Abstract
Ethnopharmacological relevance
Inhibition of soluble epoxide hydrolase (sEH) has been extensively reported to be anti-inflammatory in multiple animal models. Some anti-inflammatory traditional Chinese medicines (TCMs) and a few natural compounds were also found to be inhibitory to sEH in vitro.
Aim of the study
To determine whether the active intergradient (AI) against sEH of anti-inflammatory TCMs in vitro is anti-inflammatory in vivo and the sEH inhibitory action of the AI contributes to its anti-inflammatory effect in vivo.
Materials and Methods
In vitro inhibition assay of the sEH was conducted for the methanol and ethanol extracts of 27 anti-inflammatory TCMs. Two potent extracts were subject to further separation guided by bioassay to afford promising AI against sEH in vitro [Fr.5 of the crude ethanol extract of Rhizoma coptidis (FFCERC)]. Finally, the in vivo anti-inflammatory effect and sEH inhibitory potency of FFCERC was evaluated in a lipopolysacchride (LPS)-challenged murine model of acute systemic inflammation. The inflammatory status was characterized by the inflammatory cytokines TNF-α and interleukine-6 (IL-6) and sEH inhibitory function was evaluated by the plasma levels of epoxyeicosantrienoic acids (EETs) and dihydroxyeicosantrienoic acids (DHETs), which are the sEH mediated substrates and products, respectively.
Results
At the concentration of 25µg/mL, the crude ethanol extracts of 6 TCMs including Herba Asari, Radix Polygalae, Fructus Amomi, Radix Astragali, Radix Scutellariae, and Rhizoma Coptidis were potent against sEH. The crude extracts of Herba Asari and Rhizoma Coptidis were selected for further separation to afford FFCERC as the most promising AI for in vivo evaluation. Oral administration of FFCERC attenuated the significant increase in TNF-α and IL-6 caused by LPS challenge in a dose-dependent manner. In parallel, oral administration of FFCERC shifted the changes in plasma levels of EETs and DHETs caused by LPS-challenge like a synthetic sEH inhibitor.
Conclusions
A sEH inhibitory AI from Rhizoma Coptidis is anti-inflammatory and the inhibition of sEH contributes to this biological effect, indicating that sEH may be at least one of multiple therapeutic targets for relevant TCMs.
Keywords: Rhizoma Coptidis, soluble epoxide hydrolase (sEH), anti-inflammation, eicosanoids, cytokines
Graphic abstract
1. Introduction
Epoxide hydrolases (sEH) are hydrolytic enzymes that widely present in living organisms including plants and animals, mediating the metabolism of epoxides to form vicinal diols by the addition of a water molecule (Newman et al., 2005). The inhibitors of soluble EH (sEH) in mammals have been extensively reported to be anti-inflammatory (Hung et al., 2015; Yang et al., 2015a), analgesic (Hammock et al., 2011), anti-hypertensive (Charles et al., 2014; Honetschlagerova et al., 2013; Varcabova et al., 2013), anti-hypotrophy (Ai et al., 2009; Althurwi et al., 2013; Xu et al., 2006), anti-diabetic (Zuloaga et al., 2014), anti-fibrosis (Sirish et al., 2013) and reno-protective (Honetschlagerova et al., 2013; Kim et al., 2015) mainly through the stabilization of the endogenous epoxyeicosantrienoic acids (EETs) that are vasodialators, platelet aggregation inhibitors, and anti-inflammatory and analgesic mediators involved in the modulation of NFκB and IκB kinase system (Liu et al., 2012; Xu et al., 2006). Numerous sEH inhibitors were synthesized in laboratory for the pharmacological use in multiple diseases. However, as so far, no compound is clinically used specifically/selectively targeting sEH inhibition although both the multikinase inhibitor sorafenib and antimicrobial triclocarban were reported to be potent sEHIs (Liu et al., 2009a; Liu et al., 2011).
In contrast to the synthesized sEH inhibitors, natural products such as isolated compounds or extract formula from traditional Chinese medicines (TCMs) represent valuable pools for the development of sEH inhibitors for clinical use. However, only a few studies were reported regarding the discovery and development of sEH inhibitors from natural materials. For example, Kitamura et al a potent sEH inhibitor (1, 3-bis (4-methoxybenzyl) urea) from the root of the plant Pentadiplandra brazzeana with a IC50 of 92 nM against human sEH (Kitamura et al., 2015). Shi et al reported that the ethanol extract of Sophora flavescens root (Fabaceae) exhibits sEH inhibition with a IC50 of 2 µg/mL (Shi et al., 2008). Bai et al found seven compounds from the methanol/water (v/v, 1/1) extract of the leaves of Eucommia ulmoides Oliver with IC50s against human recombinant sEH less than 100 µM (Bai et al., 2015). The lack of the extensive studies conducted to discover natural sEH inhibitors mainly account for two facts: first, no compound has been clinically used to specifically/selectively target sEH inhibition; and second, only few natural medicines have validated their pharmacological effects with sEH inhibition in vivo. Here, we present evidences that some extracts of anti-inflammatory TCMs have sEH inhibitory potency, which contributes to the anti-inflammatory effect of the plant active ingredient (AI).
2. Material and methods
2.1. TCMs materials, chemicals and reagents
The TCM materials used in present study were purchased from a certificated TCM store in Shanghai, China. The voucher specimens were deposited in the herbarium of Department of TCM, Shanghai Tenth People’s Hospital. The voucher specimen numbers for all deposited voucher specimen were presented in Table 2. Methanol (MeOH), ethanol (EtOH), acetonitrile (ACN), dichloromethane (DCM) and water were purchased from Fisher Scientific (Pittsburgh, PA). Formic acid, acetic acid, and lipopolysacchride (LPS, Escherichia coli serotype 0111:B4) were purchased from Sigma-Aldrich (St. Louis, NJ). The HyperSep C18 SPE columns for fractionation of crude extracts were purchased from the local distributor of Thermo Fisher Scientific (Pittsburgh, PA). Mice were purchased from Shanghai Lab. Animal Research Center (Shanghai, China) and experiments were performed according to protocols approved by the Animal Use and Care Committee of Shanghai Tenth People’s Hospital, Tongji University School of Medicine.
Table 2.
