Abstract
PSMD10, also known as gankyrin, is associated with the proteasome and has been shown to be an oncoprotein in the liver. Here, we report that PSMD10 expression is stimulated by the histone demethylase JMJD2A/KDM4A and its interaction partner, the ETV1 transcription factor, in LNCaP prostate cancer cells. Global analysis of expression patterns revealed that PSMD10 mRNA levels are positively correlated with those of both JMJD2A and ETV1. In human prostate tumors, PSMD10 is highly overexpressed at the protein level and correlates with JMJD2A overexpression; further, PSMD10 expression is enhanced in the prostates of transgenic JMJD2A mice. Moreover, PSMD10 is particularly overexpressed in high Gleason score prostate tumors. Downregulation of PSMD10 in LNCaP prostate cancer cells impaired their growth, indicating that PSMD10 may exert a pro-oncogenic function in the prostate. Lastly, we observed that PSMD10 expression is correlated to YAP1, a component of the Hippo signaling pathway and whose gene promoter is regulated by JMJD2A, and that PSMD10 can cooperate with YAP1 in stimulating LNCaP cell growth. Altogether, these data indicate that PSMD10 is a novel downstream effector of JMJD2A and suggest that inhibition of the JMJD2A histone demethylase by small molecule drugs may be effective to curtail the oncogenic activity of PSMD10 in various PSMD10-overexpressing tumors.
Keywords: ETV1, gankyrin, JMJD2A, histone demethylase, KDM4A, prostate cancer, PSMD10
Introduction
Many epigenetic regulators have been identified during the last decade that function to suppress or to enhance tumor formation [1, 2]. One of the most versatile epigenetic marks is the modification of various histone lysine residues by methylation, which is controlled by the antagonism between histone methyltransferases and demethylases [3]. Over 20 different histone demethylases are currently known and the vast majority of them belongs to the JmjC domain-containing (JMJD) protein family [4]. Amongst the JMJD proteins, also called KDM4 (lysine demethylase 4) proteins, the JMJD2 subfamily is the largest one with 6 respective genes in the human genome [5–7]. The first JMJD2 protein characterized was JMJD2A/KDM4A that was reported to act as a transcriptional corepressor [8, 9]. However, the subsequent characterization of JMJD2A as an enzyme capable of removing the repressive H3K9me3 and H1.4K26me3 marks revealed that it can also function as a transcriptional coactivator [10–13].
Bioinformatical analyses as well as immunohistochemistry have shown that JMJD2A is overexpressed in prostate tumors and that high JMJD2A expression correlates with increased Gleason score and metastasis [14, 15]. Furthermore, transgenic mice that prostate-specifically overexpress JMJD2A develop prostatic intraepithelial neoplasia, demonstrating that JMJD2A overexpression is an underlying cause for the initiation of prostate cancer [15]. Mechanistically, JMJD2A can interact with two prostate cancer relevant proteins, the androgen receptor and ETV1 (ETS variant 1), and affect their transcriptional potential [15, 16]. The androgen receptor is abnormally activated or overexpressed in prostatic malignancies and a prime target for their therapy [17]. Like the androgen receptor, ETV1 is a DNA-binding transcription factor highly regulated through post-translational modification [18–23] and overexpressed in about 5–10% of all human prostate tumors [24, 25]. Furthermore, ETV1 transgenic mice develop prostatic intraepithelial neoplasia and combining ETV1 overexpression with complete loss – but not with inactivation of just one allele – of the PTEN tumor suppressor leads to adenocarcinomas [26–28]. In addition, joint overexpression of ETV1 and JMJD2A combined with PTEN haploinsufficiency results in the progression of prostatic intraepithelial neoplasia to the carcinoma stage, indicating that the JMJD2A-ETV1 complex has oncogenic properties [15].
PSMD10 (proteasome 26S subunit, non-ATPase, 10), also called gankyrin, was originally cloned as a component of the 26S proteasome [29, 30]. In particular, PSMD10 binds to the S6 ATPase of the proteasome, yet this interaction seems to be not stable and suggests that PSMD10 may interact with other protein complexes aside from the proteasome [31]. The yeast ortholog of PSMD10 is not required for cell growth and viability, implying that mammalian PSMD10 might not be an essential protein [30, 31]. Notably, PSMD10 is an oncoprotein and appears to play an important role in liver cancer. It is overexpressed in hepatocellular carcinomas, predicts a poor outcome and is required for efficient liver cancer cell growth and invasion in vitro as well as tumorigenicity and metastasis in vivo [32–34]. However, the role of PSMD10 in prostate cancer has remained unexplored. In this report, we describe that PSMD10 is one downstream effector of the histone demethylase JMJD2A, thereby implicating PSMD10 as a potential promoter of prostate tumorigenesis.
Materials and methods
Knockdown experiments
All shRNAs were cloned into pSIREN-RetroQ (Clontech) and targeted the following sequences: PSMD10 #1, 5′-TCGAATAACTGTTGAGATT-3′; PSMD10 #2, 5′-GAGAATGGTGGAAGGTTAA-3′; YAP1, 5′-AGTAATAGTTGGTTGTGAA-3′; JMJD2A #3, 5′-GTTGAGGATGGTCTTACCT-3′; JMJD2A #5, 5′-GGACTTAGCTTCATAACTA-3′. ETV1 shRNA #1 and shRNA #5 were described before [35]. The resulting retroviral vectors were cotransfected with two packaging plasmids encoding VSVG and Gag-Pol into human 293T cells, which were grown in DMEM media supplemented with 10% fetal bovine serum [36], by the calcium phosphate coprecipitation method [37, 38]. Resulting virus was harvested as described before [39]. Then, LNCaP cells were infected with retrovirus for two or three times and selected by incubation with 1 μg/ml puromycin [40].
