SUMMARY
Dopamine (DA) neurons in the ventral tegmental area (VTA) are heterogeneous and differentially regulate ingestive and locomotor behaviors that impact energy balance. Identification of which VTA DA neurons mediate behaviors that limit weight gain has been hindered, however, by the lack of molecular markers to distinguish VTA DA populations. Here, we identified a specific subset of VTA DA neurons that express neurotensin receptor-1 (NtsR1) and preferentially comprise mesolimbic, but not mesocortical, DA neurons. Genetically targeted ablation of VTA NtsR1 neurons uncouples motivated feeding and physical activity, biasing behavior toward energy expenditure and protecting mice from age-related and diet-induced weight gain. VTA NtsR1 neurons thus represent a molecularly-defined subset of DA neurons that are essential for the coordination of energy balance. Modulation of VTA NtsR1 neurons may therefore be useful to promote behaviors that prevent the development of obesity.
Keywords: Neurotensin Receptor, Dopamine, Nucleus Accumbens, Locomotor Activity, Feeding, Body Weight, Metabolism, Obesity, Mesolimbic
eTOC BLURB
Woodworth et al. identify a subset of VTA dopamine neurons that express neurotensin receptor-1. Ablation of these neurons leads to enhanced physical activity and energy expenditure that protects mice from diet-induced obesity, revealing an important role for VTA NtR1 neurons in the regulation of body weight.
Obesity is caused by overconsumption of calorie-dense food combined with insufficient physical activity, and predisposes individuals to myriad chronic conditions that shorten life span. Proper diet and exercise are important for maintaining healthy weight but these lifestyle modifications are difficult to maintain long-term. Consequently, most individuals that lose weight through changes in diet and activity tend to regain it over time (Soleymani et al., 2016). Therapeutic strategies to suppress motivated feeding and encourage physical activity would therefore be helpful to prevent weight gain and combat the obesity epidemic.
The motivated feeding and locomotor behaviors that influence body weight are regulated, in part, by dopamine (DA) neurons in the ventral tegmental area (VTA) that release DA to the ventral striatum and prefrontal cortex (PFC) (Day et al., 2007; McCullough et al., 1993). DA itself is essential for energy balance, demonstrated by the aphagia, hypolocomotion, reduced body weight, and early lethality of mice that genetically lack DA (Zhou and Palmiter, 1995). Disruptions in DA signaling are also observed in obese rodents and humans, suggesting that inappropriate regulation of DA circuits contributes to weight gain (Beeler et al., 2015; Johnson and Kenny, 2010; Stice et al., 2008). Yet, the mechanisms by which VTA DA neurons orchestrate energy balance remain poorly understood. One emerging theory is that VTA DA neurons are not homogenous, but in fact consist of subsets of neurons that coordinate distinct aspects of feeding and locomotor activity. Indeed, VTA DA neurons can be differentiated according to their electrophysiological firing properties (Lammel et al., 2008) or their anatomical inputs and projections (Lammel et al., 2011; Lammel et al., 2012). Mesolimbic VTA DA neurons project to the ventral striatum (consisting of the nucleus accumbens [NA] and olfactory tubercle [OFT]) and are primarily activated by “rewarding” stimuli, whereas mesocortical DA neurons project to the PFC and are regulated by “aversive” cues (Lammel et al., 2011). Some VTA DA neurons also contain the classical neurotransmitters GABA or glutamate (Root et al., 2014; Stamatakis et al., 2013) that can promote or suppress feeding in the NA (Ho and Berridge, 2014; Reynolds and Berridge, 2003). Collectively, these data suggest the possibility that distinct VTA DA populations modify obesogenic behaviors. Unfortunately, no criteria to date identify which specific VTA DA populations may be leveraged to treat energy balance disorders. Molecular markers to differentiate subsets of VTA DA neurons would be useful in this regard. While the transcription factor Neurod6 distinguishes the subset of lateral septum-projecting VTA DA neurons (Khan et al., 2017), markers for mesolimbic or mesocortical VTA subsets remain to be identified.
Neuropeptides such as orexin-A/B, neuropeptide-Y, corticotropin releasing factor (CRF), glucagon-like peptide-1 (GLP-1), and neurotensin (Nts) differentially modify DA-dependent behaviors (Anderberg et al., 2014; Kalivas et al., 1983; Vittoz and Berridge, 2006; Wanat et al., 2008), thus we reasoned that VTA DA neurons might be distinguished by their expression of neuropeptide receptors. Of these systems, Nts holds particular promise for modifying obesogenic behaviors since pharmacologic administration of Nts in the VTA restrains feeding, increases locomotor activity and induces weight loss in obese rodents (Cador et al., 1986; Elliott and Nemeroff, 1986; Feifel et al., 2010). Nts signals via neurotensin receptors -1 and -2 (NtsR1 and NtsR2) that are expressed in the VTA (Lepee-Lorgeoux et al., 1999; Palacios et al., 1988), and Nts enhances the activation of VTA DA neurons and DA release in the NA (Kalivas et al., 1983; Sotty et al., 1998). The lack of reagents to identify cells expressing NtsRs, however, has hampered understanding of how Nts mechanistically engages DA neurons. To overcome this challenge, we used Cre-lox technology to selectively visualize NtsR1 or NtsR2-expressing cells in mice and identified a unique subset of VTA DA neurons that express NtsR1. Disrupting these VTA NtsR1 neurons alters DA-mediated behaviors to prevent weight gain, suggesting that this subset of VTA neurons may represent a cellular target for preventing the development of obesity.
RESULTS
NtsR1 and NtsR2 Identify Distinct VTA Cell Types
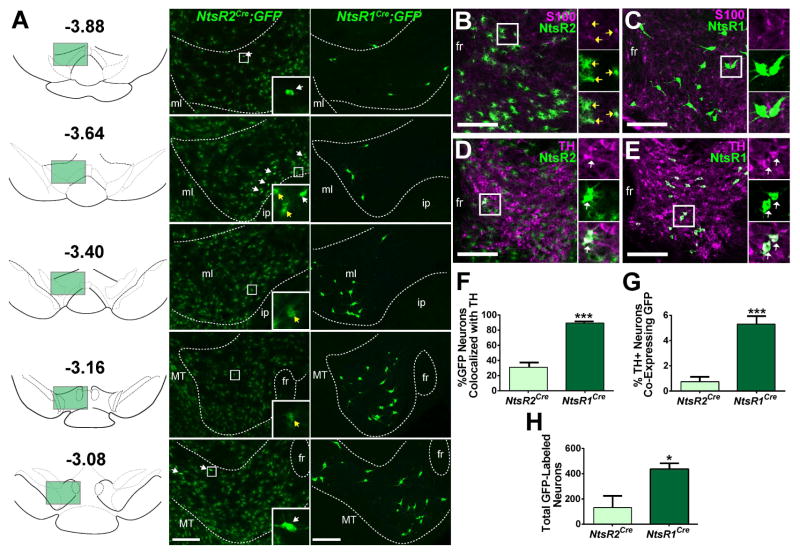
We crossed existing transgenic NtsR1Cre mice and newly generated knock-in NtsR2Cre mice onto a Cre-inducible-GFP reporter line, such that progeny expressed GFP selectively in NtsR1 or -2 expressing cells, respectively (Fig. S1, S2A). These models revealed striking differences in the morphology, number and distribution of NtsR1-GFP and NtsR2-GFP cells throughout the rostro-caudal axis of the VTA (Fig. 1A). While a few NtsR2-GFP cells appeared to be neurons (Fig. 1A white arrows), the vast majority were stellate-shaped cells consistent with glial morphology (Fig. 1A yellow arrows). By contrast, NtsR1-GFP cells were exclusively neuronal (Fig. 1A) and more numerous than NtsR2-GFP neurons. The NtsR1-GFP neurons were predominantly located in the rostral VTA (bregma-3.40 to -3.05), while the few NtsR2-GFP neurons clustered along the ventral-medial border of the caudal VTA (bregma -3.64), a region enriched in GABAergic neurons (Mazei-Robison and Nestler, 2012).