Yields and sEH inhibitory potency of extracts of 27 anti-inflammatory TCMs
| TCMs (voucher specimen number) |
Source materials with full scientific name |
Yields (%) | Inhibition of hsEH at 25 µg/ml (%)* |
IC50 against hsEH (µg/mL) |
Major traditional use relating to anti- inflammation |
|||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MeOH Extract |
EtOH Extract |
MeOH Extract |
EtOH Extract |
MeOH Extract |
EtOH Extract |
|||
| Herba Sarcandra (SY-CNCM-2015-SG-01) | Aerial parts of Sarcandra glabra (Thunb.) Nakai | 3.9 | 0.8 | 27.5 | 57.3 | -# | - | Anti-stress, antioxidant, anti-inflammatory, detoxifying and blood activating (Yu, 1982a) |
| Radix Paeoniae alba (SY-CNCM-2015-CO-02) | Rhizomes of Cynanchum otophyllum | 9.5 | 0.7 | 38.4 | 48.0 | - | - | Analgesia (Yu, 1977a) |
| Radix Glycyrrhizae (SY-CNCM-2015-GU-01) | Rhizomes of Glycyrrhiza uralensis Fisch. | 14.8 | 3.5 | 47.6 | 95.3 | - | 5.6 | Analgesia (Chen and Chen, 2004) |
| Radix Bupleuri (SY-CNCM-2015-BC-01) | Rhizomes of Bupleurum chinense DC. | 5.6 | 3.0 | 36.0 | 79.0 | - | 12.7 | Anti-inflammatory, antipyretic, analgesia (Yu, 1979d) |
| Herba Asari (SY-CNCM-2015-AS-01) | Aerial parts of Asarum sieboldii Miq | 7.0 | 2.5 | 85.3 | 114.6 | 6.9 | 4.6 | Anti-inflammatory, antipyretic (Yu, 1988a) |
| Rhizoma Cimicifugae (SY-CNCM-2015-CF-01) | Rhizomes of Cimicifuga foetida L | 20.0 | 3.1 | 30.0 | 59.8 | - | - | Relieving swelling and pain (Yu, 1979a) |
| Radix Polygalae (SY-CNCM-2015-PT-01) | Root of Polygala tenuifolia | 22.9 | 5.2 | 44.3 | 110.1 | - | 11.6 | Treating pain, cough and bronchitis (Wu, 1997a) |
| Fructus Amomi (SY-CNCM-2015-AV-01) | Fruits of Amomum villosum Lour. Var.xanthioides (Wwall.ex Bak.)T.L.Wu et Senjen | 1.4 | 1.6 | 54.9 | 103.1 | - | 6.2 | Treating diarrhea and vomiting (Yu, 1981) |
| Fructus Forsythiae (SY-CNCM-2015-FS-01) | Fruits of Forsythia suspensa | 6.6 | 10.2 | 76.8 | 55.5 | 30.0 | - | Antipyretic, anti-hypertension (Yu, 1992) |
| Radix Notoginseng (SY-CNCM-2015-PN-01) | Root of Panax notoginseng (Bulk.) F. H. Chen | 50.7 | 5.5 | 19.2 | 35.6 | - | - | Relieving swelling and pain (Yu, 1978) |
| Radix Platycodonis (SY-CNCM-2015-PG-01) | Root of Platycodon grandiflorus | 15.6 | 1.2 | 26.4 | 33.0 | - | - | Anti-inflammation, treating cough (Yu, 1983) |
| Radix Pseudostellariae (SY-CNCM-2015-PH-01) | Root of Pseudostellaria heterophylla (Miq.)Pax ex Pax et Hoffm | 1.7 | 0.2 | 51.2 | 22.5 | - | - | Tonifying Qi, nourishing blood and saliva (Wu, 1996c) |
| Radix Astragali (SY-CNCM-2015-AM-01) | Root of Astragalus membranaceus (Fisch.) Bunge. | 10.1 | 2.8 | 19.6 | 104.0 | - | 13.0 | Antihypertension, anti-oxidant (Wu, 1993) |
| Radix Scrophulariae (SY-CNCM-2015-SN-01) | Root of Scrophularia ningpoensis Hemsl | 28.8 | 1.3 | 29.2 | 15.4 | - | - | Relieving swelling and detoxicating (Yu, 1979b) |
| Semen Cassiae (SY-CNCM-2015-SO-01) | Seeds of Senna obtusifolia | 7.2 | 4.3 | 35.8 | 18.1 | - | - | Live detoxicating and vision improving (Yu, 1988b) |
| Radix Isatidis (SY-CNCM-2015-BC-01) | Roots of Baphicacanthus cusia (Nees) Bremek | 1.9 | 1.0 | 25.9 | 23.0 | - | - | antipyretic, detoxicating, analgesic (Yu, 1987) |
| Salvia prionits (SY-CNCM-2015-SP-01) | Entire plant of Salvia Prionitis Hance | 4.0 | 2.8 | 68.9 | 86.4 | 10.2 | 9.6 | Treating cold and Abdominal pain (Yu, 1977c) |
| Flos lonicerae (SY-CNCM-2015-LJ-01) | Flowers of Lonicera japonica | 21.1 | 3.7 | 60.8 | 85.8 | - | 13.2 | Anti-inflammatory, antipyretic, detoxicating, analgesic (Yu, 1988c) |
| Herba Houttuyniae (SY-CNCM-2015-HC-01) | Aerial parts of Houttuynia cordata Thunb. | 4.5 | 2.3 | 72.8 | 90.4 | 13.1 | 6.9 | Anti-inflammatory, antipyretic (Yu, 1982b) |
| Fructus Aurantii (SY-CNCM-2015-CA-01) | Fruits of Citrus aurantium L. or C. sinensis Osbeck | 14.1 | 3.3 | 5.0 | 57.4 | - | - | Relieving arthritic pain (Wu, 1997b) |
| Radix Scutellariae (SY-CNCM-2015-SB-01) | Roots of Scutellaria baicalensis | 7.6 | 4.5 | 46.9 | 109.0 | - | 7.6 | Anti-inflammatory (Yu, 1977b) |
| Herba Taraxaci (SY-CNCM-2015-TM-01) | Entire plants of Taraxacum mongolicum Hand | 6.1 | 3.4 | 41.9 | 94.2 | - | 4.1 | Antipyretic, relieving swelling (Wu, 1999a) |
| Rhodiola dumulosa (SY-CNCM-2015-RD-01) | Rhizomes of Rhodiola dumulosa (Franch.) S. H. Fu | 19.0 | 18.0 | 54.6 | 36.0 | - | - | Relieving swelling (Yu, 1984) |
| Fructus Gardeniae (SY-CNCM-2015-GJ-01) | Fruits of Gardenia jasminoides Ellis | 8.7 | 10.5 | 45.6 | 28.8 | - | - | Antipyretic (Wu, 1999b) |
| Rhizoma Coptidis (SY-CNCM-2015-CC-01) | Rhizomes of Coptis chinensis Franch | 15.7 | 2.4 | 60.9 | 127.4 | - | 4.7 | Treating acute conjunctivitis (Yu, 1979c) |
| Radix Stephaniae Tetrandra (SY-CNCM-2015-ST-01) | Roots of Stephania tetrandra S. Moore | 3.0 | 0.2 | 28.0 | 20.6 | - | - | Treating arthritis and hypertension (Wu, 1996b) |
| Fructus Schisandrae (SY-CNCM-2015-SC-01) | The dried mature fruits of Schisandra chinensis (Turcz.) Baill. or S. sphenanthera Rehd. et Wils | 9.9 | 25.9 | 15.2 | 63.8 | - | - | Treating cough (Wu, 1996a) |
Note:
sEH inhibitory potency was determined at the final concentration of 25 ug/mL.
