Abstract
Acute effects of individual and complex mixtures of polycyclic aromatic hydrocarbons (PAHs) are well documented in vertebrate species. Hypoxia in fish reduces metabolic rate and reproduction. However, less is known about the later life consequences stemming from early-life exposure to PAHs or hypoxia, particularly their co-exposure. To address this, medaka (Oryzias latipes) embryos were exposed to a complex PAH mixture sediment extract from the Elizabeth River, VA (ERSE) at concentrations of 0.1, 0.5, or 1.0% or to one of three different hypoxia scenarios: continuous, nocturnal, or late stage embryogenesis hypoxia. Co-exposures with 0.1% ERSE and each of the hypoxia scenarios were conducted. Results included decreased survival with ERSE, hatching delays with hypoxia, and higher occurrences of deformities with each. The continuous hypoxia scenario caused the most significant changes in all endpoints. These early-life exposures altered later-life growth, impaired reproductive capacity, and reduced the quality of their offspring. ERSE alone resulted in a female-biased sex ratio while continuous or nocturnal hypoxia produced significantly greater numbers of males; and co-exposure produced an equal sex ratio. Exposure to a PAH mixture and hypoxia during early life stages has meaningful later-life and next generational consequences.
Keywords: PAH mixture, hypoxia, co-exposure, later-life consequences, growth, reproductive success
1. Introduction
Because polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants (Latimer and Zheng, 2003) and hypoxic zones are widespread (Diaz and Rosenberg, 2008) interactions are likely especially in highly populated estuarine areas (Buchheister et al., 2013; Turner et al., 2006; Zhai et al., 2012). To date, however, studies of such interactions are few in the aquatic toxicology literature.
PAHs are components of petroleum and products of incomplete combustion of organic compounds. In the aquatic environment, they may be present as complex mixtures binding to and persisting in organic matter and sediments (Fernandes et al., 1997; Hylland, 2006; Latimer and Zheng, 2003). The latter, in particular, constitutes a major sink for PAHs (Cachot et al., 2006; Di Giulio and Clark, 2015; Yanagida et al., 2012). Numerous fish species lay their eggs on or bury them in sediments of river- or seabeds (Baumard et al., 1998; Cachot et al., 2007). When eggs are exposed directly via contaminated water or sediments, PAHs pass through the chorions, where they may be taken up by the embryo proper (Cachot et al., 2006; McElroy et al., 2006; Mu et al., 2014) and adversely affect development (Vignet et al., 2014).
A United States Environmental Protection Agency (USEPA) Superfund site, located in the Elizabeth River estuary (Portsmouth, VA, USA), has high levels of PAH contamination. The historic use of creosote, a complex mixture containing numerous PAHs, contaminated the river at the former Atlantic Wood Industries (AWI) facility and nearby wood treatment facilities (Clark et al., 2013; Walker and Dickhut, 2001). Prolonged use of creosote at this site resulted in one of the highest reported total sediment PAH concentrations, 100–500 μg/g dry sediment (Fang et al., 2014; Walker et al., 2004). This sediment was collected, porewater extracted and chemically characterized resulting in Elizabeth River Sediment Extract (ERSE) (Fang et al., 2014). Resident adult Atlantic killifish (Fundulus heteroclitus) collected near the AWI site exhibited a variety of alterations in multiple organs including liver and pancreatic neoplasms (Vogelbein et al., 1990; Vogelbein and Unger, 2006) and DNA adducts in spleen and anterior kidney (Rose et al., 2000).
Following laboratory exposure to ERSE dilutions (0.1 and 1% corresponding to 5.04 μg/L and 50.4 μg/L total PAHs, respectively), Atlantic killifish larvae had significantly altered behavior and locomotor activity (Brown et al., 2016) and adults revealed alterations in swimming performance (Brown et al., in press). In pink salmon (Oncorhynchus gorbuscha) and rainbow trout (Oncorhychus mykiss), dissolved PAHs from crude oil caused increased embryo mortality, altered time to hatch, produced spinal and jaw deformities and yolk sac edema, and later, was shown to reduce growth and impair swimming performance (Carls et al., 2008; Hawkins et al., 2002; Incardona et al., 2009; Wills et al., 2009).
In a laboratory exposure, zebrafish (Danio rerio) embryos responded to PAH-spiked sediment. Associated larvae displayed behavioral changes that extended into adulthood along with disruption of growth and reproduction (Vignet et al., 2014). Furthermore, multigenerational effects on survival and developmental deformities were seen in zebrafish larvae of parents exposed to benzo(a)pyrene (BaP) (Corrales et al., 2014). Marine medaka (Oryzias melastigma), maternally exposured to phenanthrene (PHE) had increased accumulation in adults and their eggs, enhanced heart rate and diminished hatching success in offspring (Sun et al., 2015).
Aquatic hypoxia (defined as ≤2.8 mg O2 L−1) is one of the most pressing and ubiquitous problems in marine- and freshwater. More than 400 “dead zones” are known worldwide, and climate change is expected to exacerbate this problem (Diaz and Rosenberg, 2008; John et al., 2002). Under laboratory conditions, prolonged, continuous hypoxia (1.7–2.0 mg O2 L−1, 10–15 wks) depressed reproduction in Altantic croaker (Micropogonias undulatus) (Thomas and Rahman, 2011; Thomas et al., 2006) and Gulf killifish (Fundulus grandis) (1.34 mg O2 L−1, 30 d) (Landry et al., 2007). And, recently, such impairments were shown to extend from F0 to F1 and F2 generations in marine medaka (1.4 mg O2 L−1, 3 mo) (Wang et al., 2016).
Hypoxia in estuaries is characterized by two temporal scales, persistent and cyclic (Timmerman and Chapman, 2004). The former may develop seasonally due to water column stratification and subsequent oxygen depletion in bottom water. These conditions frequently coincide with annual recruitment and growth of benthic and pelagic fish species (Rabalais et al., 2010; Vanderplancke et al., 2015). Such a scenario impaired reproductive output and skewed sex ratios towards males in Atlantic croaker (cf. field component of Thomas and Rahman, 2011). Cyclic hypoxia may develop daily in shallow estuaries and tidal creeks due to diel cycles in the water column, sediment, photosynthesis, and respiration (Tyler et al., 2009). During summer, dissolved oxygen (DO) concentrations in tidal creeks can fall below 2 mg/L from several minutes to several hours daily (Tyler et al., 2009; Yang et al., 2013). Effects arising from hypoxia include: growth and reproductive impairments; altered gene expression; altered energy metabolism; increased resistance of offspring to hypoxia; and disruption of sex hormones (Cheek, 2011; Cheung et al., 2014; Ho and Burggren, 2012; Lai et al., 2016; Pincetich et al., 2005; Shang et al., 2006; Wu et al., 2003; Yu et al., 2015; Yu et al., 2012; Yu et al., 2008).
Because oxygen saturation requirements exist for biodegradation of PAHs, hypoxia may prolong the persistence of PAHs resulting in synergistic, antagonistic or additive effects (Boyd et al., 2005; Fleming and Di Giulio, 2011; Matson et al., 2008; Wassenberg and Di Giulio, 2004). Hypoxia should be considered in evaluations of natural waterways because of the possibility of underestimating ecotoxicity of PAHs.
The choice of Japanese medaka (Oryzias latipes) as the aquarium model fish species for this study was based on several life history characteristics. Relative to the time of hatching for zebrafish (2–3 days) (Kimmel et al., 1995), the longer time to hatch (7–10 days) of medaka (Iwamatsu, 2004; Kinoshita et al., 2009) enabled extended observations of normal and abnormal development while providing increased duration of embryo exposure (Perrichon et al., 2014). In addition, medaka reach sexual maturity at approximately 8 weeks, allowing relatively rapid trangenerational studies (Lawrence et al., 2012). While previous ERSE studies were conducted with the estuarine Atlantic killifish and zebrafish (Brown et al., 2016; Colton et al., 2014; Fang et al., 2014), medaka is a euryhaline teleost with a well-characterized role in laboratory investigations (Inoue and Takei, 2002) including PAHs (Farwell et al., 2006; Hawkins et al., 1990; Le Bihanic et al., 2014) and hypoxia (Cheung et al., 2014).
The present study evaluated early development and later-life consequences after single- or co-exposure to ERSE and/or hypoxia. For the latter, three different scenarios (continuous; nocturnal; and late stage embryogenesis) assessed effects of initiation and duration of hypoxia on development. Following early life exposure, embryo hatching rate, morphological alterations, growth, and sex ratios were measured. Reproductive assays with these adults, exposed as embryos, were used to assess offspring quality in the next generation.
2. Materials and methods
2.1 Medaka culture
Orange-red (OR) strain medaka (Oryzias latipes) were maintained under animal care and maintenance protocols approved by the Duke University Institutional Animal Care and Use Committee. Our colony has been maintained for over 20 years and used extensively in studies of development and reproduction (e.g., Chernick et al., 2016; Davis et al., 2002; Koger et al., 2000; Miller et al., 2012). Adult fish were maintained in an AHAB recirculating water system (Pentair Aquatic Habitats, Apopka, FL, USA) at 24°C under a 14:10 light:dark cycle and fed three times per day with Otohime β1 commercial dry diet (Pentair Aquatic Eco-systems, Apopka, FL, USA) and supplemented with Artemia nauplii (90% GSL stain, Pentair Aquatic Eco-systems) during the first two feedings. Chorionated embryos were collected by siphoning tanks approximately 30 minutes after morning- and afternoon feedings. Embryos were cleaned and separated by rolling them on a moistened paper towel and then disinfected using a 15-minute immersion in 0.05% methylene blue (Sigma-Aldrich, St. Louis, MO, USA) followed by thorough rinsing in MilliQ water (Millipore, Billerica, MA, USA) (Padilla et al., 2009). Embryos were staged according to Iwamatsu (2004) and transferred to vials for testing.
2.2 Sediment extraction and chemical characterization
Sediment samples were collected from the AWI Superfund site (36°48′ 27.2″N, 76°17′ 38.1″W) on the Elizabeth River, VA, USA. A representative extract was collected in the form of supernatant and fully characterized as described by Fang et al. (2014). This Elizabeth River sediment extract (ERSE) is a complex mixture of water and suspended solids constituting an environmentally accurate representation compared to whole sediment (Fang et al., 2014). Mean concentrations of 36 selected PAHs were 5.1 ± 0.4 μg/ml and a 1% dilution of ERSE corresponds to 50.45 μg/L of total PAHs.
2.3 Preliminary assays
Initial range-finding studies determined embryo mortality and developmental alterations upon ERSE exposure. Initiation at developmental stage 16 (21 hours post fertilization (hpf); late gastrula stage) (Iwamatsu, 2004) was selected because it is an early stage that provided ample time to select and divide individuals from a uniform population into treatment groups. Embryos were placed in 20 mL borosilicate scintillation vials (Wheaton, VWR International, Radnor, PA), with 5 individuals in each of 3 vials per treatment group (Oxendine et al., 2006). Each vial containing 2 mL of ERSE diluted with MilliQ water to 0% (control), 0.1%, 0.5%, 1.0%, 5.0%, or 10.0% and 18 mL headspace was placed in an incubator (Fisher Scientific, Pittsburg, PA) at 27°C and a 14:10 light:dark cycle and ERSE solutions were renewed daily. After we encountered 100% mortality at 5% and 10% (Fig. S2), concentrations of 0.1%, 0.5%, or 1.0% were selected for further testing. Preliminary assays also identified 0.1% ERSE as our selected concentration for co-exposures.
Initial hypoxia assays exposed embryos to 2.4 mg O2 L−1 (6.3% O2) or 2.8 O2 mg/L (7.4% O2), each in 1 of 3 scenarios: H1) continuous hypoxia for 24 hours per day from 21 hpf (stage 16) until 14 days post fertilization (dpf) H2) nocturnal hypoxia from 21:00–09:00 and normoxia from 09:00–21:00 from 21 hpf until 14 dpf; or H3) late stage embryogenesis, continuous hypoxia, initiated at 100 hpf (stage 32) until 14 dpf. For the latter scenario, stage 32 was chosen because it coincided with onset of formation of the swim bladder and initiation of blood circulation (Iwamatsu, 2004). In addition, we have shown several metabolic changes to be influenced by hypoxia in medaka at or near this developmental stage (Pincetich et al., 2005). All scenarios were run with 5 embryos in each of 3 replicates, and exposures were performed in a Heraeus Heracell 150 Tri-Gas Cell Culture Incubator (Thermo Scientific, Waltham, MA) with oxygen adjustment by nitrogen injection. As described by Fleming and Di Giulio (2011), initial oxygen adjustment of this incubator takes approximately 6–8 hours. Therefore, all equipment and materials were prepared in advance in preparation for rapid placement of vials in the incubator. At the same time, normoxic controls (8.0 mg O2 L−1) were maintained in a separate incubator (Fisher Scientific, USA) at 27°C. For the normoxic hours of the H2 scenario, vials were removed from the hypoxia incubator and rapidly transferred to the incubator containing controls. At the nocturnal start time, the process was reveresed, and the vials returned to the previously calibrated hypoxia incubator.