Figure 1. Distribution and neurochemical phenotype of VTA NtsR1 and NtsR2 neurons.
VTA NtsR1 and NtsR2 neurons were visualized by crossing NtsR1Cre and NtsR2Cre mice to a Cre-inducible GFP reporter line. See Fig. S1 and S2A for more detail. A) Rostrocaudal distribution of VTA NtsR1 and NtsR2 neurons with bregma positions per (Paxinos and Franklin, 2001). White arrows denote neurons, yellow arrows denote glial cells. B) Colocalization of glial cell marker S100 (purple) in the VTA with NtsR2 cells (green) and C) NtsR1 cells (green). D) Colocalization of tyrosine hydroxylase (TH, purple) in the VTA with NtsR2 cells (green) and E) NtsR1 cells (green). F) Percentage of NtsR1 and NtsR2 neurons that colocalize with TH. G) Percentage of TH positive neurons that colocalize with NtsR1 or NtsR2. H) Total number of GFP-identified NtsR1 and NtsR2 neurons in the VTA. NtsR1Cre;GFP n=4, NtsR2Cre;GFP n=6. Data represent mean ± SEM, *p < 0.05, ***p < 0.001 determined by unpaired t-test. Scale bars = 100 μm. Abbreviations: ml = medial lemniscus; ip = interpeduncular nucleus; fr = fasciculus retroflexus; MT = medial terminal nucleus of the accessory optic tract.
Given the distinct morphology and distribution of NtsR1-GFP and NtsR2-GFP cells, we next used immunolabeling to determine if they represent distinct cellular populations. The NtsR2-GFP cells with stellate morphology co-localized with the astrocyte marker S100, confirming that most NtsR2 cells are glia (Fig. 1B, yellow arrows). By contrast, none of the NtsR1-GFP cells or the few NtsR2-GFP cells with neuronal morphology co-localized with S100 (Fig. 1B, C). Instead >90% of NtsR1-GFP neurons co-expressed tyrosine hydroxylase (TH), a marker of DA neurons (Fig 1. E–H), consistent with our previous findings (Opland et al., 2013). Only ~30% of the much smaller population of NtsR2-GFP neurons co-expressed TH, representing ≤1% of all VTA DAergic neurons (Fig. 1D, F–H). Collectively, these data reveal that NtsR1 and NtsR2 are expressed in non-overlapping VTA subpopulations, that NtsR1 is the predominant receptor isoform expressed on VTA neurons, and that VTA NtsR1 neurons are a subset of all VTA DA neurons.
VTA NtsR1 Neurons are a Projection-Defined Subset of VTA DA Neurons
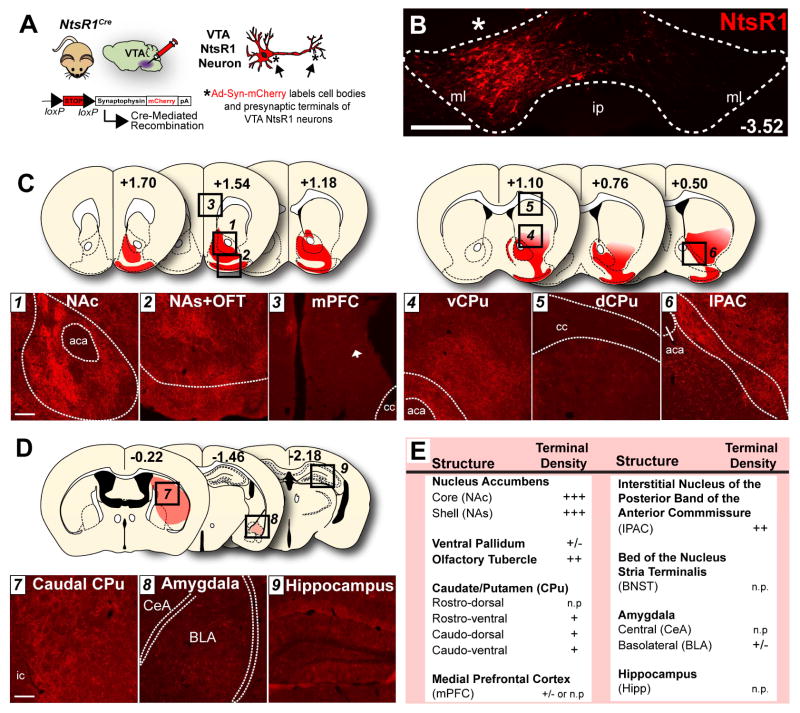
We next investigated whether the subset of VTA NtsR1 neurons are a mesolimbic- or mesocortical-specified population of VTA DA neurons by injecting the Cre-mediated anterograde tract tracer Ad-Syn-mCherry (Opland et al., 2013) into the VTA of NtsR1Cre mice (Fig. 2A, B, Fig. S2B–D). This method revealed that VTA NtsR1 neurons densely project to the ventral striatum, including the NA core and shell (NAc and NAs) and the olfactory tubercle (OFT) (Fig. 2C, E). Numerous VTA NtsR1 terminals were also observed within NA-adjacent regions such as the interstitial nucleus of the posterior limb of the anterior commissure (IPAC, Fig. 2C, E) (Heimer et al., 1997) and the ventral caudate-putamen (CPu) (Fig. 2C, E). There was, however, a notable absence of VTA NtsR1 terminals in the PFC (Fig. 2C, E). We also examined other projection targets of the VTA, and observed minor terminals within the caudal CPu and basolateral amygdala (BLA) but none within the central amygdala (CeA) or the hippocampus (Hipp) (Fig. 2D). Thus, NtsR1 defines a subset of VTA DA neurons that predominantly project to the ventral striatum and contiguous regions rather than the PFC. This indicates that VTA NtsR1 neurons preferentially define a subset of mesolimbic, not mesocortical VTA DA neurons.
Figure 2. VTA NtsR1 neurons project to the ventral striatum.
A) NtsR1Cre mice were unilaterally injected in the VTA with Ad-Syn-mCherry, resulting in syn-mCherry expression in the cell bodies and presynaptic terminals of VTA NtsR1 neurons (n=5). B) VTA injection site (asterisk) with mCherry-labeled NtsR1 cell bodies. Scale bar = 200 μm. C, D) Efferent targets of VTA NtsR1 neurons. Numbered dashed-boxes in coronal schematic images correspond to microscopy images below. Bregma positions are according to (Paxinos and Franklin, 2001). Scale bars in C, D = 100 μm. E) Table summarizing relative NtsR1 terminal density across multiple brain areas with emphasis on previously established VTA efferents. KEY: +++ heavy; ++ moderate; +light, +/-; sparse; n.p.= not present. Abbreviations: aca=anterior commissure, cc=corpus callosum, ic=internal capsule, ml=medial lemniscus, ip=interpeduncular nucleus, CeA= central amygdala, BLA= basolateral amygdala.
