Abstract
Background
As treatment options for atrial fibrillation (AF) increase, more attention is focused on patients’ experiences and quality of life (QoL). However, little is known about the factors associated with these outcomes.
Methods
The Atrial Fibrillation Effect on QualiTy-of-life (AFEQT) is a disease-specific QoL tool for AF, with domain and summary scores ranging from 0 (the worst QoL) to 100. Using multivariable linear regression, we evaluated factors associated with baseline AFEQT Summary and Subscale Scores in ORBIT AF, a large, community-based AF registry. Independent associations were reported as coefficient estimates in scores and 95% confidence intervals (CI).
Results
Overall, AFEQT was assessed in 2,007 AF outpatients from 99 sites. Median age (IQR) was 76 years (67-82) and 43% were female. The median AFEQT summary score was 82 (67-94). Female sex, younger age, new onset AF, higher heart rate, obstructive sleep apnea, symptomatic heart failure (HF), chronic obstructive pulmonary disease and coronary artery disease were all independently associated with reduced QoL. Female sex [Estimate −7.03, 95% CI (−9.31, −4.75)] and new onset versus permanent AF [Estimate −7.44, 95% CI (−11.03, −3.84)] were independently associated with increased symptoms. NYHA Class III or IV HF [Estimate -14.44, 95% CI (−19.46, −8.76)] and female sex [Estimate −7.91, 95% CI (−9.95, −5.88)] were most independently associated with impaired daily activities.
Conclusions
QoL in patients with AF varies widely and is associated with several patient factors. Understanding patient factors independently associated with worse QoL can be a foundation for tailoring treatment.
Keywords: Quality of life, atrial fibrillation
Background
Atrial fibrillation (AF) significantly impairs quality of life (QoL) in many patients.1,2 This impairment in QoL can be comparable to that observed in heart failure (HF) and is due to both the symptoms of the disease and end-organ complications, such as stroke.2, 3 At present, the major goal of rhythm management treatment is to improve patients’ symptom burden and QoL. Given the fundamental importance of QoL considerations for AF patients, and its role in selecting AF treatment, there is an increasing emphasis on AF-related QoL measures and assessments.4, 5 However, despite the importance of QoL in AF, few data are available identifying patient-level factors associated with QoL in AF.
The Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) is a validated QoL instrument that assesses 4 conceptual domains in AF-related QoL.6 Although there are alternative symptom assessment tools for patients with AF,7-10 AFEQT is the only validated instrument that assesses treatment, symptoms, QoL, physical limitations and abilities. The purpose of this analysis is to describe patient characteristics across the range of QoL scores in patients with AF as well as identify clinical characteristics associated with global AF-related QoL.
Methods
The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT AF) is a large, nationwide observational cohort study with longitudinal follow-up of two years or more. The rationale and design of ORBIT AF has been described previously.11 In brief, ORBIT AF enrolled patients with AF from a variety of diverse clinical practice environments, including internal medicine, cardiology, and cardiac electrophysiology clinics. Patients with electrocardiographically confirmed AF were eligible, provided they did not have AF due to a transient, reversible cause such as pulmonary embolism or hyperthyroidism. Consecutive eligible patients were enrolled and followed every 6 months for at least two years, and data were submitted to the registry via Web-enabled case report forms using standardized definitions. All subjects provided written, informed consent. Institutional review boards of the Duke Clinical Research Institute and the participating enrollment sites approved the study.
The present analysis focuses on the patient reported outcomes substudy in ORBIT AF. Patient QoL data were obtained using patient-reported outcome questionnaires that were administered as a prospective substudy (n=2007 patients from 99 sites; 20% of total ORBIT AF cohort). All patients enrolled in the main cohort were approached to complete the questionnaire on a voluntary basis until the QoL subsample enrollment goal was reached. Patients were enrolled from June 2010 to August 2011. Baseline health status and patient-reported outcomes were assessed in this population at entry into the registry.
AF-related QoL was measured at baseline, 12 months, and 24 months. AF-related QoL was measured using the previously validated AFEQT questionnaire (St Jude Medical, St Paul, MN, http://afeqt.org/).6 The AFEQT is a 20-item questionnaire assessing four domains of AF-related QoL, including daily activities, symptoms, treatment concerns, and treatment satisfaction. An overall Summary Score (AFEQT score) can be calculated from the first three domains (Symptoms, Daily Activities, and Treatment Concern) and was the primary outcome of interest in this analysis. The main objective was to identify the determinants of AF-related QoL as reflected by the overall AFEQT score and for each of the four domains. AFEQT scores range from 0-100, with 0 representing the worst possible QoL and 100 representing the best (no impairment). AFEQT scores were also compared to physician-assessed AF symptom severity and burden documented using the European Heart Rhythm Association (EHRA) symptom classification defined as: asymptomatic (EHRA 1), mild symptoms (EHRA 2), severe symptoms (EHRA 3), and disabling symptoms (EHRA 4).12, 13 Finally, individual patient-reported symptoms of palpitations, syncope or fainting, dyspnea on exertion, exercise intolerance, lightheadedness or dizziness, dyspnea at rest, fatigue, and chest tightness or discomfort were also compared across quartiles of AFEQT score.