In the 2.8 mg O2 L−1 H1 scenario, survival was 83.7 ± 9.2% and hatching success was 80.1 ± 12.7% versus a survival of 69.2 ± 14.1% and hatching of 46.7 ± 30.6 % in 2.4 O2 mg/L; survival was 93.3 ± 11.5% and hatching 93.3 ± 3.85% in controls. Control embryo survival and rate of abnormalities was not significantly different from that of the breeding colony (95.63% and 3.21%, respectively). Therefore, the 2.8 mg O2 L−1 concentration was chosen for all successive tests. Control, 1.0% ERSE, and H1 (2.8 mg O2 L−1) hatched at 8–9 dpf, 7–9 dpf, and 12–14 dpf, respectively.Termination of exposures at 14 dpf provided time for hatching of individuals in the H1 scenario where delays occurred.
2.4 Experimental design:Assessment of development in F0 embryos
Stage 16 embryos were randomly distributed into vials containing solution as described in section 2.3, with 7 embryos per vial and 5 replicates per treatment (n = 35). ERSE dilutions of 0% (control), 0.1%, 0.5%, or 1.0% or 3 hypoxia (2.8 mg O2 L−1) scenarios (H1, H2, and H3) were tested. Co-exposures of 0.1% ERSE with each of the hypoxia scenarios were conducted. All embryos were observed daily for mortalities and hatching.
At 14 dpf, all larvae were transferred to clean 1X embryo rearing medium (ERM; 1g NaCl, 0.03g KCl, 0.04g CaCl2·2H2O, 0.16g MgSO4·7H2O in 1 L MilliQ water) in a Petri dish (VWR International) and the number of survivors counted. Next, 15 viable individuals were randomly selected per exposure group. For analyses, individual larvae were anesthetized in 150 mg/L tricaine methanesulfonate (MS-222) (Sigma-Aldrich) and then transferred to a 70% methyl cellulose solution to minimize tilting and maintain orientation (head directed to the left and the organism in right lateral recumbency) (Chernick et al., 2016). Altered anatomical structures were rapidly identified, counted (Chernick et al., 2016), and scored for severity (Corrales et al., 2014; Mu et al., 2014) using a Nikon SMZ150 dissecting stereomicroscope (Nikon Instruments Inc., Melville, NY, USA). These included craniofacial abnormality (Cb), pericardial (Pc) edema, swim bladder (Sb) non-inflation, and blood pooling (Bp) (Table S1, Fig. 1, Fig. S3. After imaging and scoring, larvae were allowed to recover in 1X ERM and then returned to dishes with cohorts from their original treatment group. Immediately following scoring, digital photographs were taken with a Nikon SMZ1500 stereomicroscope equipped with a Nikon DXM 1200 camera and NIS Elements 3.20.01 software (Nikon Instruments Inc.). Later, this image was used to produce areal analyses. Technical precision enabled completion of evaluation within minutes, minimizing stress for each individual.
Fig. 1.
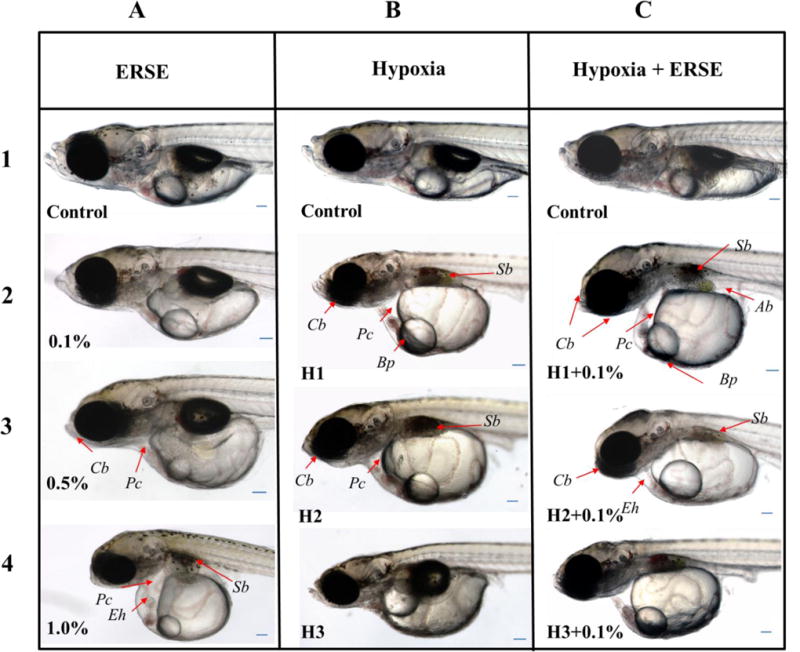
Representative examples of larval abnormalities observed in vivo at 14 dpf (n = 15). Treatment groups are in columns: (A) ERSE, (B) hypoxia, and (C) hypoxia+0.1% ERSE. Concentrations/scenarios are arranged in rows (1–4). Each image can be read as a combination of the column letter and row number (e.g., 1.0% ERSE exposed larva is A4). Arrows indicate abnormalities: pericardial edema (Pc), abdominal edema (Ab), craniofacial abnormality (Cb), swim bladder partially inflated or absent (Sb), elongated heart (Eh), blood pooling (Bb). Scale bars are 10 μm.
Data were collected to create a deformity index as previously described (Corrales et al., 2014; Mu et al., 2014). Due to the fact that multiple alterations characterized individuals, the use of an index ranked total response per individual. Scores within a given endpoint were based on presence/absence (0–1) and severity (0–3, 3 as highest severity). Pericardial edema (Pc: 0–3), craniofacial abnormality (Cb: 0–1), spinal deformity (Sd: 0–1), swim bladder (Sb, 0–3), fin abnormality (Fr: 0–1), spleen (Sc: 0–1), blood pooling (Bp: 0–1), and elongated heart (Eh, 0–1). The deformity index was calculated by dividing the total score (the sum of the severity scores for an individual) by the maximum score possible (Table S1, Table S2, and Fig. S3).
From the digital images of each individually labeled larva, the following parameters were measured: standard length (distance between the rostral margin of the snout and the caudal peduncle) and areas of pericardial cavity, swim bladder, and yolk sac (Fig. S3-A. These parameters were measured digitally and quantified using Image Pro Plus software (Fig. S3-A) (IPP 6.0, Media Cybernetics, Inc, Rockville, MD, USA). The margin of the pericardial cavity (parietal pericardium) was traced and the area of the heart plus surrounding space (cavity) determined. Swim bladder area was used as a measure of development and presence of clear space within the organ (i.e., lumen) provided evidence of insufflation (Iwamatsu, 2004) (Fig. S3-A). Degree of absorption (i.e., utilization) of the yolk was estimated by tracing yolk sac margin and normalized to standard body length (Corrales et al., 2014).
Larvae were maintained in Petri dishes and fed live Paramecium caudatum. With continued growth, fish were transferred to larger vessels and fed dry diet, initially Labo 130 (<130 μm particle size) (Kyorin, Himeji, Japan) then, upon further growth, Otohime β1 (200–360 μm particle size) supplemented with live Artemia nauplii. At 30 dpf, to avoid overcrowding, juveniles from each treatment were transferred to 3 liter tanks, in our AHAB (Pentair Aquatic Ecosystems) system, until adulthood (120 dpf). These individuals were used for the later life assessments described below. This generation is hereafter referred to as F0 (Fig. S1).
2.5 Survival, abnormality, growth, and sex ratios of F0 adult fish
At 120 dpf, adults were evaluated for percent affected individuals including body curvature, craniofacial and/or axial skeletal defects, buoyancy in water column, or any signs of erratic swimming behavior. Phenotypic sex was determined based on specific secondary sexual characteristics (Kinoshita et al., 2009); males differed from females in dorsal and anal fin morphology. Dorsal fins of presumptive males had a saw-toothed distal margin with a cleft at the caudal-most portion, and anal fins were larger and rectangular with papillary processes in caudal portion and a ragged (saw-toothed) ventral margin. Females had smooth fin margins, and neither cleft nor papillary processes. Next, a randomly selected subset of 10 fish per treatment was anesthetized with MS-222, transferred to a 100 mm diameter Petri dish containing a millimeter scale for measurement of standard body length. After completion of this assessment, fish were euthanized with an overdose of MS-222 and sex confirmed by gonadal identification at necropsy. For this, an incision was made along the ventral midline and the abdominal viscera were separated to enable their identification under the stereomicroscope with emphasis on ovaries or testes. Identification of sex by secondary sexual characteristics and internal examination matched in 100% of fish. Six fish (3 males, 3 females) from each treatment group were euthanized via rapid cooling and the caudal peduncle was removed and frozen −80°C for future analysis. The remainder of the body was fixed in GPHS fixative (0.05% glutaraldehyde, 2% paraformaldehyde, 80% Histochoice MB fixative (Sigma-Aldrich), 1% sucrose and 1% CaCl2) for future histological analysis. Any remaining fish not selected for bone staining (below) were fixed as intact carcasses using the same methods and stored in Holt’s gum sucrose solution (1% gum arabic, 30% sucrose) at 4°C for later analyses (n=226).
Twenty fish (5 each from: control, 1% ERSE, H1, and H1+0.1% ERSE) with skeletal abnormalities were selected for bone staining with Alizarin Red S (Sigma-Aldrich) by modification of published methods (Baker et al., 2013; Padilla et al., 2009; Shanthanagouda et al., 2014). Briefly, fixed specimens were rinsed in 1X PBST overnight and internal organs removed through the ventral incison. The specimens were then bleached with 3% hydrogen peroxide (Sigma-Aldrich) for 24 h, neutralized in 30% aqueous sodium borate for 12 h, and stained in a 25% (w/v) solution of Alizarin red S in ethanol overnight. Soft tissues were digested with 0.1% trypsin (Sigma-Aldrich) overnight and individuals were tranferred through a series of overnight suspensions in KOH/glycerol solutions (3:1, 1:1, 1:3). Resultant specimens were stored in 100% glycerol until digital imaging. Skeletal images were compared to those used in whole skeleton (Bird and Mabee, 2003), vertebra and caudal fin (Bensimon-Brito et al., 2010; Bensimon-Brito et al., 2012) in zebrafish as well as vertebral column in medaka (Inohaya et al., 2007). Quantification was restricted to caudal vertebral elements using ImageJ 1.46 (Rasband, 2014) and included the number of vertebral bodies within a 100 μm region, vertebral body length (μm), and intervertebral ligament length (μm).
2.6 Reproductive capacity of F0 adult fish and quality of F1 offspring
At 120 dpf, F0 fish with no obvious abnormalities were used for spawning experiments in groups of 6 (3 males, 3 females). Fish were placed together the night before the assessment in 3 L AHAB semi-static tanks containing 2 L water. The fish were maintained under our breeding colony conditions described in section 2.1. Eggs were collected each morning for 7 days, and the number of fertilized eggs counted and normalized by the number of females in each tank to calculate average fecundity and fertilization success. Resulting embryos from exposed parents (F0) are hereafter referred to as F1.
To determine the next generation effects of parental exposure on offspring (F1), stage 16 embryos (n=50) were randomly selected from each F0 spawning group and maintained in vials containing 1X ERM in a 27°C incubator with a 14:10 light:dark cycle until hatch. Mortality and resultant developmental deformities (described in section 2.4) of embryos and eleutheroembryos were monitored and recorded until 14 dpf.