Loss of VTA NtsR1 Neurons Alters Energy Balance to Promote Leanness
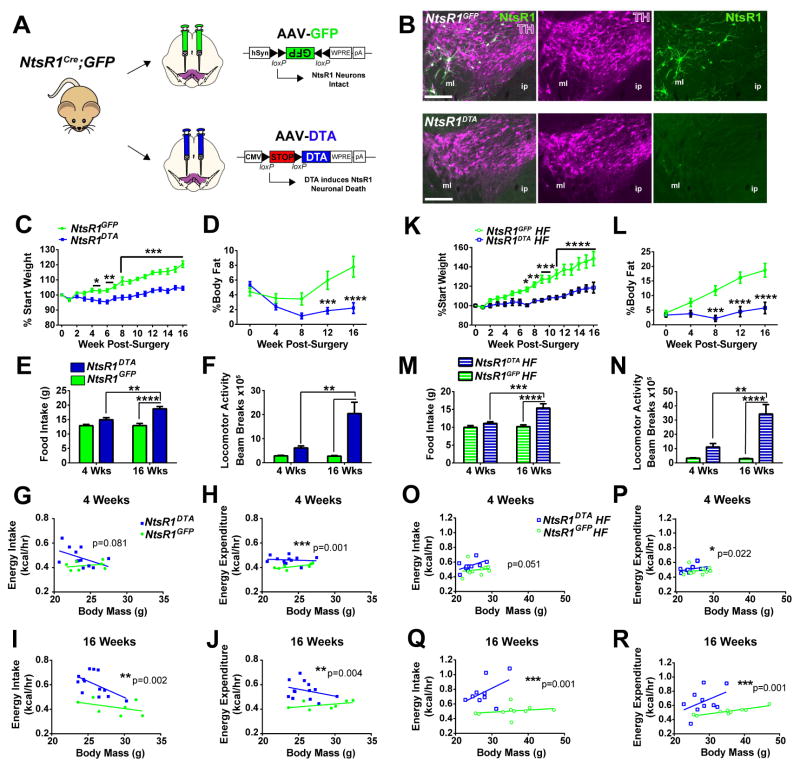
To determine if VTA NtsR1 neurons are required for the control of energy balance, we genetically lesioned them (Pei et al., 2014) (Fig. 3A). Adult NtsR1Cre;GFP mice received bilateral injections of an adeno-associated virus (AAV) expressing Cre-dependent Diphtheria Toxin A subunit (AAV-DTA) into the VTA, and DTA expression selectively kills VTA NtsR1 neurons (NtsR1DTA mice). Neighboring cells lacking Cre remain intact since they neither express the toxic DTA nor the B subunit required to internalize it. Separate NtsR1Cre;GFP mice received bilateral VTA injections of AAV-GFP and retained intact NtsR1 neurons (NtsR1GFP controls). We verified that AAV-DTA sufficiently depleted VTA NtsR1 neurons as early as two weeks post-surgery, but the remaining TH-positive DA neurons confirmed that this method does not ablate DA neurons that do not express NtsR1 (Fig. 3B). We therefore compared NtsR1DTA mice and controls to reveal the role of VTA NtsR1 neurons in energy balance. NtsR1DTA mice exhibited significantly reduced body weight compared to NtsR1GFP controls by 4 wks post-surgery and remained leaner (Fig. 3C) with decreased body fat percentage (Fig. 3D). Surprisingly, NtsR1DTA mice consumed increasingly more chow (Fig. 3E) and drank more water over the study compared to NtsR1GFP controls (Fig. S3A), suggesting that loss of VTA NtsR1 neurons promotes ingestive behavior. While the increased energy intake of NtsR1DTA mice was surprising given their decreased body weight (Fig. 3I, J), we wondered if augmented energy expenditure might explain why these mice remain lean. To this end, we noted hyperactivity of NtsR1DTA mice in their home cages (Video S1, S2), and metabolic analysis confirmed increased locomotor activity compared to NtsR1GFP controls (Fig. 3F). Increased locomotion generally demands a commensurate increase in respiration to support the activity, and indeed the hyperlocomotive NtsR1DTA mice had increased VO2 and VCO2 (Supp. Fig. 3C, D and F, G) and exhibited increasingly higher rates of energy expenditure over the course of the study compared to controls (Fig. 3H, J). The respiratory exchange ratio (RER) was unaffected (Fig. S3E, H) indicating that loss of VTA NtsR1 neurons does not alter energy substrate usage. Collectively, these data indicate that loss of VTA NtsR1 neurons biases energy balance toward increased physical activity and energy expenditure to promote leanness.
Figure 3. Loss of VTA NtsR1 neurons disrupts energy balance.
A) 8 wk old male NtsR1Cre;GFP mice received bilateral injections of AAV-DTA or AAV-GFP in the VTA. B) Images show representative NtsR1-GFP (green) and TH (purple) expression from NtsR1GFP and NtsR1DTA mice 2 weeks after surgery. Scale bars =200μm. Abbreviations: ml=medial lemniscus, ip=interpeduncular nucleus. C–J) Metabolic assessment of NtsR1GFP and NtsR1DTA mice on chow diet over 4 days (3.1 kcal/g) (NtsR1GFP n=7, and NtsR1DTA n=13). C) Percentage change in body weight from starting weight and D) percentage of body fat determined via NMR spectroscopy. E) Chow intake and F) total locomotor activity as measured in TSE metabolic cages. G) Weight-adjusted energy intake and H) energy expenditure at 4 wk post surgery and I) weight-adjusted energy intake and J) energy expenditure measured at 16 wk post surgery. K–R) Metabolic assessment of NtsR1GFP and NtsR1DTA mice on HF diet (4.7 kcal/g) (NtsR1GFP HF n=10 and NtsR1DTA HF n=10) over 4 days. K) Percentage change in body weight from starting weight, L) percentage of body fat, M) HF diet intake and N) total locomotor activity. O) Energy intake and P) energy expenditure at 4 wk post surgery, and Q) energy intake and R) energy expenditure at 16 wk post surgery, normalized to body mass. For C–F and K–N, graphed data represent mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001 analyzed by two-way ANOVA with Bonferroni post-tests. For scatterplots in G–J and O–R, data were analyzed using ANCOVA to account for body weight as a covariate and p values are indicated on graphs. See Fig. S3 for additional metabolic data.
Given the lean phenotype of mice lacking VTA NtsR1 on a normal chow diet, we hypothesized that they would be protected from diet-induced obesity. NtsR1DTA mice gained significantly less weight on high fat (HF) diet and were completely protected from diet-induced increase in body fat percentage compared to control mice (Fig. 3K, L), which persisted for at least 10–13 months post-surgery (Fig. S3O). Although NtsR1DTA mice consumed more HF diet (Fig. 3M) and had higher weight-adjusted energy intake than NtsR1GFP controls (Fig. 3O, Q), they also exhibited escalating increases in locomotor activity (Fig. 3N), VO2, VCO2 (Figure S3 I, J and L, M) and energy expenditure (Fig. 3P, R) over the study. Thus, NtsR1DTA mice remain lean in spite of their obesogenic diet, and this was due to chronically increased locomotor activity and energy expenditure.
