Abstract
Due to the advent of antiretroviral therapy HIV is no longer a terminal disease and the HIV infected patients are becoming increasingly older. While this is a major success, with increasing age comes an increased risk for disease. The age-related comorbidities that HIV infected patients experience suggest that they suffer from accelerated aging. One possible contributor to this accelerated aging is cellular senescence, an age-associated response that can occur prematurely in response to stress, and that is emerging as a contributor to disease and aging. HIV patients experience several stressors such as the virus itself, antiretroviral drugs and to a lesser extent, substance abuse that can induce cellular senescence. This review summarizes the current knowledge of senescence induction in response to these stressors and their relation to the comorbidities in HIV patients. Cellular senescence may be a possible therapeutic target for these comorbidities.
Keywords: Senescence, HIV, HAART, HIV-comorbidities, Premature aging, Substance abuse
1. Introduction
HIV-1 infection is a world-wide pandemic that affects more than 36 million people. In the United States alone it is estimated that more than 1 million people are infected with HIV (Nguyen and Holodniy, 2008). The HIV infected population is becoming increasingly older, mainly due to the introduction of highly active antiretroviral therapy (HAART) (Bhatia et al., 2012). The Center for Disease Control CDC has estimated that by 2015, more than 50% of the HIV infected population in the United States will be 50 years of age or greater (Effros et al., 2008). While this heralds transforming of HIV from a terminal to a manageable disease, the increased age remains a problem because aging is a risk factor for a variety of diseases (Niccoli and Partridge, 2012). In fact, HIV-infected patients suffer from many similar ailments experienced in the elderly, equivalently affecting their quality of life. This has led to the theory that patients experience a premature aging phenotype (Guaraldi et al., 2011). Aging is considered a loss of physiological integrity, leading to systemic decline and impaired homeostasis (Lopez-Otin et al., 2013). On a mechanistic level this is thought to occur via the effects of chronic inflammation, oxidative stress and mitochondrial dysfunction (Jenny, 2012). The ultimate result is a decline in various organ systems such as cardiovascular, renal, skeletal and nervous (Jenny et al., 2012). It is therefore relevant that HIV infected patients suffer from comorbidities associated with these systems such as atherosclerosis, diabetes, renal failure, osteoporosis and neurological deficits (Guaraldi et al., 2011; Letendre, 2011). Growing evidence reveals a physiological role for cellular senescence; an age-related phenomenon, in age-associated pathology (Campisi, 2013; Campisi et al., 2011) and the presence of senescent cells may have implications for the premature aging phenotype that accompanies HIV-infected patients. While premature aging in general has been discussed in relation to HIV-infected patients and their comorbidities, there has been no overview of cellular senescence as a possible contributor to premature aging in HIV patients. This review aims to evaluate the role of cellular senescence in the aging of HIV-infected patients. We describe the premature senescence inducing capabilities of three factors experienced by HIV patients: the virus itself, antiretroviral drugs, and substance abuse. We propose that one or more of these factors may contribute to cellular senescence and consequently to premature aging in HIV infected patients.
2. Senescence
Cellular senescence is an age-related phenotype originally discovered to occur in vitro after extensive cell passaging (Hayflick and Moorhead, 1961). Subsequent studies have demonstrated that it is associated with the telomere attrition that occurs with successive rounds of DNA replication (Bodnar et al., 1998). More recently senescence has been found to occur prematurely in response to a variety of insults. Oncogene-induced senescence (OIS) can occur via the activation of tumorigenic signals such as oncogenic RAS and Myc (Lee et al., 2011; Serrano et al., 1997). Stress-induced premature senescence (SIPS) can occur in response to cytotoxic stimuli such as proteasome inhibition and oxidative stress (Bitto et al., 2010; Chen et al., 1995; Wan et al., 2014a). Regardless of the inducer, there are several phenotypes generally shared among senescent cells including cell cycle arrest, increased senescence-associated beta galactosidase (SA-β Gal) activity, expression of the cell cycle inhibitors p16 and p21, DNA damage, heterochromatin formation, and the secretion of pro-inflammatory cytokines and proteases known as the senescence-associated secretory phenotype (SASP) (Rodier and Campisi, 2011).