Statistical Analysis
Baseline characteristics of the 2007 patients participating in the ORBIT AF sub-study were described across quartiles of AFEQT scores. Patient characteristics were presented as frequencies and percentages for categorical variables and as median (interquartile range) for continuous variables. The baseline characteristics were compared across AFEQT score quartiles using chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables. AFEQT scores were also assessed across the following key subgroups: age (<65, 65-80, >80), sex, and type of AF (paroxysmal, persistent, permanent and new-onset), with AFEQT scores presented as median (IQR) and compared using the Kruskal-Wallis non-parametric tests.
A multivariable linear regression model was built for the overall baseline AFEQT score using generalized estimating equations method with exchangeable working correlation matrix to account for within site clustering of patients. This method produces estimates similar to those from regular linear regression, but variances are adjusted for the correlation of outcomes within a site and are robust against departures from normality. The pre-specified covariates based on clinically relevance listed in the appendix were used as potential candidate variables to build the model using backward selection with a stay criteria of 0.05. Continuous covariates were evaluated for linearity with overall AFEQT score and non-linearity was evaluated with piecewise splines.
All candidate variables had < 2% missingness except eGFR (9%), hematocrit (12%), left ventricular ejection fraction (18%), and left atrial diameter (22%). In order to account for missing data, multiple imputation was used. Five imputed datasets were created with values imputed for all variables included in the modeling. Imputed values were obtained by the Markov chain Monte Carlo method or regression methods. Model selection was performed on the first imputed dataset to obtain a set of factors in which each factor was independently associated, holding all other variables constant, with overall AFEQT score. For each imputed dataset, a model with this set of factors was fit. The results from each model were then combined to produce statistically valid inferences when imputed datasets are used. The same process was used to build models for the AFEQT subscales. Frailty, was defined by three or more of the following symptoms: 10 pound unintentional weight loss in the past year, self-reported exhaustion, weakness on grip strength, slow walking speed or low physical activity.
Two additional regression methods were used to build a model identifying factors associated with overall AFEQT score: linear regression with log transformed AFEQT score and pseudo-binomial regression which is used for the modeling of proportions that follow an unknown distribution. The results were similar across the three regression methods in terms of significant variables and p-values, therefore simple linear regression as described above was used for ease in interpretation.
Independent associations with outcomes were displayed as coefficient estimates (95% confidence intervals [CI]). All analyses were performed using SAS software (version 9.4, SAS Institute, Cary, North Carolina, USA).
This research was sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents
Results
Baseline Characteristics
Median age was 76 (67-82), 43% were female, and the overall median CHA2DS2-VASC score was 4 (3-5). As shown in Table 2, physicians’ assessments of patient symptoms were documented using the EHRA functional status assessment: 34% had no symptoms (EHRA class I), while 48%, 16%, and 2% had mild, severe, and disabling symptoms (EHRA classes II –IV respectively). Overall, 10% had first-detected AF; 48% had paroxysmal, 15% had persistent, and 28% had permanent AF. AFEQT score was lowest among patients with new-onset/first detected AF 76.9 (54.6-88.0), as compared with patients having paroxysmal 83.3 (67.6-93.5), persistent 80.1 (61.1-94.0), or permanent 83.3 (68.5-93.5) AF (p<0.0001). The majority of patients were cared for by cardiologists (84%) followed by internal medicine/primary care physicians (65%) and electrophysiologists (14%).
Table 2.