The crude extracts yielding more than 70% inhibition of hsEH at the final concentration of 25 µg/mL were subject to IC50 measurement. “-”indicates that the IC50 was not determined.
Peer reviewed scientific papers reported the anti-inflammatory effect of target TCMs.
2.2. Preparation of the crude extracts for in vitro sEH inhibitory assay
Dried TCM materials were chopped or pulverized into small pieces with the length in any direction no longer than 5 mm. The chopped or pulverized material (2 g) was mixed with 95% EtOH (10 mL) and sit under room temperature over night. Then the mixture was filtered under vacuum. The residue was extracted a 2nd time with 10 mL 95% EtOH. The filtrate was combined and then evaporated under vacuum at room temperature to give the EtOH extract. The second chopped or pulverized material (2 g) was then extracted with MeOH (10 mL×2) using the same procedure as the extraction with EtOH. The filtrate from 2 times of extraction with MeOH was combined and then evaporated under vacuum at room temperature to give the MeOH extract. Each extract (2.5 mg) was added into a clean 2 mL eppendorf tube and then mixed with DMSO (1.0 mL). Under room temperature, the mixture was mixed on a Vortex mixer at 1500 rpm for 4 h. The mixture was then centrifuged at 15000 rpm for 5 min and the supernatant (500 uL) was transferred into a clean tube for in vitro sEH inhibitory assay.
2.3. Preparation of fraction of the crude extract for in vitro sEH inhibitory assay
The crude EtOH extract of Rhizoma Coptidis, the most potent one were subject to further fractionation by using a SPE column. The crude extract (2 g) was loaded to a SPE column mixed with MeOH/water (v/v, 2/8, 4 mL). After eluted under vacuum, the eluent was dried on a Speedvac under vacuum to give Fr.1. Then a series solution [MeOH/water (v/v, 4/6), MeOH/water (v/v, 6/4), MeOH/water (v/v, 2/8), MeOH, and MeOH/DCM (v/v, 1/1), each 4 mL] was used to elute the column sequentially. The eluents were evaporated on a Speedvac under vacuum to give Fr.2, Fr.3, Fr.4, Fr.5, and Fr. 6, respectively. Each fractionation (0.250 mg) was formulated in DMSO (1 mL) following the procedure mentioned in 2.2 for in vitro sEH inhibitory assay.
2.4. In vitro sEH Inhibitory assay
The in vitro sEH inhibition and IC50 values were determined by using a fluorescent assay method reported previously (Jones et al., 2005; Liu et al., 2009b).
2.5. In vivo pharmacological effect of the active intergradient (AI)
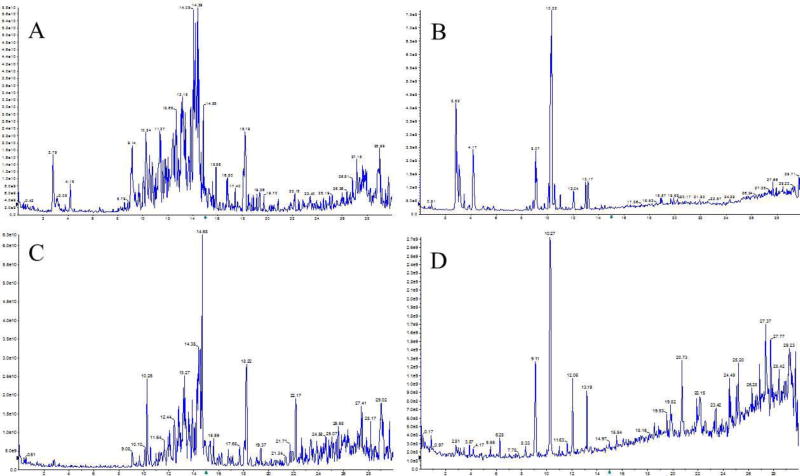
Fr.5 of the crude ethanol extract of Rhizoma Coptidis (FFCERC) was grounded carefully using a pestle and mortal and then suspended into saline (50 mg/mL). Mice (C57BL/6, male, 8-week old) were assigned at random into 4 groups, each group containing 4 or 5 animals receiving respective treatments. Specifically, the animals in group 1 received saline orally (p.o.) and intraperitoneally (i.p.) as negative control. The animals in group 2 received saline (p.o.) and LPS (10 mg/mL, i.p.) as positive control. The animals in the group 3 received LPS (10 mg/mL, i.p.) and FFCERC (100 mg/kg, p.o.). FFCERC was administered immediately and 12 hr after LPS injection, respectively. The animals in the group 4 received saline (i.p.) and FFCERC (250 mg/kg, p.o.). FFCERC was administered as described in group 3. Mice were sacrificed 24 hours after treatment. The blood was collected to separate plasma for further analysis as previously reported protocol (Liu et al., 2009b). In addition, the chemical fingerprints of the EtOH extract of Rhizoma Coptidis and its resulted fraction FFCERC were presented as Figure 1 by using an Agilent 1260 HPLC-AB SCIEX QTrap6500 system.
Figure 1.
The chemical fingerprints of the EtOH extract of Rhizoma Coptidis (A and B) and its resulted fraction FFCERC (C and D) recorded by positive (A and C) and negative modes (B and D), respectively.