Loss of VTA NtsR1 Neurons Alters Hedonic and Motivated Sucrose Intake
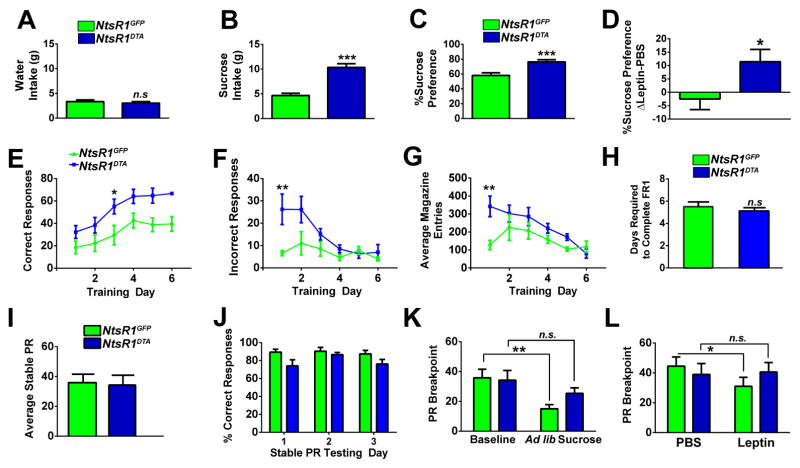
Loss of VTA NtsR1 neurons could also promote leanness by reducing the hedonic value of food, leading to insufficient caloric intake needed to balance increased energy expenditure. Surprisingly, NtsR1DTA mice preferred and over-consumed sucrose compared to control mice (Fig. 4A–C). We reasoned that if NtsR1DTA over-consume sucrose to try and meet homeostatic need, then their intake should be suppressed by treatment with the appetite-suppressing hormone, leptin (Halaas et al., 1995). While leptin mildly diminished sucrose preference in NtsR1GFP controls, it did not suppress the sucrose preference of NtsR1DTA mice (Fig. 4D). These data suggest that loss of VTA NtsR1 neurons prevents appropriate response to leptin, compromising the ability to adapt caloric intake and locomotor behaviors as needed to maintain normal weight.
Figure 4. VTA NtsR1 neurons modulate hedonic and motivated ingestive behavior.
A) Water intake, B) 0.5% sucrose intake and C) sucrose preference in untreated NtsR1GFP and NtsR1DTA mice over 4 days. D) Difference in average sucrose preference while mice received PBS or leptin (5mg/kg, ip) at the onset of the dark cycle. NtsR1GFP n=7, and NtsR1DTA n=13, n.s. = not significant, *p < 0.05, **p < 0.01 and ***p < 0.001 by unpaired t-test. E–H) NtsR1GFP and NtsR1DTA mice were trained to nose-poke for sucrose pellets and assessed for response accuracy and magazine entries during FR1 training. E) Number of correct responses, F) number of incorrect responses, G) number of magazine entries, and H) days required to complete FR1 training. I–L) NtsR1GFP and NtsR1DTA mice were tested on a PR schedule until the number of rewards earned varied <10% over 3 consecutive days. I) Average stable PR breakpoint and J) percentage of correct responses over the three stable PR days. K) PR breakpoint was assessed after animals received ad lib access to sucrose rewards the night before testing. L) NtsR1GFP and NtsR1DTA mice were tested one hour after PBS or leptin treatment (5mg/kg, ip). NtsR1GFP n=6, and NtsR1DTA =10, n.s. = not significant, *p < 0.05, **p < 0.01, analyzed via repeated measures two-way ANOVA with Bonferroni post-tests. All graphed data represent mean ± SEM.
We next examined whether loss of VTA NtsR1 neurons might have uncoupled motivated feeding required to offset increased energy expenditure. Despite being hyperactive, NtsR1DTA mice correctly learned to self-administer sucrose in a time frame similar to that of control mice (Fig. 4E, H), confirming that loss of VTA NtsR1 neurons does not compromise learning or attention processes required for motivated intake. Furthermore, NtsR1DTA and NtsR1GFP mice worked equally to obtain sucrose (Fig. 4I, J), thus loss of VTA NtsR1 neurons does not prevent sucrose “wanting”. Given the extreme energy demands of NtsR1DTA mice, however, their lack of increased motivated responding is counterintuitive: these mice should be more motivated than controls to consume calories (Fulton et al., 2000). We therefore reasoned that VTA NtsR1 neurons may be required to coordinate signals of energy status with appropriate adaptations in motivated behavior needed to maintain body weight. If this were true, then NtsR1DTA mice lacking VTA NtsR1 neurons should be unable to adjust their motivated intake in response to peripheral energy cues. As expected, NtsR1GFP control mice were less motivated to nose-poke for sucrose in response to cues of energy sufficiency such as sucrose pre-feeding (Scheggi et al., 2013) or leptin (Sharma et al., 2012), but NtsR1DTA mice did not adjust their intake in response to these cues (Fig. 4K, L). Taken together, these findings reveal an important role for VTA NtsR1 neurons in linking metabolic status and motivated feeding necessary to maintain body weight.
Loss of VTA NtsR1 Neurons Modifies DA-Mediated Locomotor Activity
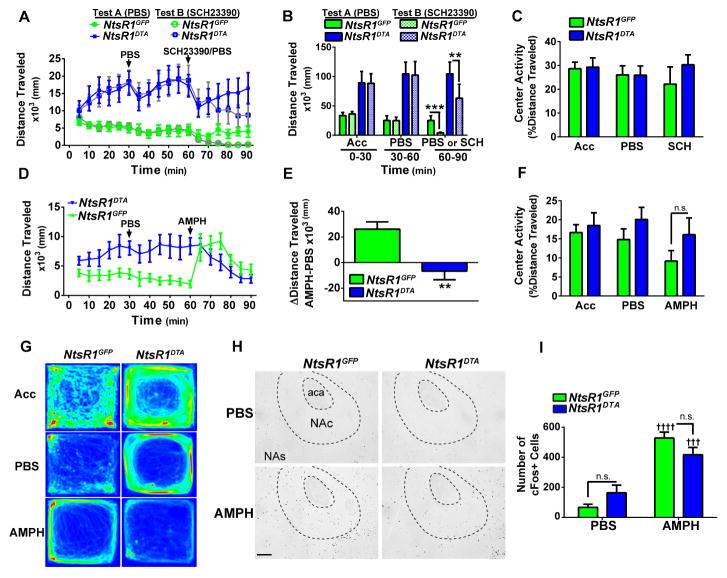
The hyperactivity of NtsR1DTA mice suggested that they retained DA-mediated locomotor behavior. Consistent with this, open field locomotor activity of NtsR1DTA and NtsR1GFP mice was not disrupted by PBS-injection stress (Fig. 5A, B, Test A), whereas treatment with the D1 receptor antagonist SCH23390 (0.1mg/kg ip) blunted locomotor activity in both groups (Fig. 5A, B, Test B). We therefore used amphetamine (AMPH)-induced locomotor activity to assess the integrity of the mesolimbic DA system in NtsR1DTA mice. As expected, AMPH treatment (4mg/kg ip) significantly increased locomotor activity in NtsR1GFP mice, but NtsR1DTA mice did not respond with increased activity (Fig 5D–G). Instead, we anecdotally observed that AMPH-treated NtsR1DTA mice engaged in stationary, stereotypic movements typical with ceiling effects of DA signaling, which account for their apparent decrease in ambulatory activity (Fig. 5D–G). Since NtsR1DTA mice lack a subset of VTA DA neurons that projects heavily to the NA, we examined the ability of AMPH to induce DA release in the NA using AMPH-induced cFos immunoreactivity to identify activated NA neurons. AMPH treatment significantly increased numbers of cFos-positive cells in the NA of both NtsR1DTA and control mice, although the response was non-significantly blunted in NtsR1DTA mice, consistent with loss of some, but not all NA-projecting VTA DA neurons (Fig. 5H, I). Together, these data indicate that loss of VTA NtsR1 neurons does not completely abolish mesolimbic signaling. In fact, the high baseline locomotor activity and AMPH-induced stereotypy observed in NtsR1DTA mice may indicate enhanced DA signaling, similar to compensatory increases in extracellular DA that occur with partial loss of DA neurons (Robinson et al., 1994). Importantly, although hyperactivity and excessive DA have been linked with increased anxiety states, there were no differences in anxiety-like behavior between NtsR1GFP and NtsR1DTA mice as assessed via open field center activity (Fig. 5C, F) and elevated plus maze (EPM) (Fig. S4).
