB) congeners and dichlorodiphenyldichloroethylene (DDE)), and five perfluoroalkyl substances (PFASs), and hair and cord blood mercury concentrations were measured. We used regression (single-pollutants) and structural equation models (SEMs) (multiple-pollutant analyses using latent constructs of OCs, PFASs and mercury) to estimate the associations with GDM and birth size measures, accounting for mediation and/or effect modification by GDM.
Results
Serum-DDE and hair-mercury concentrations were associated with GDM (adjusted OR per concentration doubling: 1.29; 95% CI: 0.94, 1.77 for DDE, and 0.79; 95% CI: 0.62, 0.99 for mercury), but in multiple pollutant-adjusted SEMs only a positive association between OC exposure and GDM remained significant (change in GDM odds per OC doubling: 0.45; 95% CI: 0.05, 0.86). PCB and overall OC exposure were positively associated with head circumference (SEM; mean change per OC doubling: 0.13 cm; 95% CI, 0.01. 0.25). Overall PFAS exposure was inversely associated with birth weight (SEM; mean change per PFAS doubling: -169 g; 95% CI: -359, 21), and for many single-PFASs we found a pattern of inverse associations with birth weight and head circumference in boys, and positive or null associations in girls. None of the environmental pollutants was associated with offspring length. GDM neither modified nor mediated the associations with birth size measures.
Conclusions
We found associations with GDM and offspring birth size to be specific to the environmental pollutant or pollutant group. Associations with birth size measures appear to be independent of GDM occurrence.
Introduction
The prevalence of gestational diabetes mellitus (GDM) is on the rise, currently affecting between 3% and 25% of pregnancies, depending on the population and the clinical criteria used for diagnosis (Zhu and Zhang 2016). GDM is associated with a higher risk of fetal macrosomia (i.e. increased fetal growth and body fat deposition) (Kc et al. 2015) as well as, with higher risks of metabolic abnormalities in later life in the mothers and also their offsprings (Bellamy et al. 2009: Ehrlich et al. 2013; Zhu and Zhang 2016). The etiology of GDM is multifactorial, and modifiable risk factors likely include exposures to environmental pollutants that can act as endocrine and metabolism disruptors in promoting weight gain and insulin resistance (Janesick and Blumberg 2016; Lee et al. 2014; Magliano et al. 2014; Taylor et al. 2013). Exposures to persistent organic pollutants (POPs), such as organochlorine compounds (OCs) and perfluoroalkyl substances (PFASs), and exposures to metals, such as mercury, have been associated with increased risk for type 2 diabetes (reviewed in Lee et al. 2014; Kuo et al. 2013; Magliano et al. 2014; Taylor et al. 2013). However, only a few previous studies, with inconclusive findings, have specifically focused on risk of GDM (Jaacks et al. 2016; Peng et al. 2015; Shapiro et al. 2015 and 2016; Smarr et al. 2016; Vafeiadi et al. 2016; Zhang et al. 2015).
Exposure to POPs and metals may also interfere with intrauterine growth and adversely affect birth size (Casas et al. 2015; Govarts et al. 2012; Vrijheid et al. 2016; Bach et al. 2015; Murcia et al. 2016). Because the exposure passes from the mother to the fetus through the placenta (Kim et al. 2014; Needham et al. 2011), early life health outcomes may be affected through the direct action and toxicity of the pollutants to fetal tissues and the placenta, or they may be indirectly affected through alterations in hormone balance and tissue functions of the mother. However, whether potentially diabetogenic effects of environmental pollutants in the mother may mediate health outcomes seen in the offspring has not been previously examined.
We evaluated the associations of maternal exposures to several pollutants (OCs, PFASs and mercury) in regard to GDM occurrence and offspring birth size measures in a Faroese birth cohort, where a wide range of exposures occur through the consumption of fish and seafood (Weihe et al. 1996 and 2008). Given the suspected association of environmental pollutant exposures with GDM occurrence, and the causal role of maternal hyperglycemia in regard to fetal metabolic programming and macrosomia (Kahraman et al. 2014; Kc et al. 2015), we hypothesized that the occurrence of GDM may mediate the associations of diabetogenic environmental pollutants with birth size measures. An alternate and plausible hypothesis is that the increased availability of glucose and other macronutrients in fetal circulation through the placenta in GDM cases (Araujo et al. 2015) may change the metabolic responses of the fetus to intrauterine chemical exposures (Goran et al. 2013; La Merrill et al. 2014; Valvi et al. 2012). Thus, we have also tested GDM status as a potential modifier of the association between environmental pollutant exposures and birth size measures.
Methods
Study population and data collection
We used information from 604 of the mother-child pairs recruited at 34 weeks of gestation at the National Hospital in Torshavn in the Faroe Islands between 1997 and 2000 (92% of the 656 mother-child pairs initially enrolled with complete data on key covariates). The ethical review committee of the Faroe Islands and the institutional review board at the Harvard T.H. Chan School of Public Health approved the study protocol, and written informed consent was obtained from all pregnant women.
Only singleton births were included. Information about maternal age at delivery, gestational age and child sex was extracted from the obstetric and medical records. Additional information was collected through interviews with the mothers at 14 days postpartum and included maternal education, parity, pre-pregnancy body mass index (BMI, i.e. weight in kg/[height in m]2), gestational weight gain, family history of diabetes and smoking during pregnancy. Offspring weight (nearest 0.1 kg) and head circumference (nearest 0.1 cm) were measured at birth, and length (nearest 0.5 cm) was measured at postpartum day 14 by the midwife.
GDM diagnosis was extracted from the medical records. Following standard clinical guidelines (Berger et al. 2003) women with elevated fasting blood glucose concentrations and/or those considered at elevated risk for GDM based on their age, pre-pregnancy BMI, family history of diabetes, GDM in previous pregnancy, previous stillbirth, macrosomia in previous delivery and polyhydramnios were identified at 24-28 weeks of gestation and given a 2h-oral glucose tolerance test (OGTT) (13% of the analysis population) to establish a possible GDM diagnosis (Berger et al. 2003; Dalgård et al. 2016). The reference group (GDM-free category) consisted of women at low risk who did not undergo an OGTT, and women non-diagnosed for GDM based on the OGTT results. Information from medical records was extracted also in regard to related pregnancy comorbidities including preeclampsia, which was not frequent in this cohort (prevalence equal to 1.5%).
Assessment of environmental pollutant exposures
Maternal serum was obtained at gestational week 34. Cord blood and maternal hair were collected at parturition, and transition milk 4-5 days later. All blood and milk samples were stored at -80° C until chemical analyses were performed at the University of Southern Denmark, as previously detailed (Grandjean et al. 2012; Heilmann et al. 2010).
OC concentrations in maternal serum were measured using gas chromatography with electron capture detection as the standard at the time. The OCs quantified included the major PCB congeners 138, 153 and 180, p,p′-dichlorodiphenyldichloroethylene (DDE) and p,p′-dichlorodiphenyltricloroethane (DDT). OC concentrations measured in breast milk were used to estimate serum concentrations for 20% of the mothers who did not have measured OC concentrations in serum (Pearson r between milk and serum concentrations ≥0.87 depending on OC) (Needham et al. 2011; Tang-Peronard et al. 2014). We substituted serum concentrations below the limit of detection (LOD) of 0.03 ng/mL by a value equal to half of the LOD. We calculated the sum of PCB congeners in maternal serum (ΣPCB) as the sum of PCB congeners 138, 153 and 180 multiplied by 2, because these were the most commonly detected congeners representing close to 50% of the total serum PCB concentrations (Grandjean et al. 1995). Because OCs are highly lipophilic concentrations were divided by the serum lipid concentrations and are expressed in μg/g lipid. The serum lipid content was calculated from cholesterol and triglyceride concentrations (Phillips et al. 1989) as determined by a kit-based analysis on a Konelab 20 Clinical Chemistry Analyzer (Thermo Fischer Scientific, Waltham, MA, US). In complimentary sensitivity analyses associations of OC concentrations uncorrected for lipids (in ng/mL) were adjusted by including the lipid content as a separate covariate in the models. Maternal serum PFAS concentrations were measured using high-pressure liquid chromatography with tandem mass spectrometry. The quantified substances were: perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), perfluorodecanoic acid (PFDA) and perfluorononanoic acid (PFNA). Concentrations in all samples were above the LOD (>0.03 ng/mL) and are expressed in ng/mL.
Hair and cord-blood mercury analyses have been described previously (Grandjean and Budtz-Jorgensen 2007; Kim et al. 2014). Total mercury concentrations were measured in the proximal 1-cm hair segment (expressed in μg/g hair) that mainly reflects methylmercury exposure in particular during the second and third trimesters (Grandjean et al. 1999). Mercury in umbilical cord blood (expressed in μg/L) is almost entirely found in its methylated form, which can pass the placental barrier, and it is considered a better proxy of recent fetal exposure as compared to hair mercury (Grandjean et al. 1999). All measured concentrations in hair and cord blood were above the LODs.