2.6. Quantitative analysis of sEH-mediated lipid signaling mediators
Plasma samples were prepared for quantitative analysis according to the previously reported method (Liu et al., 2010a). The separation of sEH-mediated lipid signaling mediators, such as EETs and DHETs was conducted on Agilent 1260 equipped with an Agilent Eclipse plus C18 RRHT column (1.8 u, 2.1×150 mm) according to previous method (Yang et al., 2009) while the LSMs were monitored by an AB SCIEX QTrap6500 mass spectrometer. The detailed MS parameters were presented in Table 1.
Table 1.
MS Optimum tandem mass conditions of SHE-mediated lipid signaling mediators
| Analytes | Precursor ions m/z [M− H]− |
Predominant product ion m/z |
Declustering potential (V) |
Collision Energy (V) |
Retention time (Min) |
Detection range (nM) |
|---|---|---|---|---|---|---|
| CUDAa | 338.8 | 214.3 | −24 | −28 | 8.35 | |
| 14(15)-EET-d11b | 330.5 | 232.7 | −10 | −19 | 15.92 | 0.2–500 |
| 11,12-DHET-d11b | 348.4 | 166.9 | −14.0 | −24.5 | 10.35 | 0.5–1000 |
| 14,15-DHET | 336.8 | 207.1 | −17.0 | −22.0 | 9.72 | 0.5–1000 |
| 11,12-DHET | 336.8 | 166.9 | −13.0 | −24.0 | 10.40 | 0.5–1000 |
| 8,9-DHET | 336.8 | 268.9 | −14.0 | −17.0 | 11.16 | 0.5–1000 |
| 5,6-DHET | 336.9 | 268.9 | −17.0 | −17.0 | 12.34 | 0.5–1000 |
| 14(15)-EET | 319.1 | 219.0 | −30.0 | −16.0 | 15.96 | 0.2–500 |
| 11(12)-EET | 318.8 | 167.0 | −28.0 | −20.0 | 16.60 | 0.2–500 |
| 8(9)-EET | 319.0 | 274.8 | −14.0 | −17.0 | 16.69 | 0.2–500 |
| 5(6)-EET | 319.0 | 191.0 | −25 | −20 | 16.82 | 0.2–500 |
12-(3-cyclohexan-1-yl-ureido) dodecanoic acid (CUDA) is the internal standard with a fixed concentration of 100 nM;
14(15)-EET-d11 and 11,12-DHET-d11 are the surrogates for epoxides and diols, respectively. The surrogates are used to examine the extraction efficiency of analytes and other errors in sample processing. The recovery of surrogates was validated to be more than 80%. The LQT and accuracy of analytes are the similar to or better than those in the literature (Yang et al., 2009).
2.7. ELISA assay of TNF-α and IL-6
Plasma levels of TNF-α and IL-6 were measured by using ELISA kits according to the previously reported method (Liu et al., 2010b).
2.8. Statistical analysis
Data are presented as mean ± SD. Statistical analyses were conducted by ANOVA followed with Tukey's (variance homogeneity) or Games-Howell's (variance heterogeneity) post ad hoc comparison test using the software SPSS 22.0 (SPSS Inc., Chicago, IL) with P<0.05 as the significant level.
3. Results
3.1. The yields and sEH inhibitory potency of crude extracts of TCMs
As shown in Table 2, the ethanol and methanol crude extracts yields of raw materials were at the range between 0.2 and 50.7%. Among tested TCMs, the ethanol crude extracts yields were usually lower than those of methanol crude extracts except the extracts of Fructus Amomi, Fructus Forsythiae, Fructus Gardeniae and Fructus Schisandrae chinensis.
At the tested concentration of 0.025 mg/mL, the ethanol extracts of most tested TCMs usually yield the higher inhibition of the human sEH than methanol extracts. In addition, the crude ethanol extracts of 6 TCMs, including Herba Asari, Radix Polygalae, Fructus Amomi, Radix Astragali, Radix Scutellariae, and Rhizoma Coptidis had the sEH inhibitory potency of more than 100% at the tested concentration of 0.025 mg/mL.
3.2 sEH inhibitory potency of fractions of promising extracts of TCMs
As shown in Table 2, the ethanol crude extracts of Rhizoma Coptidis and Herba Asari were the most promising ones among all the extracts. Therefore, they were further fractioned by flash chromatography over SPE columns, each to give 6 fractions as presented in Table 3. At the tested concentration of 2.5 µg/mL, the Fr.5 of the ethanol crude extract of Rhizoma Coptidis (FFCERC) was the most potent against sEH, with inhibition over 80%. Unexpectedy, the six fractions of the ethanol crude extract of Herba Asari did not show any strong inhibition of the human sEH (< 40%).
Table 3.
sEH inhibitory potency of six fractions of crude ethanol extracts of Rhizoma Coptidis and Herba Asari
| TCMs | Fr.1 | Fr.2 | Fr.3 | Fr.4 | Fr.5 | Fr.6 | |
|---|---|---|---|---|---|---|---|
| Rhizoma Coptidis | Yield (mg/g dried TCM) | 2.0 | 3.5 | 4.4 | 1.5 | 4.3 | 2.2 |
| sEH inhibition (%)* | 26.2 | 36.0 | 59.9 | 14.2 | 82.2 | 43.5 | |
| Herba Asari | Yield (mg/g dried TCM) | 5.4 | 1.8 | 0.4 | 0.8 | 4.9 | 7.0 |
| sEH inhibition (%)* | 35.5 | < 5 | < 5 | < 5 | < 5 | < 5 |
Note:
sEH inhibition was determined at the final concentration of 2.5 µg/mL.
3.3 Anti-inflammatory effect of FFCERC
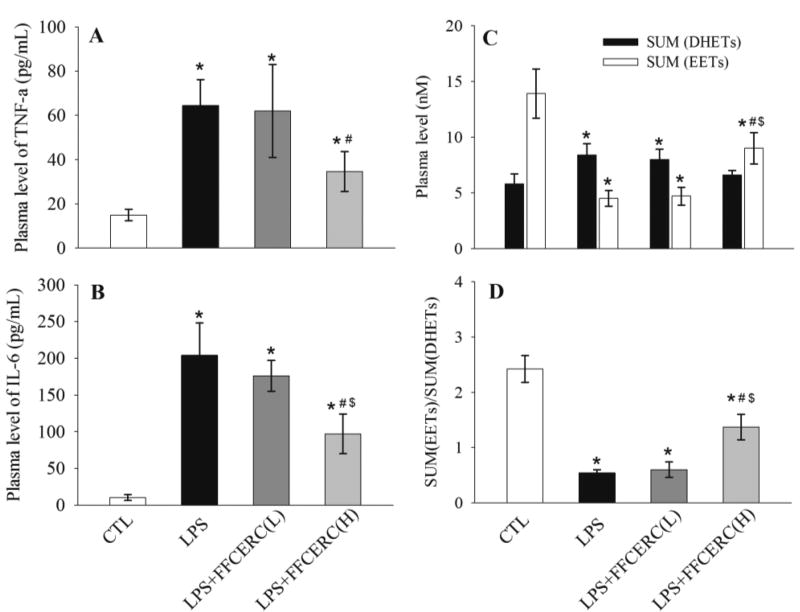
To test the anti-inflammatory effect of FFCERC, we administered FFCERC suspension to a murine model of acute systemic inflammation caused by LPS. As expected, in the animals received LPS only, the plasma levels of pro-inflammatory cytokines TNF-α and IL-6 were significantly increased. In the test animals, this inflamed state was significantly attenuated by the oral administration of FFCERC in a dose dependent manner (Table 4 and Figure 2A and 2B).