There are several mechanisms proposed for how senescence leads to disease in vivo. Since skeletal muscle and fat tissue progenitor cells are highly prone to senescence in BubR1 progeroid mice (Baker et al., 2013), it has been proposed that senescence acts in a cell autonomous manner to deplete the pool of cycling cells available to an organism. This is thought to occur by depleting stem and progenitor cells as well as by disrupting the stem cell niche. The tissue homeostasis and regenerative capacity of an organism can subsequently become impaired. In a study that involved a construct to clear senescent cells in aged mice, adipose progenitor cells were dramatically decreased in white adipose tissue of senescence cleared mice compared to uncleared control mice (Baker et al., 2016). Thus, there is in vivo evidence of senescence affecting stem cells.
Senescence may also contribute to disease in a cell non-autonomous manner through the SASP. The myriad of proteases and pro-inflammatory cytokines secreted by senescent cells are thought to have detrimental effects on the local microenvironment. These effects include perturbation of tissue structure by the cleavage of membrane-bound receptors and extracellular matrix proteins (Coppe et al., 2008), stimulation of tissue fibrosis (Laberge et al., 2012), agitation of the stem cell niche (Pricola et al., 2009) and paracrine senescence by induction of senescence in healthy neighboring cells (Acosta et al., 2013). These inflammatory processes are important because inflammation has been implicated in many age-related diseases including cardiovascular, Alzheimer’s and Parkinson’s diseases (Jabbari Azad et al., 2014; Libby, 2006; Yan et al., 2014). Accordingly, a mouse model of low-grade chronic inflammation has even been shown to result in increased senescent cells and accelerated aging (Jurk et al., 2014).
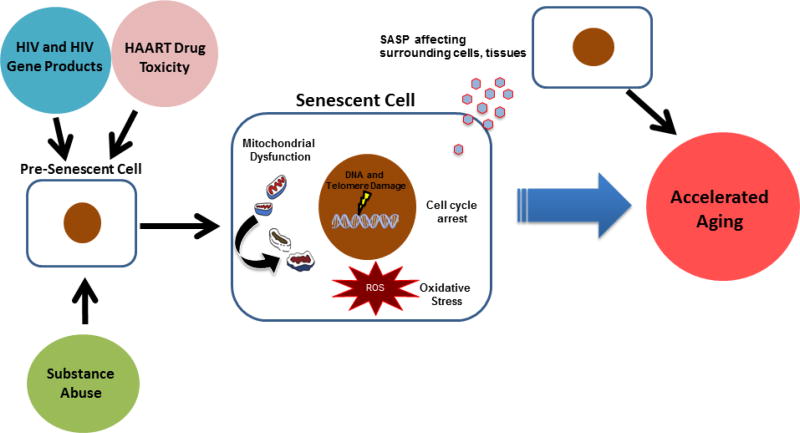
Senescence may therefore be playing a role in the various co-morbidities experienced by HIV infected patients. Indeed, a literature-based gene analysis found a very strong association between HIV and senescence genes (Zhao et al., 2016). However, little is known about what specifically induces senescence in HIV-infected patients. These patients are exposed to a variety of stressors including HIV gene products, HAART drugs and to a lesser extent, addictive compounds via substance abuse that likely all contribute to the induction of senescence and consequentially premature aging (Figure 1). The next sections will discuss what is known for each of these stimuli as inducers of the senescence program.
Fig. 1.