2.7 Statistical analyses
Statistical analyses were performed comparing exposed animals to their treatment control using SPSS Student Version 16.0 (Chicago IL, USA) software. To compare time to hatch, percent differences were calculated as: (observed – expected)/expected. Expected values were mean time to hatch in controls. All data are means ± SEM. Levene’s test was used as a preliminary screen for homogeneity of variances between or among groups with equal n values and the Brown-Forsythe test was used for groups with unequal n values. All data were tested for normal distribution by the Kolmogorov-Smirnov test. When necessary, natural log data transformations were used to maintain normal distribution between or among groups. Depending on the homogeneity test results, either parametric one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons or non-parametric t-test followed by Kruskal-Wallis post-hoc test was used to identify differences between two groups. Treatment groups were compared to controls run concurrently. A difference of p < 0.05 was considered to be significant.
3. Results
3.1 Embryo survival, time to hatch, and phenotypic alterations
The survival of embryos exposed to 0.1- or 0.5% ERSE alone did not differ from that of controls (Table 1). Exposure to ERSE at 1.0% resulted in 73.6 ± 10.2% survival, significantly less (p =0.043) than control embryos (94.3 ±7.8%) (Table 1). For fish exposed to ERSE alone, time to hatch ranged from 7–10 dpf and was not different from controls across the range of concentrations used (Table 1). Only the 1.0% exposure resulted in a statistically increased number of altered individuals and deformity index scores compared to control (p = 0.04) (Table 1). Of the various phenotypic responses, the single statistically significant change was the presence of Pc edema in this treatment (p = 0.042) (Table 1), reflected in the increase of pericardial cavity area (p < 0.001) (Fig. 1-A4, Table 2). Moderate to severe Pc edema was sometimes associated with abdominal edema (Ab). Ab edema was identified by a flattened dorsal margin of the yolk sac and a clear space within the dorsal surface of the abdominal cavity (Fig. 1-C2).
Table 1.
Summary of survival and developmental deformities observed in F0 embryos at 14 dpf following exposure to ERSE, hypoxia, or co-exposure (n = 25 ~ 34). Treatments compared to their concurrant controls.
| Treatments | Time to hatch (dpf) | Survival (%) | Total altered individuals (%) | Pericardial edema (Pc) (%) | Swim bladder non-inflation (Sb) (%) | Craniofacial abnormality (Cb) (%) | Deformity index |
|---|---|---|---|---|---|---|---|
| 0.0% ERSE | 8.8 ± 1.1 | 94.3 ± 7.8 | 2.8 ± 6.3 | 0 | 0 | 2.8 ± 6.3 | 0.022 ± 0.05 |
| 0.1% ERSE | 8.3 ± 0.6 | 97.1 ± 6.4 | 5.7 ± 7.8 | 3.5 ± 7.1 | 0 | 0 | 0.033 ± 0.07 |
| 0.5% ERSE | 8.7 ± 1.1 | 93.3 ± 10.2 | 9.6 ± 6.7 | 6.1 ± 7.2 | 6.1 ± 7.2 | 3.5 ± 7.1 | 0.07 ± 0.08 |
| 1.0% ERSE | 8.6 ± 1.5 | 73.6 ± 10.2a | 26.7 ± 12.2a | 26.7 ± 12.2a | 11.3 ± 10.4 | 7.3 ± 10.11 | 0.22 ± 0.31a |
| H1 | 12.7 ± 0.8a,b | 80.1 ± 12.8 | 39.9 ± 15.4a | 9.0 ± 13.1 | 35.9 ± 10.8 | 9.0 ± 13.1 | 0.28 ± 0.21a,b |
| H1+0.1%ERSE | 13.1 ± 0.8a,b | 68.6 ± 6.4a | 64.0 ± 21.9a,b | 30.0 ± 14.1a,b | 59.1 ± 12.4a,b | 34.0 ± 13.4a,b | 0.41 ± 0.3a,b |
| H2 | 10.8 ± 0.7a,b,c,d | 80.0 ± 23.9 | 36.5 ± 12.9a | 9.8 ± 12.1 | 32.4 ± 16.5a | 9.8 ± 12.2 | 0.09 ± 0.03c |
| H2+ 0.1% ERSE | 10.8 ± 0.6a,b,c,d | 85.7 ± 17.5 | 35.7 ± 23.3a | 8.3 ± 14.4 | 29.0 ± 25.8a | 24.0 ± 14.6 | 0.19 ± 0.14d |
| H3 | 10.9 ± 1.2a,b | 88.6 ± 11.9 | 22.6 ± 8.1a | 0 | 6.2 ± 8.5 | 9.5 ± 8.7 | 0.07 ± 0.05c |
| H3+0.1% ERSE | 11.0 ± 1.0a,b,c | 82.1 ± 21.4 | 29.6 ± 14.2a | 8.6 ± 10.2 | 16.1 ± 23.6 | 9.8 ± 12.2 | 0.15 ± 0.12d |
indicates significant difference from control (p < 0.05);
indicates significant difference from 0.1% ERSE (p < 0.05);
indicates significant difference from H1 (p < 0.05);
indicates significant difference from H1+0.1 % ERSE (p < 0.05).
Table 2.
Areas of yolk sac, pericardial cavity, and swim bladder in F0 larvae at 14 dpf following exposure to ERSE, hypoxia, or co-exposure as embryos (n = 15). All data are means ± SEM. Before averaging, values have been normalized to standard body length for each individual. Treatments compared to their concurrant controls.
| Treatments | Standard length (μm) | Yolk sac area (μm2) | Pericardial cavity area (μm2) | Swim bladder area (μm2) |
|---|---|---|---|---|
| 0.0% ERSE | 472.4 ± 29.8 | 813.2 ± 198.1 | 201.6 ± 31.4 | 719.9 ± 112.4 |
| 0.1% ERSE | 460.4 ± 37.1 | 945.5 ± 453.7 | 211.3 ± 104.5 | 843.5 ± 201.7 |
| 0.5% ERSE | 455.9 ± 12.9 | 637.8 ± 400.3 | 316.2 ± 74.5 | 859.9 ± 197.2 |
| 1.0% ERSE | 447.0 ± 10.1 | 897.0 ± 284.1 | 478.3 ± 138.6a | 958.2 ± 126.3 |
| H1 | 422.6 ± 20.2a | 2340.8 ± 556.3a | 268.2 ± 61.5 | 571.2 ± 67.1a |
| H1+0.1%ERSE | 394.5 ± 24.4a | 3530.2 ± 921.4a | 305.8 ± 163.1a | 422.9 ± 121.1a |
| H2 | 414.0 ± 11.7a | 1482.7 ± 775.2 | 330.5 ± 43.9 | 537.5 ± 136.8 |
| H2+ 0.1% ERSE | 419.3 ± 33.9a | 1751.5 ± 490.3a | 257.9 ± 70.0 | 458.7 ± 187.4a |
| H3 | 449.7 ± 24.9 | 1334.5 ± 408.2a | 195.9 ± 36.4 | 643.6 ± 153.5 |
| H3+0.1% ERSE | 436.8 ± 29.9a | 2097.0 ± 884.1a | 278.3 ± 138.6 | 559.9 ± 185.6a |
indicates that there is a significant difference from control (p < 0.05).
Note: Example of tracing for areas given in Fig. S2A
Representative larval phenotypes are shown in Fig. 1 with controls (top row) having normal development of the mandible and maxilla, well-formed inner ear apparatus with otoliths, normal appearing pericardial cavity, lipid droplet or vesicle, and limited remnants of the yolk sac. Additionally, the swim bladder was represented by the dark oval body, dorsal to the yolk sac and lipid vesicle, with a clear spot within the center representing insufflation. Larvae exposed to 0.1% ERSE as embryos (Fig. 1-A2), most closely resembled the control, including the clear spot within the center of the developing swim bladder. Taken together, these larvae scored nearly zero in the deformity index (Table 1, Table S1). Although not statistically significant, the most apparent condition in larvae exposed to 0.5% ERSE (Fig. 1-A3) was the greater area of the yolk sac, indicative of reduced yolk resorption (Table 2). In some individuals, the rostral most portion of the head indicated craniofacial deformity (Cb) in the form of a lack of growth in the maxilla and mandible.
Hypoxia alone caused no significant difference in embryo survival compared to normoxic controls. However, H1+0.1% co-exposure (p = 0.041) (Table 1) decreased survival. The normoxic controls hatched at approximately 9 days (Table 1). Hypoxia prolonged time to hatch in H1, H2, and H3 by 56.2%, 27.6%, and 30.1%, respectively (p < 0.001, p < 0.001, and p < 0.001) (Table 1). While H2 and H3 delayed hatching, each was significantly less than H1 (27.6 and 30.1% shorter, respectively) (Table 1). At 14 dpf, the percent of total altered larvae exposed to hypoxia as embryos, regardless of the scenario or co-exposure, was significantly increased compared to normoxic controls (p < 0.001, Table 1).
When effects of hypoxia alone under each of the three scenarios were considered, H1 scored the highest in the deformity index (Table 1, Fig. 1-B) and had increased yolk sac area and decreased swim bladder area (Table 2). This is illustrated in the H1 representative larva (Fig. 1-B2) where retention of the yolk sac, minimal swim bladder development, and Pc edema were observed. In larvae exposed to H2, incidence of Sb non-inflation was increased (= 0.012) but no decreases in mean swim bladder area were seen (Table 1, Table 2, Fig. 1-B3). While not statistically significant, we observed Cb abnormalities and retention of much of the yolk sac in some individuals in this group (Fig. 1-B3). Examination of the larva from H3 showed increases only in yolk sac area (Table 2, Fig. 1-B4). Among the three different hypoxic scenarios, H1 had the most numerous and severe phenotypic alterations (i.e., deformity indices) compared to controls (p = 0.021, Table 1).
Hypoxia (all scenarios) and 0.1% ERSE co-exposures resulted in the same delays in hatching seen in hypoxia alone (Table 1), but decreased survival was only observed in H1+0.1% ERSE (Table 1). The H1+0.1% ERSE co-exposure required the greatest number of days (12–13 dpf, 54.3% longer) for hatching compared to controls, but was not significantly different from H1 alone. This suggests that hypoxia was responsible for the delayed hatching in the co-exposure.
Co-exposure was associated with alterations in larvae in all of the scenarios (Fig. 1-C, Table 1, Table 2). The total number of altered individuals was significantly increased by co-exposure in all scenarios, most notably in the H1+0.1% ERSE (p = 0.024) where occurrences were higher than H1 alone (Table 1, Fig. 1). The deformity index in H1+0.1% ERSE was increased relative to control, H2 + 0.1% ERSE and H3 + 0.1% ERSE (< 0.001, p = 0.025 and p = 0.006, respectively) (Table 1). Specific phenotypic alterations after H1+0.1% ERSE included increased Cb abnormalities (p = 0.017), increased Pc edema (p = 0.037) and pericardial cavity area (p = 0.017), increased yolk sac area (p < 0.001), and increased Sb non-inflation (p < 0.001), and decreased area (p < 0.001) (Table 1; Table 2). These changes are shown in the representative larvae in Fig. 1-C2, where Pc cavity edema was apparent, deforming the adjacent edge of the yolk sac, and the swim bladder was not inflated. In addition, the rostral-most portion of the head showed little development beyond the eye and an angle was seen between the orientation of the head and the dorsal margin of the trunk. Larvae exposed to H2+0.1% ERSE as embryos (Fig. 1-C3) had increased yolk sac area and decreased swim bladder inflation and area (Table 1, Table 2, Fig. 1-C3). While not statistically significant, there was a trend towards more Cb abnormalities. This is evident in Fig. 2-C3 where head structures are like those of the larva immediately above (Fig. 1-C2), including delayed development of the inner ear. Larvae exposed to H3+0.1% ERSE as embryos had increased yolk sac area and decreased swim bladder area (Table 2). The representative larva in Fig. 1-C4 shows that much of the yolk sac had yet to be resorbed and a small swim bladder anlagen was present.
Fig. 2.