Table 4.
Murine plasma levels of key lipid signal mediators and cytokines
| Group | 1 (N = 4) | 2 (N = 4) | 3 (N = 5) | 4 (N = 5) |
|---|---|---|---|---|
| LPS (mg/kg) | - | 10 | 10 | 10 |
| FFCERC (mg/kg) | - | - | 100 | 250 |
| TNF-α (pg/mL) | 14.9 ± 2.7b | 64.5 ± 11.7a | 62.0 ± 21.7a | 34.6 ± 9.0a, b |
| IL-6 (pg/mL) | 10.4 ± 4.2b | 203.8 ± 43.6a | 175.6 ± 21.2a | 97.4 ± 26.7a, b, c |
| 14,15-EET (nM) | 2.12 ± 0.17b | 0.88 ± 0.26a | 0.85 ± 0.23a | 1.49 ± 0.27a, b, c |
| 11,12-EET (nM) | 2.34 ± 0.29b | 0.76 ± 0.21a | 0.84 ± 0.17a | 1.55 ± 0.26a, b, c |
| 8,9-EET (nM) | 1.88 ± 0.56b | 0.46 ± 0.21a | 0.53 ± 0.32a | 0.80 ± 0.23 |
| 5,6-EET (nM) | 7.56 ± 2.0b | 2.45 ± 0.64a | 2.48 ± 0.54a | 5.2 ± 0.84b, c |
| 14,15-DHET (nM) | 1.65 ± 0.73 | 2.18 ± 0.66 | 1.98 ± 0.58 | 1.73 ± 0.49 |
| 11,12-DHET (nM) | 1.29 ± 0.38 | 2.00 ± 0.50 | 1.83 ± 0.32 | 1.43 ± 0.22 |
| 8,9-DHET (nM) | 2.04 ± 0.38 | 2.22 ± 0.43 | 2.13 ± 0.42 | 2.02 ± 0.39 |
| 5,6-DHET (nM) | 0.80 ± 0.14b | 2.02 ± 0.28a | 2.02 ± 0.30a | 1.46 ± 0.20a, b, c |
, Significantly different (p < 0.05) from group 1;
, significantly different (p < 0.05) from group 2;
, significantly different (p < 0.05) from group 3 determined by ANOVA followed with Tukey's (variance homogeneity) or Games-Howell's (variance heterogeneity) post ad hoc comparison test using the software SPSS 22.0.
Figure 2.
Administration of FFCERC dose-dependently inhibited the release of proinflammatory cytokines TNF-α (A) and IL-6 (B), and altered the plasma levels of EETs (C, white bar) and DHETs (C, black bar), resulting in an alteration in the ratio of EETs to DHETs (D). LPS (10 mg/kg) and FFCERC (100 and 250 mg/kg) were administered to male C57BL/6 mice (8-week old) with i.p. injection and oral gavage, respectively. Animals were sacrificed 24 h after treatment. Data represent mean ± SD of 4–5 mice. The total EETs column sums 14, 15-, 11, 12-, 8, 9- and 5, 6-EETs and their corresponding diols compose the total DHETs column. The detailed EET and DHET data are presented in Table 4. *significantly different (P<0.05) from control group (CTL), # significantly different (P<0.05) from LPS group, $ significantly different (P<0.05) from LPS + FFCERC (low dose) group determined by ANOVA followed with Tukey’s or Games-Howell’s test.
3.4 FFCERC mediated epoxide lipids like sEH inhibitors
To test whether FFCERC can inhibit sEH in vivo, we measure the plasma levels of anti-inflammatory lipid signaling mediator EETs, which are hydrolyzed by sEH to their corresponding metabolites DHETs. The plasma levels of four EETs and four corresponding DHETs are presented in Table 4. As illustrated in Figure 2C, LPS-challenge significantly decreased plasma level of EETs while significantly increased plasma level of DHETs, resulting in a significant decrease in the ratio of EETs to DHETs (Figure 2D). High dose of FFCERC significantly shifted the changes caused by LPS in plasma levels of EETs and DHETs, as well as the corresponding change in EETs/DHETs ratio while low dose of FFCERC slightly changed plasma levels of EETs and DHETs.
4. Discussion
The sEH has been documented to be a potential intervention target for many inflammation-associated morbid conditions like pain (Pillarisetti and Khanna, 2012; Wagner et al., 2011), cardiovascular and renal diseases (Fleming, 2014; Imig and Hammock, 2009; Kim et al., 2015). In contrast to the data available for synthetic sEH inhibitors, only a few studies were reported regarding natural sEH inhibitors and sEH inhibition of natural materials (Bai et al., 2015; Kitamura et al., 2015; Lee et al., 2014; Li et al., 2015b; Shi et al., 2008; Sun et al., 2014). We also note that, in most studies the natural sEH inhibitors and sEH inhibition of natural materials were only evaluated in vitro except for 1, 3-bis (4-methoxybenzyl) urea, a compound from the root of the plant Pentadiplandra brazzeana, whose analgesic effect as a sEH inhibitor was evaluated in a rodent model of nociceptive pain (Kitamura et al., 2015). Different from most previous studies, we tested the in vitro sEH inhibitory activity of crude extracts of 27 TCMs first. And then we performed fractionation of two promising extracts and selected the most promising fraction guided by the bio-assay for further in vivo anti-inflammatory investigation.