Senescence-associated accelerated aging in response to HIV-related factors. HIV infected patients are exposed to a variety of stressors that can induce premature cellular senescence. These factors include the virus itself, HAART drugs and addictive compounds from substance abuse. Cellular senescence can contribute to disease in a cell autonomous or cell non-autonomous manner. Autonomously a senescent cell undergoes permanent growth arrest, depleting an organism’s pool of cycling cells and impairing its regenerative capacity. Senescent cells can also experience a variety of internal stressors such as DNA and telomere damage, mitochondrial dysfunction and oxidative stress due to increased ROS production. In a non-autonomous manner, senescent cells secrete a variety of pro-inflammatory cytokines and proteases known as the SASP regardless of whether they are immune or non-immune cells. The SASP is able to induce senescence in neighboring cells and create a pro-inflammatory microenvironment, disrupting the overall homeostasis of the surrounding tissue. Induction of the senescence program in HIV-infected patients may consequently lead to the accelerated aging phenotype observed in these patients. HAART, Highly Active Antiretroviral Therapy; SASP, Senescence Associated Secretory Phenotype.
3. HIV and senescence
3.1 HIV life cycle
HIV infection is a multi-step process that involves the cooperation of many viral proteins (Simon et al., 2006). Infection primarily occurs in CD4+ cells expressing the co-receptors CXCR4 or CCR5. The virus utilizes these receptors with the envelope proteins gp120 and gp41(Wilen et al., 2012). gp120 binds to CD4 and undergoes a conformational change that allows gp41 to penetrate the cell membrane. The viral and cell membranes are then able to fuse, allowing the contents of the viral capsid to be deposited into the cell. Once inside the cell, HIV reverse transcriptase converts the viral single stranded RNA into double stranded DNA in an error bound process that may account for the drug resistance and immune avoidance capabilities of the virus (Sarafianos et al., 2009). Once transcribed, the viral genome is incorporated into the host genome using the viral integrase protein (Craigie and Bushman, 2012). HIV can then use host machinery for transcription of the virus but several viral regulatory proteins are still important and produced early during the lifecycle. One of these early proteins, nef, is not directly involved with HIV gene expression but has multiple functions including downregulation of CD4, perturbation of T-cell activation and increasing viral infectivity(Brady et al., 1993; Collins et al., 1998; Kestler et al., 1991). Other early proteins include tat, a transcriptional transactivator which increases expression of all HIV genes (Das et al., 2011)and rev, which helps to export incompletely spliced transcripts into the cytoplasm, allowing translation of structural proteins (Pollard and Malim, 1998). Viral proteases cleave these proteins into their mature forms. The structural proteins include gp160 which is cleaved into the envelope proteins gp120 and gp41 mentioned above (Wilen et al., 2012), and gag which is cleaved into the viral capsid and matrix (Freed, 1998). Once in their mature forms, the viral proteins are assembled into a mature HIV virion which can then go on to infect another cell. With such a variety of proteins, HIV infection can be very stressful on a cell; several studies have thus looked to see if this could induce senescence.
3.2. HIV-induced senescence and co-morbidities
In a recent study, bone marrow mesenchymal stem cells (MSCs) were treated with the HIV proteins tat and nef. Both proteins induced premature MSC senescence which was associated with oxidative stress and mitochondrial dysfunction (Beaupere et al., 2015). Interestingly, tat and nef seem to induce senescence by alternate mechanisms, as tat was associated with NF-κB while nef was associated with autophagy inhibition. Moreover, both proteins impaired the osteoblastic potential of MSCs, suggesting that MSC senescence could be a contributor to osteoporosis in HIV infected patients.
Other studies take an in vivo approach by measuring senescence in cohorts of HIV infected patients. In one such cohort, corneal endothelial cells of HIV+ subjects had increased features of aging and senescence such as decreased cell density and variation in cell size and shape compared to the uninfected (Pathai et al., 2013b). Importantly levels of p16, a marker of senescence, were increased in peripheral blood leukocytes of the patients that had decreased endothelial cell density. In a sub-Saharan Africa cohort, p16 levels were also higher in peripheral blood leukocytes of HIV infected patients compared to uninfected controls (Pathai et al., 2013a). Additionally, these cells had decreased telomere length, suggesting a correlation between senescence and accelerated aging.
This accelerated aging phenotype appears to be related to HIV disease progression. CD8+ T-cell telomerase activity in HIV-infected patients was compared to CD38 levels, a measure of disease progression (Chou et al., 2013). Decreases in telomerase activity and other biomarkers such as adenosine deaminase were strongly associated with an increase in CD38. Therefore, CD8+ T-cell senescence may be a potential biomarker of immune status in HIV infected patients.