Atrial Fibrillation History Across AFEQT Quartiles
| Overall N=2007 |
Quartile 1 (Worst QoL) N=497 |
Quartile 2 N=507 |
Quartile 3 N=501 |
Quartile 4 (Best QoL) N=502 |
P-Value | |
|---|---|---|---|---|---|---|
| Type of AF | 0.0005 | |||||
| First Detected/New Onset | 197 (9.8) | 71 (14.3) | 46 (9.1) | 50 (10.0) | 30 (6.0) | |
| Paroxysmal | 955 (47.6) | 216 (43.5) | 248 (48.9) | 246 (49.1) | 245 (48.8) | |
| Persistent | 300 (15.0) | 88 (17.7) | 74 (14.6) | 58 (11.6) | 80 (15.9) | |
| Permanent | 555 (27.7) | 122 (24.6) | 139 (27.4) | 147 (29.3) | 147 (29.3) | |
| EHRA Symptom Level | <0.0001 | |||||
| No Symptoms | 686 (34.2) | 65 (13.1) | 153 (30.2) | 189 (37.7) | 279 (55.6) | |
| Mild | 961 (47.9) | 235 (47.3) | 277 (54.6) | 266 (53.1) | 183 (36.5) | |
| Severe | 327 (16.3) | 179 (36.0) | 69 (13.6) | 44 (8.8) | 35 (7.0) | |
| Disabling | 32 (1.6) | 18 (3.6) | 8 (1.6) | 1 (0.2) | 5 (1.0) | |
| CHADS2 Risk Score | 0.7263 | |||||
| 0 | 135 (6.7) | 31 (6.2) | 28 (5.5) | 41 (8.2) | 35 (7.0) | |
| 1 | 436 (21.7) | 101 (20.3) | 121 (23.9) | 106 (21.2) | 108 (21.5) | |
| ≥ 2 | 1436 (71.6) | 365 (73.4) | 358 (70.6) | 354 (70.7) | 359 (71.5) | |
| Prior Treatment with Antiarrhythmic Drug | 833 (41.5) | 236 (47.5) | 207 (40.8) | 202 (40.3) | 188 (37.5) | 0.0112 |
| Prior Cardioversion | 497 (24.8) | 142 (28.6) | 131 (25.8) | 120 (24.0) | 104 (20.7) | 0.0326 |
| Catheter Ablation of AF | 97 (4.8) | 34 (6.8) | 31 (6.1) | 16 (3.2) | 16 (3.2) | 0.0072 |
| Atrial Flutter Ablation | 60 (3.0) | 17 (3.4) | 13 (2.6) | 10 (2.0) | 20 (4.0) | 0.2561 |
| AV Node/HIS Bundle Ablation | 43 (2.1) | 17 (3.4) | 10 (2.0) | 13 (2.6) | 3 (0.6) | 0.0174 |
| Any Surgical Intervention | 36 (1.8) | 6 (1.2) | 14 (2.8) | 9 (1.8) | 7 (1.4) | 0.2490 |
| Provider Specialty | <0.0001 | |||||
| Cardiology | 1686 (84.0) | 402 (80.9) | 431 (85.0) | 423 (84.4) | 430 (85.7) | 0.1670 |
| Internal Medicine or Primary Care | 1295 (64.5) | 327 (65.8) | 327 (64.5) | 308 (61.5) | 333 (66.3) | 0.3764 |
| Electrophysiology | 274 (13.7) | 78 (15.7) | 68 (13.4) | 58 (11.6) | 70 (13.9) | 0.3021 |
All values are presented as N(%)
Baseline AFEQT Scores and Associated Patient Characteristics
The median baseline AFEQT score for the overall population was 81.5 (66.7-93.5). Median AFEQT score was 79.6 (62.0-91.7) for women compared with 83.3 (69.4-94.4) for men, (p<0.0001). Patients who were younger than 65 had a median AFEQT score of 78.7 (58.3-90.7) compared with 81.5 (65.7-92.6) in ages 65-80 and 85.2 (69.6-95.4) for age >80 (p<0.0001). Table 1 details the baseline characteristics across quartiles of AFEQT scores. Compared with the highest quartile, patients with the lowest quartile of AFEQT scores (worst AF-related QoL) were younger, more likely female, and had more comorbid diseases. Patients with the lowest quartile AFEQT scores also had higher heart rates and BMI than those with the highest quartile scores. They were more likely to report symptoms at baseline such as palpitations, dyspnea on exertion or rest, exercise intolerance, lightheadedness, fatigue or chest tightness than patients with the highest quartile of AFEQT scores (p<0.0001 for all).
Table 1.