This study evaluated the in vitro inhibitory potency against human sEH of MeOH and EtOH extracts of 27 anti-inflammatory TCMs, respectively. The major traditional use of these TCMs relating to anti-inflammation was presented in Table 2. Usually, the EtOH extract is more potent than the MeOH extract of the same TCM, probably due to the former contains less polar components than the latter, which is also supported by the structurally similar synthetic sEH inhibitors, showing the less polar one with stronger potency against sEH (Kim et al., 2007). Previously reported active pure natural compounds or natural component mixture against sEH usually yields IC50 values in the µM range (Bai et al., 2015; Lee et al., 2014; Li et al., 2015b; Shi et al., 2008; Sun et al., 2014). In this study, we found the EtOH extracts from eight TCMs, including Radix Glycyrrhizae, Herba Asari, Fructus Amomi, Salvia prionits, Herba Houttuyniae, Radix Scutellariae, Herba Taraxaci and Rhizoma Coptidis, have potent inhibitory activity against sEH, suggesting AIs with IC50 values in the low nM range (Table 2). Among them, Herba Asari is the only one whose MeOH and EtOH were very potent both with the IC50 values below 10 µg/ml (Table 2). Therefore, Herba Asari and Rhizoma Coptidis, which EtOH extract was the most potent against sEH, were selected for further purification.
Interestingly, the further fractionation of the EtOH extract of Herba Asari did not yield any fraction with high potency (table 3). On the other end, the EtOH extract of Rhizoma Coptidis provided two fractions with high sEH inhibitory potency; with fraction 5 (FFCERC) showing higher potency than fraction 2 against sEH. Thus FFCERC was selected for further in vivo test.
We used a murine model of acute systemic inflammation that was previously used to evaluate synthetic sEH inhibitors (Liu et al., 2013; Liu et al., 2009a; Liu et al., 2011; Liu et al., 2009b), to test the biological property of FFCERC. It should be noted that the TCM extract is much different than the synthetic sEHIs. For the synthetic sEHIs with the IC50 values of 1–10 nM in vitro, for example, AUDA-BE, t-AUCB, and TPPU, the in vivo effective oral doses in a murine model of inflammation were of 0.3–10 mg/kg depending on the oral bioavailability of these sEHIs (Liu et al., 2013; Liu et al., 2009a; Liu et al., 2011; Liu et al., 2009b). However, in this study the FFCERC is much different. The FFCERC has a similar in vitro IC50 value to the synthetic sEHIs t-AUCB, but in the pilot study, even 10 mg/kg oral dose of FFCERC didn’t work on the inflamed animals. The favorite effects (TNF-alpha and IL-6 plasma levels) weren’t observed in the inflamed animals until the oral dose of FFCERC was elevated to 250 mg/kg. Specifically, after LPS challenge, the plasma levels of pro-inflammatory TNF-α and pleiotropic IL-6 significantly increased in comparison to the control group. Oral administration of FFCERC significantly decreased these markers of inflammation in a dose –dependent manner (Figure 2A and 2B). In response to the LPS-challenge, when compared to the control groups, the plasma level of EETs significantly decreased while the corresponding DHETs increased significantly, resulting in a significant decrease in plasma ratio of EETs to DHETs (Figure 2C and 2D). Oral administration of FFCERC revoked some of the changes in plasma levels of EETs and DHETs caused by LPS-challenge, in a similar way observed previously with synthetic sEH inhibitor (Liu et al., 2013; Liu et al., 2009a). These data suggest the anti-inflammatory effect of FFCERC in the tested animal model is in part, if not totally, due to its in vivo inhibition of sEH.
Rhizoma Coptidis, well known as “Huanglian” in China, is the dried rhizomes of C. chinensis Franch, C. deltoidea C. Y. cheng et Hsiao, and C. teeta Wall. Rhizoma Coptidis has been widely reported to be anti-inflammatory as both a single drug and an ingredient of a TCM formula (Choi et al., 2013; Friedemann et al., 2014; Li et al., 2015a; Lower-Nedza et al., 2013; Wu et al., 2012; Yang et al., 2015b). Previous studies showed that Huang-Lian-Jie-Du-Tang, a formula of TCMs containing Rhizoma Coptidis, as well as some natural compounds obtained from Rhizoma Coptidis like coptisine, inhibits the production of arachidonic acid-derived eicosanoids by directly inhibiting 5-lipoxygenase and down-regulation of cyclooxygenase-2 (Li et al., 2012; Zeng et al., 2011). This study reported the sEH inhibitory activity of Rhizoma Coptidis for the first time, resulting in a better understanding of the pharmacological effects of Rhizoma Coptidis including but not limited to anti-inflammation, cardiovascular protection, lowering glycemia and hyperlipidemia, which are associated with and contributed, at least in part, by inhibition of sEH evidenced by synthetic sEH inhibitors (Lorthioir et al., 2012; Morisseau and Hammock, 2013; Pillarisetti and Khanna, 2012). However, an obvious limitation for this study is the active compounds against sEH have not been identified yet from the EtOH extract of Rhizoma Coptidis.The metabolites from the extract of Rhizoma Coptidis were previously reported (Jiang et al., 2012; Wang et al., 2014), in particular, the MS data of some metabolites were included in the online databases like hmdb.ca and foodb.ca. These published data facilitate the tentative structural identification of the chemicals in FFCREC. Based on the comparing the molecular mass and the key fragments with the previously reported data, 10 molecules in FFCREC were tentatively identified as in Table S1. However, it should be noted that these identification need further confirmation, such as comparison with authentic reference and isolation of the pure compounds for 1H- and 13C-NMR analysis. Berberine, a well-known active compound of the TCM Rhizoma Coptidis, is a quaternary ammonium salt with the similar structure to coptisine, both belonging to a class of isoquinoline alkaloid (Tang et al., 2009). Berberine has been clinically used in China to treat gastroenteritis, and bacterial dysentery intestinal infection, eye conjunctivitis, suppurative otitis media and cardiovascular diseases like arrhythmia, hypertension and congestive heart failure. Berberine was also found to be anti-inflammatory in many in vitro and in vivo models, perhaps involved in AMPK/mTOR signaling pathway (Fan et al., 2015; Mo et al., 2014). In current study, berberine was obtained from FFCERC. However, berberine is not the active ingredient against hsEH in EtOH extract of Rhizoma Coptidis because the IC50 value of berberine against hsEH is more than 100 uM, much weaker than FFCERC when co-assayed with the fluorescent method. And unfortunately, the activity of other sub-fractions of FFCERC became much weaker than FFCERC, suggesting a profound synergism among the chemicals in FFCERC. In addition, as shown in Table S1, most compounds have the similar structures to berberine. All these information indicate meaningless to isolate the pure active compounds from FFCERC.