Other cohorts have studied the relationship between senescence and HIV-associated co-morbidities. For instance, one study examined chronic obstructive pulmonary disease (COPD). CD4+ T-cell senescence and circulating levels of IL-6, a component of the SASP, were correlated with decreased pulmonary function (Fitzpatrick et al., 2014). The study also examined telomere length, a marker of replicative senescence, in peripheral blood mononuclear cells (PBMCs) and found that this too was associated with pulmonary dysfunction in HIV-infected patients. Another HIV-infected cohort was examined to find the relationship between COPD and senescence in peripheral leukocytes. In this study, leukocyte telomere length was shorter in HIV-infected patients compared to control and shorter telomeres were once again associated with decreased pulmonary function (Liu et al., 2015a).
Taken together, these studies demonstrate that HIV factors can induce senescence and that there is an association between senescence, aging and disease. However, there are very few in vitro studies being performed to examine HIV-associated senescence. Since More HIV gene products need to be tested as well as viral infection in vitro. While patient cohort studies are important, there is homogeneity to the cell types examined. More studies need to evaluate senescence in non-immune cells of HIV-infected patients. In addition, since most HIV-infected patients are on HAART, these drugs constitute another potential inducer of the senescence program. The next section examines a link between HAART drugs and senescence.
4. HAART drug induced senescence
4.1. Classes of HAART Drugs
HIV infection used to be considered a terminal disease primarily due to the fact that early on in the epidemic only one class of antiretroviral drugs existed, the nucleoside reverse transcriptase inhibitors (NRTIs), leading to drug resistant strains of HIV. Eventually a new class of antiretroviral drugs called protease inhibitors (PIs) were created and the combination of multiple classes of drugs to treat HIV became known as highly active antiretroviral therapy (HAART), leading to the modern era of HIV infection as a chronic but manageable disease (Sued et al., 2016). There are now five classes of antiretroviral drugs used in HAART that target different facets of the HIV lifecycle. NRTIs and nucleotide reverse transcriptase inhibitors (NtRTIs) competitively inhibit reverse transcriptase by acting as nucleoside and nucleotide analogs respectively, preventing the DNA chain from being extended (Nolan and Mallal, 2004). A second class of HAART drugs are the non-nucleoside reverse transcriptase inhibitors (NNRTIs) which also inhibit the reverse transcriptase but non-competitively by binding to an allosteric region of the enzyme (Sluis-Cremer and Tachedjian, 2008). The third class is constituted by PIs that target the viral protease enzyme, therefore preventing cleavage of viral proteins to their mature form (Nolan, 2003). A fourth class, entry inhibitors (EIs) interfere with the binding and entry of the virus into the host cell and the class as a whole does not have a specific target like other classes. Some targets of EIs include co-receptors on the host cell and the viral envelope protein (Briz et al., 2006). The last class of HAART drugs are the integrase inhibitors (IIs) which target the viral integrase, preventing incorporation of the viral DNA into the host genome (Messiaen et al., 2013). Toxicities due to the above classes of drugs have been implicated in many of the co-morbidities associated with HIV (Nolan, 2003; Nolan and Mallal, 2004). There are various lines of evidence supporting that HAART drugs accelerate aging in HIV patients (Smith et al., 2012), and studies that explore the relationship between HAART drug induced senescence and disease are therefore starting to emerge.
4.2. HAART drug induced senescence and co-morbidities
PIs have been demonstrated to inhibit prelamin A maturation into lamin A (Caron et al., 2003). Since impairment of the mature lamin is a mechanism for lipodystrophies, there is a link between PIs and lipodystrophy (Mattout et al., 2006). Prelamin A accumulation also has been linked to age-related disease due to it being the underlying factor for Hutchinson-Gilford progeria syndrome (HGPS), a premature aging syndrome of which lipodystrophy is one symptom (Young et al., 2005). Human fibroblasts treated with the PIs indinavir or nelfinavir had prelamin A accumulation, premature senescence and signs of oxidative stress (Caron et al., 2007). Interestingly, inhibition of prelamin A farnesylation decreases both oxidative stress and senescence in these cells, suggesting that accumulation of unprocessed prelamin A due to PIs induces senescence via oxidative damage. Adipose tissue from lipodystrophic HIV-infected patients who were on these PIs showed an increase in the senescence marker p16, providing an in vivo relationship between senescence and lipodystrophy (Caron et al., 2007).