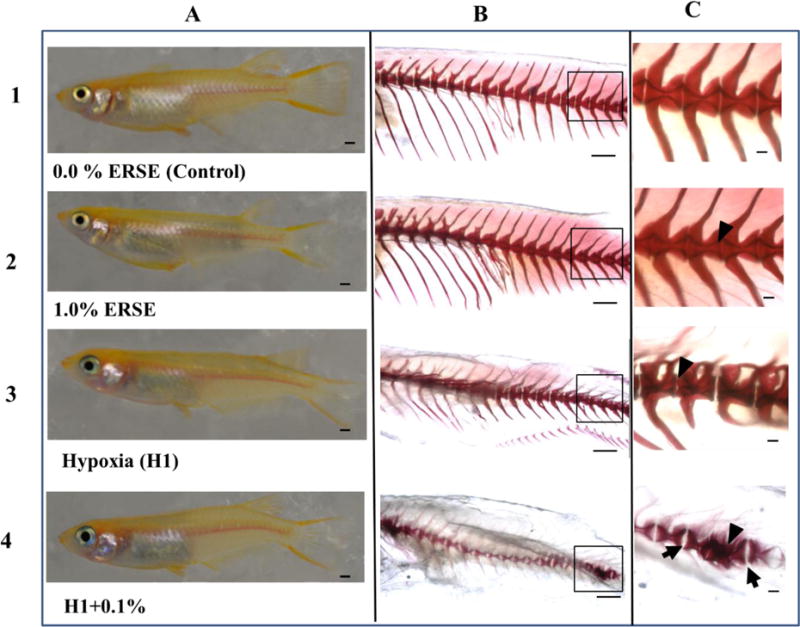
Representative axial skeleton of F0 adults at 120 dpf. Different views/magnifications of representative individuals are in columns: (A) Digital images of anesthetized, laterally oriented control and exposed fish, scale bar is 1 mm., (B) Alizarin red stained axial skeletons of control and exposed fish seen in column A, scale bar is 1 mm, and (C) vertebrae in insets from column B magnified, scale bar is 100 μm. Treatment groups are in rows: (1) Control, (2) 1.0% ERSE, (3) H1 scenario, (4) H1+0.1% ERSE co-exposure. Black arrowheads point to reduced intervertebral ligament spaces, and black arrows point to enlarged intervertebral ligament spaces.
H1 and H2 and all co-exposures reduced standard length of larvae (Table 2) and individuals revealed less overall development (Fig. 1). Yolk sac area and Sb non-inflation were the most common changes.
3.2 Survival, growth, and deformities in F0 adult fish
The numbers of surviving F0 fish at 120 dpf from all ERSE only exposure groups were not significantly different from controls. However, the incidence of altered individuals was significantly increased in the 1.0% ERSE group (p = 0.037) (Table 3). At 120 dpf, only fish from H1 and H1+0.1% ERSE groups showed significantly decreased survival compared to its control (p = 0.037 and p = 0.013, respectively), and higher incidences of deformities (p = 0.001 and p = 0.047, respectively) (Table 3).
Table 3.
Survival, deformities, and standard body length of F0 adult fish at 120 dpf (n = 18–34). All data are means ± SEM. Treatments compared to their concurrant controls.
| Treatments | Survival rate (%) | Total altered individuals (%) | Body Length (cm) |
|---|---|---|---|
| 0.0% ERSE | 95.5 ± 2.9 | 0 | 2.41 ± 0.11 |
| 0.1% ERSE | 89.7 ± 5.4 | 0 | 2.43 ± 0.07 |
| 0.5% ERSE | 90.4 ± 5.3 | 4.5 ± 6.4 | 2.37 ± 0.12 |
| 1.0% ERSE | 83.7 ± 12.2 | 11.2 ± 1.8a,b | 2.17 ± 0.23a |
| H1 | 78.0 ± 9.2a | 14.2 ± 8.3a,b | 2.04 ± 0.13a,b |
| H1+0.1% ERSE | 70.8 ± 5.8a | 18.3 ± 5.6a,b | 2.07 ± 0.16a,b |
| H2 | 92.8 ± 0.7 | 3.3 ± 4.7 | 2.12 ± 0.11a,b |
| H2+0.1% ERSE | 81.0 ± 16.7 | 3.6 ± 5.0 | 2.10 ± 0.26a,b |
| H3 | 83.9 ± 3.8 | 3.8 ± 5.4 | 2.10 ± 0.13a,b |
| H3+0.1% ERSE | 87.5 ± 8.8 | 3.6 ± 5.0 | 2.07 ± 0.09a,b |
indicates that there is a significant difference from control (p < 0.05);
indicates that there is a significant difference from 0.1% ERSE (p < 0.05).
Note. The incidences of survival were reported as 1 minus the cumulative incidence of mortality from 14 dpf to 120 dpf.
The standard body lengths (cm) of adults in all treatment groups except 0.1% and 0.5% ERSE were significantly shorter relative to controls (p = 0.001) (Table 3), with standard length decreased from 2.41 ± 0.11 cm in the control groups to 2.04 ± 0.13 cm in the H1 group (Table 3). After co-exposure to hypoxia (all scenarios) and 0.1% ERSE, adults also showed significant decreases in body length compared to 0.1% ERSE (p < 0.001).
Bone staining of vertebrae in control fish showed a regular “hour-glass”-like appearance, with widened end plates and narrowed bodies (Fig. 2-B1, Fig. 2-C1). Vertebrae of the 1.0% ERSE group resembled controls. Intervertebral ligaments, not typically ossified, were stained by Alizarin but measurements of the structures remained possible (Fig. 2-B2, Fig. 2-C2, Table S3). Conversely, the intervertebral spaces in H1+0.1% ERSE appeared wider than normal (Fig. 2-C3) and the vertebral bodies shortened (Table S3). Hypoxia treatments also deformed vertebral bodies, with some thinned and others bent and/or collapsed (caudal vertebrae) and hemal and neural arches were truncated (Fig. 2 rows 3–4). These alterations were exacerbated in H1+0.1% ERSE, with deformed and compressed vertebral bodies (Fig. 2-B4, Fig. 2-C4, Table S3). The abdominal hemal arches in this adult showed the greatest difference relative to control or to the 1.0% ERSE group.
3.2 Sex ratios and reproductive capacity in F0 adult fish
ERSE only exposures increased the ratio of females to males in F0 fish in both the 0.5% ERSE (73.3 ± 0.4% female; p = 0.028) and 1.0% ERSE (77.5 ± 1.5% female; p = 0.08) groups compared to control (61.8 ± 0.9% female) (Fig. 3-A). In contrast, hypoxia exposures caused an opposite effect in sex ratio. The percentage of female fish from H1 exposures and H2 exposures decreased to 39.5 ± 2.9% and 43.1 ± 4.4%, respectively, relative to 61.8 ± 1.1% of control (p = 0.07 and p = 0.02, respectively). There was no significant difference between H3 exposures and control (Fig. 3-B). Surprisingly, after co-exposure, sex ratios were not significantly different relative to control. In the H1+0.1% ERSE group, the percentage of females in the population had a significant shift relative to H1 exposures (p = 0.016) (Fig. 3-B), the female ratio in H1+0.1% ERSE increased to 59.1 ± 6.4% relative to 39.6 ± 2.9% in H1.
Fig. 3.
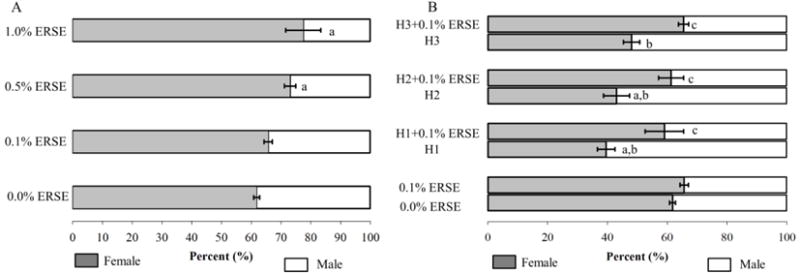
Sex ratios (as determined by secondary sex characteristics and confirmed by internal examination) of F0 adults exposed to ERSE and/or hypoxia as embryos. The sex ratios per treatment are reported as mean ± SEM (A: 0.0% (Control) n = 32, 0.1% n = 29, 0.5% n = 26, 1.0% n = 18; B: Normoxia (Control) n = 34, 0.1% n = 28, H1 n = 19, H2 n = 23, H3 n = 25, H1+0.1% n = 16, H2+0.1% n = 21, H3+0.1 n = 27 individuals). For panels A and B, controls are on the bottom. Grey bars represent females and white bars represent males. “a” indicates that there is a significant difference from control (p < 0.05); “b” indicates that there is a significant difference from 0.1% ERSE (p < 0.05); “c” indicates that there is a significant difference from H1 (p < 0.05).
ERSE early-life exposure showed a trend toward increased egg production, with the number of eggs per female in the 1.0% ERSE group significantly higher than control (p = 0.024) (Fig. 4-A). Regardless of concentration, fertilization success did not differ (Fig. 4-B). Hypoxia early-life exposures negatively impacted egg production, the number of eggs per female from H1-exposed and H2-exposed fish was decreased by 35.3 ± 8.2% and 21.7 ± 4.4% compared to the control (p = 0.012 and p = 0.05, respectively). This decrease was reflected in significant drops in percentages of fertilized eggs in H1 and H2 (p = 0.002 and p = 0.014, respectively) (Fig. 4-D). However, no significant difference in egg production between the different hypoxia scenarios was seen (Fig. 4-C). Co-exposure (H1+0.1% ERSE) decreased egg production and fertilization (Fig. 4-C,D). While all co-exposures significantly reduced fertilized eggs relative to the control (H1 p < 0.001, H2 p < 0.001, and H3 p < 0.001) (Fig. 4-D), H1+0.1% ERSE had the fewest fertilized eggs (p = 0.048) (Fig. 4-D).
Fig. 4.
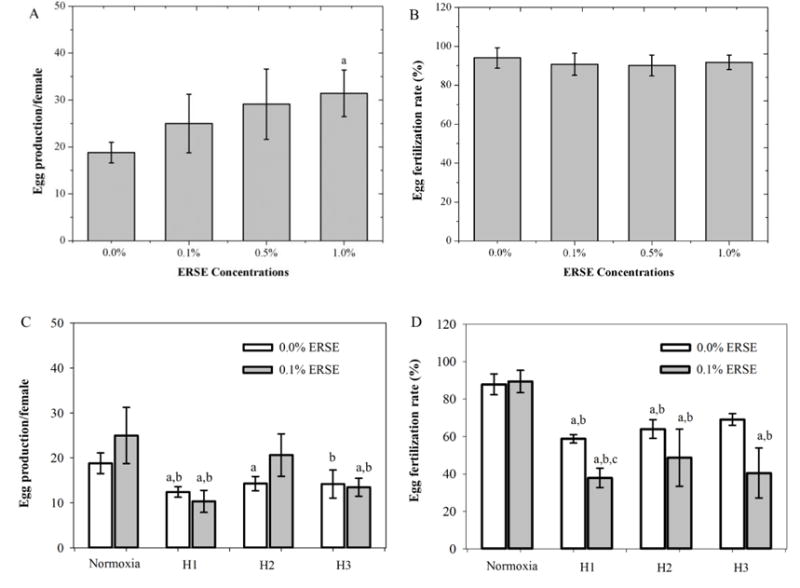
Reproductive capacity of F0 adults at 120 dpf. (A) Effects of early-life ERSE exposure on F0 egg production normalized by the number of females; (B) Effects of early-life ERSE exposure on F0 egg fertilization rate; (C) Effects of early-life hypoxia exposures on F0 egg production normalized by the number of females; (D) Effects of early-life hypoxia exposures on F0 egg fertilization rate. Bars represent means ± SEM for 7 day collection period. “a” indicates that there is a significant difference from control (p < 0.05); “b” indicates that there is a significant difference from 0.1% ERSE (p < 0.05); “c” indicates that there is a significant difference from H1 (p < 0.05).
3.3 Hatch, survival, and deformities in F1 offspring
Although early-life exposure to ERSE only in F0 adults was not associated with changes in fertilization success, significant differences were seen in F1 embryo survival (Table 4; 0.5% ERSE, p < 0.001; 1.0% ERSE, p = 0.004), percent of altered individuals (Table 4; 1.0% ERSE, p = 0.006), and hatching success (i.e., number of individuals hatched at 14 dpf) (Table 4; 0.5% ERSE, p = 0.041; 1.0% ERSE, p < 0.001). Unlike results in F0 embryos, F1 embryos of 0.5% ERSE exposed parents, showed significant decreases in hatching and survival (Table 1, Table 4). In all but H3 groups, hypoxia exposure significantly decreased the hatching success (Table 4; H1, p = 0.006; H1+0.1% ERSE, p < 0.001; H2, p = 0.014; H2+0.1% ERSE, p = 0.005) compared to controls. In the H1 groups and H2+0.1% ERSE, decreased survival (Table 4; H1, p = 0.002; H1+0.1% ERSE, p < 0.001; H2+0.1% ERSE p = 0.005) and increased incidences of altered individuals (Table 4; H1, p = 0.015; H1+0.1% ERSE, p < 0.001; H2+0.1% ERSE, p = 0.046) was observed. Because the exposure duration ended at 14 dpf, it is important to note that survival rate (Table 4) includes both embryo and eleutheroembryo mortality.