RT-PCR
Total RNA was isolated from LNCaP cells with Trizol (Invitrogen) and reverse transcribed utilizing pd(N)6 random primers. Then, PSMD10 mRNA levels were quantitated using iQ SYBR Green Supermix (BioRad) and employing a real-time PCR machine. PSMD10 mRNA levels were normalized to those of GAPDH by the comparative cycle time method [41]. Primers specific for PSMD10 were 5′-GGCCGATAAATCCCTGGCTA-3′ and 5′-CAGGCTAAGTGTAGAGGAGTG-3′ (yielding a 459 bp product), while primers for amplifying a 226 bp GAPDH cDNA fragment were 5′-GAGCCACATCGCTCAGACACC-3′ and 5′-TGACAAGCTTCCCGTTCTCAGC-3′.
Western blotting
Protein extracts were run on SDS polyacrylamide gels [42], proteins transferred to PVDF membrane (Millipore) and then challenged with antibodies [43]. Next, secondary antibodies coupled to horseradish peroxidase were employed for incubation of the membranes [44] and signals detected with enhanced chemiluminescence and exposure to film [45].
Immunohistochemistry
A human tissue microarray encompassing 31 matching normal and cancerous prostate tissue cores (AccuMax A302IV, slide #139) was treated for 20 min with Bond Epitope Retrieval Solution I and then stained with a Leica Bond-III apparatus. Mouse monoclonal PSMD10 antibody was from Santa Cruz Biotechnology (gankyrin 3A6C2, sc-101498) and employed at a dilution of 1:100. Staining intensity was scored on a scale of 0–3, while a score of 1–4 was employed to determine the percentage of stained cells. The final staining index was calculated as the product of these two scores. Since the tissue microarray contained two cores for tumor tissue, but only one core for normal tissue, the staining index for tumor tissue was defined as the average from each pair of tumor cores. For staining of mouse tissue derived from syngeneic wild-type and JMJD2A transgenic mice [15], 40 min of treatment with Bond Epitope Retrieval Solution I was followed by staining with rabbit polyclonal PSMD10 antibodies (Santa Cruz Biotechnology, gankyrin H-231, sc-8991) at a 1:100 dilution.
Cell growth assay
LNCaP cells stably expressing shRNA or HA-tagged PSMD10 (or empty vector pQCXIH) were seeded into 96-wells at a density of 3000 cells per well [46]. Then, cells were grown for the indicated number of days, after which growth was determined with the PrestoBlue cell viability kit (Invitrogen) by measuring fluorescence at 590 nm.
Statistical analysis
Statistical tests are described in the respective figure legends. R values are Pearson correlation coefficients. A P value of less than 0.05 was considered statistically significant.
Results
Stimulation of PSMD10 expression by JMJD2A and ETV1
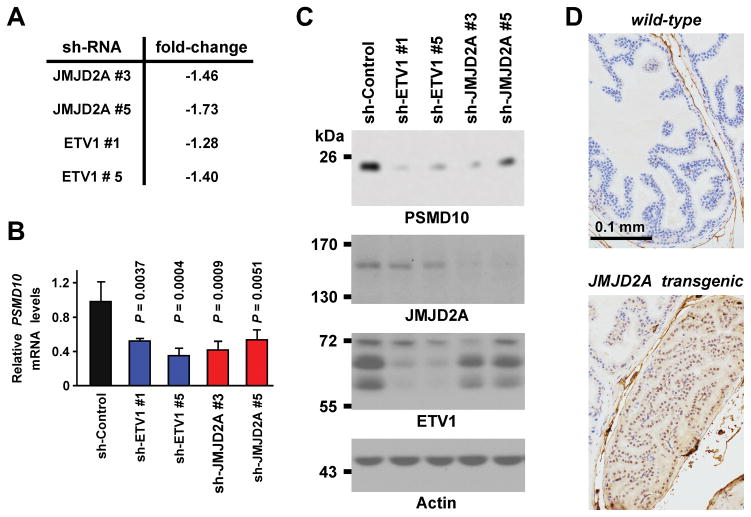
To understand the physiological role of the histone demethylase JMJD2A and its interaction partner ETV1, we previously performed mRNA microarray experiments with LNCaP prostate cancer cells in order to detect genes commonly regulated by JMJD2A and ETV1 [15]. Analysis of these microarray data revealed that among the 256 genes becoming downregulated by >1.4-fold upon JMJD2A shRNA expression was PSMD10, yet PSMD10 did not meet the criterion of >1.4-fold downregulation with ETV1 shRNA (Figure 1A). However, when we performed quantitative RT-PCR to validate the microarray data, we found that both ETV1 and JMJD2A shRNAs led to more than 1.8-fold downregulation of PSMD10 mRNA levels (Figure 1B), indicating that the microarray data underestimated the degree of PSMD10 regulation by JMJD2A and ETV1. Altogether, these data suggest that PSMD10 transcription is regulated by both JMJD2A and ETV1.
Figure 1.
PSMD10 as a target of JMJD2A. A: Analysis of published microarray experiments (Gene Expression Omnibus GSE47750) with LNCaP prostate cancer cells. Shown are changes (compared to control shRNA) of PSMD10 mRNA levels upon downregulation of JMJD2A or ETV1. B: Quantitative RT-PCR results for PSMD10 mRNA after downregulation of JMJD2A or ETV1 in LNCaP cells. Shown are averages (n = 3) with standard deviations. Statistical significance was determined by one-way ANOVA with Bonferroni correction. C: Corresponding western blots. D: Immunohistochemical staining for PSMD10 in the prostate from a wild-type or JMJD2A transgenic mouse.