Another age-related HIV co-morbidity that is linked to prelamin A accumulation is cardiovascular disease (McClintock et al., 2006). The senescence link was explored in a study that treated vascular smooth muscle cells (VSMCs) with the PIs atazanavir, lopinavir and ritonavir. Treated VSMCs not only had increased prelamin A accumulation, oxidative damage and senescence induction, but also calcification (Afonso et al., 2015), a contributor to atherosclerosis (Kalra and Shanahan, 2012). A separate study treated human coronary artery endothelial cells (HCAECs) with the PIs lopinavir and ritonavir. Similar to the studies mentioned above, PI treated HCAECs had increases in prelamin A accumulation, senescence markers and oxidative stress which were prevented by a farnesylation inhibitor (Lefevre et al., 2010). However, this study also demonstrated that the statin class of drugs used to treat cardiovascular disease also inhibited PI induced premature senescence due to their own farnesylation inhibitory activity. Moreover, PBMCs from HIV-infected patients on ritonavir had increased levels of the senescence markers p53 and p21 which were lower in patients that were also on a statin (Lefevre et al., 2010).
Osteoporosis is another age associated co-morbidity suffered by HIV patients which seems to partly be caused by PIs (Duvivier et al., 2009). The mechanism behind this decrease in bone mineral density is not well known but one study proposed a dysfunction in precursors due to senescence. Bone marrow mesenchymal stem cells treated with combinations of the PIs atazanavir, lopinavir and ritonavir underwent premature senescence in a farnesylated prelamin A-dependent manner (Hernandez-Vallejo et al., 2013). Most importantly, these senescent MSCs had decreased osteoblastic and adipogenic differentiation potential which was reversed by pravastatin. Thus, statins may be a potential therapy to use in prelamin A senescence based HIV co-morbidities.
Senescence has been examined in other classes of HAART drugs such as NRTIs because they have been shown to be highly toxic to mitochondria (Johnson et al., 2001) and mitochondrial dysfunction is an inducer of cellular senescence (Moiseeva et al., 2009; Velarde et al., 2012; Wiley et al., 2016). Senescence was induced in medulloblastoma cell lines treated with the NRTI abacavir (Rossi et al., 2009). However, being an immortalized cell line the senescence induction appeared to be related to the inhibition of telomerase. In a study involving non-immortalized cells, fibroblasts and preadipocytes treated with the NRTIs stavudine and zidovudine displayed mitochondrial dysfunction accompanied by the induction of premature senescence (Caron et al., 2008). Interestingly, other NRTIs including abacavir, tenofovir and lamivudine were unable to induce these phenotypes, suggesting that NRTIs have differential effects on senescence induction. The same study showed that adipose tissue from lipodystrophic patients on an NRTI-based therapy that includes stavudine or zidovudine but no PIs had increased markers of senescence and mitochondrial dysfunction. NRTI-induced senescence may therefore be a contributor to lipodystrophy in HIV patients.
Lung and cardiac fibroblasts treated with the NRTIs tenovovir and emtricitabine also underwent premature senescence that was associated with mitochondrial dysfunction (Nacarelli et al., 2016). This senescence induction was associated with increased mTORC1 signaling and rapamycin, an inhibitor of mTORC1, attenuated NRTI-mediated senescence. Thus some NRTIs are able to induce senescence in an mTORC1-dependent manner. Since these results were observed in both lung and cardiac fibroblasts, NRTI-induced senescence may be a contributor to pulmonary and cardiac disease in HIV patients.