In addition to Rhizoma Coptidis, we also found the MeOH and EtOH extracts of several other TCMs display sEH inhibition (Table 2). It suggests the possibility to find active compounds and ingredients with the functions associated with sEH, including but not limited to anti-inflammation, anti-hypertension, anti-hypertrophy, renal-protection. It also cautions us to consider the adverse effects of sEH inhibition when we prescribe TCMs with sEH inhibitory activity for some diseases like cancer (Zhang et al., 2013).
5. Conclusions
This study demonstrated that a promising ingredient, FFCREC from Rhizoma Coptidis, has some anti-inflammatory activity linked to its ability to inhibit sEH in vivo, indicating that sEH may be a therapeutic target, at least one of the multiple targets for relevant TCMs.
Supplementary Material
Acknowledgments
This work was supported in part by NIEHS Grant (R01 ES02710), NIEHS Superfund Grant (P42 ES04699), NIH/NHLBI grant (R01 HL59699-06A1), and a Translational Technology Grant from the UC Davis Medical Center, as well as NSFC 81470588.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
JYL (jyliutongji.edu.cn), HZH (hzhuang1yahoo.com) and BDH (bdhammockucdavis.edu) designed the study. JYL, CM (chmorisseauucdavis.edu), and HZH conducted the research and analyzed the data. JYL, CM and BDH wrote the paper.
References
- Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, Shyy JY, Hammock BD, Zhu Y. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althurwi HN, Tse MM, Abdelhamid G, Zordoky BN, Hammock BD, El-Kadi AO. Soluble epoxide hydrolase inhibitor, TUPS, protects against isoprenaline-induced cardiac hypertrophy. Br J Pharmacol. 2013;168:1794–1807. doi: 10.1111/bph.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai MM, Shi W, Tian JM, Lei M, Kim JH, Sun YN, Kim YH, Gao JM. Soluble Epoxide Hydrolase Inhibitory and Anti-inflammatory Components from the Leaves of Eucommia ulmoides Oliver (Duzhong) J Agric Food Chem. 2015 doi: 10.1021/acs.jafc.5b00055. [DOI] [PubMed] [Google Scholar]
- Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Chen TT. Chinese Medical Herbology and Pharmacology Textbook. Art of Medicine Press; 2004. [Google Scholar]
- Choi YY, Kim MH, Cho IH, Kim JH, Hong J, Lee TH, Yang WM. Inhibitory effect of Coptis chinensis on inflammation in LPS-induced endotoxemia. Journal of Ethnopharmacology. 2013;149:506–512. doi: 10.1016/j.jep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Fan X, Wang J, Hou J, Lin C, Bensoussan A, Chang D, Liu J, Wang B. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015;13:92. doi: 10.1186/s12967-015-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev. 2014;66:1106–1140. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- Friedemann T, Otto B, Klatschke K, Schumacher U, Tao Y, Leung AKM, Efferth T, Schroder S. Coptis chinensis Franch. exhibits neuroprotective properties against oxidative stress in human neuroblastoma cells. Journal of Ethnopharmacology. 2014;155:607–615. doi: 10.1016/j.jep.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Wagner K, Inceoglu B. The soluble epoxide hydrolase as a pharmaceutical target for pain management. Pain Manag. 2011;1:383–386. doi: 10.2217/pmt.11.47. [DOI] [PubMed] [Google Scholar]
- Honetschlagerova Z, Kitada K, Huskova Z, Sporkova A, Kopkan L, Burgelova M, Varcabova S, Nishiyama A, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Kujal P, Vernerova Z, Cervenka L. Antihypertensive and renoprotective actions of soluble epoxide hydrolase inhibition in ANG II-dependent malignant hypertension are abolished by pretreatment with L-NAME. J Hypertens. 2013;31:321–332. doi: 10.1097/HJH.0b013e32835b50aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YW, Hung SW, Wu YC, Wong LK, Lai MT, Shih YH, Lee TS, Lin YY. Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav Immun. 2015;43:118–129. doi: 10.1016/j.bbi.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Huang L-F, Wu L-B, Wang Z-H, Chen S-L. UPLC-QTOF/MS Analysis of Alkaloids in Traditional Processed Coptis chinensis Franch. Evidence-Based Complementary and Alternative Medicine. 2012 doi: 10.1155/2012/942384. Article ID 942384, 942386 Pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal Biochem. 2005;343:66–75. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Nishi K, Tsai HJ, Bradford T, Koda Y, Watanabe T, Morisseau C, Blanchfield J, Toth I, Hammock BD. Design of bioavailable derivatives of 12-(3-adamantan-1-yl-ureido)dodecanoic acid, a potent inhibitor of the soluble epoxide hydrolase. Bioorg Med Chem. 2007;15:312–323. doi: 10.1016/j.bmc.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308:F131–139. doi: 10.1152/ajprenal.00531.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Morisseau C, Inceoglu B, Kamita SG, De Nicola GR, Nyegue M, Hammock BD. Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana baillon: synthesis, quantification, and measurement of biological activities in vitro and in vivo. PLoS One. 2015;10:e0117438. doi: 10.1371/journal.pone.0117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Oh SJ, Lee SY, Lee JY, Ma JY, Kim YH, Kim SK. Discovery of soluble epoxide hydrolase inhibitors from natural products. Food Chem Toxicol. 2014;64:225–230. doi: 10.1016/j.fct.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Li JY, Wang XB, Luo JG, Kong LY. Seasonal Variation of Alkaloid Contents and Anti-Inflammatory Activity of Rhizoma coptidis Based on Fingerprints Combined with Chemometrics Methods. Journal of Chromatographic Science. 2015a;53:1131–1139. doi: 10.1093/chromsci/bmu175. [DOI] [PubMed] [Google Scholar]
- Li L, Zeng HW, Shan L, Yuan X, Li YS, Liu RH, Zhang WD. The different inhibitory effects of Huang-Lian-Jie-Du-Tang on cyclooxygenase 2 and 5-lipoxygenase. Journal of Ethnopharmacology. 2012;143:732–739. doi: 10.1016/j.jep.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Li W, Kim JH, Zhou W, Shim SH, Ma JY, Kim YH. Soluble epoxide hydrolase inhibitory activity of phenolic components from the rhizomes and roots of Gentiana scabra. Biosci Biotechnol Biochem. 2015b:1–5. doi: 10.1080/09168451.2014.1002451. [DOI] [PubMed] [Google Scholar]