Since mRNA levels are not always indicative of protein levels, we explored if PSMD10 protein levels were also reduced upon either JMJD2A or ETV1 downregulation in LNCaP cells. We indeed observed that this was the case (Figure 1C). In addition, we performed immunohistochemistry on prostates from transgenic mice that prostate-specifically overexpressed JMJD2A [15] and found robustly enhanced PSMD10 protein expression in JMJD2A transgenic compared to syngeneic wild-type mice (Figure 1D). This supports the notion that JMJD2A overexpression results in a concomitant increase of PSMD10 protein levels.
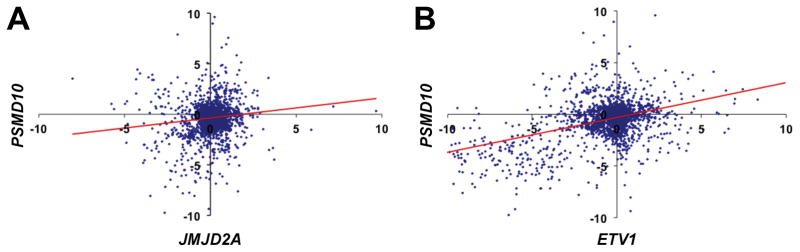
We then interrogated PSMD10 expression in a collection of 3949 mRNA microarrays representing a multitude of different tissues and experimental conditions [47]. In this huge dataset, we observed a strong positive correlation between JMJD2A and PSMD10 mRNA expression levels (Figure 2A). An even stronger correlation existed between ETV1 and PSMD10 (Figure 2B). Collectively, these data indicate that PSMD10 gene transcription is regulated by JMJD2A and ETV1 not only in the prostate but rather globally.
Figure 2.
Global correlation between PSMD10 and either JMJD2A or ETV1 mRNA expression. A: PSMD10 versus JMJD2A mRNA levels. R = 0.12, P = 2.3x10−9. Trendline is indicated in red color. B: Likewise for PSMD10 and ETV1. R = 0.42, P = 10−110.
PSMD10 overexpression in human prostate tumors
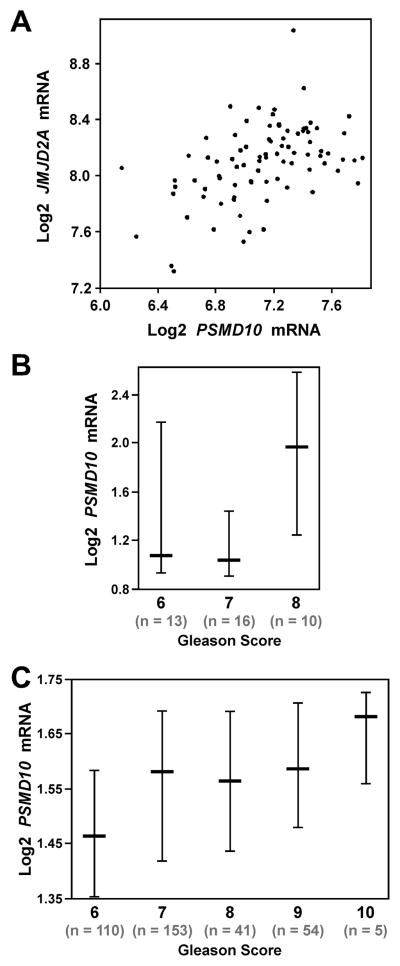
Since both JMJD2A and ETV1 are highly implicated in prostate tumorigenesis, we next analyzed the expression of PSMD10 in human prostate tumors. First, we employed published microarray data [48] and found that PSMD10 mRNA levels correlated with those of JMJD2A in human prostate tumors (Figure 3A), further supporting the notion that JMJD2A stimulates PSMD10 expression. Possibly due to the fact that ETV1 is only overexpressed in 5–10% of prostate tumors [24, 25], we did not detect any significant correlation between ETV1 and PSMD10 in this microarray data set because the number of samples (n = 85) might be too low. Analysis of other published microarray data [49, 50] indicated that PSMD10 mRNA levels correlate with the Gleason score (Figure 3B and 3C); please note that a Gleason score of 6 or less implies a good prognosis, while Gleason scores above 6 are associated with progressively poorer outcome. This suggests that PSMD10 expression increases with the aggressiveness of prostate cancer and may thus contribute to the transition from indolent to advanced disease.
Figure 3.
PSMD10 mRNA levels in human prostate cancer. A: Analysis of published microarray data [48] with cBioPortal (www.cbioportal.org) showing a correlation between JMJD2A and PSMD10 mRNA levels (n = 85). R = 0.47, P = 2.9x10−6. B: Increased PSMD10 mRNA levels in Gleason score 8 prostate tumors; P = 0.027 (Student’s t-test). Shown are log2-transformed mRNA levels (median and respective 25–75 percentile range). Data were derived from published microarray data [49] and analyzed with Oncomine (www.oncomine.org). C: Analogous; P = 4.5x10−6 (Student’s t-test). Data were derived from published microarray data [50].