Statistical analysis
A total of 604 mother-child pairs had complete information about maternal serum PFAS concentrations and the study outcomes, and fewer mother-child pairs had information about OC concentrations (N=564), and hair (N=561) and cord blood (N=538) mercury concentrations.
Environmental pollutant exposure variables were log2-transformed to normalize the right-skewed distributions, and analyzed both continuously and categorically using tertile groups. Generalized additive models (GAMs) were used to assess the linearity of dose-response relationships between continuous exposures and outcome measures (Royston and Ambler 1998). In single-pollutant analyses, we used logistic regression to determine the associations with GDM (no/yes), and linear regression to evaluate the associations with continuous birth size measures (i.e. weight, length and head circumference). Effect modification by GDM was evaluated by including interaction cross product terms (GDM*exposure) in the birth size models and both overall and GDM stratified coefficients are shown. We evaluated effect modification by sex using the same approach, as sex-dimorphic associations between exposure to metabolism-disrupting chemicals and growth patterns are possible (Bach et al. 2015; Casas et al. 2015; Heindel et al. 2016; Valvi et al. 2012 and 2014).
All associations of interest were assessed in both unadjusted models and multivariable-adjusted models including important confounders. Potential confounders were selected based on previous literature using directed acyclic graphs. The GDM models were initially adjusted for maternal age, education, parity, pre-pregnancy BMI and smoking during pregnancy. The birth size models were adjusted for the same set of covariates along with child sex, as this is an important determinant of birth size and a hypothesized effect modifier. Further, we evaluated in these initial multivariable-adjusted models confounding by gestational weight gain, family history of diabetes (i.e. self-reports of type 1 and 2 diabetes in lineal ascendant relatives), and concentrations of 25-hydroxy vitamin D. Fish and seafood intake is a common source of vitamin D and the environmental pollutants under study, and a positive association between cord blood vitamin D and offspring length has been reported in this cohort (Dalgård et al. 2016), whereas vitamin D insufficiency during pregnancy has been associated with a higher risk for GDM in previous studies (Wei et al. 2013). The inclusion of gestational weight gain, family history of diabetes, and vitamin D concentrations in the models did not change the coefficients for the exposure-outcome pairs of interest by more than 10%, and these covariates were therefore not retained in the final statistical models. Further, in restricted analysis of 126 mother-child pairs with measured maternal serum concentrations of fatty acids (Bjerve et al. 1987), concentrations of docosahexaenoic acid (i.e. the main indicator of n-3 polyunsaturated fatty acids that similar to environmental pollutant exposures occur from the consumption of fish and seafood), which are associated with offspring birth weight (Grandjean et al. 2001; Oken et al. 2004), did not confound the associations with birth size measures (coefficient change<10%). Gestational age is an important birth size determinant that could also be a mediator in the association between environmental pollutants and birth size measures; however, adjustment for gestational age in the models did not materially change the magnitude or precision of coefficients.
In multiple-pollutant analyses, we used structural equation models (SEMs) to assess the joint associations of environmental pollutant exposures with GDM or birth size measures, and to examine mediation effects. The conceptual framework of the SEM analysis is shown in the Supplementary Material (Figure S1). In contrast to common regression analysis, SEMs permit the modeling of covariance matrices and provide parameter estimates by minimizing the differences between the observed covariances and those predicted by the model. SEM analyses include a measurement part in which the observed variables are linked to a limited number of latent functions (latent constructs, hereafter), and a structural part describing potential causal dependencies among the latent constructs and with other observed variables (Budtz-Jorgensen, Keiding et al. 2002). For the measurement part, we specified latent constructs describing the overall exposure to three groups of environmental pollutants as previously reported (Oulhote et al. 2017): (1) a latent construct for OCs based on the concentrations of ΣPCB, DDE and DDT quantified in maternal serum (DDT was not analyzed as a single-pollutant because of its high percentage of values below the LOD, but the inclusion of DDT as the third parameter in the confirmatory factor analyses improved the model fit); (2) a latent construct for PFASs based on the concentrations of the five PFAS compounds in maternal serum; and (3) a latent construct for mercury based on maternal hair and cord blood concentrations. All environmental pollutants showed significant correlations with their latent construct (Supplementary Material, Table S1). The structural model part identified the association dependencies between the three latent exposure constructs and GDM or birth size measures, with adjustment for the same set of covariates applied in regression analysis. We accounted for GDM as a mediator in the SEMs for birth size measures and estimated the total (i.e. unadjusted for any mediator), direct (adjusted for mediator) and indirect effects (i.e. ∼difference between total and direct effects). SEMs exhibited an acceptable to very good fit to the data, indicated by a high comparative fit index (CFI between 0.918 and 0.999) and a low root mean square error of approximation (RMSEA between 0.05 and 0.01) for all study outcomes. Information in additional covariates was missing in less than 1% of observations and we used Full Information Maximum Likelihood estimation, which utilizes all available information and minimizes bias compared to conventional methods for handling missing data, such as list-wise deletion (Allison 2003).
SEM analyses were performed using the “lavaan” package in R statistical computing software version 3.2.3 that calculates diagonally weighted least square estimators with robust variance for the associations with dichotomous outcomes (i.e., GDM), and maximum likelihood estimators with robust variance for the associations with continuous outcomes (i.e., birth size measures) (Rosseel 2012). The SEM estimators presented for GDM reflect an increase (if estimator>0) or a decrease (if estimator<0) in the odds (probit function) of GDM diagnosis per doubling of the exposures accounted in the latent construct. The estimators for continuous birth outcomes reflect the change in the offspring size measure per doubling of the exposures accounted in the latent construct. Statistical analyses other than SEMs were performed using STATA 14. All statistical tests were two-sided and the level of significance was set at a P-value<0.05 for all associations including interactions. Results are interpreted based on the consistency in the associations found among the single- and multiple-pollutant statistical approaches applied, along with the magnitude and precision of effect estimates rather than solely relying on statistical significance.
Results
The prevalence of GDM diagnosis in this population was 8% (N=49). Mothers diagnosed with GDM compared to mothers without GDM diagnosis were of older age, had on average higher pre-pregnancy BMIs, were more likely to report a family history of diabetes, and their offspring presented on average higher birth weights (Table 1). Maternal serum POP concentrations and hair and cord-blood mercury concentrations did not significantly differ according to GDM status (Table 2). The highest correlations among environmental pollutant concentrations were seen between hair and cord blood mercury (Pearson r=0.83), and between ΣPCB and DDE in serum (r=0.88), while poorer correlations were observed for pairs of PFASs (r ranged from 0.08 for PFHxS-PFNA to 0.63 for PFOS-PFNA) (Supplementary Material, Table S2).
Table 1. Characteristics of the 604 Faroese mother-child pairs overall and according to GDM diagnosis, with p for difference between the groups.
| Characteristic | Overall N=604 Mean±SD or N(%) | GDM – No N=555 (92%) Mean±SD or N(%) | GDM – Yes N=49 (8%) Mean±SD or N(%) | pa |
|---|---|---|---|---|
| Maternal age at delivery, years | 29.2 ± 5.2 | 29.1 ± 5.1 | 30.7 ± 5.4 | 0.03 |
| Family history of diabetes | ||||
| No | 321 (53.2) | 310 (55.9) | 11 (22.5) | |
| Yes | 283 (46.8) | 245 (44.2) | 38 (77.6) | <0.001 |
| Pre-pregnancy BMI, kg/m2 | 23.8 ± 4.0 | 23.7 ± 4.0 | 24.9 ± 4.5 | 0.05 |
| Pre-pregnancy BMI status b | ||||
| Underweight | 21 (3.5) | 20 (3.6) | 1 (2.0) | |
| Normal weight | 404 (66.9) | 375 (67.6) | 29 (59.2) | |
| Overweight | 133 (22.0) | 121 (21.8) | 12 (24.5) | |
| Obese | 46 (7.6) | 39 (7.0) | 7 (14.3) | 0.25 |
| Gestational weight gain, kg | 14.8 ± 5.2 | 14.8 ± 5.2 | 14.1 ± 4.7 | 0.33 |
| Parity | ||||
| Nulliparous | 162 (26.8) | 149 (26.8) | 13 (26.5) | |
| Multiparous | 442 (73.2) | 406 (73.2) | 36 (73.5) | 0.96 |
| Education | ||||
| Primary | 36 (6.0) | 35 (6.3) | 1 (2.0) | |
| Secondary | 134 (22.2) | 125 (22.5) | 9 (18.4) | |
| Terciary | 434 (71.8) | 395 (71.2) | 39 (79.6) | 0.34 |
| Smoking during pregnancy | ||||
| No | 434 (71.8) | 394 (71.0) | 40 (81.6) | |
| Yes | 170 (28.2) | 161 (29.0) | 9 (18.4) | 0.11 |
| Cord serum Vitamin D, nmol/L | 28.2 ± 18.3 | 28.6 ± 18.5 | 23.4 ± 15.6 | 0.08 |
| Duration of gestation, weeks | 39.6 ± 1.3 | 39.6 ± 1.3 | 39.8 ± 1.1 | 0.19 |
| Preterm birthc | ||||
| No | 594 (98.3) | 545 (98.2%) | 49 (100%) | |
| Yes | 10 (1.7%) | 10 (1.8%) | 0 (0%) | 0.48d |
| Child sex | ||||
| Male | 316 (52.3) | 290 (52.2) | 26 (53.1) | |
| Female | 288 (47.4) | 265 (47.8) | 23 (46.9) | 0.91 |
| Birth size measures | ||||
| Weight, g | 3712 ± 497 | 3695 ± 497 | 3907 ± 461 | 0.004 |
| Macrosomia (birth weight≥ 4500g) | ||||
| No | 563 (93.2) | 520 (93.7) | 43 (87.8) | |
| Yes | 41 (6.8) | 35 (6.3) | 6 (12.2) | 0.11 |
| Length (at age 14 days), cm | 54.3 ± 2.1 | 54.3 ± 2.1 | 54.7 ± 2.0 | 0.27 |
| Head circumference, cm | 36.9 ± 1.4 | 36.9 ± 1.4 | 37.0 ± 1.3 | 0.99 |
Chi-square for categorical variables if not indicated otherwise; Student's t-test for continuous variables (all variables shown in this table are normally distributed).