Table 4.
Effects of early-life exposure on F1 individuals: hatching success, survival, and alterations. All data are means ± SEM (n = 50). Treatments compared to their concurrant controls.
| Parental (F0) Treatments | Hatching success (%) | Survival (%) | Total altered individuals (%) |
|---|---|---|---|
| 0.0% ERSE | 91.2 ± 10.2 | 85.4 ± 9.8 | 2.2 ± 4.9 |
| 0.1% ERSE | 83.0 ± 6.7 | 77.0 ± 14.5 | 2.5 ± 5.5 |
| 0.5% ERSE | 77.2 ± 6.5* | 63.3 ± 11.7* | 15.2 ± 11.9 |
| 1.0% ERSE | 67.0 ± 12.1* | 55.7 ± 8.8* | 27.4 ± 8.2* |
| H1 | 73.3 ± 5.1* | 53.7 ± 7.8* | 25.0 ± 13.7* |
| H1+0.1% ERSE | 70.7 ± 6.3* | 44.9 ± 9.8* | 48.3 ± 17.1* |
| H2 | 74.8 ± 5.7* | 64.1 ± 8.3 | 11.5 ± 6.6 |
| H2+0.1% ERSE | 73.6 ± 10.1* | 56.6 ± 14.3* | 21.9 ± 13.8* |
| H3 | 82.2 ± 5.8 | 75.1 ± 6.3 | 9.3 ± 5.4 |
| H3+0.1% ERSE | 79.9 ± 5.2 | 64.9 ± 10.4 | 11.8 ± 13.9 |
indicates significant differences from the control (p < 0.05).
All data represent the entire study period (from 21 hpf to 14 dpf). Time to hatch (dpf) was not significantly different between groups and thus we report only hatching success (%). The cumulative number of hatched larvae was recorded for the determination of hatching success, and the cumulative mortality of embryos and larvae was recorded for the determination of survival.
4. Discussion
Our purpose was to characterize medaka embryo toxicity after exposure to a real world mixture of PAHs and/or hypoxia and to determine later life consequences in adult survivors and their offspring. The PAH mixture, ERSE (Fang et al., 2014), and/or hypoxia resulted in embryo mortality, altered development, and delayed hatching. Continued observation revealed later life consequences including diminished growth, altered sex ratios, impaired reproduction and decreases in the number and quality of offspring. Co-exposure, particularly using a continuous hypoxia scenario, proved especially harmful. Timing of initiation and duration of hypoxia were important.
4.1 Embryo toxicity after exposure to ERSE and hypoxia
1.0% ERSE alone increased developmental alterations and decreased embryo survival, but 5% ERSE produced 100% mortality. Interestingly, the Fang et al. (2014) study, using zebrafish embryos, required an 8% concentration of the same ERSE to produce 80% mortality. We consulted the comparison of development for each species and found that developmental landmarks, at time of initiation, were nearly identical (Furutani-Seiki and Wittbrodt, 2004). Since a longer time is normally required for hatching in medaka, they were exposed longer, and this may partially explain the interspecies differences in embryo mortality. Numerous investigations of survivors of embryo toxicity in fish exposed to PAHs have shown that the cardiovascular system is a common target. When Vines et al. (2000) studied Pacific herring (Clupea pallasi) embryos, those attached to creosote-treated wood failed to survive beyond three days while those exposed to aqueous solution of creosote showed higher survival but altered cardiac function. This included reduced heart rate, arrhythmia, and pericardial edema. Incardona et al. (2004) tested individual PAHs in zebrafish embryos. Fluorene, dibenzothiophene, phenanthrene, or pyrene, resulted in pericardial edema, bradycardia and arrhythmias, apparently associated with the number of rings in the PAH compounds (Incardona et al., 2004).
The effects of hypoxia proved distinct from those of ERSE. Each of our three hypoxic scenarios had no effect upon embryo mortality but delayed time to hatch with greatest effect, over 50% longer, seen under continuous hypoxia (H1). Delayed hatching is common in fish embryos reared under hypoxic conditions. Examples include brown trout (Salmo trutta) (Roussel, 2007), zebrafish (Bagatto, 2005; Shang and Wu, 2004), and medaka (Cheung et al., 2014). Hatching abnormalities may arise from delays in development such as the failure to form sufficient hatching enzyme and/or diminished jaw movement necessary for rupture of hatching gland cells and enzyme with slowed or incomplete digestion of the chorion (Korwin-Kossakowski, 2012).
Morphological indicators of delayed development included retained yolk sac and decreased swim bladder insufflation in the H1 scenario. The latter was also observed with nocturnal hypoxia (H2). We and others regard delay as a sign of developmental toxicity (Marty et al., 1995b). After hatching, medaka eleutheroembryos (Embry et al., 2010) rely on yolk reserves for up to five days (Yamamoto, 1975). Just prior to hatch the rate of yolk absorption increases rapidly, likely in response to increases in absorptive surface area and metabolic activity of the yolk syncytium (Heming and Buddington, 1988). During development and immediately post-hatch, the yolk is the major source of energy for formation of new tissue and respiration (Kamler, 2008). However, we found that late hatching individuals were smaller and appeared less developed, with larger yolk areas. This finding is in agreement with reviews of resource allocation and reduced yolk absorption observed with hypoxia and xenobiotics (Heming and Buddington, 1988; Kamler, 2008).
Similar to other physostomic fishes (cf. Doroshev and Cornacchia, 1979), recently hatched medaka inflate their swim bladder enabling buoyancy with continued body growth (Lindsey et al., 2010). When larvae have reduced- or non-inflated swim bladders, they cannot maintain position in the water column and must expend energy to maintain buoyancy, leading to emaciation and eventual mortality (González-Doncel et al., 2003; Marty et al., 1995a).
While exposure to either 0.1% ERSE or the H1 scenario alone resulted in no pericardial edema, craniofacial abnormalities, or swim bladder non-inflation; co-exposure produced all of these alterations. When coupled, abnormalities and hatching delays render these larvae at a disadvantage (c.f. McKim, 1977). Similar hypoxic conditions (2.7 mg O2 L−1) in zebrafish increased pericardial edema at all dilutions of ERSE (1.0% ~ 20.0%) (Fleming and Di Giulio, 2011). Several studies of both pyrolytic and petrogenic PAHs and some hypoxia studies have linked cardiac function and swimming ability to larval survival and growth (Incardona et al., 2009; Incardona et al., 2004; Le Bihanic et al., 2014; Roussel, 2007; Yang et al., 2013). Our results are supported by the few other studies of co-exposure including: exacerbation of embryotoxicity by hypoxia following BaP and benzo[k]fluoranthen (BkF) exposure in zebrafish (Fleming and Di Giulio, 2011); decreased survival of sheepshead minnow larvae after exposure to dispersed crude oil under hypoxic conditions (Dasgupta et al., 2015); and hypoxia inhibited pathways that detoxify oil and exacerbate oil induced DNA damage (Dasgupta et al., 2016). Investigations directed at metabolic endpoints showed that the combined effects may be attributed to a shift to more toxic metabolites or increased half-life of toxic parent PAHs or their metabolites (Fleming and Di Giulio, 2011; Matson et al., 2008).
4.2 Growth and skeletal deformities in F0 adults
We extended observations on survivors of ERSE, hypoxia, and co-exposure from larvae to adults and their offspring. Our study design did not include exposure during the interval from larvae (14 dpf) to adulthood (120 dpf). Rather, all survivors of embryo toxicity tests were reared under identical, clean conditions and diet. Both H1 alone and H1+0.1% ERSE decreased the number of individuals surviving to adulthood. In adult survivors, growth was impaired by exposure. After embryonic exposure to ERSE or hypoxia, sublethal effects in adult medaka included reduced body length and increased incidence of skeletal deformities. While larvae from the 1.0% ERSE and H3 groups were of normal length, they were smaller as adults. Both short- and long-term continuous hypoxia were found to significantly reduce growth in Gulf killifish (Landry et al., 2007), Atlantic killifish (Stierhoff et al., 2003), and zebrafish (Shang et al., 2006). The long-term impact on fish growth after early exposure to hypoxia is likely to involve metabolic alteration. For example, a review by Richards (2009) associated the activation of anaerobic metabolism in organisms exposed to hypoxia as potentially limiting the energy-consuming metabolic pathways in the liver. Regardless of cause, restriction to short-term evaluation of PAHs or hypoxia may underestimate important long-term impacts.
Reduced body length may also be due to skeletal alterations as observed in our stains of vertebrae and intervertebral spaces. Based on our findings, a linkage between reduced growth and impaired skeletal development is suggested. Individual PAHs or 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) caused skeletal deformities in adult fish (Baker et al., 2013; Dong et al., 2012; Incardona et al., 2004) as well as transgenerational bone impairments (Baker et al., 2014; Corrales et al., 2014; Seemann et al., 2015). These compounds are associated with the aryl hydrocarbon receptor (AhR) in perichordal ossification of vertebrae in medaka (Watson et al., 2016) and endochondrial bone formation via mediation of Sonic hedgehog (Shh) by the AhR signaling pathway in rockfish (Sebastiscus marmoratus) (He et al., 2011). ERSE and hypoxia resulted in different effects on the spinal column. Consecutive vertebral segments in ERSE exposed individuals were normal but staining of intervertebral ligament spaces suggested atypical ossification. Conversely, hypoxia and co-exposure resulted in wider than normal intervertebral spaces and altered vertebral bodies (Inohaya et al., 2007). From an ecotoxicological perspective, these changes may impair the ability of the host to avoid predation, capture food, and attract a mate (Seemann et al., 2015).
4.3 Sex ratios and reproductive capacity
Both 0.5% and 1.0% ERSE exposure groups had greater numbers of females than males. In the 0.5% ERSE group, this altered sex ratio was in adults that appeared normal as larvae, underscoring the need for later life observations. In zebrafish, early dioxin exposure caused alterations in sex ratios, i.e., more than 60% of the population were females (normal ratio is approximately 2:1 males to females) (Baker et al., 2013). Similar results were seen after early-life exposure to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in fathead minnows (Pimephales promelas). Those authors regarded altered estrogenic activity of BDE-47 as a cause (Thornton et al., 2016). During medaka embryogenesis, sex hormones direct the developmental form of estrogens thereby mediating development of female sex organs and their functional characteristics (Matsuda, 2005). For example, upon monohydroxylation, PAHs can induce estrogenic effects by directly binding estrogen receptors (Fertuck et al., 2001). Our observations may reflect endocrine disruption by PAHs interfering with normal estrogen signaling. Vignet et al. (2014) found a trend toward lower reproduction in zebrafish after embryonic exposure to PAH-spiked sediment. Results with TCDD in zebrafish are similar; exposure during critical ontogenetic periods caused reduced fitness and reproductive capacity later in life (Baker et al., 2013; King-Heiden et al., 2012). In addition to fecundity, the decrease in F1 offspring survival, hatching, and development observed herein may be related to the reduced growth and fitness of F0 adult fish.
We found significant decreases in fertilization, hatching success, and larval survival in hypoxia scenarios. Severe hypoxia (0.5 mg O2 L−1, 48–288 hpf) upset the balance of sex hormones during embryonic development (Shang and Wu, 2004) and led to changes in sex differentiation and ratio of zebrafish at later developmental stages (Shang et al., 2006). Relative to ERSE exposure of this study, hypoxia exposures (H1 and H2) caused an opposite effect in sex ratio, with a significantly greater number of males than females. However, this was not seen in the H3 treatment group. Perhaps H3 was late enough that it did not disrupt the formation of undifferentiated gonads (Schartl, 2004); or, phenotypic sex determination had already begun (Kanamori et al., 1985). In zebrafish, hypoxia can disrupt sex hormones and sex differentiation as early as 48 hpf (Shang and Wu, 2004; Shang et al., 2006). In medaka, Cheung et al. (2014) demonstrated that hypoxia directly acted on genes that regulate sex determination and differentiation, thereby turning genotypic females into phenotypic males and leading to a male-dominant F1 population. The environmental relevance of altered sex ratio was affirmed in a field survey in the Gulf of Mexico and Mississippi River Delta, USA, which showed sex ratio of natural populations of Atlantic croaker to be skewed towards males at hypoxic sites (Thomas and Rahman, 2011).