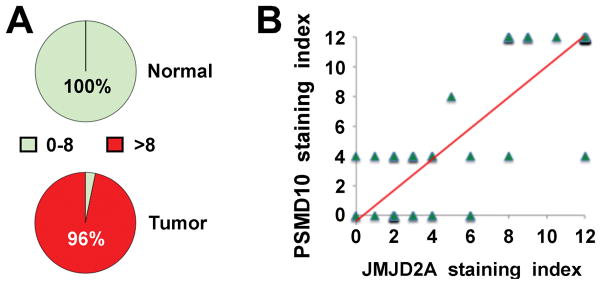
To complement the bioinformatics analysis, we also stained a human tissue microarray containing matching normal and cancerous prostate tissues. We found that PSMD10 protein expression was highly overexpressed in the vast majority of human prostate tumors (Figure 4A). Moreover, since we previously stained the same tissues with JMJD2A antibodies [15], we were able to correlate PSMD10 with JMJD2A staining. A highly significant correlation between JMJD2A and PSMD10 protein levels was thereby uncovered (Figure 4B), which further corroborates that PSMD10 expression is stimulated by JMJD2A. Unfortunately, we could not find a suitable ETV1 antibody for immunohistochemistry, therefore not allowing us to assess if likewise ETV1 and PSMD10 protein levels might be correlated in prostate tissue.
Figure 4.
Overexpression of PSMD10 in human prostate tumors. A: Nuclear PSMD10 staining in 31 matching normal prostates and tumors was graded on a scale of 0–12. Staining was discriminated between grades 0–8 and >8; P = 1.4x10−16 (two-tailed Fisher’s exact probability test). B: Correlation between nuclear JMJD2A and PSMD10 staining across the 31 matching normal and cancerous prostate specimens. R = 0.87, P = 5.1x10−21. Trendline is indicated in red color.
PSMD10 as a cell growth regulator
To determine if and how PSMD10 affects prostate cancer cells, we downregulated PSMD10 with two different shRNAs in LNCaP cells. Growth of these cells was then assessed and found to be significantly reduced after six days (Figure 5A). We also overexpressed PSMD10 in LNCaP cells, yet this did not result into any change of cell growth (Figure 5B), possibly because endogenous PSMD10 amounts were already at saturating levels. Regardless, our experiments demonstrate that PSMD10 is a positive regulator of growth in LNCaP prostate cancer cells, which is consistent with its original identification as an oncoprotein [32].
Figure 5.
Role of PSMD10 in cell proliferation. A: Downregulation of PSMD10 with two different shRNAs in LNCaP prostate cancer cells reduces their growth. Shown are averages (n = 3) with standard deviations. *, P < 0.0001 (one-way ANOVA with Bonferroni correction). Corresponding western blots are shown on the top. B: Analogous, overexpression of HA-tagged PSMD10 in LNCaP cells. Arrow points at the endogenous PSMD10 protein, which runs at a slightly lower apparent molecular weight than the ectopic HA-tagged PSMD10.
Cooperation between PSMD10 and YAP1
We previously identified the YAP1 gene, which encodes for a transcriptional cofactor in the Hippo signaling pathway [51], as a target of the JMJD2A-ETV1 complex [15]. Interestingly, Ingenuity pathway analysis of our published microarray data [15] revealed that PSMD10 and YAP1 participate in a common network of JMJD2A-regulated genes in LNCaP prostate cancer cells (Figure 6A). This prompted us to study if PSMD10 and YAP1 are coregulated on a global scale. And indeed, there was a significant correlation between PSMD10 and YAP1 mRNA levels across 3949 microarray data sets (Figure 6B).
Figure 6.
Relationship between PSMD10 and YAP1. A: Ingenuity pathway analysis revealing a gene network containing both YAP1 and PSMD10 upon JMJD2A downregulation in LNCaP cells. B: Global coexpression of PSMD10 and YAP1 mRNA. R = 0.44, P = 4.6x10−114. Trendline is indicated in red color. C: Joint downregulation of YAP1 and PSMD10 in LNCaP prostate cancer cells. Shown are indicated western blots. D: Corresponding cell growth assay. Averages (n = 3) and standard deviations are depicted. *, P < 0.0001 (one-way ANOVA with Bonferroni correction).
We then asked the question if this coregulation of PSMD10 and YAP1 might be biologically relevant. To this end, we downregulated either PSMD10 or YAP1 alone in LNCaP prostate cancer cells, or both together. Efficient reduction of PSMD10 and/or YAP1 protein levels was obtained with our shRNAs (Figure 6C). Then, we examined how this would affect LNCaP cell growth. As expected from published data for YAP1 [15, 52] or from the results for PSMD10 shown in Figure 5A, individual depletion of YAP1 or PSMD10 reduced prostate cancer cell growth (Figure 6D). Excitingly, joint downregulation of YAP1 and PSMD10 resulted in a significant further reduction of cell growth, indicating that YAP1 and PSMD10 cooperate in facilitating LNCaP cell growth.
Discussion
In this report, we provide evidence that PSMD10 expression is regulated by the JMJD2A histone demethylase, which likely does so in conjunction with the ETV1 transcription factor. Furthermore, we show for the first time that PSMD10 is overexpressed in prostate tumors and its expression appears to correlate with the severity of the disease. Lastly, our data have uncovered that PSMD10 is a growth promoting protein in prostate cancer cells and may cooperate with YAP1 in this regard.
Although our findings demonstrate that JMJD2A and ETV1 can stimulate the expression of PSMD10 in LNCaP prostate cancer cells, it is unclear whether or not this involves the direct binding of these two transcription factors to the PSMD10 gene promoter and its subsequent stimulation. For instance, we cannot exclude an indirect mechanism by which JMJD2A and ETV1 upregulate expression of another transcription factor that then binds to and stimulates the PSMD10 promoter. Regardless, our discovery that JMJD2A and ETV1 expression levels are globally correlated with those of PSMD10 suggests that PSMD10 is not only an effector of JMJD2A and ETV1 in prostate cancer cells, but potentially in many other cancers. In particular, this may be relevant in breast cancer, since overexpression or dysregulation of JMJD2A and ETV1 has been reported for breast tumors [53–57] and overexpressed PSMD10 appears to promote especially the metastasis of breast cancer cells [58, 59].