BMI status defined using the World Health Organization recommended cutoffs for white adults.
Preterm birth defined as <37 weeks of gestational duration.
Fisher exact test instead of chi-square because of the low expected cell frequencies of preterm children.
GDM: gestational diabetes mellitus
BMI: body mass index
Table 2. Concentrations of environmental pollutants in maternal biological samples and cord blood, overall and according to GDM diagnosis, with p for difference between the groups.
| Environmental pollutant | Overall | GDM - No | GDM - Yes | pb | ||
|---|---|---|---|---|---|---|
| N | % < LOD a | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Serum ΣPCB, μg/g-lipidc | 564 | 0% | 1.23 (0.79, 2.00) | 1.22 (0.79, 1.95) | 1.33 (0.79, 2.23) | 0.65 |
| Serum DDE, μg/g-lipid | 564 | 0% | 0.54 (0.33, 0.94) | 0.52 (0.33, 0.92) | 0.72 (0.41, 1.20) | 0.11 |
| Serum DDT, μg/g-lipid | 564 | 58% | 0.00 (0.00, 0.02) | 0.00 (0.00, 0.02) | 0.00 (0.00, 0.02) | 0.91 |
| Serum PFOS, ng/mL | 604 | 0% | 27.2 (23.1, 33.1) | 27.4 (23.2, 33.2) | 26.1 (23.0, 30.6) | 0.37 |
| Serum PFOA, ng/mL | 604 | 0% | 3.31 (2.54, 3.99) | 3.34 (2.54, 4.04) | 3.17 (2.45, 3.79) | 0.23 |
| Serum PFHxS, ng/mL | 604 | 0% | 4.54 (2.24, 8.52) | 4.52 (2.20, 8.49) | 4.90 (2.60, 9.21) | 0.77 |
| Serum PFDA, ng/mL | 604 | 0% | 0.28 (0.22, 0.38) | 0.28 (0.22, 0.38) | 0.30 (0.23, 0.35) | 0.77 |
| Serum PFNA, ng/mL | 604 | 0% | 0.59 (0.46, 0.79) | 0.60 (0.46, 0.79) | 0.58 (0.45, 0.73) | 0.37 |
| Hair mercury, μg/g | 561 | 0% | 2.21 (1.30, 4.03) | 2.23 (1.30, 4.12) | 1.90 (1.28, 3.71) | 0.30 |
| Cord blood mercury, μg/L | 538 | 0% | 11.9 (7.2, 20.9) | 12.1 (7.2, 21.5) | 10.6 (7.8, 17.9) | 0.35 |
Values below LOD were substituted by LOD/2.
K-sample equality-of-medians test for the comparison of concentration medians between GDM categories.
The ΣPCB is calculated by summing the concentrations of PCB congeners 138, 153 and 180 multiplied by 2.
GDM: gestational diabetes mellitus
LOD: limit of detection
IQR: interquartile range
In single-pollutant regression analyses (Table 3), maternal serum DDE concentrations were associated with higher odds of GDM in the unadjusted model (OR per doubling of DDE concentrations: 1.33; 95% CI: 1.00, 1.77). This association was slightly attenuated and nonsignificant in the multivariable-adjusted model (adjusted OR per doubling of DDE concentrations: 1.29; 95% CI: 0.94, 1.77). We also observed a non-significant and nonmonotonic association between maternal serum PFDA concentrations and GDM diagnosis (adjusted OR in exposure tertile group 2 versus 1: 1.97; 95% CI: 0.94, 4.12). Effect estimates for the associations between other POPs and GDM were non-significant and closer to unity. Maternal hair-mercury concentrations were associated with lower odds of GDM in both the unadjusted and adjusted models (OR per doubling of hair mercury concentrations: 0.81; 95% CI: 0.65, 1.01, and adjusted OR: 0.79; 95%CI: 0.62, 0.99). The associations of cord-blood mercury concentrations with GDM diagnosis were in the same direction and non-significant.
Table 3. Odds of GDM per increases in environmental pollutant concentrations in biological samples (calculated in regard to doubled exposure and for tertile groups).
| Environmental pollutant | Concentration range | OR (95%CI) | |
|---|---|---|---|
| Unadjusted models | Adjusted modelsa | ||
| Serum ΣPCB | per doubling of exposure | 1.11 (0.83, 1.49) | 0.97 (0.71, 1.33) |
| 0.06-0.93 μg/g-lipid | 1 | 1 | |
| 0.94-1.70 μg/g-lipid | 1.18 (0.55, 2.55) | 1.08 (0.49, 2.39) | |
| 1.71-11.8 μg/g-lipid | 1.61 (0.78, 3.34) | 1.26 (0.57, 2.75) | |
| Serum DDE | per doubling of exposure | 1.33 (1.00, 1.77) | 1.29 (0.94, 1.77) |
| 0.04-0.37 μg/g-lipid | 1 | 1 | |
| 0.38-0.73 μg/g-lipid | 1.26 (0.49, 3.30) | 1.17 (0.44, 3.09) | |
| 0.74-11.4 μg/g-lipid | 2.11 (0.88, 5.10) | 1.89 (0.75, 4.76) | |
| Serum PFOS | per doubling of exposure | 0.89 (0.44, 1.80) | 0.86 (0.43, 1.70) |
| 9.3-24.3 ng/mL | 1 | 1 | |
| 24.4-30.8 ng/mL | 0.83 (0.42, 1.65) | 0.85 (0.43, 1.70) | |
| 30.9-68.8 ng/mL | 0.57 (0.27, 1.21) | 0.56 (0.26, 1.19) | |
| Serum PFOA | per doubling of exposure | 0.79 (0.45, 1.36) | 0.79 (0.44, 1.41) |
| 0.82-2.79 ng/mL | 1 | 1 | |
| 2.80-3.80 ng/mL | 1.06 (0.54, 2.09) | 1.01 (0.50, 2.06) | |
| 3.81-8.43 ng/mL | 0.65 (0.30, 1.39) | 0.66 (0.30, 1.48) | |
| Serum PFHxS | per doubling of exposure | 1.05 (0.82, 1.34) | 1.03 (0.80, 1.33) |
| 0.62-2.90 ng/mL | 1 | 1 | |
| 2.91-7.32 ng/mL | 0.99 (0.48, 2.03) | 0.98 (0.47, 2.05) | |
| 7.33-26.4 ng/mL | 1.06 (0.52, 2.15) | 1.00 (0.48, 2.07) | |
| Serum PFDA | per doubling of exposure | 1.29 (0.81, 2.06) | 1.20 (0.73, 1.96) |
| 0.03-0.22 ng/mL | 1 | 1 | |
| 0.23-0.33 ng/mL | 2.08 (1.00, 4.32) | 1.97 (0.94, 4.12) | |
| 0.34-1.22 ng/mL | 1.17 (0.53, 2.59) | 1.02 (0.45, 2.30) | |
| Serum PFNA | per doubling of exposure | 0.99 (0.61, 1.63) | 0.88 (0.53, 1.47) |
| 0.12-0.50 ng/mL | 1 | 1 | |
| 0.51-0.70 ng/mL | 0.70 (0.34, 1.45) | 0.62 (0.30, 1.30) | |
| 0.71-2.51 ng/mL | 0.81 (0.40, 1.62) | 0.65 (0.31, 1.36) | |
| Hair mercury | per doubling of exposure | 0.81 (0.65, 1.01) | 0.79 (0.62, 0.99) |
| 0.02-1.51 μg/g | 1 | 1 | |
| 1.52-3.43 μg /g | 0.93 (0.45, 1.90) | 0.92 (0.44, 1.90) | |
| 3.44-32.8 μg/g | 0.75 (0.35, 1.59) | 0.73 (0.34, 1.59) | |
| Cord blood mercury | per doubling of exposure | 0.88 (0.67, 1.15) | 0.87 (0.66, 1.15) |
| 1.57-8.61 μg/L | 1 | 1 | |
| 8.62-17.8 μg/L | 1.09 (0.53, 2.23) | 1.08 (0.52, 2.26) | |
| 17.9-192.8 μg/L | 0.74 (0.34, 1.61) | 0.73 (0.33, 1.62) | |
Models adjusted for maternal age at delivery, education, parity, pre-pregnancy BMI (continuous) and smoking during pregnancy.