Baseline Characteristics Across AFEQT Summary Score Quartiles
| Overall N=2007 |
Quartile 10.0-65.7 N=497 (Worst QoL) |
Quartile 266.7-81.5 N=507 |
Quartile 381.9-93.1 N=501 |
Quartile 493.5-100.0 N=502 (Best QoL) |
P-Value | |
|---|---|---|---|---|---|---|
| Age (years)* | 76 (67-82) | 73 (66-81) | 74 (67-81) | 75 (67-82) | 78 (70-83) | <0.0001 |
| Female | 866 (43.2) | 251 (50.5) | 222 (43.8) | 212 (42.3) | 181 (36.1) | <0.0001 |
| Race | 0.0369 | |||||
| White | 1805 (89.9) | 449 (90.3) | 453 (89.4) | 453 (90.4) | 450 (89.6) | |
| Black | 116 (5.8) | 29 (5.8) | 25 (4.9) | 28 (5.6) | 34 (6.8) | |
| Hispanic | 53 (2.6) | 7 (1.4) | 21 (4.1) | 9 (1.8) | 16 (3.2) | |
| Other | 28 (1.4) | 10 (2.0) | 8 (1.6) | 9 (1.8) | 1 (0.2) | |
| Past Medical History | ||||||
| Hypertension | 1657 (82.6) | 429 (86.3) | 423 (83.4) | 402 (80.2) | 403 (80.3) | 0.0317 |
| Diabetes | 554 (27.6) | 159 (32.0) | 133 (26.2) | 135 (27.0) | 127 (25.3) | 0.0819 |
| OSA | 405 (20.2) | 131 (26.4) | 119 (23.5) | 88 (17.6) | 67 (13.4) | <0.0001 |
| CAD | 631 (31.4) | 172 (34.6) | 154 (30.4) | 164 (32.7) | 141 (28.1) | 0.1337 |
| Heart Failure | 548 (27.3) | 168 (33.8) | 151 (29.8) | 119 (23.8) | 110 (21.9) | <0.0001 |
| Stroke | 178 (8.9) | 43 (8.7) | 39 (7.7) | 51 (10.2) | 45 (9.0) | 0.5793 |
| Implanted Device | ||||||
| ICD | 98 (4.9) | 27 (5.4) | 27 (5.3) | 20 (4.0) | 24 (4.8) | 0.7019 |
| CRT-P | 15 (0.8) | 4 (0.8) | 5 (1.0) | 3 (0.6) | 3 (0.6) | 0.8701 |
| CRT-D | 63 (3.1) | 15 (3.0) | 16 (3.2) | 17 (3.4) | 15 (3.0) | 0.9829 |
| BMI (kg/m2) | 28.8 (25.4, 33.7) | 30.2 (26.3, 36.3) | 29.0 (25.1, 34.3) | 28.8 (25.3-33.1) | 28.0 (25.0-32.4) | <0.0001 |
| Heart Rate (bpm) | 70 (63-79) | 72 (64-80) | 70 (64-80) | 70 (63-76) | 70 (61-76) | 0.0167 |
| Systolic Blood Pressure (mmHg) | 126 (118-138) | 125 (114-136) | 126 (118-138) | 124 (116-138) | 128 (118-138) | 0.0788 |
| Diastolic Blood Pressure (mmHg) | 72 (67-80) | 72 (66-80) | 72 (66-80) | 72 (67.5-80) | 72 (68-80) | 0.6847 |
| Calculated CrCl (mL/min/1.73m2) | 68.2 (50.0-94.9) | 72.3 (52.3-104.0) | 68.6 (49.2-94.9) | 68.0 (49.9-93.7) | 65.0 (49.3-87.0) | 0.0062 |
| LVEF (%) | 56 (50-61) | 55 (50-60) | 55 (50-62) | 58 (50-62) | 60 (50-64) | 0.0210 |
Values are presented as N (%) or median (interquartile range) unless otherwise specified.
Creatinine clearance calculated using the Cockroft-Gault formula and does not include patients on dialysis.
QoL: Quality of Life; OSA: Obstructive Sleep Apnea; CAD: Coronary Artery Disease; ICD: Implantable Cardioverter Defibrilation; CRT-P: Cardiac Resynchronization Therapy Pacemaker; CRT-P Cardiac Resynchronization Therapy Defibrillator; CrCl: creatinine clearance; LVEF: left-ventricular ejection fraction
Associations between AFEQT and Prior AF Treatments
Patients with higher AFEQT scores were least likely to have prior attempts at rhythm control (DCCV, antiarrhythmic medications, atrial fibrillation ablation). (Table 2) More specifically, the use of catheter ablation was most common among patients with lower QoL (6.8% in the lowest quartile vs. 3.2% in the highest quartile of AFEQT; p=0.0072).
Adjusted Associations of Patient Factors with AFEQT
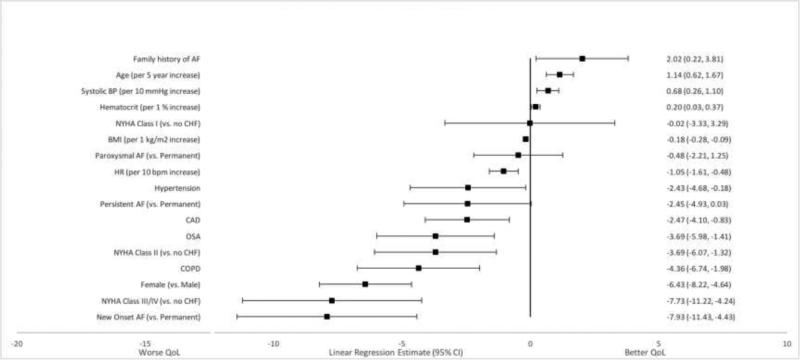
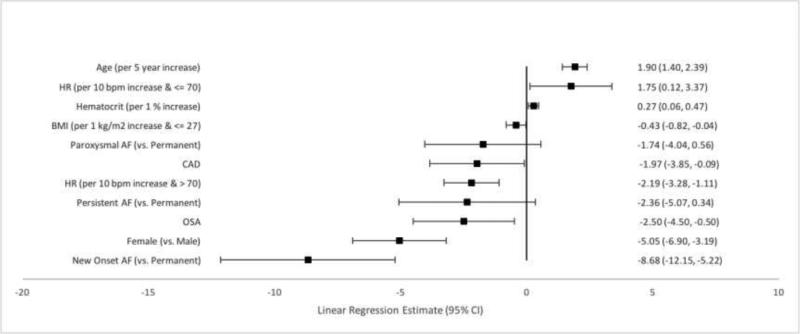
Several patient characteristics were independently associated, while holding all other variables constant, with overall AFEQT score. Older age was associated with higher scores. Holding other variables constant, for every 5-year increase in age the AFEQT score increased by 1 point [Linear Regression Estimate 1.14 (95% CI 0.62, 1.67), p<0.0001]. (Figure 1) Patients with a family history of AF also had higher AFEQT scores (Estimate 2.02; 95% CI 0.22, 3.81; p=0.0274). Female sex was associated with lower scores as was each 10 bpm increase in heart rate. Comorbid diseases were also associated with lower AFEQT summary scores. Chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), coronary artery disease (CAD) and NYHA class II-IV HF were each associated with lower scores.