Interestingly, PSMD10 is also overexpressed in human cholangiocarcinomas and a predictor for overall survival [60]. Likewise, YAP1, which is a versatile transcriptional regulator [61, 62], was reported to be overexpressed in this cancer and phenocopied PSMD10 with regard to its ability to promote tumorigenesis and metastasis [63]. Similar to our data showing a correlation between PSMD10 and YAP1 mRNA levels, there was significant coexpression of YAP1 and PSMD10 at the protein level in cholangiocarcinomas. Further, YAP1 and PSMD10 were each able to stimulate the expression of the other, which seems to occur through indirect transcriptional mechanisms [63]. However, this is different from our results in LNCaP cells, since there was no impact on the expression of the respective other protein when YAP1 or PSMD10 were downregulated (see Figure 6C). Hence, it may be that there are cell type-specific differences in how transcription of the YAP1 and PSMD10 genes are regulated.
Several mechanisms by which PSMD10 contributes to liver oncogenesis have been described. These include the activation of the CDK4 protein kinase, possibly by blocking the interaction of the cell cycle inhibitor p16 with CDK4, which leads to enhanced phosphorylation of the retinoblastoma tumor suppressor and its subsequent degradation [31, 32, 64]. Likewise, the stability of the p53 tumor suppressor is compromised by PSMD10, since PSMD10 binds to the MDM2 ubiquitin ligase and thereby increases MDM2-mediated ubiquitylation of p53 [65]. Further, PSMD10 facilitates the degradation of the transcription factor C/EBPα when it is phosphorylated on S193, which promotes carcinogen-induced liver tumorigenesis [66]. On the other hand, PSMD10 protects OCT4, a transcription factor playing important roles in stem cell maintenance, from degradation by sequestering WWP2, an E3 ligase normally targeting OCT4 for proteasomal destruction. The consequence is an expansion of tumor-initiating cells in the liver, which is predicted to aggravate hepatocarcinogenesis [67]. It remains to be studied if these or other mechanisms pertain to PSMD10’s function as a growth promoter in prostate cancer cells.
Despite the fact that PSMD10 is an established oncoprotein, there are currently no drugs available to inhibit its activity. Our study implicates that alternative routes of reducing PSMD10 activity should be considered, namely the inhibition of either JMJD2A or ETV1 since that would reduce PSMD10 expression. In fact, multiple small molecule drugs have been developed to inhibit the enzymatic activity of the histone demethylase JMJD2A, but their utility has not yet been tested in the clinic [68–75]. Similarly, some progress has been made to develop inhibitors of the ETV1 transcription factors [76, 77]. Another possibility of counteracting PSMD10 overexpression may entail targeting the YAP1 protein, since YAP1 might be stimulating PSMD10 transcription in some cell types and/or the co-overexpressed YAP1 protein could aggravate the oncogenic impact of PSMD10. Similar to JMJD2A and ETV1, potential inhibitors of YAP1 were discovered, but have not yet been clinically tested for cancer therapy [78, 79].
In conclusion, we have identified PSMD10 as a downstream effector of the histone demethylase JMJD2A and the DNA-binding ETV1 protein. Hence, together with YAP1, which is likewise regulated by these two oncogenic transcription factors, PSMD10 overexpression may contribute to tumor formation in the prostate and other organs.
Acknowledgments
We thank the Stephenson Cancer Center Biospecimen and Tissue Pathology Core, which has been supported by a grant from the National Institute of General Medical Sciences (P20 GM103639), for performing immunohistochemistry. This work was also supported by grant R01 CA154745 from the National Cancer Institute. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Footnotes
Disclosure of conflict of interest
Ralf Janknecht is a scientific advisor for Chrysalis Therapeutics. The other authors have declared that no conflict of interest exists.
References
- 1.Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 3.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol. 2012;13:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 5.Katoh M, Katoh M. Identification and characterization of JMJD2 family genes in silico. Int J Oncol. 2004;24:1623–1628. [PubMed] [Google Scholar]
- 6.Berry WL, Janknecht R. KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res. 2013;73:2936–2942. doi: 10.1158/0008-5472.CAN-12-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labbe RM, Holowatyj A, Yang ZQ. Histone lysine demethylase (KDM) subfamily 4: structures, functions and therapeutic potential. Am J Transl Res. 2013;6:1–15. [PMC free article] [PubMed] [Google Scholar]
- 8.Gray SG, Iglesias AH, Lizcano F, Villanueva R, Camelo S, Jingu H, Teh BT, Koibuchi N, Chin WW, Kokkotou E, Dangond F. Functional characterization of JMJD2A, a histone deacetylase- and retinoblastoma-binding protein. J Biol Chem. 2005;280:28507–28518. doi: 10.1074/jbc.M413687200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Yoon HG, Wong J. JMJD2A is a novel N-CoR-interacting protein and is involved in repression of the human transcription factor achaete scute-like homologue 2 (ASCL2/Hash2) Mol Cell Biol. 2005;25:6404–6414. doi: 10.1128/MCB.25.15.6404-6414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- 12.Shin S, Janknecht R. Diversity within the JMJD2 histone demethylase family. Biochem Biophys Res Commun. 2007;353:973–977. doi: 10.1016/j.bbrc.2006.12.147. [DOI] [PubMed] [Google Scholar]