GDM: gestational diabetes mellitus
GAMs indicated linear dose-response relationships between log2-transformed single-pollutant exposures and birth size measures (i.e., p gain for linearity >0.10 for all pairs of exposure-outcomes evaluated). Linear regression analyses (Table 4) in regard to birth weight (in g) showed a non-significant inverse association for maternal serum PFOS concentrations (adjusted β per doubling of PFOS concentrations: -81; 95% CI: -173, 11), and inverse associations of smaller magnitude were also seen for other PFASs. Further, for PFOA and less clearly PFOS, we found evidence that associations with birth weight may differ according to sex (P-sex interaction=0.04 and 0.08, respectively) (Supplemental Material, Table S3). Concentrations of OCs and mercury were not associated with birth weight, and we did not find clear association patterns between the environmental pollutants examined and offspring length (Table 4). In regard to birth head circumference (in cm), positive associations were seen for serum ΣPCB (adjusted β per doubling of ΣPCB: 0.15; 95% CI: 0.03, 0.26) and less evidently for DDE concentrations (Table 4). Associations of maternal serum PFAS concentrations with head circumference were positive for some compounds (PFDA, PFNA and PFHxS) and statistically significant for PFHxS (adjusted β per doubling of PFHxS: 0.11; 95% CI: 0.01, 0.20). Further, for some PFAS compounds, we found evidence that the associations with head circumference may differ according to sex (P-sex interaction<0.05 for PFOS, PFHxS and PFDA). Non-significant positive associations with head circumference were also seen for hair and cord blood mercury concentrations. We did not find clear evidence for effect modification by GDM in the associations between environmental pollutants and birth size measures (P-GDM interaction>0.05 for all exposure-outcome pairs).
Table 4. Change in birth size measures per doubling of environmental pollutant concentrations in maternal biological samples and cord blood, overall and according to GDM diagnosis.
| Birth size measure/ Exposure variable | Overall | GDM-No | GDM-Yes | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted models β (95%CI) | Adjusted modelsa | Adjusted modelsa β (95%CI) | Adjusted modelsa β (95%CI) | p for GDM interaction | ||
| β (95%CI) | p for sex interaction | |||||
|
| ||||||
| Weight (g) | ||||||
| Serum ΣPCB | 15 (-25, 55) | 2 (-40, 43) | 0.71 | -10 (-54, 34) | 102 (-38, 242) | 0.13 |
| Serum DDE | -6 (-44, 33) | -21 (-60, 18) | 0.39 | -30 (-70, 11) | 6 (-154, 166) | 0.31 |
| Serum PFOS | -72 (-167, 24) | -81 (-173, 11) | 0.08 | -83 (-178, 13) | 149 (-248, 547) | 0.73 |
| Serum PFOA | -85 (-161, -10) | -11 (-88, 67) | 0.04 | 5 (-74, 85) | -60 (-448, 327) | 0.40 |
| Serum PFHxS | 11 (-23, 45) | 15 (-18, 47) | 0.75 | 17 (-16, 51) | -16 (-144, 111) | 0.49 |
| Serum PFDA | -20 (-81, 41) | -41 (-102, 18) | 0.81 | -49 (-111, 13) | 170 (-109, 450) | 0.47 |
| Serum PFNA | -18 (-85, 49) | -42 (-108, 25) | 0.96 | -43 (-111, 26) | 93 (-177, 364) | 0.78 |
| Hair mercury | 12 (-20, 44) | 2 (-30, 33) | 0.61 | 7 (-26, 41) | 17 (-77, 111) | 0.54 |
| Cord blood mercury | 14 (-22, 51) | 5 (-30, 41) | 0.63 | 3 (-34, 39) | 103 (-43, 250) | 0.56 |
| GDM (yes vs no)b | 214 (68, 360) | 183 (41, 325) | na | na | na | na |
|
| ||||||
| Length (cm) | ||||||
| Serum ΣPCB | 0.08 (-0.10, 0.26) | 0.08 (-0.10, 0.26) | 0.21 | 0.07 (-0.12, 0.26) | 0.26 (-0.38, 0.91) | 0.70 |
| Serum DDE | -0.00 (-0.16, 0.16) | -0.02 (-0.18, 0.14) | 0.38 | -0.04 (-0.22, 0.13) | 0.12 (-0.62, 0.87) | 0.22 |
| Serum PFOS | 0.09 (-0.31, 0.49) | 0.05 (-0.33, 0.43) | 0.17 | 0.10 (-0.30, 0.50) | 0.08 (-1.61, 1.77) | 0.58 |
| Serum PFOA | -0.28 (-0.60, 0.03) | 0.03 (-0.29, 0.35) | 0.64 | 0.10 (-0.24, 0.42) | -0.58 (-2.23, 1.07) | 0.19 |
| Serum PFHxS | -0.12 (-0.26, 0.02) | -0.10 (-0.24, 0.03) | 0.50 | -0.11 (-0.25, 0.03) | -0.10 (-0.65, 0.46) | 0.86 |
| Serum PFDA | 0.03 (-0.22, 0.29) | -0.01 (-0.26, 0.24) | 0.22 | -0.04 (-0.30, 0.22) | 0.75 (-0.42, 1.93) | 0.30 |
| Serum PFNA | 0.06 (-0.22, 0.34) | 0.01 (-0.26, 0.29) | 0.72 | -0.01 (-0.30, 0.28) | 0.54 (-0.59, 1.67) | 0.47 |
| Hair mercury | 0.06 (-0.08, 0.21) | 0.02 (-0.12, 0.16) | 0.29 | 0.00 (-0.14, 0.15) | 0.26 (-0.29, 0.81) | 0.49 |
| Cord blood mercury | 0.08 (-0.08, 0.24) | 0.03 (-0.13, 0.18) | 0.18 | 0.00 (-0.16, 0.16) | 0.56 (-0.13, 1.24) | 0.18 |
| GDM (yes vs no)b | 0.24 (-0.39, 0.88) | 0.13 (-0.48, 0.74) | na | na | na | na |
|
| ||||||
| Head circumference (cm) | ||||||
| Serum ΣPCB | 0.16 (0.05, 0.27) | 0.15 (0.03, 0.26) | 0.39 | 0.12 (0.01, 0.25) | 0.33 (0.03, 0.63) | 0.47 |
| Serum DDE | 0.11 (-0.00, 0.22) | 0.08 (-0.03, 0.20) | 0.60 | 0.07 (-0.05, 0.19) | 0.26 (-0.17, 0.70) | 0.48 |
| Serum PFOS | 0.07 (-0.21, 0.35) | 0.00 (-0.28, 0.27) | 0.01 | 0.09 (-0.20, 0.38) | -0.47 (-1.33, 0.39) | 0.08 |
| Serum PFOA | -0.06 (-0.28, 0.16) | 0.00 (-0.22, 0.23) | 0.90 | 0.06 (-0.18, 0.29) | -0.37 (-1.23, 0.49) | 0.11 |
| Serum PFHxS | 0.11 (0.01, 0.21) | 0.11 (0.01, 0.20) | 0.04 | 0.10 (-0.00, 0.19) | 0.15 (-0.14, 0.44) | 0.47 |
| Serum PFDA | 0.14 (-0.04, 0.33) | 0.11 (-0.07, 0.29) | 0.03 | 0.13 (-0.06, 0.32) | -0.13 (-0.76, 0.49) | 0.42 |
| Serum PFNA | 0.13 (-0.07, 0.32) | 0.06 (-0.14, 0.25) | 0.30 | 0.12 (-0.09, 0.32) | -0.26 (-0.87, 0.36) | 0.09 |
| Hair mercury | 0.08 (-0.00, 0.17) | 0.06 (-0.03, 0.15) | 0.64 | 0.05 (-0.04 (0.15) | 0.02 (-0.22, 0.27) | 0.86 |
| Cord blood mercury | 0.10 (-0.00, 0.20) | 0.07 (-0.03, 0.17) | 0.76 | 0.07 (-0.04, 0.17) | 0.01 (-0.38, 0.41) | 0.59 |
| GDM (yes vs no)b | 0.00 (-0.41, 0.41) | -0.09 (-0.49, 0.31) | na | na | na | na |
Models adjusted for maternal age at delivery, education, parity, pre-pregnancy BMI (continuous), smoking during pregnancy and child sex.