Figure 1. Factors Associated with Overall AFEQT Score.
The estimate represents the difference in overall AFEQT score for each binary variable while holding all other variables constant. For example, patients with a family history of AF have a two point higher AFEQT score than patients without a family history of AF, holding all other covariates constant.
Additionally, patients with new-onset/first-detected AF had lower scores compared with those with permanent AF. This group had an AFEQT score 8 points lower than patients with permanent AF, while patients with paroxysmal and persistent AF did not have demonstrable differences in AFEQT summary scores compared with patients with permanent AF.
Adjusted Associations of Patient Factors with AFEQT Subscales
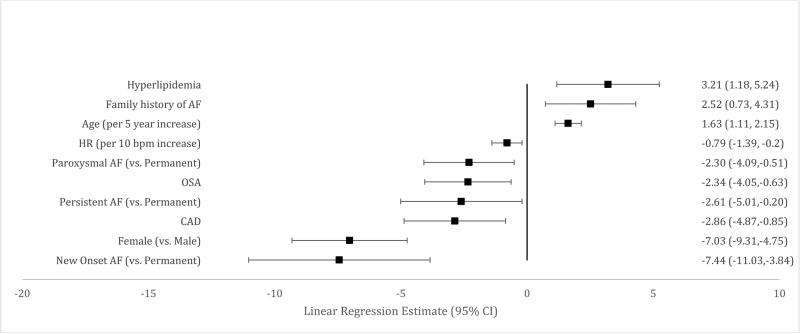
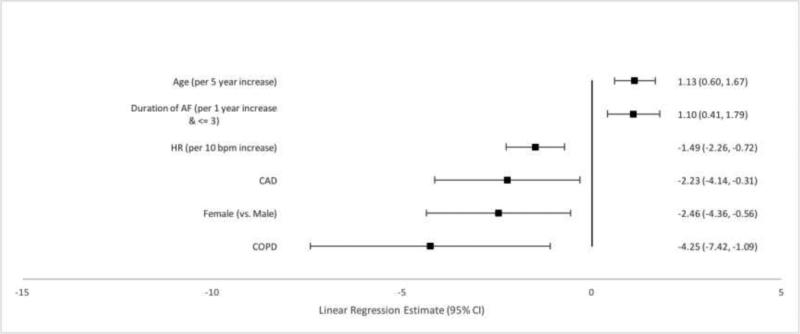
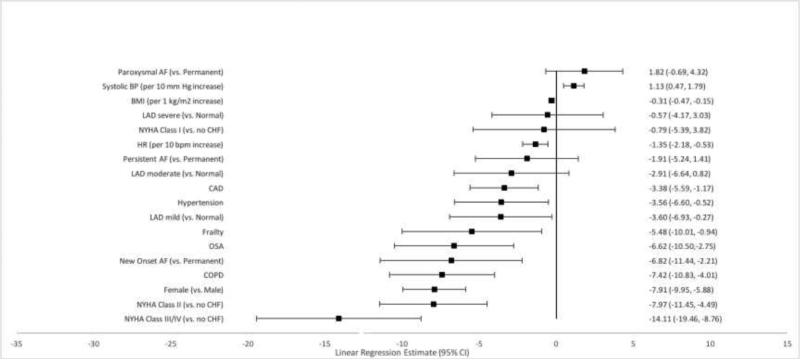
Independently associated factors were also identified for each of the three subscales that comprise the overall AFEQT score (symptoms, daily activities and treatment concern) as well as a treatment satisfaction subscale. Female sex and higher heart rate were consistently associated with worse scores across each subscale. (Figures 2-5). Older patients had fewer symptoms, less treatment concern and greater treatment satisfaction, but age was not associated with daily activity scores. New onset AF was associated with worse symptoms, less daily activity, and greater treatment concern. Treatment satisfaction increased by one point each year up to three years after AF diagnosis.
Figure 2.