- 13.Trojer P, Zhang J, Yonezawa M, Schmidt A, Zheng H, Jenuwein T, Reinberg D. Dynamic Histone H1 Isotype 4 Methylation and Demethylation by Histone Lysine Methyltransferase G9a/KMT1C and the Jumonji Domain-containing JMJD2/KDM4 Proteins. J Biol Chem. 2009;284:8395–8405. doi: 10.1074/jbc.M807818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 15.Kim TD, Jin F, Shin S, Oh S, Lightfoot SA, Grande JP, Johnson AJ, van Deursen JM, Wren JD, Janknecht R. Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. J Clin Invest. 2016;126:706–720. doi: 10.1172/JCI78132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin S, Janknecht R. Activation of androgen receptor by histone demethylases JMJD2A and JMJD2D. Biochem Biophys Res Commun. 2007;359:742–746. doi: 10.1016/j.bbrc.2007.05.179. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 18.Brown TA, McKnight SL. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992;6:2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- 19.Janknecht R. Analysis of the ERK-stimulated ETS transcription factor ER81. Mol Cell Biol. 1996;16:1550–1556. doi: 10.1128/mcb.16.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosc DG, Goueli BS, Janknecht R. HER2/Neu-mediated activation of the ETS transcription factor ER81 and its target gene MMP-1. Oncogene. 2001;20:6215–6224. doi: 10.1038/sj.onc.1204820. [DOI] [PubMed] [Google Scholar]
- 21.Goel A, Janknecht R. Acetylation-mediated transcriptional activation of the ETS protein ER81 by p300, P/CAF, and HER2/Neu. Mol Cell Biol. 2003;23:6243–6254. doi: 10.1128/MCB.23.17.6243-6254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goueli BS, Janknecht R. Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol Cell Biol. 2004;24:25–35. doi: 10.1128/MCB.24.1.25-35.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel A, Janknecht R. Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J Biol Chem. 2004;279:14909–14916. doi: 10.1074/jbc.M400036200. [DOI] [PubMed] [Google Scholar]
- 24.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 25.Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6:429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- 26.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 27.Shin S, Kim TD, Jin F, van Deursen JM, Dehm SM, Tindall DJ, Grande JP, Munz JM, Vasmatzis G, Janknecht R. Induction of prostatic intraepithelial neoplasia and modulation of androgen receptor by ETS variant 1/ETS-related protein 81. Cancer Res. 2009;69:8102–8110. doi: 10.1158/0008-5472.CAN-09-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baena E, Shao Z, Linn DE, Glass K, Hamblen MJ, Fujiwara Y, Kim J, Nguyen M, Zhang X, Godinho FJ, Bronson RT, Mucci LA, Loda M, Yuan GC, Orkin SH, Li Z. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev. 2013;27:683–698. doi: 10.1101/gad.211011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson S, Hastings R, Takayanagi K, Reynolds S, Low P, Billett M, Mayer RJ. The 26S-proteasome: regulation and substrate recognition. Mol Biol Rep. 1997;24:39–44. doi: 10.1023/a:1006800522814. [DOI] [PubMed] [Google Scholar]
- 30.Hori T, Kato S, Saeki M, DeMartino GN, Slaughter CA, Takeuchi J, Toh-e A, Tanaka K. cDNA cloning and functional analysis of p28 (Nas6p) and p40. 5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene. 1998;216:113–122. doi: 10.1016/s0378-1119(98)00309-6. [DOI] [PubMed] [Google Scholar]
- 31.Dawson S, Apcher S, Mee M, Higashitsuji H, Baker R, Uhle S, Dubiel W, Fujita J, Mayer RJ. Gankyrin is an ankyrin-repeat oncoprotein that interacts with CDK4 kinase and the S6 ATPase of the 26 S proteasome. J Biol Chem. 2002;277:10893–10902. doi: 10.1074/jbc.M107313200. [DOI] [PubMed] [Google Scholar]
- 32.Higashitsuji H, Itoh K, Nagao T, Dawson S, Nonoguchi K, Kido T, Mayer RJ, Arii S, Fujita J. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med. 2000;6:96–99. doi: 10.1038/71600. [DOI] [PubMed] [Google Scholar]
- 33.Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang W, Ren YB, Su B, Cao GW, Yang Y, Yan YQ, Shen F, Wu MC, Feng GS, Wang HY. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology. 2011;53:181–192. doi: 10.1002/hep.24015. [DOI] [PubMed] [Google Scholar]
- 34.Jing H, Zhang G, Meng L, Meng Q, Mo H, Tai Y. Gradually elevated expression of Gankyrin during human hepatocarcinogenesis and its clinicopathological significance. Sci Rep. 2014;4:5503. doi: 10.1038/srep05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh S, Shin S, Lightfoot SA, Janknecht R. 14–3-3 proteins modulate the ETS transcription factor ETV1 in prostate cancer. Cancer Res. 2013;73:5110–5119. doi: 10.1158/0008-5472.CAN-13-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowdy SC, Mariani A, Janknecht R. HER2/Neu- and TAK1-mediated up-regulation of the transforming growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol Chem. 2003;278:44377–44384. doi: 10.1074/jbc.M307202200. [DOI] [PubMed] [Google Scholar]