Figure 5. Loss of VTA NtsR1 neurons alters response to amphetamine.
A) Locomotor activity of NtsR1GFP and NtsR1DTA mice in open field boxes during acclimation, treatment with PBS, and either PBS (Test A) or D1R antagonist SCH23390 (0.1 mg/kg, ip) (Test B). Data represent average distance traveled per 5 min interval ± SEM (NtsR1GFP n=6, and NtsR1DTA n=10). B) Mean distance traveled during treatment period ± SEM, analyzed by two-way ANOVA. C) Percentage of distance traveled in the center zone of boxes over each testing period. D) Open field locomotor activity of NtsR1GFP and NtsR1DTA mice after treatment with PBS and AMPH (4 mg/kg, ip). Data represent average distance traveled per 5 min interval ± SEM (NtsR1GFP n=6, and NtsR1DTA n=10). E) Mean difference between locomotor activity after AMPH treatment relative to PBS ± SEM, **p=0.003. F) Percentage of distance traveled in the center zone over each period ± SEM, n.s. = not significant. See Fig. S4 for additional anxiety data. G) Representative heat maps demonstrating mouse location during acclimation (Acc), PBS, and AMPH treatments. H) cFos expression in the NA (+1.34 bregma) of NtsR1GFP and NtsR1DTA treated with PBS or AMPH 90 minutes prior to perfusion. Abbreviations: aca = anterior commissure; NAc = nucleus accumbens core; NAs = nucleus accumbens shell. I) Number of cFos positive (cFos+) cells in NA. Graphed data represent mean ± SEM, analyzed by two-way ANOVA. Ɨ Ɨ Ɨ Ɨ p<0.0001, Ɨ Ɨ Ɨ p<0.001, indicates significant difference between PBS and amphetamine treatment within each group, (NtsR1GFP PBS n=3, AMPH=3; NtsR1DTA PBS n=3, AMPH n=5).
Loss of VTA NtsR1 Neurons Modifies the Mesolimbic DA System
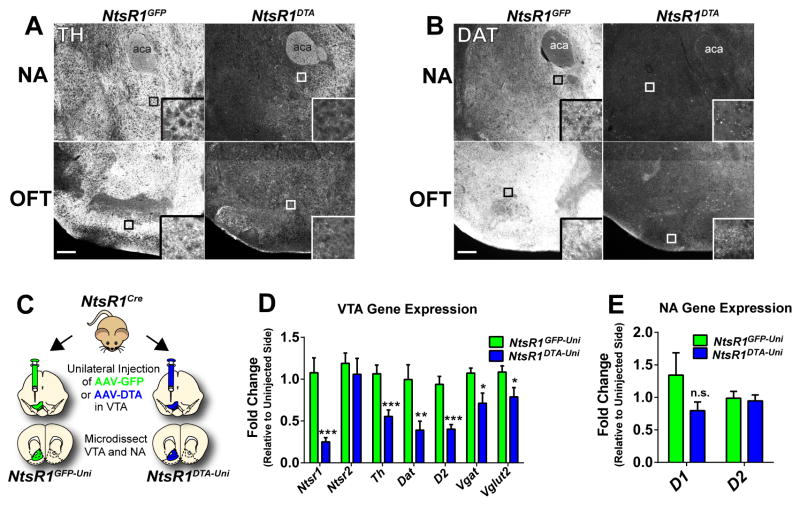
Lastly, we examined how loss of VTA NtsR1 neurons impacts the integrity of the mesolimbic DA system. NtsR1DTA mice have reduced TH and DAT immuo-labeled terminals in the NA and OFT compared to control mice (Fig. 6A, B) but residual immunoreactivity suggests preservation of non-NtsR1 expressing mesolimbic neurons. To assess molecular alterations in the VTA, we administered unilateral VTA injections of AAV-DTA or AAV-GFP to NtsR1Cre;GFP mice and analyzed the fold difference in gene expression between the injected and uninjected sides; we refer to these as NtsR1GFP-Uni and NtsR1DTA-Uni mice (Fig. 6C). As expected, NtsR1 and DA-associated transcripts such as Th, Dat and D2 were significantly reduced in the VTA of NtsR1DTA-Uni mice, consistent with the loss of DAergic VTA NtsR1 cell bodies (Fig. 6D). The slight reduction in Vglut2 and Vgat in NtsR1DTA-Uni mice suggests that VTA NtsR1 neurons may also contain glutamate and/or GABA in addition to DA. Ntsr2 expression, however, did not differ between NtsR1DTA-Uni mice and controls, consistent with our finding that NtsR1 and NtsR2 are expressed on distinct VTA cell populations (Fig. 1). As expected, the fold change in expression between injected and uninjected sides was close to 1.0 in the in NtsR1GFP-Uni controls, validating the use of the uninjected side as an internal comparison for each mouse. No differences in NA expression of D1 or D2 were observed between groups (Fig. 6E). In sum, loss of VTA NtsR1 neurons results in structural and molecular adaptations in the mesolimbic DA system but does not abrogate the entirety of DA-mediated signaling that is required for survival.
Figure 6. Ablation of VTA NtsR1 neurons decreases markers of DA signaling.
Post-hoc analysis of A) tyrosine hydroxylase (TH) and B) dopamine transporter (DAT) immunoreactivity in the NA and OFT (+1.50 bregma) of NtsR1GFP and NtsR1DTA mice perfused 10–13 months after surgery. Scale bars = 100 μm. Abbreviation: aca = anterior commissure. White boxes identify digitally enlarged areas from each image. C) NtsR1Cre mice were injected unilaterally with AAV-DTA to ablate VTA NtsR1 neurons or AAV-GFP as a control. The VTA and NA were dissected separately from each side of the brain and expression of DA-related genes was assessed at 16 wks post-surgery in the D) VTA and E) NA. Fold change was determined for each animal relative to the uninjected side. Data are expressed as mean ± SEM (NtsR1GFP n=6–7, NtsR1DTA n=8–9). Significant differences were determined by unpaired t-test *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
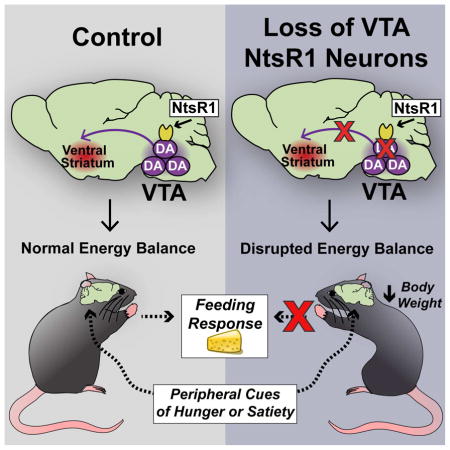
The lack of molecular markers to differentiate functionally heterogeneous and projection-specified VTA DA neurons has impeded understanding of how these neurons orchestrate behavior and body weight. We show that NtsR1 expression identifies a subset of VTA DA neurons that projects preferentially to the ventral striatum compared to the PFC, and thus represents a molecular marker of primarily mesolimbic, not mesocortical VTA DA neurons. Loss of this VTA NtsR1 population alters DA-mediated behaviors to protect against weight gain without compromising DA-mediated signaling necessary for survival (Zhou and Palmiter, 1995). Collectively, our data identify VTA NtsR1 neurons as important coordinators of energy intake and output behaviors that determine body weight.