Coefficients for the association between GDM diagnosis and birth size measures are shown for comparative purposes.
na: not applicable
GDM: gestational diabetes mellitus
SEM estimates adjusted simultaneously for the three exposure latent constructs (OCs, PFASs and mercury) and additional covariates are presented in Table 5, where for comparison purposes we also show the estimates for maternal smoking status in pregnancy (no/yes) obtained from the same models. OC exposure was associated with increased odds of GDM (change in GDM probit per doubling of OC exposure: 0.45; 95%CI: 0.05, 0.86), while no clear association was shown for PFAS and mercury exposures. Interaction terms between any of the three exposure latent constructs and GDM were non-significant (P-GDM interaction>0.30 in all SEM models). Further, we did not find evidence that GDM may mediate the associations of environmental pollutant exposures with birth size outcomes (i.e., estimates for indirect effects per doubling of exposure close to 0 for all exposure latent constructs and birth size measures) (Table 5). In agreement with the results of the single-PFAS linear regression analyses, we found a non-significant inverse association between PFAS exposure and birth weight using SEMs (mean change per doubling of the PFAS exposure: -169g; 95% CI: -359, 21), which was similar in magnitude as the association between maternal smoking and birth weight (-144g). A nonsignificant inverse association was seen with head circumference, while no association was found between PFAS exposure and offspring length. Exposure to OCs was associated with an increase in head circumference (mean change in head circumference per doubling of OC exposure: 0.13 cm; 95%CI: 0.01, 0.25) and unrelated to offspring weight or length. Mercury exposure was not associated with birth size measures.
Table 5. SEM estimates for the associations with GDM diagnosis or birth size measures per doubling of environmental pollutant exposures (latent constructsa), and direct and indirect effects from mediation analyses.
| Outcome / Exposure | Total Effectb | Direct Effectb | Indirect Effectb | |||
|---|---|---|---|---|---|---|
| Estimate (95% CI)c | P | Estimate (95% CI) c | P | Estimate (95% CI)c | P | |
|
| ||||||
| GDM (no/yes) | ||||||
| OCs | 0.45 (0.05, 0.86) | 0.03 | na | na | na | na |
| PFASs | -0.25 (-1.06, 0.56) | 0.72 | na | na | na | na |
| Mercury | -0.10 (-0.32, 0.11) | 0.34 | na | na | na | na |
| Smoking (yes vs no)d | -0.01 (-1.07, 1.05) | 0.65 | na | na | na | na |
| Weight (g) | ||||||
| OCs | 16 (-27, 58) | 0.48 | 15 (-27, 57) | 0.48 | 0.7 (-4, 5) | 0.77 |
| PFASs | -169 (-359, 21) | 0.11 | -177 (-375, 21) | 0.09 | 8 (-22, 38) | 0.48 |
| Mercury | 19 (-26, 64) | 0.82 | 22 (-23, 66) | 0.31 | -2 (-8, 4) | 0.48 |
| Smoking (yes vs no)d | -144 (-226, -62) | 0.001 | na | na | na | na |
| Length (cm) | ||||||
| OCs | 0.05 (-0.13, 0.23) | 0.57 | 0.06 (-0.12, 0.24) | 0.55 | 0.00 (-0.01, 0.01) | 0.75 |
| PFASs | -0.06 (-0.96, 0.84) | 0.91 | -0.05 (-0.97, 0.86) | 0.93 | -0.01 (-0.05, 0.04) | 0.54 |
| Mercury | 0.02 (-0.18, 0.23) | 0.83 | 0.02 (-0.19, 0.22) | 0.88 | 0.01 (-0.01, 0.02) | 0.31 |
| Smoking (yes vs no)d | -0.58 (-0.95, -0.20) | 0.003 | na | na | na | na |
| Head circumference (cm) | ||||||
| OCs | 0.13 (0.01, 0.25) | 0.02 | 0.13 (0.01, 0.25) | 0.03 | 0.00 (-0.01, 0.01) | 0.74 |
| PFAS | -0.25 (-0.87, 0.37) | 0.43 | -0.24 (-0.86, 0.37) | 0.44 | -0.01 (-0.03, 0.02) | 0.63 |
| Mercury | 0.05 (-0.07, 0.17) | 0.40 | 0.05 (-0.07, 0.17) | 0.44 | 0.00 (-0.01, 0.01) | 0.41 |
| Smoking (yes vs no)d | -0.21 (-0.43, 0.03) | 0.09 | na | na | na | na |
The latent construct of OC exposures is estimated based on measured maternal serum concentrations of ΣPCB, DDE and DDT. The latent construct of PFAS exposures is estimated based on measured maternal serum concentrations of PFOS, PFOA, PFHxS, PFDA and PFNA. The latent construct of mercury exposures is estimated based on measured mercury concentrations in maternal hair and cord blood.
The total effect reflects the estimated association between exposure and outcome unadjusted for any mediator. The direct effect reflects the estimated association adjusted for GDM as a mediator. The indirect effect approximates the difference between the total and direct effects.
All estimates are adjusted simultaneously for the three latent constructs of environmental pollutant exposures, and maternal age at delivery, education, parity, pre-pregnancy BMI (continuous), smoking during pregnancy and child sex. Estimates for the associations with GDM reflect the change in GDM odds (probit) per doubling of environmental pollutant exposures OR in the group of mothers who smoked during pregnancy compared to the reference exposure category (i.e. non-smokers: mean estimate=0). Estimates for the associations with the birth size measures reflect the mean change of the birth size measure per doubling of environmental pollutant exposures OR in the group of newborns whose mothers smoked during pregnancy compared to the reference exposure group (i.e. newborns of non-smokers: mean estimate=0). Estimates above 0 indicate positive associations, and estimates below 0 indicate inverse associations for all exposure-outcome associations shown in this table.
The estimates for smoking are extracted from the same outcome model as the latent constructs of environmental pollutant exposures and are shown for comparative purposes.
na: not applicable
SEM: structural equation model
GDM: gestational diabetes mellitus
In analyses stratified by child sex, we found evidence of an inverse association with birth weight for PFOS, and less clearly PFOA, in boys, but not in girls (Figure 1A and Supplementary Material, Table S3). Similarly, in regard to head circumference we found a pattern of inverse or null associations in boys, and mainly positive associations in girls, for all PFAS compounds with the exception of PFHxS for which the association was positive in boys only (Figure 1B and Supplementary Material, Table S3). Sex did not significantly modify the associations between PFAS compounds and offspring length, or the associations of OCs and mercury with birth size measures (Supplementary Material, Table S3). In subsequent sensitivity analysis for OCs (data not shown), analysing the OC concentrations uncorrected for lipids and including maternal lipid concentrations as a separate covariate in the models, did not significantly change the shown associations between OCs and GDM or birth size measures.
Figure 1. (A-B). Linear regression and SEM estimatesa for the associations with birth weight (A) and head circumference (B) per doubling of maternal serum PFAS concentrations, according to sex.
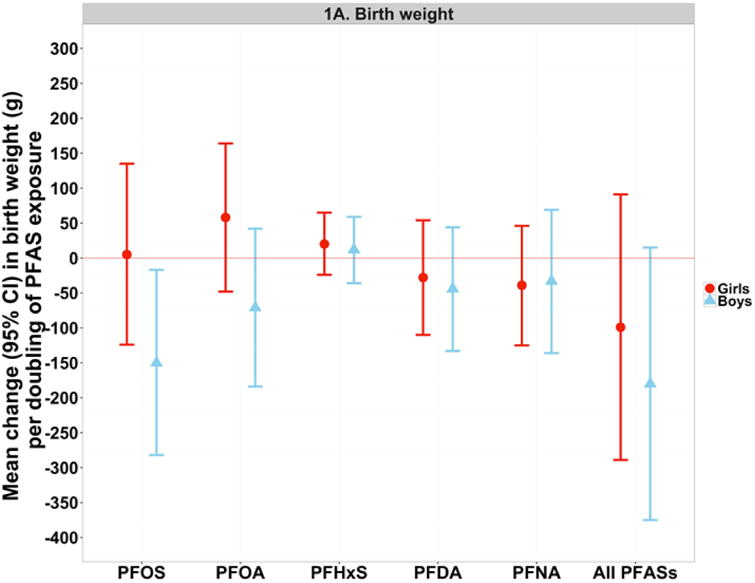
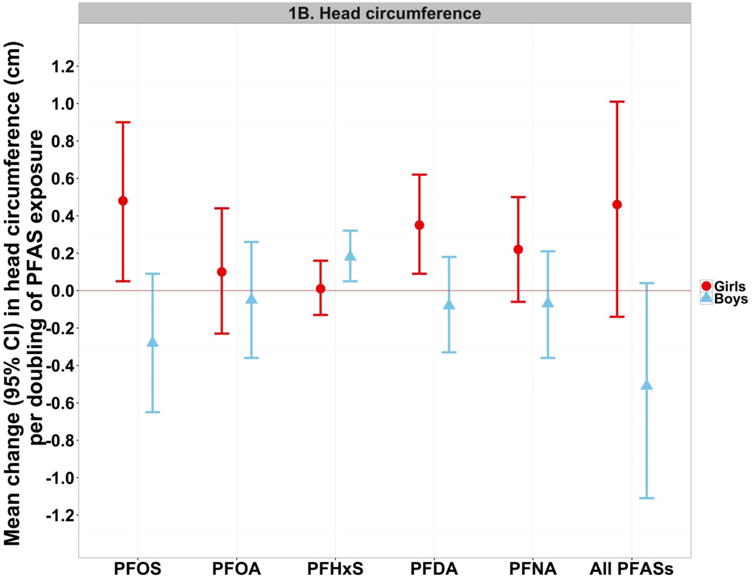
aThe single-PFAS estimates shown in this figure reflect the mean change in the birth size measure per doubling of measured PFAS concentrations in maternal serum, after adjustment for maternal age at delivery, education, parity, pre-pregnancy BMI (continuous) and smoking during pregnancy. The multiple-PFAS estimates reflect the mean change in the birth size measure (total effect from SEMs) per doubling of PFAS exposure (i.e. latent construct of all five PFAS compounds), after adjustment for the latent constructs of OC and mercury exposures, and maternal age at delivery, education, parity, pre-pregnancy BMI (continuous) and smoking during pregnancy.