- Liu JY, Li N, Yang J, Qiu H, Ai D, Chiamvimonvat N, Zhu Y, Hammock BD. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci U S A. 2010a;107:17017–17022. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Lin YP, Qiu H, Morisseau C, Rose TE, Hwang SH, Chiamvimonvat N, Hammock BD. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci. 2013;48:619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Park SH, Morisseau C, Hwang SH, Hammock BD, Weiss RH. Sorafenib has soluble epoxide hydrolase inhibitory activity, which contributes to its effect profile in vivo. Mol Cancer Ther. 2009a;8:2193–2203. doi: 10.1158/1535-7163.MCT-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Qiu H, Morisseau C, Hwang SH, Tsai HJ, Ulu A, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase contributes to the anti-inflammatory effect of antimicrobial triclocarban in a murine model. Toxicol Appl Pharmacol. 2011;255:200–206. doi: 10.1016/j.taap.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Tsai HJ, Hwang SH, Jones PD, Morisseau C, Hammock BD. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol. 2009b;156:284–296. doi: 10.1111/j.1476-5381.2008.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010b;79:880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Webb HK, Fukushima H, Micheli J, Markova S, Olson JL, Kroetz DL. Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor kappaB signaling. J Pharmacol Exp Ther. 2012;341:725–734. doi: 10.1124/jpet.111.191247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorthioir A, Guerrot D, Joannides R, Bellien J. Diabetic CVD--soluble epoxide hydrolase as a target. Cardiovasc Hematol Agents Med Chem. 2012;10:212–222. doi: 10.2174/187152512802651042. [DOI] [PubMed] [Google Scholar]
- Lower-Nedza AD, Kuess C, Zhao HY, Bian BL, Brantner AH. In vitro Anti-inflammatory and Antioxidant Potential of Si-Miao-San, its Modifications and Pure Compounds. Natural Product Communications. 2013;8:1137–1141. [PubMed] [Google Scholar]
- Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, Xiao H. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20:574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Pillarisetti S, Khanna I. Targeting soluble epoxide hydrolase for inflammation and pain - an overview of pharmacology and the inhibitors. Inflamm Allergy Drug Targets. 2012;11:143–158. doi: 10.2174/187152812800392823. [DOI] [PubMed] [Google Scholar]
- Shi DH, Xu C, Guo BX, Wang XT, Chen YX, Tan RX. Inhibition of soluble epoxide hydrolase by extracts derived from inflammation-treating Chinese medicinal herbs. Phytother Res. 2008;22:1264–1268. doi: 10.1002/ptr.2326. [DOI] [PubMed] [Google Scholar]
- Sirish P, Li N, Liu JY, Lee KS, Hwang SH, Qiu H, Zhao C, Ma SM, Lopez JE, Hammock BD, Chiamvimonvat N. Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proc Natl Acad Sci U S A. 2013;110:5618–5623. doi: 10.1073/pnas.1221972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YN, Li W, Kim JH, Yan XT, Kim JE, Yang SY, Kim YH. Chemical constituents from the root of Polygonum multiflorum and their soluble epoxide hydrolase inhibitory activity. Arch Pharm Res. 2014 doi: 10.1007/s12272-014-0520-4. [DOI] [PubMed] [Google Scholar]
- Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. Journal of Ethnopharmacology. 2009;126:5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Varcabova S, Huskova Z, Kramer HJ, Hwang SH, Hammock BD, Imig JD, Kitada K, Cervenka L. Antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats is mediated by suppression of the intrarenal renin-angiotensin system. Clin Exp Pharmacol Physiol. 2013;40:273–281. doi: 10.1111/1440-1681.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Gill SS, Hammock BD. Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction. J Agric Food Chem. 2011;59:2816–2824. doi: 10.1021/jf102559q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang S, Chen L, X-J H, Q-W Z, Jiang R-W, F Y, Ye W-C. New enantiomeric isoquinoline alkaloids from Coptis chinensis. Phytochemistry Letters. 2014;7:89–92. [Google Scholar]
- Wu YH, Chuang SY, Hong WC, Lai YJ, Chang YL, Pang JHS. In vivo and in vitro inhibitory effects of a traditional Chinese formulation on LPS-stimulated leukocyte-endothelial cell adhesion and VCAM-1 gene expression. Journal of Ethnopharmacology. 2012;140:55–63. doi: 10.1016/j.jep.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1993. p. 131. [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1996a. p. 252. [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1996b. p. 052. [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1996c. p. 67. [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1997a. [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1997b. p. 194. [Google Scholar]
- Wu ZY. Flora of China. China Science Press; Beijing: 1999a. p. 32. [Google Scholar]
- Wu ZY. Flora of China. China, Beijing: 1999b. p. 332. [Google Scholar]
- Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, Chiamvimonvat N. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, Hwang SH, Kenyon NJ, Hammock BD. Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 2015a;52:46–55. doi: 10.1165/rcmb.2013-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ning YM, Zhu XZ, Li RL, Ye HJ, Zhao L, Jin LH, Zhou XM. Antifungal and Anti-Inflammatory Effects of Coptidis Rhizoma Extract against Candida Albicans. African Journal of Traditional Complementary and Alternative Medicines. 2015b;12:161–168. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1977a. p. 377. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1977b. p. 194. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1977c. p. 151. [Google Scholar]
- Yu DJ. Flora of China. Beijing: 1978. p. 183. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1979a. p. 101. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1979b. p. 55. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1979c. p. 593. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1979d. p. 290. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1981. p. 125. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; 1982a. p. 79. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1982b. p. 8. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1983. p. 77. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1984. p. 188. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1987. p. 65. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1988a. p. 176. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1988b. p. 126. [Google Scholar]
- Yu DJ. Flora of China. China Science Press; Beijing: 1988c. p. 236. [Google Scholar]
- Yu DJ. Flora. Beijing: 1992. p. 42. [Google Scholar]
- Zeng HW, Dou SS, Zhao J, Fan SY, Yuan X, Zhu SL, Li L, Zhang WD, Liu RH. The inhibitory activities of the components of Huang-Lian-Jie-Du-Tang (HLJDT) on eicosanoid generation via lipoxygenase pathway. Journal of Ethnopharmacology. 2011;135:561–568. doi: 10.1016/j.jep.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga KL, Krasnow SM, Zhu X, Zhang W, Jouihan SA, Shangraw RE, Alkayed NJ, Marks DL. Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS One. 2014;9:e97529. doi: 10.1371/journal.pone.0097529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.