Factors Associated with Symptom Subscale Score
Figure 5.
Factors Associated with Treatment Satisfaction Subscale Score
Comorbid diseases were associated with AFEQT subscales. Coronary artery disease was associated with worse scores in each of the four subscales. Obstructive sleep apnea was associated with increased symptoms, decreased daily activities and increased treatment concern. New York Heart Association Class II-IV HF was associated with decreased daily activities (p<0.0001 for all), as was hypertension. Frailty was also associated with decreased daily activity.
Discussion
In this dedicated analysis of AF-related QoL in over 2000 ambulatory patients, there are two important findings. First, many patient characteristics and comorbid diseases were independently associated with QoL in AF patients. Second, as expected, AFEQT scores were associated with patient-reported symptom assessments.
Association of patient factors with QoL
Our analysis demonstrated that several non-modifiable patient characteristics are associated with QoL in patients with AF. Women and younger patients had lower AFEQT summary and subscale scores, and each of these characteristics was associated with worse QoL. This finding is consistent with prior studies demonstrating higher EHRA scores in women14 and lower EHRA scores among older patients.15 This information should be considered when determining management strategies, as rhythm management is most indicated in patients who are symptomatic in AF.16, 17 Interestingly, female sex has been associated with worse QoL in patients with coronary heart disease,18, 19 but sex-based associations between QoL among patients with HF remain unclear.20-22 Unlike our study, older age is associated with worse QoL in heart failure.22
Our analysis also reveals that the comorbid conditions, such as CAD, COPD, OSA, or advanced HF, are associated with poor QoL in patients with AF. Conceptually, this makes sense, as patients with these comorbidities may have less physical reserve, may have independent associations with QoL, and therefore become more symptomatic when they are in AF. Furthermore, hypertension,24 OSA25 and HF26, 27 are all risk factors for the development of AF. The combination of HF and AF can become a self-fulfilling prophecy, as poorly controlled AF is associated with tachycardia-induced cardiomyopathy28-30 as well as worsening of chronic HF.31 For this reason, the 2014 AHA/ACC/HRS guidelines for the management of patients with AF issued a Class IIa recommendation for rhythm control strategy in patients with AF and HF who remain symptomatic after attempting rate control.16 Our finding that comorbid diseases are associated with poor QoL in AF patients is consistent with prior literature and suggests that aggressive rate and/or rhythm control for AF and optimal management of comorbid diseases may be paramount in improving QoL for this patient population.
Also, family history of AF was associated with improved QoL. Up to 30% of AF patients have a family history of the disease.23 Further investigation is needed to determine whether the association between family history and AF QoL is due to genetic variation, psychosocial factors or altered expectations in patients’ whose family members also suffered from the disease.
Restoration of sinus rhythm
Consistent with guideline recommendations, therapies designed to restore sinus rhythm are most frequently utilized in patients with the worst QoL. This analysis suggests that patients with the worst QoL are most likely to be treated with antiarrhythmic therapy or undergo a procedure to restore sinus rhythm. Treatment to restore sinus rhythm is not associated with a mortality benefit.32-34 However, in patients with symptomatic AF, it is associated with an improvement in QoL.33 Our finding that patients with the highest AFEQT score were less likely to be treated with an antiarrhythmic, undergo cardioversion or atrial fibrillation catheter ablation, indicates that these therapies are appropriately reserved for patients who are most likely to benefit. Given that each of these interventions is associated with both morbidity and mortality, it is paramount that they be implemented in patients with an optimal risk to benefit profile.
Association of patient symptoms with AFEQT scores
Similar to findings previously published by our group, this study demonstrated that both EHRA scores and patient-reported symptoms were associated with AFEQT scores.35 The original derivation cohort had a mean AFEQT score of 62, indicating a worse overall QoL compared with our cohort with a mean score of 77. The finding that the AFEQT questionnaire is associated with both patient and physician symptom assessments indicates that the AFEQT questionnaire, which has been previously developed and validated among a population of 214 AF patients, also performed well among our population of over 2000 patients with better QoL than the derivation cohort.6 It also empowers physicians to assess symptoms among their patient populations, as these assessments are likely an accurate reflection of patient's overall QoL and may help guide management strategies.
Limitations
One potential limitation to this study is the possible omission of significant explanatory variables from our model. Despite consideration of 44 clinically relevant candidate variables, there may be other factors that are associated with QoL in patients with AF that were not included in the model, and therefore cannot be assessed in this study. The AFEQT, as with any disease-specific QoL instrument can be influenced by the general health status of a given patient. Additionally, the use of the AFEQT questionnaire is subject to recall bias, which may alter the way respondents with increased symptoms respond to questions relative to the way participants with few symptoms respond. However, the questionnaire was validated and our findings were consistent with other validated scoring systems, such as the EHRA. Although there are some limitations to this study, this is the largest known investigation assessing quality of life predictors among patients with AF.