H1 and H2 clearly reduced reproductive capacity as evidenced by significant reductions in egg number, fertilization rate, and decreased F1 larval survival. Waterborne BaP has been to shown modify the global and gene specific DNA methylation during zebrafish development (Fang et al., 2013). Recently Wang et al. (2016) demonstrated another pathway in which hypoxia could trigger epigenetic changes in the methylome of sperm, and alter expression of genes and proteins, leading to a reduction in motility and quantity of sperm in the F0, F1, and F2 generations of marine medaka. It is possible that hypoxia could target the egg, sperm or both, resulting in changes we observed. Future study should include next generation tests of sperm quality, fertilization success, and ability to elicit spawning behavior in females.
In our study, co-exposure to hypoxia and ERSE did not significantly alter the sex ratio. While this suggests a return to normality, it was associated with significant decreases in fertilization and larval survival in F1 offspring. Similarly, Hedgpeth and Griffitt (2016) reported that co-exposure to crude- or dispersed oil and hypoxia in sheepshead minnows (Cyprinodon variegatus) decreased egg production and both embryo hatching success as well as larval survival.
4.4 Initiation and duration of hypoxia
Initiation and duration of hypoxia was influential in perturbing both early- and later-life development. F0 embryos exposed to late stage embryogenesis hypoxia (H3) had fewer observable malformations and greater F1 larval survival than their counterparts exposed to either of the other two scenarios. Conceivably, due to axial patterning during development (McGinnis and Krumlauf, 1992), hypoxia initiated at early developmental stages may affect some structures while not affecting others. Different “critical windows” exist where specific sites may be more sensitive or susceptible at different times (Burggren, 1999; Cheung et al., 2014; Pincetich et al., 2005).
Our three hypoxia scenarios all affected normal development; however, only H1 and H2 affected reproductive success. Nocturnal hypoxia incorporated a repeated pattern of exposure and warrants further consideration. Perhaps, repeated short periods of nocturnal hypoxia might have a cumulative impact resulting in impairment of reproductive capacity (Cheek, 2011). For example, juvenile southern catfish (Silurus meridionalis) maintained under nocturnal hypoxia (3.0 mg O2 L−1) had lower final body mass and length than normoxic fish (Yang et al., 2013). It would be interesting to determine which organs and tissues were affected in that study. Field observations in Week- and Pensacola Bays, USA have shown that exposure to diel hypoxia was associated with significantly impaired reproductive capacity in Gulf killifish (Cheek et al., 2009). Possibly, episodic hypoxia alters both normal development of embryos and interrupts sex steroid production in adults, thereby decreasing the recruitment of populations (Cheek et al., 2009; Thomas and Rahman, 2011; Tyler et al., 2009).
5. Conclusions
ERSE and hypoxia affected different aspects of development and co-exposures resulted in greater adverse effects than either alone. Initiation and duration of hypoxia proved important, with initiation at later stages of development associated with the fewest changes while continuous hypoxia resulted in the most pronounced effects. Altered growth, sexual differentiation and reduced reproductive capacity were later life effects of early life exposure. The developmental alterations, diminished survival, hatching delays, and morphological change in both exposed individuals and their subsequent offspring have real world significance.
Supplementary Material
Highlights.
Co-exposures resulted in decreased survival, hatching delays, and deformities
Among three scenarios, continuous hypoxia caused the most significant changes
Hypoxia onset earlier in development resulted in more developmental changes
PAHs alone caused adult female-bias; hypoxia male-bias; no bias with both
Offspring of co-exposed parents were most severely affected
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences Superfund Research Program (P42-ES-10356), Duke University Integrated Toxicology Program (T32ES007031), and the National Natural Science Foundation of China (41476096). We would like to thank AtLee Watson for his help with skeletal analyses. We would also like to thank Casey Lindberg for discussion and helpful review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagatto B. Ontogeny of cardiovascular control in zebrafish (Danio rerio): Effects of developmental environment. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2005;141:391–400. doi: 10.1016/j.cbpb.2005.07.002. http://dx.doi.org/10.10167j.cbpb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Baker TR, Peterson RE, Heideman W. Early dioxin exposure causes toxic effects in adult zebrafish. Toxicological Sciences. 2013;135:241–250. doi: 10.1093/toxsci/kft144. http://dx.doi.org/10.1093/toxsci/kft144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TR, Peterson RE, Heideman W. Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicological Sciences. 2014;138:403–411. doi: 10.1093/toxsci/kfu006. http://dx.doi.org/10.1093/toxsci/kfu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumard P, Budzinski H, Garrigues P, Sorbe JC, Burgeot T, Bellocq J. Concentrations of PAHs (polycyclic aromatic hydrocarbons) in various marine organisms in relation to those in sediments and to trophic level. Marine Pollution Bulletin. 1998;36:951–960. http://dx.doi.org/10.1016/S0025-326X(98)00088-5. [Google Scholar]
- Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion. Journal of Applied Ichthyology. 2010;26:235–238. http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x. [Google Scholar]
- Bensimon-Brito A, Cardeira J, Cancela ML, Huysseune A, Witten PE. Distinct patterns of notochord mineralization in zebrafish coincide with the localization of Osteocalcin isoform 1 during early vertebral centra formation. BMC Developmental Biology. 2012;12:28–41. doi: 10.1186/1471-213X-12-28. http://dx.doi.org/10.1186/1471-213X-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae) Developmental Dynamics. 2003;228:337–357. doi: 10.1002/dvdy.10387. http://dx.doi.org/10.1002/dvdy.10387. [DOI] [PubMed] [Google Scholar]
- Boyd TJ, Montgomery MT, Steele JK, Pohlman JW, Reatherford SR, Spargo BJ, Smith DC. Dissolved oxygen saturation controls PAH biodegradation in freshwater estuary sediments. Microbial Ecology. 2005;49:226–235. doi: 10.1007/s00248-004-0279-0. http://dx.doi.org/10.1007/s00248-004-0279-0. [DOI] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicology and Teratology. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. http://dx.doi.org/10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Thompson J, Chernick M, Hinton DE, di Giulio RT. Later life swimming performance and persistent heart damage following subteratogenic PAH mixture exposure in the Atlantic killifish (Fundulus heteroclitus) Environmental Toxicology and Chemistry. doi: 10.1002/etc.3877. in press. http://dx.doi.org/10.1002/etc.3877. [DOI] [PMC free article] [PubMed]
- Buchheister A, Bonzek CF, Gartland J, Latour RJ. Patterns and drivers of the demersal fish community of Chesapeake Bay. Marine Ecology Progress Series. 2013;481:161–180. http://dx.doi.org/10.3354/meps10253. [Google Scholar]
- Burggren W. Genetic, environmental and maternal influences on embryonic cardiac rhythms. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1999;124:423–427. doi: 10.1016/s1095-6433(99)00134-8. http://dx.doi.org/10.1016/S1095-6433(99)00134-8. [DOI] [PubMed] [Google Scholar]
- Cachot J, Geffard O, Augagneur S, Lacroix S, Le Menach K, Peluhet L, Couteau J, Denier X, Devier MH, Pottier D, Budzinski H. Evidence of genotoxicity related to high PAH content of sediments in the upper part of the Seine estuary (Normandy, France) Aquatic Toxicology. 2006;79:257–267. doi: 10.1016/j.aquatox.2006.06.014. http://dx.doi.org/10.1016/j.aquatox.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Cachot J, Law M, Pottier D, Peluhet L, Norris M, Budzinski H, Winn R. Characterization of toxic effects of sediment-associated organic pollutants using the λ transgenic medaka. Environmental Science & Technology. 2007;41:7830–7836. doi: 10.1021/es071082v. http://dx.doi.org/10.1021/es071082v. [DOI] [PubMed] [Google Scholar]
- Carls MG, Holland L, Larsen M, Collier TK, Scholz NL, Incardona JP. Fish embryos are damaged by dissolved PAHs, not oil particles. Aquatic Toxicology. 2008;88:121–127. doi: 10.1016/j.aquatox.2008.03.014. http://dx.doi.org/10.1016/j.aquatox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Cheek AO. Diel hypoxia alters fitness in growth-limited estuarine fish (Fundulus grandis) Journal of Experimental Marine Biology and Ecology. 2011;409:13–20. http://dx.doi.org/10.1016/jjembe.2011.07.006. [Google Scholar]
- Cheek AO, Landry CA, Steele SL, Manning S. Diel hypoxia in marsh creeks impairs the reproductive capacity of estuarine fish populations. Marine Ecology Progress Series. 2009;392:211–221. http://dx.doi.org/10.3354/meps08182. [Google Scholar]
- Chernick M, Ware M, Albright E, Kwok KWH, Dong W, Zheng N, Hinton DE. Parental dietary seleno-L-methionine exposure and resultant offspring developmental toxicity. Aquatic Toxicology. 2016;170:187–198. doi: 10.1016/j.aquatox.2015.11.004. http://dx.doi.org/10.1016/j.aquatox.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CHY, Chiu JMY, Wu RSS. Hypoxia turns genotypic female medaka fish into phenotypic males. Ecotoxicology. 2014;23:1260–1269. doi: 10.1007/s10646-014-1269-8. http://dx.doi.org/10.1007/s10646-014-1269-8. [DOI] [PubMed] [Google Scholar]
- Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound- and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River Estuary (Virginia, USA) Environmental Science & Technology. 2013;47:10556–10566. doi: 10.1021/es401604b. http://dx.doi.org/10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton MD, Kwok KWH, Brandon JA, Warren IH, Ryde IT, Cooper EM, Hinton DE, Rittschof D, Meyer JN. Developmental toxicity and DNA damage from exposure to parking lot runoff retention pond samples in the Japanese medaka (Oryzias latipes) Marine Environmental Research. 2014;99:117–124. doi: 10.1016/j.marenvres.2014.04.007. http://dx.doi.org/10.1016/j.marenvres.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J, Thornton C, White M, Willett KL. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquatic Toxicology. 2014;148:16–26. doi: 10.1016/j.aquatox.2013.12.028. http://dx.doi.org/10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Di Giulio RT, Drollette BD, L Plata D, Brownawell BJ, McElroy AE. Hypoxia depresses CYP1A induction and enhances DNA damage, but has minimal effects on antioxidant responses in sheepshead minnow (Cyprinodon variegatus) larvae exposed to dispersed crude oil. Aquatic Toxicology. 2016;177:250–260. doi: 10.1016/j.aquatox.2016.05.022. http://dx.doi.org/10.1016/j.aquatox.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Huang IJ, McElroy AE. Hypoxia enhances the toxicity of corexit EC9500A and chemically dispersed Southern Louisiana Sweet Crude Oil (MC-242) to sheepshead minnow (Cyprinodon variegatus) larvae. PLOS ONE. 2015;10:e0128939. doi: 10.1371/journal.pone.0128939. http://dx.doi.org/10.1371/journal.pone.0128939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CR, Okihiro MS, Hinton DE. Effects of husbandry practices, gender, and normal physiological variation on growth and reproduction of Japanese medaka, Oryzias latipes. Aquatic Toxicology. 2002;60:185–201. doi: 10.1016/s0166-445x(02)00004-8. http://dx.doi.org/10.1016/S0166-445X(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Di Giulio RT, Clark BW. The Elizabeth River story: A case study in evolutionary toxicology. Journal of Toxicology and Environmental Health, Part B. 2015;18:259–298. doi: 10.1080/15320383.2015.1074841. http://dx.doi.org/10.1080/15320383.2015.1074841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. http://dx.doi.org/10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Dong W, Hinton DE, Kullman SW. TCDD disrupts hypural skeletogenesis during medaka embryonic development. Toxicological Sciences. 2012;125:91–104. doi: 10.1093/toxsci/kfr284. http://dx.doi.org/10.1093/toxsci/kfr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshev SI, Cornacchia JW. Initial swim bladder inflation in the larvae of Tilapia mossambica (Peters) and Morone saxatilis (Walbaum) Aquaculture. 1979;16:57–66. http://dx.doi.org/10.1016/0044-8486(79)90172-8. [Google Scholar]