Since Nts actions via NtsR1 and NtsR2 are implicated in control of distinct physiology (Geisler et al., 2006), we hypothesized that these receptors might identify functionally distinct DA neurons. We therefore used Cre-mediated reporter mice to identify the cells expressing each receptor isoform, confirming that NtsR1 is almost exclusively expressed by DA neurons while NtsR2 is primarily expressed by non-DAergic neurons and astrocytes in the VTA. Since NtsR1 is a Gαq-coupled GPCR (Tanaka et al., 1990), our findings indicate a mechanism for Nts to directly enhance the activation of NtsR1-expressing DA neurons, consistent with the established roles for NtsR1 in promoting DA release to the striatum and DA-mediated locomotor activity (Remaury et al., 2002; Steinberg et al., 1994), anorexia (Remaury et al., 2002), and reward (Kempadoo et al., 2013; Rouibi et al., 2015) Our data do not, however, rule out roles for the non-DAergic populations of NtsR2 cells to indirectly promote DA signaling. The TH-negative NtsR2 neurons found primarily within the ventromedial, GABA-rich portion of the VTA may disinhibit local DA neurons and promote their activation (Kempadoo et al., 2013; Piccart et al., 2015). Nts may also act via the numerous NtsR2-expressing astrocytes within the VTA to indirectly modify DA-mediated behavior, consistent with reports of glial cells mediating NtsR2-dependent fear behavior (Yamauchi et al., 2007). Going forward, it will be important to define the neural circuits by which Nts engages these distinct NtsR1 and NtsR2-expressing cell types and their individual contributions to DA-mediated behaviors and energy balance.
In situ hybridization predicts a larger population of NtsR1-expressing neurons in the VTA than were identified using transgenic NtsR1Cre-GFP mice (Allen Brain Atlas - (Lein et al., 2007) and Fig. 1). This discrepancy may be due to low Cre expression common to transgenic lines, diminishing the intensity of Cre-mediated GFP expression necessary to identify NtsR1 cells. Increasing the concentration of Cre-inducible transcripts can enhance recombination, and indeed, injecting NtsR1Cre mice with AAV-GFP identifies additional NtsR1 neurons in the VTA. The AAV-identified VTA NtsR1-GFP neurons exclusively co-label with a subpopulation of TH cells, confirming that VTA NtsR1 neurons are a subset of all DA neurons (Fig. S5). In sum, these data suggest that the VTA NtsR1 neuronal population is larger than depicted in Fig. 1, but that subsequent AAV manipulations were able to transduce and modulate most VTA NtsR1 neurons.
Although generalized depletion of VTA DA neurons does not alter locomotor activity or energy intake (Drui et al., 2014; Ouachikh et al., 2013; Pioli et al., 2008), ablation of the specific subset of VTA NtsR1 neurons altered both behaviors and prevented age-associated and diet-induced weight gain. Since aging and obesogenic environment are two significant instigators of human weight gain, our findings suggest roles for VTA NtsR1 neurons in countering the development of obesity. Intriguingly, the pronounced physical activity and energy expenditure of NtsR1DTA mice appears to be the primary source of protection from weight gain. Increased physical activity is similarly impactful in humans, as individuals who exercise are more likely to avoid weight gain with age (Lee et al., 2010; Shiroma et al., 2012). Although the NtsR1DTA mice eat slightly more than controls, their energy intake is only sufficient to compensate for elevated levels of activity and energy expenditure, and did not promote weight gain. Thus, mesolimbic VTA NtsR1 neurons are not essential drivers of feeding per se, but they coordinate energy intake required to support physical activity.
Given that VTA NtsR1 neurons project heavily to the ventral striatum, where DA release regulates the motivation for palatable foods (Day et al., 2007; McCullough et al., 1993), we were surprised that NtsR1DTA mice did not differ from controls in their willingness to work for sucrose rewards. Furthermore, NtsR1DTA mice demonstrated intact liking and wanting of palatable foods. These findings suggest that VTA NtsR1 neurons are not required for DA-mediated ingestive behavior, and that other non-NtsR1 expressing VTA neurons exert this control. It may be argued, however, that NtsR1DTA mice with low body weight and adiposity should display increased motivation for sucrose (Fulton et al., 2000), and thus have a deficit in coordinating intake behavior to meet energy demands. This is consistent with our findings that NtsR1DTA mice also lack the ability to adapt motivated intake in the face of satiety cues such as sucrose pre-feeding and leptin treatment (Sharma et al., 2012). Taken together, these data suggest that VTA NtsR1 neurons are not required for the execution of motivated food intake, but rather serve to tune it in response to peripheral energy needs.
VTA NtsR1 neurons must receive information about peripheral energy status to coordinate metabolic state and DA-mediated behavior. The adipocyte-derived hormone leptin modulates energy balance in part via the actions of Nts and NtsR1 (Kim et al., 2008; Opland et al., 2013; Sahu et al., 2001), and we demonstrate an obligate role of VTA NtsR1 neurons in this process. It is unlikely that VTA NtsR1 neurons are direct targets of leptin because VTA NtsR1- and leptin receptor (LepRb) expressing populations have distinct anatomical distributions and projection sites (Fig. 2 and (Leshan et al., 2010)), suggesting that they comprise separate neuronal populations. Leptin does, however, activate LepRb neurons of the lateral hypothalamic area (LHA) that project to and release Nts into the VTA, promoting NtsR1-dependent release of DA in the NA (Opland et al., 2013; Patterson et al., 2015). LHA LepRb neurons may thus be the neural hubs linking leptin action with Nts and DA signaling.
We used NtsR1 as a marker to identify VTA NtsR1 neurons, but it remains to be determined which signals from these neurons are critical for regulation of energy balance. VTA NtsR1 neurons may co-express both GABA and/or glutamate to modify activation of striatal targets. Nts action via NtsR1 expressed on VTA NtsR1 neurons may also contribute to DA-mediated behaviors. Indeed, the phenotype of NtsR1DTA mice resembles whole-body NtsR1 knockout mice with hyperactivity, increased sucrose preference, and lack of adaptive response to leptin (Opland et al., 2013). Together, these models argue that chronic disruption of Nts signaling via VTA NtsR1 neurons biases homeostasis toward energy expenditure. Perhaps the most important signal from VTA NtsR1 neurons may be DA itself. Although NtsR1DTA mice exhibit substantial loss of VTA NtsR1 neurons and diminished DAergic projections to the NA, their intact AMPH-induced cFos confirms that at least some DA neurons remain and are functional. If anything, chronic loss of VTA NtsR1 neurons may lead to enhanced DA signaling. Indeed, mice lacking VTA NtsR1 neurons display increased general ambulatory behavior, but decreased AMPH-induced locomotor activity accompanied by stereotypy, a behavioral response typically associated with high-dose AMPH and ceiling-levels of DA efflux (Yates et al., 2007). We thus speculate that loss of VTA NtsR1 neurons leads to increased striatal DA signaling, and AMPH further increases DA to levels that promote stereotypy instead of augmenting ambulatory activity. Numerous mechanisms could enhance DA action, such as altered balance of D1/D2 protein expression or impaired DAT kinetics, and will be important to define in the future.