Of the NNRTIs, nevirapine is particularly toxic (Robertson et al., 2012) which makes it a very likely senescence inducer. HeLa cells treated with nevirapine underwent premature senescence that was associated with activation of the DNA damage response (Stefanidis et al., 2008). Nevirapine appears to have differential senescence induction capabilities because while it was able induce a growth arrest in both HepG2 cells and THLE2, an immortalized human liver cell line, senescence markers were only present in THLE2 cells (Fang and Beland, 2013). The relevance of these results towards HIV co-morbidities is uncertain because of the use of immortalized cell lines but to date no study has examined NNRTI senescence in primary cells.
No study has examined if entry or integrase inhibitors induce cellular senescence. This is likely due to the fact that these two classes are not usually included in the first line of defense against HIV (Sued et al., 2016). Nevertheless, the senescence inducing ability of these two classes should be studied since this may reveal less senescence-inducing alternatives to the current therapies. For all of the drug classes there has been a sore lack of studies examining senescence induction in central nervous system (CNS) cells. Senescence in the CNS is a novel concept but emerging evidence suggests that CNS senescence may contribute to neurological disorders (Bhat et al., 2012; Wan et al., 2014a). HAART drugs may therefore be contributing to HIV-associated neurocognitive disorders (HAND) via senescence of CNS cells.
Since HIV patients on HAART take a combination of drugs, it can be difficult to attribute in vivo effects to a specific drug or class of drugs, especially when the virus by itself may constitute an additional variable. Studies should therefore be performed using animal models to determine if HAART drugs can induce senescence in vivo. Nevertheless, the in vitro evidence paired with senescence markers displayed in related tissue of HIV patients represents strong support for HAART drug mediated senescence and disease.
5. Substance abuse as a contributor to the induction of senescence in HIV infection
5.1. Accelerated aging in substance abusers
HIV infected individuals have a higher probability of abusing addictive substances (Chang et al., 2007). Consequently, drug abusers make a large fraction of HIV infected individuals. The exact proportion is uncertain due to a reliance on self-report data but some reports estimate as high as 35% (Murrill et al., 2001). There are a variety of complications arising from substance abuse that range from neurological to immunological impediments (Tiwari et al., 2013). Drug abusers are thought to undergo accelerated aging since many of these complications are found in the elderly as well (Reece, 2007). Substance abusers even tend to look older after a period of use (Tiwari et al., 2013). It is therefore possible that substance abuse can be a contributor to the age related co-morbidities of HIV patients through the induction of senescence.
5.2. Substance abuse-induced senescence and disease
In a study of heroin users, PBMCs from these individuals had markedly lower telomerase activity compared to healthy controls (Cheng et al., 2013). MRI analysis found that for the heroin users but not the controls, there was an association between lower telomerase activity and grey matter loss in the right dorsolateral prefrontal cortex (DLPC), a region of the brain associated with age-related decline. Thus, heroin appears to be accelerating aging, and while markers of senescence were not explicitly examined in these patients, the decreased telomerase activity suggests shortened telomeres, a feature of senescent cells (van Deursen, 2014).
Senescence was more explicitly examined in a study of methamphetamine. Mouse embryonic fibroblasts (MEFs) treated with methamphetamine underwent premature cellular senescence in culture (Astarita et al., 2015). This senescence induction was suppressed by a ceramide inhibitor, suggesting that methamphetamine induced senescence is mediated by sphingolipid signaling. In the same study, mice that were treated with methamphetamine had ceramide dependent transcriptional upregulation of senescent markers in skeletal muscle, confirming the in vitro results (Astarita et al., 2015). Methamphetamine induced senescence may therefore be a consideration for the accelerated aging phenotype of HIV patients who abuse this drug.
One of the most commonly abused drugs is alcohol, abuse of which can contribute to liver disease (Tiwari et al., 2013). Hepatocytes from ethanol fed mice showed increased levels of senescence markers compared to control fed mice (Wan et al., 2014b). The study also demonstrated that alcohol-induced inflammation from macrophages enhanced the senescence induction of hepatocytes. Since inflammation is a component of HIV infection (Sued et al., 2016), it is possible that alcohol-medicated hepatocyte senescence could be even more pronounced in the HIV-infected.