- Embry MR, Belanger SE, Braunbeck TA, Galay-Burgos M, Halder M, Hinton DE, Leonard MA, Lillicrap A, Norberg-King T, Whale G. The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquatic Toxicology. 2010;97:79–87. doi: 10.1016/j.aquatox.2009.12.008. http://dx.doi.org/10.1016/j.aquatox.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Fang M, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Di Giulio RT, Ferguson PL, Stapleton HM. Effect-directed analysis of Elizabeth River porewater: Developmental toxicity in zebrafish (Danio rerio) Environmental Toxicology and Chemistry. 2014;33:2767–2774. doi: 10.1002/etc.2738. http://dx.doi.org/10.1002/etc.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Thornton C, Scheffler BE, Willett KL. Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development. Environmental Toxicology and Pharmacology. 2013;36:40–50. doi: 10.1016/j.etap.2013.02.014. http://dx.doi.org/10.1016/j.etap.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell A, Nero V, Croft M, Bal P, Dixon DG. Modified Japanese medaka embryo-larval bioassay for rapid determination of developmental abnormalities. Archives of Environmental Contamination and Toxicology. 2006;51:600–607. doi: 10.1007/s00244-005-0319-x. http://dx.doi.org/10.1007/s00244-005-0319-x. [DOI] [PubMed] [Google Scholar]
- Fernandes MB, Sicre MA, Boireau A, Tronczynski J. Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Marine Pollution Bulletin. 1997;34:857–867. http://dx.doi.org/10.1016/S0025-326X(97)00063-5. [Google Scholar]
- Fertuck KC, Kumar S, Sikka HC, Matthews JB, Zacharewski TR. Interaction of PAH-related compounds with the α and β isoforms of the estrogen receptor. Toxicology Letters. 2001;121:167–177. doi: 10.1016/s0378-4274(01)00344-7. http://dx.doi.org/10.1016/S0378-4274(01)00344-7. [DOI] [PubMed] [Google Scholar]
- Fleming CR, Di Giulio RT. The role of CYP1A inhibition in the embryotoxic interactions between hypoxia and polycyclic aromatic hydrocarbons (PAHs) and PAH mixtures in zebrafish (Danio rerio) Ecotoxicology. 2011;20:1300–1314. doi: 10.1007/s10646-011-0686-1. http://dx.doi.org/10.1007/s10646-011-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani-Seiki M, Wittbrodt J. Medaka and zebrafish, an evolutionary twin study. Mechanisms of Development. 2004;121:629–637. doi: 10.1016/j.mod.2004.05.010. http://dx.doi.org/10.1016/j.mod.2004.05.010. [DOI] [PubMed] [Google Scholar]
- González-Doncel M, de la Pena E, Barrueco C, Hinton DE. Stage sensitivity of medaka (Oryzias latipes) eggs and embryos to permethrin. Aquatic Toxicology. 2003;62:255–268. doi: 10.1016/s0166-445x(02)00090-5. http://dx.doi.org/10.1016/s0166-445x(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Hawkins SA, Billiard SM, Tabash SP, Brown RS, Hodson PV. Altering cytochrome P4501A activity affects polycyclic aromatic hydrocarbon metabolism and toxicity in rainbow trout (Oncorhynchus mykiss) Environmental Toxicology and Chemistry. 2002;21:1845–1853. http://dx.doi.org/10.1002/etc.5620210912. [PubMed] [Google Scholar]
- Hawkins WE, Walker WW, Overstreet RM, Lytle JS, Lytle TF. Carcinogenic effects of some polycyclic aromatic hydrocarbons on the Japanese medaka and guppy in waterborne exposures. Science of The Total Environment. 1990;94:155–167. doi: 10.1016/0048-9697(90)90370-a. http://dx.doi.org/10.1016/0048-9697(90)90370-A. [DOI] [PubMed] [Google Scholar]
- He C, Zuo Z, Shi X, Li R, Chen D, Huang X, Chen Y, Wang C. Effects of benzo(a)pyrene on the skeletal development of Sebastiscus marmoratus embryos and the molecular mechanism involved. Aquatic Toxicology. 2011;101:335–341. doi: 10.1016/j.aquatox.2010.11.008. http://dx.doi.org/10.1016/j.aquatox.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Hedgpeth BM, Griffitt RJ. Simultaneous exposure to chronic hypoxia and dissolved polycyclic aromatic hydrocarbons results in reduced egg production and larval survival in the sheepshead minnow (Cyprinodon variegatus) Environmental Toxicology and Chemistry. 2016;35:645–651. doi: 10.1002/etc.3207. http://dx.doi.org/10.1002/etc.3207. [DOI] [PubMed] [Google Scholar]
- Heming TA, Buddington RK. Yolk absorption in embryonic and larval fishes. In: Hoar WS, Randall DJ, editors. Fish Physiology. Academic Press; San Diego, CA: 1988. pp. 407–446. [Google Scholar]
- Ho DH, Burggren WW. Parental hypoxic exposure confers offspring hypoxia resistance in zebrafish (Danio rerio) The Journal of experimental biology. 2012;215:4208–4216. doi: 10.1242/jeb.074781. http://dx.doi.org/10.1242/jeb.074781. [DOI] [PubMed] [Google Scholar]
- Hylland K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. Journal of Toxicology and Environmental Health, Part A. 2006;69:109–123. doi: 10.1080/15287390500259327. http://dx.doi.org/10.1080/15287390500259327. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Day HL, Sloan CA, Bolton JL, Collier TK, Scholz NL. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupeapallasi) exposed to crude oil during weathering. Environmental Science & Technology. 2009;43:201–207. doi: 10.1021/es802270t. http://dx.doi.org/10.1021/es802270t. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicology and Applied Pharmacology. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. http://dx.doi.org/10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Inohaya K, Takano Y, Kudo A. The teleost intervertebral region acts as a growth center of the centrum: In vivo visualization of osteoblasts and their progenitors in transgenic fish. Developmental Dynamics. 2007;236:3031–3046. doi: 10.1002/dvdy.21329. http://dx.doi.org/10.1002/dvdy.21329. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takei Y. Diverse adaptability in Oryzias species to high environmental salinity. Zoological Science. 2002;19:727–734. doi: 10.2108/zsj.19.727. http://dx.doi.org/10.2108/zsj.19.727. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mechanisms of Development. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. http://dx.doi.org/10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- John SG, Rudolf Shiu-sun W, Ying Ying O. Effects of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series. 2002;238:249–279. http://dx.doi.org/10.3354/meps238249. [Google Scholar]
- Kamler E. Resource allocation in yolk-feeding fish. Rev Fish Biol Fisheries. 2008;18:143–200. http://dx.doi.org/10.1007/s11160-007-9070-x. [Google Scholar]
- Kanamori A, Nagahama Y, Egami N. Development of the tissue architecture in the gonads of the medaka Oryzias latipes. Zoological science. 1985;2:695–706. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. http://dx.doi.org/10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- King-Heiden TC, Mehta V, Xiong KM, Lanham KA, Antkiewicz DS, Ganser A, Heideman W, Peterson RE. Reproductive and developmental toxicity of dioxin in fish. Molecular and Cellular Endocrinology. 2012;354:121–138. doi: 10.1016/j.mce.2011.09.027. http://dx.doi.org/10.1016/j.mce.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Murata K, Naruse K, Tanaka M. Medaka: Biology, management, and experimental protocols. John Wiley & Sons, Ltd; Sigapore: 2009. [Google Scholar]
- Koger CS, Teh SJ, Hinton DE. Determining the sensitive developmental stages of intersex induction in medaka (Oryzias latipes) exposed to 17β-estradiol or testosterone. Marine Environmental Research. 2000;50:201–206. doi: 10.1016/s0141-1136(00)00068-4. http://dx.doi.org/10.1016/S0141-1136(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Korwin-Kossakowski M. Fish hatching strategies: A review. Rev Fish Biol Fisheries. 2012;22:225–240. http://dx.doi.org/10.1007/s11160-011-9233-7. [Google Scholar]
- Lai KP, Li JW, Tse ACK, Wang SY, Chan TF, Wu RSS. Differential responses of female and male brains to hypoxia in the marine medaka Oryzias melastigma. Aquatic Toxicology. 2016;172:36–43. doi: 10.1016/j.aquatox.2015.12.016. http://dx.doi.org/10.1016/j.aquatox.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Landry CA, Steele SL, Manning S, Cheek AO. Long term hypoxia suppresses reproductive capacity in the estuarine fish, Fundulus grandis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2007;148:317–323. doi: 10.1016/j.cbpa.2007.04.023. http://dx.doi.org/10.1016/j.cbpa.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Latimer JS, Zheng J. The sources, transport and fate of PAHs in the marine environment. In: Douben PE, editor. PAHs: An Ecotoxicological Perspective. John Willey and Sons; West Sussex, UK: 2003. pp. 9–53. [Google Scholar]
- Lawrence C, Adatto I, Best J, James A, Maloney K. Generation time of zebrafish (Danio rerio) and medakas (Oryzias latipes) housed in the same aquaculture facility. Lab Animal. 2012;41:158–165. doi: 10.1038/laban0612-158. [DOI] [PubMed] [Google Scholar]
- Le Bihanic F, Clérandeau C, Le Menach K, Morin B, Budzinski H, Cousin X, Cachot J. Developmental toxicity of PAH mixtures in fish early life stages. Part II: Adverse effects in Japanese medaka. Environ Sci Pollut Res. 2014;21:13732–13743. doi: 10.1007/s11356-014-2676-3. http://dx.doi.org/10.1007/s11356-014-2676-3. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Smith FM, Croll RP. From inflation to flotation: Contribution of the swimbladder to whole-body density and swimming depth during development of the zebrafish (Danio rerio) Zebrafish. 2010;7:85–96. doi: 10.1089/zeb.2009.0616. http://dx.doi.org/10.1089/zeb.2009.0616. [DOI] [PubMed] [Google Scholar]
- Marty GD, Hinton DE, Cech JJ. Oxygen consumption by larval Japanese medaka with inflated or uninflated swim bladders. Transactions of the American Fisheries Society. 1995a;124:623–627. http://dx.doi.org/10.1577/1548-8659(1995)124<0623:NOCBLJ>2.3.CO;2. [Google Scholar]
- Marty GD, Hinton DE, Summerfelt RC. Histopathology of swimbladder noninflation in walleye (Stizostedion vitreum) larvae: Role of development and inflammation. Aquaculture. 1995b;138:35–48. http://dx.doi.org/10.1016/0044-8486(95)01129-3. [Google Scholar]
- Matson CW, Timme-Laragy AR, Di Giulio RT. Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish. Chemosphere. 2008;74:149–154. doi: 10.1016/j.chemosphere.2008.08.016. http://dx.doi.org/10.1016/j.chemosphere.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M. Sex determination in the teleost medaka, Oryzias latipes. Annual review of genetics. 2005;39:293–307. doi: 10.1146/annurev.genet.39.110304.095800. http://dx.doi.org/10.1146/annurev.genet.39.110304.095800. [DOI] [PubMed] [Google Scholar]
- McElroy AE, Bogler A, Weisbaum D, Norris M, Mendelman LV, Setlow R, Winn R. Uptake, metabolism, mutant frequencies and mutational spectra in λ transgenic medaka embryos exposed to benzo[α]pyrene dosed sediments. Marine Environmental Research. 2006;62(Supplement 1):S273–S277. doi: 10.1016/j.marenvres.2006.04.019. http://dx.doi.org/10.1016/j.marenvres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. http://dx.doi.org/10.1016/0092-8674(92)90471-N. [DOI] [PubMed] [Google Scholar]
- McKim JM. Evaluation of tests with early life stages of fish for predicting long-term toxicity. Journal of the Fisheries Research Board of Canada. 1977;34:1148–1154. http://dx.doi.org/10.1139/f77-172. [Google Scholar]