SEM: structural equation model
Discussion
In the present study we combined single-pollutant and multiple-pollutant approaches and found associations of environmental pollutant exposures with GDM occurrence and offspring birth size measures, specific to each pollutant or pollutant group examined. The study findings support an association between OC exposures during pregnancy and higher odds of GDM diagnosis, while no clear evidence for such association was found for PFAS or mercury exposures. Moreover, maternal OC exposures were associated with a small increase in offspring head circumference at birth, and maternal PFAS concentrations showed patterns of inverse associations with birth weight and head circumference in boys but null or positive associations in girls, though effect modification by sex was significant only for some PFAS compounds. The environmental pollutants and mixtures evaluated were not associated with offspring length. Further, our findings indicate that GDM diagnosis neither modifies nor mediates the associations between environmental pollutant exposures and birth size measures, suggesting that associations with offspring size might be independent of GDM occurrence.
Important strengths of this study include the exposure assessment using biomarkers of multiple environmental pollutants, the wide list of potential confounders considered including maternal weight status and essential nutrients, and the homogeneity of the Faroese population in regard to socioeconomic and lifestyle factors which reduces the likelihood of residual confounding. The use of SEMs permitted the simultaneous adjustment for concurrent correlated exposures, while allowing for measurement error in the exposure variables, which is essential for unbiased effect estimation (Butdtz-Jorgensen et al. 2002; Carroll et al. 2006). In the absence of exposure-mediator statistical interactions, as it was the case in this study, SEMs provide an accurate estimation of mediation effects that coincides with the estimation of counterfactual approaches (Valeri and Vanderweele 2013). Study limitations include the lack of information about maternal fasting blood glucose levels, which would have allowed to identify women with impaired glucose tolerance non-diagnosed for GDM. Moreover, adjustment for maternal glomerular filtration rate, a marker of kidney function, has been proposed to attenuate the associations of maternal serum PFASs with birth weight (Verner et al. 2015) and therefore overestimation in our study of any potential true PFAS effect is possible. Finally, even though vitamin D and n-3 fatty acid concentrations did not confound the associations of interest, we cannot completely rule out confounding by maternal diet.
Extensive evidence from in vivo and in vitro studies supports obesogenic and diabetogenic effects of environmental pollutant exposures through multiple hormonal and epigenetic alterations that may induce metabolic responses in the mother and her offspring (Heindel et al. 2016; Janesick and Blumberg 2016). Exposure to certain POPs, such as DDE, PFOS and PFOA, has been previously proposed to alter the secretion and function of human sex steroids and thyroid hormones (Berg et al. 2016; Ferguson et al. 2012), induce inflammation, mitochondrial dysfunction and oxidative stress (Kim and Lee 2014; Myre and Imbeault 2014), promote adipogenesis through activation of the peroxisome proliferator activated receptor gamma (PPARγ) (Janesick and Blumberg 2016), and alter lipid peroxidation and pancreatic beta cell function (Al-Eryani et al. 2015). Findings from experimental studies also support an interference of mercury exposure to lipid peroxidation and pancreatic beta cell function (Chen et al. 2006 and 2010; Moreira et al. 2012), though data on metabolic effects of mercury are sparse (Heindel et al. 2016).
Maternal serum concentrations of OCs in this cohort are higher than those reported in a more recent Faroese cohort (Karlsen et al. 2016) and in recent pregnancy cohorts in Canada and Europe (Casas et al. 2015; Shapiro et al. 2016; Vafeiadi et al. 2014). The maternal serum concentrations of PFASs are comparable to those reported in Europe and North America though the mixture profiles vary among populations (Casas et al. 2015; Shapiro et al. 2016; Zhang et al. 2015). PFOS and PFOA have been widely used in the past 60 years in consumer products and industrial applications due to their oil and water repellent properties (Grandjean and Clapp 2014), and while PFOS exposure has decreased in the past decade, exposure to other PFASs (e.g., PFNA and PFHxS) has emerged (Kato et al. 2011; Oulhote et al. 2016). In regard to mercury exposure, 30% to 65% of mothers in our study have hair concentrations above the recommended safety limits of 1.0 μg/g and 0.58 μg/g, which is a lower proportion compared to Southern European countries, but higher than in most other EU regions (Bellanger et al. 2013).
One recent study in US pregnant women, with a range of serum DDE concentrations similar to the Faroese mothers, reported a non-significant positive association with GDM risk (Smarr et al. 2016), in agreement with our findings. However, no association with GDM was seen in Canadian and Greek pregnant women at lower DDE levels (Shapiro et al. 2016; Vafeiadi et al. 2016). Two studies in Canada and US reported no association between PCB concentrations and GDM in line with our findings (Jaacks et al. 2016; Shapiro et al. 2016), but serum PCB concentrations were associated with increased odds of GDM in one study in Greece (Vafeiadi et al. 2016). In our study, the association between OC exposure and GDM persisted in multi-pollutant adjusted SEMs, and our findings overall therefore support an association with increased GDM risk, in agreement with the OC associations with the risk of type 2 diabetes reported in studies of adults (Lee et al. 2014; Kuo et al. 2013; Magliano et al. 2014; Taylor et al. 2013).
Evidence in regard to the association of PFAS and mercury exposures with GDM is limited and inconclusive. One smaller study in the US (N=272) at similar serum concentrations of PFOA and lower concentrations of PFOS and PFNA compared to our study found higher odds of GDM at increased PFOA concentrations (Zhang et al. 2015). However, a study in Canada with twice the sample size compared to our study found no clear association for PFOA or other PFASs (Shapiro et al. 2016). Contrary to the findings of the US study we did not find an association between PFAS exposures and GDM, which may be due in part to differences in the PFAS mixture and characteristics of the US population (e.g., lower education and higher prevalence of obesity and GDM compared to the Faroese mothers). Non-significant positive associations for blood-mercury and odds of GDM have been reported in Canada (Shapiro et al. 2015), and for meconium mercury in one study in China (Peng et al. 2015). In our study, maternal hair mercury was associated with lower odds of GDM, but this association did not remain significant in the multipollutant-adjusted models and it was attenuated in particular after adjustment for OC exposures. However, non-significant associations with GDM in this and previous studies that have relied on GDM diagnosis from medical registries should be interpreted with caution, as OGTTs are routinely administered to women considered at higher risk only, possibly leading to an underestimation of GDM cases, and misclassification error that may most likely attenuate the associations toward the null.
Previous meta-analyses support an inverse association between maternal PCB-153 concentrations and birth weight (Casas et al. 2015; Govarts et al. 2012), which was not clearly shown in our study. This may be due to reduced power and/or the higher PCB exposures in this population compared to others, as non-monotonic dose-response relationships for endocrine disruptors are likely (Vandenberg et al. 2012). However, maternal PCB and DDE concentrations have been associated with higher offspring BMI later in childhood in this (Tang-Peronard et al. 2014) and other birth cohorts (Karlsen et al. 2016; Valvi et al. 2012 and 2014). Associations between maternal OC exposures and offspring head circumference have been studied at a lesser extent, with our findings and those of one previous study (de Cock et al. 2014) suggesting a positive association between maternal DDE exposure and offspring head circumference, whereas other studies have reported decreases in head circumference associated with higher maternal concentrations of DDT (Lopez-Espinosa et al. 2011), DDE and PCBs (Vafeiadi et al. 2014). An advantage of our study compared to prior literature is the multiple-pollutant approach used, however the relevance of a potentially positive association with head circumference at birth for neurodevelopmental outcomes in later life is unclear.