- 37.Goueli BS, Janknecht R. Regulation of telomerase reverse transcriptase gene activity by upstream stimulatory factor. Oncogene. 2003;22:8042–8047. doi: 10.1038/sj.onc.1206847. [DOI] [PubMed] [Google Scholar]
- 38.Janknecht R. Regulation of the ER81 transcription factor and its coactivators by mitogen- and stress-activated protein kinase 1 (MSK1) Oncogene. 2003;22:746–755. doi: 10.1038/sj.onc.1206185. [DOI] [PubMed] [Google Scholar]
- 39.Berry WL, Kim TD, Janknecht R. Stimulation of beta-catenin and colon cancer cell growth by the KDM4B histone demethylase. Int J Oncol. 2014;44:1341–1348. doi: 10.3892/ijo.2014.2279. [DOI] [PubMed] [Google Scholar]
- 40.Kim TD, Oh S, Shin S, Janknecht R. Regulation of tumor suppressor p53 and HCT116 cell physiology by histone demethylase JMJD2D/KDM4D. PLoS One. 2012;7:e34618. doi: 10.1371/journal.pone.0034618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiTacchio L, Bowles J, Shin S, Lim DS, Koopman P, Janknecht R. Transcription factors ER71/ETV2 and SOX9 participate in a positive feedback loop in fetal and adult mouse testis. J Biol Chem. 2012;287:23657–23666. doi: 10.1074/jbc.M111.320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mooney SM, Goel A, D’Assoro AB, Salisbury JL, Janknecht R. Pleiotropic effects of p300-mediated acetylation on p68 and p72 RNA helicase. J Biol Chem. 2010;285:30443–30452. doi: 10.1074/jbc.M110.143792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papoutsopoulou S, Janknecht R. Phosphorylation of ETS transcription factor ER81 in a complex with its coactivators CREB-binding protein and p300. Mol Cell Biol. 2000;20:7300–7310. doi: 10.1128/mcb.20.19.7300-7310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knebel J, De Haro L, Janknecht R. Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J Cell Biochem. 2006;99:319–329. doi: 10.1002/jcb.20945. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Janknecht R. Regulation of the ETS transcription factor ER81 by the 90-kDa ribosomal S6 kinase 1 and protein kinase A. J Biol Chem. 2002;277:42669–42679. doi: 10.1074/jbc.M205501200. [DOI] [PubMed] [Google Scholar]
- 46.Kim TD, Shin S, Berry WL, Oh S, Janknecht R. The JMJD2A demethylase regulates apoptosis and proliferation in colon cancer cells. J Cell Biochem. 2012;113:1368–1376. doi: 10.1002/jcb.24009. [DOI] [PubMed] [Google Scholar]
- 47.Wren JD. A global meta-analysis of microarray expression data to predict unknown gene functions and estimate the literature-data divide. Bioinformatics. 2009;25:1694–1701. doi: 10.1093/bioinformatics/btp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu P, Ramachandran S, Ali Seyed M, Scharer CD, Laycock N, Dalton WB, Williams H, Karanam S, Datta MW, Jaye DL, Moreno CS. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006;66:4011–4019. doi: 10.1158/0008-5472.CAN-05-3055. [DOI] [PubMed] [Google Scholar]
- 50.Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, Sboner A, Pawitan Y, Andren O, Johnson LA, Tang J, Adami HO, Calza S, Chinnaiyan AM, Rhodes D, Tomlins S, Fall K, Mucci LA, Kantoff PW, Stampfer MJ, Andersson SO, Varenhorst E, Johansson JE, Brown M, Golub TR, Rubin MA. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst. 2008;100:815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM, Fu K, Datta K, Palermo N, Chen Y, Dong J. The Hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015;35:1350–1362. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patani N, Jiang WG, Newbold RF, Mokbel K. Histone-modifier gene expression profiles are associated with pathological and clinical outcomes in human breast cancer. Anticancer Res. 2011;31:4115–4125. [PubMed] [Google Scholar]
- 54.Berry WL, Shin S, Lightfoot SA, Janknecht R. Oncogenic features of the JMJD2A histone demethylase in breast cancer. Int J Oncol. 2012;41:1701–1706. doi: 10.3892/ijo.2012.1618. [DOI] [PubMed] [Google Scholar]
- 55.Slee RB, Steiner CM, Herbert BS, Vance GH, Hickey RJ, Schwarz T, Christan S, Radovich M, Schneider BP, Schindelhauer D, Grimes BR. Cancer-associated alteration of pericentromeric heterochromatin may contribute to chromosome instability. Oncogene. 2012;31:3244–3253. doi: 10.1038/onc.2011.502. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Wang L, Chen Y, Li L, Yang X, Li B, Song S, Yang L, Hao Y, Yang J. ER81 expression in breast cancers and hyperplasia. Pathology Res Int. 2011;2011:980513. doi: 10.4061/2011/980513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh S, Shin S, Janknecht R. ETV1, 4 and 5: An oncogenic subfamily of ETS transcription factors. Biochim Biophys Acta. 2012;1826:1–12. doi: 10.1016/j.bbcan.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhen C, Chen L, Zhao Q, Liang B, Gu YX, Bai ZF, Wang K, Xu X, Han QY, Fang DF, Wang SX, Zhou T, Xia Q, Gong WL, Wang N, Li HY, Jin BF, Man JH. Gankyrin promotes breast cancer cell metastasis by regulating Rac1 activity. Oncogene. 2013;32:3452–3460. doi: 10.1038/onc.2012.356. [DOI] [PubMed] [Google Scholar]
- 59.Gao L, Xie H, Dong L, Zou J, Fu J, Gao X, Ou L, Xiang S, Song H. Gankyrin is essential for hypoxia enhanced metastatic potential in breast cancer cells. Mol Med Rep. 2014;9:1032–1036. doi: 10.3892/mmr.2013.1860. [DOI] [PubMed] [Google Scholar]