Conclusions
Several patient factors, both modifiable and non-modifiable, are associated with QoL in this patient population. Younger patients and women with AF had worse QoL, as did patients with comorbid diseases such as OSA, COPD, NYHA classes II-IV HF, hypertension, and obstructive coronary disease. These factors may serve both as targets for intervention as well as indicators for patients in whom a thorough QoL assessment is warranted. Armed with the knowledge of patient QoL, physicians can best advise patients about the range of treatment options for AF and employ attempts of restoring sinus rhythm among patients who are most likely to benefit.
Supplementary Material
Figure 3.
Factors Associated with Daily Activities Subscale Score
Figure 4.
Factors Associated with Treatment Concern Subscale Score
Acknowledgments
Sources of Funding
The ORBIT AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Dr. Randolph was funded by National Institutes of Health T-32 Training Grant No. T32 HL 69749-11 Al. Dr. Chan received funding from the National Heart, Lung, and Blood Institute (1R01HL123980).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Fonarow reports modest consultant support from Janssen and Medtronic. Gersh reports: consulting support from Janssen, Xenon, Cipla Limited and Armethoen, Inc.; DSMB for Mount Sinai, St. Lukes, Boston Scientific, Teva Pharmaceutical Industries, St. Jude Medical, Janssen Research and Development, Baxter Healthcare Corporation, Cardiovascular Research Foundation, and Thrombosis Research Institute; advisory board for Medtronic. Kowey reports modest consultant support from Jonhnson and Johnson. Naccarelli reports consultancies with Pfizer, Sanofi, Boehringer-Ingelheim, Bristol Myers Squibb, Otsuka, Janssen, Daiichi-Sankyo, Xention, Astra Zeneca and Glaxo Smith Kline. Peterson reports research support from Janssen and consultancies with Janssen, Boehringer Ingelheim, Sanofi, Bayer, Astra Zeneca, and Merckiccini. Piccini reports: consulting for GSK, Johnson & Johnson, Medtronic, and Spectranetics; research funding from ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, ResMed, and St. Jude Medical. Spertus reports no direct conflicts with this work. Spertus reports: consultancies with Novartis, Regeneron, Bayer, and Amgen; research grant support from Lilly, Gillea, Genetech, ACCF; ownership interest in Health Outcomes Sciences and Copyright – SAQ, KCCQ, PAQ.
References
- 1.Jenkins LS, Brodsky M, Schron E, Chung M, Rocco T, Jr., Lader E, Constantine M, Sheppard R, Holmes D, Mateski D, Floden L, Prasun M, Greene HL, Shemanski L. Quality of life in atrial fibrillation: The atrial fibrillation follow-up investigation of rhythm management (affirm) study. Am Heart J. 2005;149:112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 2.Schron EB, Jenkins LS. Quality of life in older patients with atrial fibrillation. Am J Geriatr Cardiol. 2005;14:87–90. doi: 10.1111/j.1076-7460.2005.02283.x. [DOI] [PubMed] [Google Scholar]
- 3.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: Implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 4.Darbar D, Roden DM. Symptomatic burden as an endpoint to evaluate interventions in patients with atrial fibrillation. Heart Rhythm. 2005;2:544–549. doi: 10.1016/j.hrthm.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 5.McNamara RL, Brass LM, Drozda JP, Jr., Go AS, Halperin JL, Kerr CR, Levy S, Malenka DJ, Mittal S, Pelosi F, Jr., Rosenberg Y, Stryer D, Wyse DG, Radford MJ, Goff DC, Jr., Grover FL, Heidenreich PA, Malenka DJ, Peterson ED, Redberg RF. Acc/aha key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on clinical data standards (writing committee to develop data standards on atrial fibrillation). Circulation. 2004;109:3223–3243. doi: 10.1161/01.CIR.0000131893.41821.D1. [DOI] [PubMed] [Google Scholar]
- 6.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C. Development and validation of the atrial fibrillation effect on quality-of-life (afeqt) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. doi: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 7.Coyne K, Margolis MK, Grandy S, Zimetbaum P. The state of patient-reported outcomes in atrial fibrillation : A review of current measures. PharmacoEconomics. 2005;23:687–708. doi: 10.2165/00019053-200523070-00004. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: Measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19:762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braganca EO, Filho BL, Maria VH, Levy D, de Paola AA. Validating a new quality of life questionnaire for atrial fibrillation patients. Int J Cardiol. 2010;143:391–398. doi: 10.1016/j.ijcard.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 10.Arribas F, Ormaetxe JM, Peinado R, Perulero N, Ramirez P, Badia X. Validation of the af-qol, a disease-specific quality of life questionnaire for patients with atrial fibrillation. Europace. 2010;12:364–370. doi: 10.1093/europace/eup421. [DOI] [PubMed] [Google Scholar]