Maternal serum or cord blood concentrations of PFOA and/or PFOS have been associated with decreases in birth weight in more than fourteen previous studies, although in many studies associations were non-significant (Bach et al. 2015; Vrijheid et al. 2016). In the Faroese cohort, associations with birth size measures were more evident for PFOS and in boys only, whereas previous studies have reported associations mostly for PFOA and inconclusive findings in regard to interactions by sex (Bach et al. 2015). These discrepancies could be due in part to the substantial differences in the exposure mixture profiles, and the moderate to high correlations of PFAS compounds that do not allow to disentangle their specific contributions to the observed associations. Using a multiple-pollutant approach, we found a similar trend toward reduced average birth weight (∼150 g) for a doubling of PFAS exposures, as for maternal smoking during pregnancy which is a well-known risk factor for adverse metabolic outcomes in the offspring (Bakker and Jaddoe 2011; Joubert et al. 2016; Oken et al. 2008) and effect estimates for the overall PFAS exposure did not significantly differ between boys and girls. Lower birth weight is associated with increased risk of type 2 diabetes in later life (Whincup et al. 2008), and maternal PFAS exposures have been associated with adverse metabolic outcomes in children (Hoyer et al. 2015; Karlsen et al. 2016; Tang-Peronard et al. 2014) and adults (Halldorsson et al. 2012). Thus, our findings suggest that even if associations between exposure to one single PFAS and birth weight may appear as small or insignificant, the association of PFAS exposure overall could be of clinical relevance and predict long-term adverse health outcomes. Larger studies with improved precision and accounting for multiple pollutants are needed to confirm this possibility and whether PFAS associations with birth outcomes indeed differ according to sex. In regard to mercury, findings from this and recent birth cohort studies, with reported lower mercury exposure levels compared to older birth cohorts, do not clearly support associations with birth size measures (Govarts et al. 2016; Murcia et al. 2016; Vrijheid et al. 2016); however, associations of maternal low-level mercury exposure with adverse neurodevelopmental outcomes in the offspring in recent birth cohorts are well documented (Vrijheid et al. 2016), adding to mounting evidence of mercury toxicity.
This is the first study to examine GDM as a mediator or modifier in the pollutant-related associations with offspring birth size measures. Maternal metabolic changes in response to diabetogenic environmental pollutant exposures could mediate effects in the offspring (Kahraman et al. 2014; Kc et al. 2015), while increased availability of glucose and macronutrients in fetal circulation through the placenta in GDM cases (Araujo et al. 2015) could modify the obesogenic and/or insulinogenic effects of environmental pollutants in the offspring (Goran et al. 2013; La Merrill et al. 2014). Moreover, although mechanisms are not well understood, effect modification could be also possible due to common mechanistic pathways, such as DNA methylation in imprinted genes of the placenta and/or fetal tissues involved in offspring's metabolism and growth that are thought to underlie the effects of both GDM (Kc et al. 2015; Ruchat et al. 2013) and environmental pollutant exposures (Kappil et al. 2016; Kobayashi et al. 2017) on fetal development. We did not find evidence for either a mediating or modifying role of GDM occurrence in the associations between environmental pollutant exposures and birth size measures; however, findings from this study should be interpreted with caution and require replication in larger populations, as the small GDM prevalence and sample size has reduced our study power for detecting significant associations, especially for mediating effects and interactions.
Conclusions
In pregnant women with characterized exposures of multiple environmental pollutants, we found specific associations with GDM occurrence and offspring birth size measures, according to the environmental pollutant and group of pollutants examined. Higher OC exposures during pregnancy were associated with a higher GDM occurrence and increases in offspring head circumference, while an indication of sex-dimorphic associations with birth weight and head circumference was found for PFAS exposures. GDM did not appear to either mediate or modify the associations of environmental pollutant exposures with offspring birth size measures. However, the potentially mediating or modifying role of GDM and maternal metabolic responses in the associations of environmental pollutant exposures with offspring health outcomes deserves more attention in future studies.
Supplementary Material
Highlights.
We hypothesised GDM as mediator and/or modifier of the associations of environmental chemicals with birth size.
Maternal OC exposure was associated with higher GDM odds and offspring head circumference.
Associations of maternal PFAS exposure and birth size measures may differ by sex.
Mercury exposure was not clearly associated with odds of GDM or birth size measures.
GDM status did not mediate or modify the associations with birth size measures in this Faroese birth cohort.
Acknowledgments
The authors are grateful to the study participants for their generous collaboration. This study was funded by the Danish Environmental Protection Agency (grant number 123/001-0012), the National Institute of Environmental Health Sciences of the NIH (grant numbers ES012199 and ES021477), and the US Environmental Protection Agency (R830758).
Abbreviations
- BMI
body mass index
- DDE
dichlorodiphenyldichloroethylene
- DDT
dichlorodiphenyltricloroethane
- GAM
generalized additive models
- GDM
gestational diabetes mellitus
- LOD
limit of detection
- OCs
organochlorine compounds
- PCBs
polychlorinated biphenyl
- PFASs
perfluoroalkyl substances
- PFDA
perfluorodecanoic acid
- PFNA
perfluorononanoic acid
- PFHxS
perfluorohexane sulfonic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonate
- POPs
persistent organic pollutants
- SEMs
structural equation models
Footnotes
Conflict of interest: The authors have no competing interests to declare, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Eryani L, Wahlang B, Falkner KC, Guardiola JJ, Clair HB, Prough RA, et al. Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicol Pathol. 2015;43(4):482–497. doi: 10.1177/0192623314549960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112(4):545–557. doi: 10.1037/0021-843X.112.4.545. [DOI] [PubMed] [Google Scholar]
- Araujo JR, Keating E, Martel F. Impact of gestational diabetes mellitus in the maternal-to-fatal transport of nutrients. Curr Diab Rep. 2015;15(2):569. doi: 10.1007/s11892-014-0569-y. [DOI] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit Rev Toxicol. 2015;45(1):53–67. doi: 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- Bakker H, Jaddoe VW. Cardiovascular and metabolic influences of fetal smoke exposure. Eur J Epidemiol. 2011;26(10):763–770. doi: 10.1007/s10654-011-9621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- Bellanger M, Pichery C, Aerts D, Berglund M, Castaño A, Cejchanova PC, et al. Economic benefits of methylmercury exposure control in Europe: Monetary value of neurotoxicity prevention. Environ Health. 2013;12:3. doi: 10.1186/1476-069X-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V, Nost TH, Pettersen RD, Hansen S, Veyhe AS, Jorde R, et al. Environ Health Perspect. 2016. Organic pollutants and the association with maternal and infant thyroid homeostasis: A multipollutant assessment. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H, Crane J, Farine D. Screening for gestational diabetes mellitus. J Obstet Gynaecol Can. 2003;25(2):96–106. doi: 10.1016/s1701-2163(16)30205-5. [DOI] [PubMed] [Google Scholar]
- Bjerve KS, Fischer S, Alme K. Alpha-linolenic acid deficiency in man: effect of ethyl linolenate on plasma and erythrocyte fatty acid composition and biosynthesis of prostanoids. Am J Clin Nutr. 1987;46:570–576. doi: 10.1093/ajcn/46.4.570. [DOI] [PubMed] [Google Scholar]
- Butdz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1(1):2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement error in nonlinear models: A model perspective. Second. United Kingdom: Chapman and Hall; 2006. [Google Scholar]
- Casas M, Nieuwenhuijsen M, Martinez D, Ballester F, Basagana X, Basterrechea M, et al. Prenatal exposure to PCB-153, p,p′-DDE and birth outcomes in 9000 mother-child pairs: Exposure-response relationship and effect modifiers. Environ Int. 2015;74:23–31. doi: 10.1016/j.envint.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Chen YW, Huang CF, Yang CY, Yen CC, Tsai KS, Liu SH. Inorganic mercury causes pancreatic beta-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol Appl Pharmacol. 2010;243(3):323–331. doi: 10.1016/j.taap.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, et al. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem Res Toxicol. 2006;19(8):1080–1085. doi: 10.1021/tx0600705. [DOI] [PubMed] [Google Scholar]