- Miller HD, Clark BW, Hinton DE, Whitehead A, Martin S, Kwok KW, Kullman SW. Anchoring ethinylestradiol induced gene expression changes with testicular morphology and reproductive function in the medaka. PLoS ONE. 2012;7:e52479. doi: 10.1371/journal.pone.0052479. http://dx.doi.org/10.1371/journal.pone.0052479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Wang J, Jin F, Wang X, Hong H. Comparative embryotoxicity of phenanthrene and alkyl-phenanthrene to marine medaka (Oryzias melastigma) Marine Pollution Bulletin. 2014;85:505–515. doi: 10.1016/j.marpolbul.2014.01.040. http://dx.doi.org/10.1016/j.marpolbul.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Oxendine SL, Cowden J, Hinton DE, Padilla S. Adapting the medaka embryo assay to a high-throughput approach for developmental toxicity testing. NeuroToxicology. 2006;27:840–845. doi: 10.1016/j.neuro.2006.02.009. http://dx.doi.org/10.1016/j.neuro.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Padilla S, Cowden J, Hinton DE, Yuen B, Law S, Kullman SW, Johnson R, Hardman RC, Flynn K, Au DWT. Use of medaka in toxicity testing, Current Protocols in Toxicology. John Wiley & Sons, Inc; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrichon P, Le Bihanic F, Bustamante P, Le Menach K, Budzinski H, Cachot J, Cousin X. Influence of sediment composition on PAH toxicity using zebrafish (Danio rerio) and Japanese medaka (Oryzias latipes) embryo-larval assays. Environ Sci Pollut Res. 2014;21:13703–13719. doi: 10.1007/s11356-014-3502-7. http://dx.doi.org/10.1007/s11356-014-3502-7. [DOI] [PubMed] [Google Scholar]
- Pincetich CA, Viant MR, Hinton DE, Tjeerdema RS. Metabolic changes in Japanese medaka (Oryzias latipes) during embryogenesis and hypoxia as determined by in vivo 31P NMR. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2005;140:103–113. doi: 10.1016/j.cca.2005.01.010. http://dx.doi.org/10.1016/j.cca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Rabalais NN, Díaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences. 2010;7:585–619. http://dx.doi.org/10.5194/bg-7-585-2010. [Google Scholar]
- Rasband WS. ImageJ. Vol. 1. U. S. National Institutes of Health Bethesda; Maryland, USA: 2014. p. 46. Web Site: http://imagej.nih.gov/ij/ [Google Scholar]
- Richards JG. Metabolic and Molecular Responses of Fish to Hypoxia. In: Richards JG, Farrell AP, Colin JB, editors. Fish Physiology. Academic Press; San Diego, CA: 2009. pp. 443–485. [Google Scholar]
- Rose WL, French BL, Reichert WL, Faisal M. DNA adducts in hematopoietic tissues and blood of the mummichog (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, Virginia. Marine Environmental Research. 2000;50:581–589. doi: 10.1016/s0141-1136(00)00252-x. http://dx.doi.org/10.1016/S0141-1136(00)00252-X. [DOI] [PubMed] [Google Scholar]
- Roussel JM. Carry-over effects in brown trout (Salmo trutta): Hypoxia on embryos impairs predator avoidance by alevins in experimental channels. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:786–792. http://dx.doi.org/10.1139/f07-055. [Google Scholar]
- Schartl M. A comparative view on sex determination in medaka. Mechanisms of Development. 2004;121:639–645. doi: 10.1016/j.mod.2004.03.001. http://dx.doi.org/10.1016/j.mod.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Seemann F, Peterson DR, Witten PE, Guo BS, Shanthanagouda AH, Ye RR, Zhang G, Au DWT. Insight into the transgenerational effect of benzo[a]pyrene on bone formation in a teleost fish (Oryzias latipes) Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2015;178:60–67. doi: 10.1016/j.cbpc.2015.10.001. http://dx.doi.org/10.1016/j.cbpc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Shang EHH, Wu RSS. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environmental Science & Technology. 2004;38:4763–4767. doi: 10.1021/es0496423. http://dx.doi.org/10.1021/es0496423. [DOI] [PubMed] [Google Scholar]
- Shang EHH, Yu RMK, Wu RSS. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio) Environmental Science & Technology. 2006;40:3118–3122. doi: 10.1021/es0522579. http://dx.doi.org/10.1021/es0522579. [DOI] [PubMed] [Google Scholar]
- Shanthanagouda AH, Guo BS, Ye RR, Chao L, Chiang MWL, Singaram G, Cheung NKM, Zhang G, Au DWT. Japanese medaka: Non-mammalian vertebrate model for studying sex and age-related bone metabolism in vivo. PLoS ONE. 2014;9:e88165. doi: 10.1371/journal.pone.0088165. http://dx.doi.org/10.1371/journal.pone.0088165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierhoff KL, Targett TE, Grecay PA. Hypoxia tolerance of the mummichog: The role of access to the water surface. Journal of Fish Biology. 2003;63:580–592. http://dx.doi.org/10.1046/j.1095-8649.2003.00172.x. [Google Scholar]
- Sun L, Zuo Z, Chen M, Chen Y, Wang C. Reproductive and transgenerational toxicities of phenanthrene on female marine medaka (Oryzias melastigma) Aquatic Toxicology. 2015;162:109–116. doi: 10.1016/j.aquatox.2015.03.013. http://dx.doi.org/10.1016/j.aquatox.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Thomas P, Rahman MS. Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proceedings of the Royal Society B: Biological Sciences. 2011;279:28–38. doi: 10.1098/rspb.2011.0529. http://dx.doi.org/10.1098/rspb.2011.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Rahman MS, Kummer JA, Lawson S. Reproductive endocrine dysfunction in Atlantic croaker exposed to hypoxia. Marine Environmental Research. 2006;62(Supplement 1):S249–S252. doi: 10.1016/j.marenvres.2006.04.031. http://dx.doi.org/10.1016/j.marenvres.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Path EM, Nystrom GS, Venables BJ, Sellin Jeffries MK. Early life stage exposure to BDE-47 causes adverse effects on reproductive success and sexual differentiation in fathead minnows (Pimephales promelas) Environmental Science & Technology. 2016;50:7834–7841. doi: 10.1021/acs.est.6b02147. http://dx.doi.org/10.1021/acs.est.6b02147. [DOI] [PubMed] [Google Scholar]
- Timmerman CM, Chapman LJ. Patterns of hypoxia in a coastal salt marsh: Implications for ecophysiology of resident fishes. Florida Scientist. 2004;67:80–91. [Google Scholar]
- Turner RE, Rabalais NN, Justic D. Predicting summer hypoxia in the northern Gulf of Mexico: Riverine N, P, and Si loading. Marine Pollution Bulletin. 2006;52:139–148. doi: 10.1016/j.marpolbul.2005.08.012. http://dx.doi.org/10.1016/j.marpolbul.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Tyler RM, Brady DC, Targett TE. Temporal and spatial dynamics of diel-cycling hypoxia in estuarine tributaries. Estuaries and Coasts. 2009;32:123–145. http://dx.doi.org/10.1007/s12237-008-9108-x. [Google Scholar]
- Vanderplancke G, Claireaux G, Quazuguel P, Huelvan C, Corporeau C, Mazurais D, Zambonino-Infante JL. Exposure to chronic moderate hypoxia impacts physiological and developmental traits of European sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem. 2015;41:233–242. doi: 10.1007/s10695-014-0019-4. http://dx.doi.org/10.1007/s10695-014-0019-4. [DOI] [PubMed] [Google Scholar]
- Vignet C, Devier MH, Le Menach K, Lyphout L, Potier J, Cachot J, Budzinski H, Bégout ML, Cousin X. Long-term disruption of growth, reproduction, and behavior after embryonic exposure of zebrafish to PAH-spiked sediment. Environ Sci Pollut Res. 2014;21:13877–13887. doi: 10.1007/s11356-014-2585-5. http://dx.doi.org/10.1007/s11356-014-2585-5. [DOI] [PubMed] [Google Scholar]
- Vines CA, Robbins T, Griffin FJ, Cherr GN. The effects of diffusible creosote-derived compounds on development in Pacific herring (Clupeapallasi) Aquatic Toxicology. 2000;51:225–239. doi: 10.1016/s0166-445x(00)00107-7. http://dx.doi.org/10.1016/S0166-445X(00)00107-7. [DOI] [PubMed] [Google Scholar]
- Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Research. 1990;50:5978–5986. [PubMed] [Google Scholar]
- Vogelbein WK, Unger MA. Liver carcinogenesis in a non-migratory fish: The association with polycyclic aromatic hydrocarbon exposure. Bull Eur Assoc Fish Pathol. 2006;26:11–20. [Google Scholar]
- Walker SE, Dickhut RM. Sources of PAHs to sediments of the Elizabeth River, VA. Soil Sediment Contam. 2001;10:611–632. http://dx.doi.org/10.1080/20015891109464. [Google Scholar]
- Walker SE, Dickhut RM, Chisholm-Brause C. Polycyclic aromatic hydrocarbons in a highly industrialized urban estuary: Inventories and trends. Environmental Toxicology and Chemistry. 2004;23:2655–2664. doi: 10.1897/03-628. http://dx.doi.org/10.1897/03-628. [DOI] [PubMed] [Google Scholar]
- Wang SY, Lau K, Lai KP, Zhang JW, Tse ACK, Li JW, Tong Y, Chan TF, Wong CKC, Chiu JMY, Au DWT, Wong AST, Kong RYC, Wu RSS. Hypoxia causes transgenerational impairments in reproduction of fish. Nature Communications. 2016;7:12114. doi: 10.1038/ncomms12114. http://dx.doi.org/10.1038/ncomms12114 http://www.nature.com/articles/ncomms12114#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor aonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. http://dx.doi.org/10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ATD, Planchart A, Mattingly CJ, Winkler C, Reif DM, Kullman SW. Embryonic exposure to TCDD impacts osteogenesis of the axial skeleton in Japanese medaka, Oryzias latipes. Toxicological Sciences. 2016;155:485–496. doi: 10.1093/toxsci/kfw229. http://dx.doi.org/10.1093/toxsci/kfw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Zhu S, Willett KL, Di Giulio RT. Effect of CYP1A inhibition on the biotransformation of benzo[a]pyrene in two populations of Fundulus heteroclitus with different exposure histories. Aquatic Toxicology. 2009;92:195–201. doi: 10.1016/j.aquatox.2009.01.009. http://dx.doi.org/10.1016/j.aquatox.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RSS, Zhou BS, Randall DJ, Woo NYS, Lam PKS. Aquatic hypoxia is an endocrine disruptor and impairs fish reproduction. Environmental Science & Technology. 2003;37:1137–1141. doi: 10.1021/es0258327. http://dx.doi.org/10.1021/es0258327. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Medaka (killifish): Biology and strains. Keigaku Publishing Co.; Tokyo, Japan: 1975. [Google Scholar]
- Yanagida GK, Anulacion BF, Bolton JL, Boyd D, Lomax DP, Paul Olson O, Sol SY, Willis M, Ylitalo GM, Johnson LL. Polycyclic aromatic hydrocarbons and risk to threatened and endangered Chinook salmon in the Lower Columbia River Estuary. Archives of Environmental Contamination and Toxicology. 2012;62:282–295. doi: 10.1007/s00244-011-9704-9. http://dx.doi.org/10.1007/s00244-011-9704-9. [DOI] [PubMed] [Google Scholar]
- Yang H, Cao ZD, Fu SJ. The effects of diel-cycling hypoxia acclimation on the hypoxia tolerance, swimming capacity and growth performance of southern catfish (Silurus meridionalis) Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2013;165:131–138. doi: 10.1016/j.cbpa.2013.02.028. http://dx.doi.org/10.1016/j.cbpa.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Yu RMK, Chaturvedi G, Tong SKH, Nusrin S, Giesy JP, Wu RSS, Kong RYC. Evidence for microRNA-mediated regulation of steroidogenesis by hypoxia. Environmental Science & Technology. 2015;49:1138–1147. doi: 10.1021/es504676s. http://dx.doi.org/10.1021/es504676s. [DOI] [PubMed] [Google Scholar]
- Yu RMK, Chu DLH, Tan T-F, Li VWT, Chan AKY, Giesy JP, Cheng SH, Wu RSS, Kong RYC. Leptin-mediated modulation of steroidogenic gene expression in hypoxic zebrafish embryos: Implications for the disruption of sex steroids. Environmental Science & Technology. 2012;46:9112–9119. doi: 10.1021/es301758c. http://dx.doi.org/10.1021/es301758c. [DOI] [PubMed] [Google Scholar]
- Yu RMK, Ng PKS, Tan T, Chu DLH, Wu RSS, Kong RYC. Enhancement of hypoxia-induced gene expression in fish liver by the aryl hydrocarbon receptor (AhR) ligand, benzo[a]pyrene (BaP) Aquatic Toxicology. 2008;90:235–242. doi: 10.1016/j.aquatox.2008.09.004. http://dx.doi.org/10.1016/j.aquatox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Zhai W, Zhao H, Zheng N, Xu Y. Coastal acidification in summer bottom oxygen-depleted waters in northwestern-northern Bohai Sea from June to August in 2011. Chinese Science Bulletin. 2012;57:1062–1068. http://dx.doi.org/10.1007/s11434-011-4949-2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