Our data indicate that loss of VTA NtsR1 neurons prevents diet-induced obesity in mice, hence VTA NtsR1 neurons may represent a cellular target for preventing and treating human obesity. Ablation of neurons is not a feasible therapeutic strategy, thus it will be imperative to identify pharmacological means to selectively modulate VTA NtsR1 neurons to promote behaviors that prevent weight gain. Previous work demonstrates that pharmacologic treatment of Nts or Nts agonists in the VTA suppresses motivated feeding (Kelley et al., 1989) and enhances locomotor activity in an NtsR1-dependent manner (Elliott and Nemeroff, 1986; Steinberg et al., 1994). Conversely, other studies showed suppression of locomotor activity when Nts was applied directly to the NAc (Kalivas et al., 1984), underscoring the need for circuit-specific interventions. Although developmental loss of NtsR1 promotes palatable food intake and weight gain (Opland et al., 2013), our current data show that selective ablation of VTA NtsR1 neurons in adult mice protects them from obesity, suggesting that pharmacological modulation of Nts signaling may be useful in adults with established VTA NtsR1 DA circuits. Going forward, it will be important to define how Nts and other signals engage VTA NtsR1 neurons to identify strategies that prevent obesity
EXPERIMENTAL PROCEDURES
Mice
NtsR1Cre mice were purchased from the Mutant Mouse Regional Resource Center, UC Davis (B6.FVB(Cg)-Tg(NtsR1-cre)GN220Gsat/Mmucd, Stcok number 030648-UCD). NtsR2Cre mice were generated via homologous recombination (knock-in) methods described previously (Leinninger et al., 2011). To visualize NtsR1 and NtsR2-expressing neurons, NtsR1Cre and NtsR2Cre mice were bred to a Cre-inducible RosaeGFP-L10a reporter line (Krashes et al., 2014), generating mice that express GFP selectively in NtsR1 and NtsR2 neurons (NtsR1Cre;GFP and NtsR2Cre;GFP); brains from adult male mice were analyzed for GFP. To induce Cre expression in NtsR2Cre;GFP mice, the frt-flanked Neo cassette was removed by injecting FlpO adenovirus (Vector Biolabs) into the lateral ventricles of adult animals (see Supp. Methods). Mice were bred and housed in a 12h light/12h dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee at Michigan State University in accordance with Association for Assessment and Accreditation of Laboratory Animal Care and National Institutes of Health guidelines.
To generate NtsR1GFP and NtsR1DTA study mice, 8–10 wk old NtsR1Cre;GFP males received bilateral injections of either 150nL Cre-inducible AAV-GFP(rAAV2/hSvn-DIO-eGFP, University of North Carolina Vector Core) or AAV-DTA (lox-mCherry-loxDTA-WPRE-AAV, serotype 10), which expresses the cytotoxic Subunit A of Diptheria Toxin in the presence of Cre into the VTA (A/P: −3.2, M/L: +/−0.48, D/V: −4.65). Only mice found to have injections contained within the VTA were included in the final analysis.
Metabolic Profiling
At 4, 8 and 16 wk post-surgery the mice were analyzed for body composition using an NMR-based instrument (Minispec mq7.5, Bruker Optics). At 4 and 16 wk post-surgery, mice were placed in TSE cages for metabolic phenotyping (PhenoMaster, TSE Systems). After 24 hours of acclimation, mice were continuously monitored for food and water intake, locomotor activity, and energy expenditure over 4 days. Ambient temperature was maintained at 20–23°C and the airflow rate through the chambers was adjusted to maintain an oxygen differential around 0.3% at resting conditions. Metabolic parameters including VO2, respiratory exchange ratio, and energy expenditure were assessed via indirect calorimetry by comparing O2 and CO2 concentrations relative to a reference cage.
Operant Testing
Standard Testing: NtsR1DTA and NtsR1GFP were trained to nose-poke for unflavored 20 mg sucrose pellets (TestDiet 1811555) using operant-responding chambers (Med Associates) as previously described (Sharma et al., 2012). See Supp. Methods for more detail. Sucrose pre-feeding: After achieving stable PR, mice were given ad lib access to 3g of sucrose pellets in their home cages overnight. The following morning they were re-tested on the PR schedule. Leptin Treatment: Mice with stable PR responses were injected with PBS or leptin (5mg/kg, ip) on different days and tested one hour later.
Open Field Locomotor Activity
Open field locomotor activity was assessed using a digital CCD camera and video-tracking software (Clever Sys) (Eagle et al., 2015). Mice were tested on 3 separate days; each day, mice were placed in the boxes to acclimate, followed by an ip injection of PBS 30 min later. At 60 min, mice received either a second injection of PBS, or SCH23390 (0.1mg/kg) or amphetamine (4mg/kg) and recorded for another 60 minutes.
Statistics
Student’s t-tests and 2-way ANOVA were calculated using Prism 6 (GraphPad). For all metabolic data, analysis of covariance (ANCOVA) was computed in SPSS 22 (IBM). Body weight was analyzed as a covariate to correct for any inherent differences it may have on metabolism (Tschop et al., 2012). All data were tested for homogeneity of regression, independence of the covariate (body weight), and linearity of regression prior to running the ANCOVA. For all data, *p<0.05, **p<0.01 and ***p<0.001.
Supplementary Material
Video S1. Home cage ambulatory activity of an NtsR1GFP mouse, Related to Figure 3.
Video S2. Home cage ambulatory activity of an NtsR1DTA mouse, Related to Figure 3.
HIGHLIGHTS.
NtsR1 is expressed on a subset of VTA DA neurons that projects to the NA
Loss of VTA NtsR1 neurons promotes energy use and prevents diet-induced obesity
VTA NtsR1 neurons are necessary to coordinate energy cues with ingestive behavior
Ablation of VTA NtsR1 neurons alters expression of mesolimbic DA markers
Acknowledgments
We thank the University of Michigan Transgenic Animal Model Core and the Van Andel Research Institute Transgenic and Targeting Core for assistance in generating NtsR2Cre mice. We thank Dr. Martin Myers Jr. for providing Ad-Syn-mCherry and Sandra O’Reilly for assisting with metabolic phenotyping. This research was supported by a Pilot and Feasibility Grant from the Michigan Diabetes Research Center (NIH Grant 5P60-DK020572) and the NIH (JAB: T32-ES00725527, F31-DK107081; HLW: F30-DK107163, GML: R01-DK103808.)