- Dalgard C, Petersen MS, Steuerwald U, Weihe P, Grandjean P. Umbilical cord serum 25-hydroxyvitamin D concentrations and relation to birthweight, head circumference and infant length at age 14 days. Paediatr Perinat Epidemiol. 2016;30(3):283–245. doi: 10.1111/ppe.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors – a Dutch prospective cohort study. Int J Environ Res Public Health. 2014;11:7001–7021. doi: 10.3390/ijerph110707001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich SF, Rosas LG, Ferrara A, King JC, Abrams B, Harley KG, et al. Pregnancy glycemia in Mexican-American women without diabetes or gestational diabetes and programming for childhood obesity. Am J Epidemiol. 2013;177(8):768–775. doi: 10.1093/aje/kws312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Hauser R, Altshul L, Meeker JD. Serum concentrations of p,p′-DDE, HCB, PCBs and reproductive hormones among men of reproductive age. Reprod Toxicol. 2012;34(3):429–435. doi: 10.1016/j.reprotox.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Dumke K, Bouret SG, Kayser B, Walker RW, Blumberg B. The obesogenic effect of high fructose exposure during early development. Nat Rev Endocrinol. 2013;9(8):494–500. doi: 10.1038/nrendo.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): A meta-analysis within 12 European birth cohorts. Environ Health Perspect. 2012;120(2):162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, et al. Combined effects of prenatal exposures to environmental chemicals on birth weight. Int J Environ Res Public Health. 2016;13(5) doi: 10.3390/ijerph13050495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. Int J Epidemiol. 2001;30(6):1272–1278. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E. Total imprecision of exposure biomarkers: implications for calculating exposure limits. Am J Int Med. 2007;50(10):712–719. doi: 10.1002/ajim.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150(3):301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Sampson EJ, Jr, et al. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res. 1995;71(1):29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ Health Perpect. 2012;120(5):668–673. doi: 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2016 doi: 10.1016/j.reprotox.2016.10.001. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Jorgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect. 2010;118(10):1434–1438. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer BB, Ramlau-Hansen CH, Vrijheid M, Valvi D, Pedersen HS, Zviezdai V, et al. Anthropometry in 5- to 9-year old Greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect. 2015;123(8):841–846. doi: 10.1289/ehp.1408881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks LM, Barr DB, Sundaram R, Maisog JM, Zhang C, Buck Louis GM. Pre-pregnancy maternal exposure to polybrominated and polychlorinated biphenyls and gestational diabetes: A prospective cohort study. Environ Health. 2016;15:11. doi: 10.1186/s12940-016-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. Am J Obstet Gynecol. 2016;214(5):559–565. doi: 10.1016/j.ajog.2016.01.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert BR, Felix JF, Yousefi P, Kelly MB, Just A, Breton C, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappil MA, Li Q, Li A, Dassanayake PS, Xia Y, Nanes JA, et al. In utero exposures to environmental organic pollutants disrupt epigenetic marks linked to fetoplacental development. Enviro Epigenet. 2016;2(1) doi: 10.1093/eep/dvv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y, Valvi D. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol. 2016 doi: 10.1016/j.reprotox.2016.08.002. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman S, Dirice E, De Jesus DF, Hu J, Kulkami RN. Maternal insulin resistance and transient hyperglycemia impact the metabolic and endocrine phenotypes of offspring. Am J Physiol Endocrinol Metab. 2014;307(10):E906–918. doi: 10.1152/ajpendo.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LT, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999-2008. Environ Sci Technol. 2011;45(19):8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: A literature review. Ann Nutr Metab. 2015;66(Suppl 2):14–20. doi: 10.1159/000371628. [DOI] [PubMed] [Google Scholar]
- Kim BM, Choi AL, Ha EH, Pedersen L, Nielsen F, Weihe P, et al. Effect of hemoglobin adjustment on the precision of mercury concentrations in maternal and cord blood. Environ Res. 2014;132:407–412. doi: 10.1016/j.envres.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JT, Lee HK. Metabolic syndrome and the environmental pollutants from mitochondrial perspectives. Rev Endocr Metab Disord. 2014;15(4):253–262. doi: 10.1007/s11154-014-9297-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Azumi K, Goudarzi H, Araki A, Miyashita C, Kobayashi S, et al. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: The Hokkaido Study. J Expo Sci Environ Epidemiol. 2017;27(3):251–259. doi: 10.1038/jes.2016.50. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13(6):831–849. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, et al. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One. 2014;9(7):e103337. doi: 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35(4):557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Murcia M, Iñiguez C, Vizcaino E, Llop S, Vioque J, et al. Prenatal exposure to organochlorine compounds and birth size. Pediatrics. 2011;128(1):e127–134. doi: 10.1542/peds.2010-1951. [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Loh VH, Harding JL, Botton J, Shaw JE. Persistent organic pollutants and diabetes: a review of the epidemiological evidence. Diabetes Metab. 2014;40(1):1–14. doi: 10.1016/j.diabet.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Moreira EL, De Oliveira J, Dutra MF, Santos DB, Golcalves CA, Goldfeder EM, et al. Does methylmercury-induced hypercholesterolemia play a causal role in its neurotoxicity and cardiovascular disease? Toxicol Sci. 2012;130(2):273–382. doi: 10.1093/toxsci/kfs252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia M, Ballester F, Enning AM, Iniguez C, Valvi D, Basterrechea M, et al. Prenatal mercury exposure and birth outcomes. Environ Res. 2016;151:11–20. doi: 10.1016/j.envres.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Myre M, Imbeault P. Persistent organic pollutants meet adipose tissue hypoxia: Does cross-talk contribute to inflammation during obesity? Obes Rev. 2014;15(1):19–28. doi: 10.1111/obr.12086. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Nielsen F, Patterson DG, et al. Partition of environmental chemicals between maternal and feral blood and tissues. Environ Sci Technol. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am J Epidemiol. 2004;160(8):774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Levitan EB, Gilman MW. Maternal smoking during pregnancy and child overweight: systematic review and mata-analysis. Int J Obes (London) 2008;32(3):201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Shamim Z, Kielsen K, Weihe P, Grandjean P, Ryder LP, et al. Children's white blood cell counts in relation to developmental exposures to methylmercury and persistent organic pollutants. Reprod Toxicol. 2017;68:207–214. doi: 10.1016/j.reprotox.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ Int. 2016 doi: 10.1016/j.envint.2016.09.015. Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Liu L, Zhang X, Heinrich J, Zhang J, Schramm KW, et al. A nested case-control study indicating heavy metal residues in meconium associate with maternal gestational diabetes mellitus risk. Environ Health. 2015;14:19. doi: 10.1186/s12940-015-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. Lavaan: An R package for structural equation modeling. J Stat Softw. 2012;48(2):1–3. [Google Scholar]
- Royston P, Ambler G. Generalized additive models. Stata Technical Bull. 1988;42:38–43. [Google Scholar]
- Ruchat SM, Houde AA, Voisin G, St-Pierre J, Perron P, Baillargeon JP, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013;8(9):935–943. doi: 10.4161/epi.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Ettinger AS, Fisher, et al. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Res. 2016;147:71–81. doi: 10.1016/j.envres.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int. 2015;83:63–71. doi: 10.1016/j.envint.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Tang-Peronard JL, Heilmann BL, Andersen HR, Steuerwald U, Grandjean P, Weihe P, et al. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: A prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr. 2014;99(1):5–13. doi: 10.3945/ajcn.113.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121(7):774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26(9):889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- Weihe P, Grandjean P, Debes F, White R. Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci Total Environ. 1996;186(1-2):141–148. doi: 10.1016/0048-9697(96)05094-2. [DOI] [PubMed] [Google Scholar]
- Weihe P, Kato K, Calafat AM, Nielsen F, Wanigatunga AA, Needham LL, et al. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ Sci Technol. 2008;42(16):6291–6295. doi: 10.1021/es800695m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- Vafeiadi M, Roumeliotaki T, Chalkiadaki G, Rantakokko P, Kiviranta H, Fthenou E, et al. Persistent organic pollutants in early pregnancy and risk of gestational diabetes mellitus. Environ Int. 2016 doi: 10.1016/j.envint.2016.10.005. Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Vafeiadi M, Vrijheid M, Fthenou E, Chalkiadaki G, Rantakokko P, Kiviranta H, et al. Persistent organic pollutants exposure during pregnancy, maternal gestational weight gain, and birth outcomes in the mother-child cohort in Crete, Greece (RHEA study) Environ Int. 2014;64:116–123. doi: 10.1016/j.envint.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goñi F, et al. Prenatal exposure to persistent oranic pollutants and rapid weight gain and overweight in infancy. Obesity (Silver Spring) 2014;22(2):488–496. doi: 10.1002/oby.20603. [DOI] [PubMed] [Google Scholar]
- Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, et al. Prenatal concentrations of polychlorinated biphenyls, DDT, and DDT and overweight in children : A prospective birth cohort study. Environ Health Perspect. 2012;120(3):451–457. doi: 10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DK, et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, et al. Associations of perfluoroalkyl substances (PFASs) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK) Environ Health Perspect. 2015;123:1317–1324. doi: 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health – a review of recent concerns. Int J Hyg Environ Health. 2016;219:331–342. doi: 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. A prospective study of prepregnancy serun concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril. 2015;103(1):184–189. doi: 10.1016/j.fertnstert.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