- 11.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: Rationale and design of orbit-af. Am Heart J. 2011;162:606–612. e601. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, Goette A, Hindricks G, Hohnloser S, Kappenberger L, Kuck KH, Lip GY, Olsson B, Meinertz T, Priori S, Ravens U, Steinbeck G, Svernhage E, Tijssen J, Vincent A, Breithardt G. Outcome parameters for trials in atrial fibrillation: Recommendations from a consensus conference organized by the german atrial fibrillation competence network and the european heart rhythm association. Europace. 2007;9:1006–1023. doi: 10.1093/europace/eum191. [DOI] [PubMed] [Google Scholar]
- 13.European Heart Rhythm A, European Association for Cardio-Thoracic S. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (esc). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Laroche C, Boriani G, Cimaglia P, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Popescu MI, Tica O, Hellum CF, Mortensen B, Tavazzi L, Maggioni AP. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in europe: A report from the euro observational research programme pilot survey on atrial fibrillation. Europace. 2015;17:24–31. doi: 10.1093/europace/euu155. [DOI] [PubMed] [Google Scholar]
- 15.Wutzler A, von Ulmenstein S, Attanasio P, Huemer M, Parwani AS, Boldt LH, Haverkamp W. Treatment of nonagenarians with atrial fibrillation: Insights from the berlin atrial fibrillation (baf) registry. Journal of the American Medical Directors Association. 2015;16:969–972. doi: 10.1016/j.jamda.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice G 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Guidelines ESCCfP 2012 focused update of the esc guidelines for the management of atrial fibrillation: An update of the 2010 esc guidelines for the management of atrial fibrillation. Developed with the special contribution of the european heart rhythm association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 18.Gijsberts CM, Agostoni P, Hoefer IE, Asselbergs FW, Pasterkamp G, Nathoe H, Appelman YE, de Kleijn DP, den Ruijter HM. Gender differences in health-related quality of life in patients undergoing coronary angiography. Open heart. 2015;2:e000231. doi: 10.1136/openhrt-2014-000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris CM, Spertus JA, Jensen L, Johnson J, Hegadoren KM, Ghali WA, Investigators A. Sex and gender discrepancies in health-related quality of life outcomes among patients with established coronary artery disease. Circulation. Cardiovascular quality and outcomes. 2008;1:123–130. doi: 10.1161/CIRCOUTCOMES.108.793448. [DOI] [PubMed] [Google Scholar]
- 20.Riedinger MS, Dracup KA, Brecht ML, Padilla G, Sarna L, Ganz PA. Quality of life in patients with heart failure: Do gender differences exist? Heart & lung : the journal of critical care. 2001;30:105–116. doi: 10.1067/mhl.2001.114140. [DOI] [PubMed] [Google Scholar]
- 21.Riegel B, Moser DK, Carlson B, Deaton C, Armola R, Sethares K, Shively M, Evangelista L, Albert N. Gender differences in quality of life are minimal in patients with heart failure. Journal of cardiac failure. 2003;9:42–48. doi: 10.1054/jcaf.2003.1. [DOI] [PubMed] [Google Scholar]
- 22.Gott M, Barnes S, Parker C, Payne S, Seamark D, Gariballa S, Small N. Predictors of the quality of life of older people with heart failure recruited from primary care. Age and ageing. 2006;35:172–177. doi: 10.1093/ageing/afj040. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Parise H, D'Agostino RB, Sr., Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D, Framingham Heart S. Lifetime risk for developing congestive heart failure: The framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 25.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 27.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 28.Manolis AG, Katsivas AG, Lazaris EE, Vassilopoulos CV, Louvros NE. Ventricular performance and quality of life in patients who underwent radiofrequency av junction ablation and permanent pacemaker implantation due to medically refractory atrial tachyarrhythmias. J Interv Card Electrophysiol. 1998;2:71–76. doi: 10.1023/a:1009721008761. [DOI] [PubMed] [Google Scholar]
- 29.Grogan M, Smith HC, Gersh BJ, Wood DL. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1570–1573. doi: 10.1016/0002-9149(92)90705-4. [DOI] [PubMed] [Google Scholar]
- 30.Raymond RJ, Lee AJ, Messineo FC, Manning WJ, Silverman DI. Cardiac performance early after cardioversion from atrial fibrillation. Am Heart J. 1998;136:435–442. doi: 10.1016/s0002-8703(98)70217-0. [DOI] [PubMed] [Google Scholar]
- 31.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 32.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ. Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study G. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 33.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. Atrial Fibrillation Follow-up Investigation of Rhythm Management I. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 34.Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, Essebag V, Eisenberg MJ, Wynant W, Richard H, Pilote L. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172:997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 35.Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Chang P, Peterson ED, Piccini JP. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: Results from the outcomes registry for better informed treatment of atrial fibrillation (orbit-af). Circulation. Cardiovascular quality and outcomes. 2015;8:393–402. doi: 10.1161/CIRCOUTCOMES.114.001303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.