Footnotes
AUTHOR CONTRIBUTIONS
HLW and GML designed the experiments and wrote the paper. HLW conducted the experiments and analyzed the data, with assistance from HMB, BB, RB, JAB, and GK. PMF provided AAV-DTA and read the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderberg RH, Anefors C, Bergquist F, Nissbrandt H, Skibicka KP. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiology & behavior. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Faust RP, Turkson S, Ye H, Zhuang X. Low Dopamine D2 Receptor Increases Vulnerability to Obesity Via Reduced Physical Activity Not Increased Appetitive Motivation. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Kelley AE, Le Moal M, Stinus L. Ventral tegmental area infusion of substance P, neurotensin and enkephalin: differential effects on feeding behavior. Neuroscience. 1986;18:659–669. doi: 10.1016/0306-4522(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature neuroscience. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Drui G, Carnicella S, Carcenac C, Favier M, Bertrand A, Boulet S, Savasta M. Loss of dopaminergic nigrostriatal neurons accounts for the motivational and affective deficits in Parkinson’s disease. Molecular psychiatry. 2014;19:358–367. doi: 10.1038/mp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Gajewski PA, Yang M, Kechner ME, Al Masraf BS, Kennedy PJ, Wang H, Mazei-Robison MS, Robison AJ. Experience-Dependent Induction of Hippocampal DeltaFosB Controls Learning. J Neurosci. 2015;35:13773–13783. doi: 10.1523/JNEUROSCI.2083-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PJ, Nemeroff CB. Repeated neurotensin administration in the ventral tegmental area: effects on baseline and D-amphetamine-induced locomotor activity. Neurosci Lett. 1986;68:239–244. doi: 10.1016/0304-3940(86)90149-7. [DOI] [PubMed] [Google Scholar]
- Feifel D, Goldenberg J, Melendez G, Shilling PD. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology. 2010;58:195–198. doi: 10.1016/j.neuropharm.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Geisler S, Berod A, Zahm DS, Rostene W. Brain neurotensin, psychostimulants, and stress--emphasis on neuroanatomical substrates. Peptides. 2006;27:2364–2384. doi: 10.1016/j.peptides.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. The European journal of neuroscience. 2014;40:3556–3572. doi: 10.1111/ejn.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Burgess SK, Nemeroff CB, Prange AJ., Jr Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience. 1983;8:495–505. doi: 10.1016/0306-4522(83)90195-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ., Jr Neurotensin microinjection into the nucleus accumbens antagonizes dopamine-induced increase in locomotion and rearing. Neuroscience. 1984;11:919–930. doi: 10.1016/0306-4522(84)90203-3. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Cador M, Stinus L, Le Moal M. Neurotensin, substance P, neurokinin-alpha, and enkephalin: injection into ventral tegmental area in the rat produces differential effects on operant responding. Psychopharmacology (Berl) 1989;97:243–252. doi: 10.1007/BF00442258. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, et al. Hypothalamic Neurotensin Projections Promote Reward by Enhancing Glutamate Transmission in the VTA. J Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Stott SR, Chabrat A, Truckenbrodt AM, Spencer-Dene B, Nave KA, Guillemot F, Levesque M, Ang SL. Survival of a Novel Subset of Midbrain Dopaminergic Neurons Projecting to the Lateral Septum Is Dependent on NeuroD Proteins. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:2305–2316. doi: 10.1523/JNEUROSCI.2414-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ER, Leckstrom A, Mizuno TM. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behav Brain Res. 2008;194:66–71. doi: 10.1016/j.bbr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Djousse L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA: the journal of the American Medical Association. 2010;303:1173–1179. doi: 10.1001/jama.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepee-Lorgeoux I, Betancur C, Rostene W, Pelaprat D. Differential ontogenetic patterns of levocabastine-sensitive neurotensin NT2 receptors and of NT1 receptors in the rat brain revealed by in situ hybridization. Brain research Developmental brain research. 1999;113:115–131. doi: 10.1016/s0165-3806(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harbor perspectives in medicine. 2012;2:a012070. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Cousins MS, Salamone JD. The role of nucleus accumbens dopamine in responding on a continuous reinforcement operant schedule: a neurochemical and behavioral study. Pharmacology, biochemistry, and behavior. 1993;46:581–586. doi: 10.1016/0091-3057(93)90547-7. [DOI] [PubMed] [Google Scholar]
- Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M, Jr, Leinninger G. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Molecular metabolism. 2013;2:423–434. doi: 10.1016/j.molmet.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouachikh O, Dieb W, Durif F, Hafidi A. Differential behavioral reinforcement effects of dopamine receptor agonists in the rat with bilateral lesion of the posterior ventral tegmental area. Behavioural brain research. 2013;252:24–31. doi: 10.1016/j.bbr.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Pazos A, Dietl MM, Schlumpf M, Lichtensteiger W. The ontogeny of brain neurotensin receptors studied by autoradiography. Neuroscience. 1988;25:307–317. doi: 10.1016/0306-4522(88)90028-0. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Wong JM, Leinninger GM, Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones JC, Kennedy RT, et al. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology. 2015;156:1692–1700. doi: 10.1210/en.2014-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin B. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab. 2014;3:209–215. doi: 10.1016/j.molmet.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart E, Courtney NA, Branch SY, Ford CP, Beckstead MJ. Neurotensin Induces Presynaptic Depression of D2 Dopamine Autoreceptor-Mediated Neurotransmission in Midbrain Dopaminergic Neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:11144–11152. doi: 10.1523/JNEUROSCI.3816-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioli EY, Meissner W, Sohr R, Gross CE, Bezard E, Bioulac BH. Differential behavioral effects of partial bilateral lesions of ventral tegmental area or substantia nigra pars compacta in rats. Neuroscience. 2008;153:1213–1224. doi: 10.1016/j.neuroscience.2008.01.084. [DOI] [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou JM, Le Fur G, Soubrie P, Caput D, et al. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nature neuroscience. 2014;17:1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouibi K, Bose P, Rompre PP, Warren RA. Ventral Midbrain NTS1 Receptors Mediate Conditioned Reward Induced by the Neurotensin Analog, D-Tyr [11] neurotensin. Frontiers in neuroscience. 2015;9:470. doi: 10.3389/fnins.2015.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Carraway RE, Wang YP. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res. 2001;888:343–347. doi: 10.1016/s0006-8993(00)03107-3. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Secci ME, Marchese G, De Montis MG, Gambarana C. Influence of palatability on motivation to operate for caloric and non-caloric food in non food-deprived and food-deprived rats. Neuroscience. 2013;236:320–331. doi: 10.1016/j.neuroscience.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Sharma S, Hryhorczuk C, Fulton S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J Vis Exp. 2012:e3754. doi: 10.3791/3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroma EJ, Sesso HD, Lee IM. Physical activity and weight gain prevention in older men. International journal of obesity (2005) 2012;36:1165–1169. doi: 10.1038/ijo.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymani T, Daniel S, Garvey WT. Weight maintenance: challenges, tools and strategies for primary care physicians. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2016;17:81–93. doi: 10.1111/obr.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Souliere F, Brun P, Chouvet G, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny MF. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: a combined in vivo electrochemical and electrophysiological study. Neuroscience. 1998;85:1173–1182. doi: 10.1016/s0306-4522(97)00691-x. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R, Brun P, Fournier M, Souilhac J, Rodier D, Mons G, Terranova JP, Le Fur G, Soubrie P. SR 48692, a non-peptide neurotensin receptor antagonist differentially affects neurotensin-induced behaviour and changes in dopaminergic transmission. Neuroscience. 1994;59:921–929. doi: 10.1016/0306-4522(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, et al. A guide to analysis of mouse energy metabolism. Nature methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. The Journal of physiology. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi R, Wada E, Kamichi S, Yamada D, Maeno H, Delawary M, Nakazawa T, Yamamoto T, Wada K. Neurotensin type 2 receptor is involved in fear memory in mice. J Neurochem. 2007;102:1669–1676. doi: 10.1111/j.1471-4159.2007.04805.x. [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neuroscience letters. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Home cage ambulatory activity of an NtsR1GFP mouse, Related to Figure 3.
Video S2. Home cage ambulatory activity of an NtsR1DTA mouse, Related to Figure 3.