- 60.Zheng T, Hong X, Wang J, Pei T, Liang Y, Yin D, Song R, Song X, Lu Z, Qi S, Liu J, Sun B, Xie C, Pan S, Li Y, Luo X, Li S, Fang X, Bhatta N, Jiang H, Liu L. Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology. 2014;59:935–946. doi: 10.1002/hep.26705. [DOI] [PubMed] [Google Scholar]
- 61.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H, Sun B, Yin D, Sun J, Song R, Pan S, Sun Y, Jiang H, Zheng T, Liu L. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015;6:17206–17220. doi: 10.18632/oncotarget.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahajan A, Guo Y, Yuan CH, Weghorst CM, Tsai MD, Li JN. Dissection of protein-protein interaction and CDK4 inhibition in the oncogenic versus tumor suppressing functions of gankyrin and P16. J Mol Biol. 2007;373:990–1005. doi: 10.1016/j.jmb.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higashitsuji H, Itoh K, Sakurai T, Nagao T, Sumitomo Y, Masuda T, Dawson S, Shimada Y, Mayer RJ, Fujita J. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell. 2005;8:75–87. doi: 10.1016/j.ccr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Wang GL, Shi X, Haefliger S, Jin J, Major A, Iakova P, Finegold M, Timchenko NA. Elimination of C/EBPalpha through the ubiquitin-proteasome system promotes the development of liver cancer in mice. J Clin Invest. 2010;120:2549–2562. doi: 10.1172/JCI41933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian YW, Chen Y, Yang W, Fu J, Cao J, Ren YB, Zhu JJ, Su B, Luo T, Zhao XF, Dai RY, Li JJ, Sun W, Wu MC, Feng GS, Wang HY. p28(GANK) prevents degradation of Oct4 and promotes expansion of tumor-initiating cells in hepatocarcinogenesis. Gastroenterology. 2012;142:1547–1558. doi: 10.1053/j.gastro.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 68.Hamada S, Kim TD, Suzuki T, Itoh Y, Tsumoto H, Nakagawa H, Janknecht R, Miyata N. Synthesis and activity of N-oxalylglycine and its derivatives as Jumonji C-domain-containing histone lysine demethylase inhibitors. Bioorg Med Chem Lett. 2009;19:2852–2855. doi: 10.1016/j.bmcl.2009.03.098. [DOI] [PubMed] [Google Scholar]
- 69.Hamada S, Suzuki T, Mino K, Koseki K, Oehme F, Flamme I, Ozasa H, Itoh Y, Ogasawara D, Komaarashi H, Kato A, Tsumoto H, Nakagawa H, Hasegawa M, Sasaki R, Mizukami T, Miyata N. Design, synthesis, enzyme-inhibitory activity, and effect on human cancer cells of a novel series of jumonji domain-containing protein 2 histone demethylase inhibitors. J Med Chem. 2010;53:5629–5638. doi: 10.1021/jm1003655. [DOI] [PubMed] [Google Scholar]
- 70.Rose NR, Woon EC, Kingham GL, King ON, Mecinovic J, Clifton IJ, Ng SS, Talib-Hardy J, Oppermann U, McDonough MA, Schofield CJ. Selective inhibitors of the JMJD2 histone demethylases: combined nondenaturing mass spectrometric screening and crystallographic approaches. J Med Chem. 2010;53:1810–1818. doi: 10.1021/jm901680b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo X, Liu Y, Kubicek S, Myllyharju J, Tumber A, Ng S, Che KH, Podoll J, Heightman TD, Oppermann U, Schreiber SL, Wang X. A selective inhibitor and probe of the cellular functions of Jumonji C domain-containing histone demethylases. J Am Chem Soc. 2011;133:9451–9456. doi: 10.1021/ja201597b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Chang J, Varghese D, Dellinger M, Kumar S, Best AM, Ruiz J, Bruick R, Pena-Llopis S, Xu J, Babinski DJ, Frantz DE, Brekken RA, Quinn AM, Simeonov A, Easmon J, Martinez ED. A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat Commun. 2013;4:2035. doi: 10.1038/ncomms3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim TD, Fuchs JR, Schwartz E, Abdelhamid D, Etter J, Berry WL, Li C, Ihnat MA, Li PK, Janknecht R. Pro-growth role of the JMJD2C histone demethylase in HCT-116 colon cancer cells and identification of curcuminoids as JMJD2 inhibitors. Am J Transl Res. 2014;6:236–247. [PMC free article] [PubMed] [Google Scholar]
- 74.Feng T, Li D, Wang H, Zhuang J, Liu F, Bao Q, Lei Y, Chen W, Zhang X, Xu X, Sun H, You Q, Guo X. Novel 5-carboxy-8-HQ based histone demethylase JMJD2A inhibitors: Introduction of an additional carboxyl group at the C-2 position of quinoline. Eur J Med Chem. 2015;105:145–155. doi: 10.1016/j.ejmech.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Ruger N, Roatsch M, Emmrich T, Franz H, Schule R, Jung M, Link A. Tetrazolylhydrazides as selective fragment-like inhibitors of the JumonjiC-domain-containing histone demethylase KDM4A. ChemMedChem. 2015;10:1875–1883. doi: 10.1002/cmdc.201500335. [DOI] [PubMed] [Google Scholar]
- 76.Rahim S, Minas T, Hong SH, Justvig S, Celik H, Kont YS, Han J, Kallarakal AT, Kong Y, Rudek MA, Brown ML, Kallakury B, Toretsky JA, Uren A. A small molecule inhibitor of ETV1, YK-4–279, prevents prostate cancer growth and metastasis in a mouse xenograft model. PLoS One. 2014;9:e114260. doi: 10.1371/journal.pone.0114260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pop MS, Stransky N, Garvie CW, Theurillat JP, Hartman EC, Lewis TA, Zhong C, Culyba EK, Lin F, Daniels DS, Pagliarini R, Ronco L, Koehler AN, Garraway LA. A small molecule that binds and inhibits the ETV1 transcription factor oncoprotein. Mol Cancer Ther. 2014;13:1492–1502. doi: 10.1158/1535-7163.MCT-13-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, Wang X, Guo T, Li P, Zhao Y, Ji H, Zhang L, Zhou Z. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]