The most extensive analyses of senescence induction from drug abuse are based on cigarettes. Several studies have demonstrated that cigarette smoke extract (CSE) induces senescence in lung fibroblasts (Ahmad et al., 2015; Kanaji et al., 2014; Nyunoya et al., 2006). Of these, one study demonstrated that CSE-induced lung fibroblast senescence was mediated by oxidative stress since antioxidants were able to minimize induction of senescence (Kanaji et al., 2014). Oxidative stress appears to play a role in other cell types as well. CSE not only induces senescence in murine skeletal muscle, but this is inhibited by antioxidants (Liu et al., 2015b).
Studies have also demonstrated that CSE induces senescence in epithelial cells. Alveolar epithelial cells (AECs) treated with CSE underwent senescence in a dose dependent manner (Tsuji et al., 2004). In the same study, mice made to inhale cigarette smoke had increased senescence in their AECs. A separate study demonstrated a mechanism using small airway epithelial cells (SAECs) (Ahmad et al., 2015). CSE induced senescence in SAECs due to impaired mitophagy of damaged mitochondria. Oxidative stress once again appeared to be involved as a mitochondria-targeted antioxidant both restored mitophagy and delayed senescence (Ahmad et al., 2015). Interestingly, nicotine may not be the component in CSE that induces senescence since it appears to reduce senescence of endothelial progenitor cells (Junhui et al., 2009).
No study has examined if cocaine can induce premature senescence. However, cocaine has been demonstrated to be very toxic to cells and even induce oxidative stress (Badisa and Goodman, 2012; Yang et al., 2010). It is therefore likely that a side-effect of this toxicity is premature senescence.
Just like with HAART drugs there is a lack of research looking into the senescence induction capabilities of these compounds in CNS cells. Certain drugs such as cocaine and methamphetamine are able to penetrate the blood brain barrier and can readily impact CNS cells (Kousik et al., 2012). They can even weaken the barrier, consequently making it easier for other stressors such as HAART drugs and HIV itself to penetrate the CNS. Studies should therefore be performed that look at the effects of combining these substances on senescence induction. Unlike HIV and HAART, drug abuse does not apply to every HIV-infected patient. Drugs of abuse may therefore not play as big a role in HIV associated co-morbidities as the other two factors. However, enough HIV infected patients are drug abusers that the senescence induction capabilities of these compounds should be considered.
6. Conclusion
Factors common to HIV-infected patients such as HIV, HAART drugs and substance abuse induce premature senescence both in vitro and in vivo. Senescence may therefore be a link to the HIV associated co-morbidities that give patients an accelerated aging phenotype. While there has been plenty of research on immunosenescence, very little has been done looking at senescence of other cell types in response to HIV infection. HIV gene products need to be examined in further detail as well since senescence induction has only been measured in response to nef and tat (Beaupere et al., 2015). While more cell types and organ systems have been studied for HAART drug induced senescence, little has been done in the CNS, a region that is becoming more relevant as HAND severity and prevalence increases with age. In addition, as integrase and entry inhibitors become more commonly used, their senescence induction capabilities need to be studied. There also needs to be animal studies to examine HAART drug mediated senescence in vivo since HIV-infection itself would be a confounding factor in patients. Substance abuse is not experienced in every HIV-infected patient but is prevalent enough to still be a factor to consider. Since researchers have to rely on self-report data for substance abuse, and HIV patients have a high prevalence of substance abuse, it can be difficult to try and isolate senescence induction from these compounds in HIV patients. However, these effects may still be elucidated by comparing uninfected substance abuse patients to non-abusers. Regardless of the stressor, there appears to be some common mechanisms of senescence induction. Most prominent is oxidative stress but there is also mitochondrial dysfunction, inflammation and telomere dysfunction. Targeting these pathways to minimize senescence induction may generate therapies to treat accelerated aging co-morbidities.
Highlights.
HIV infected patients experience accelerated aging
Cellular senescence is a possible contributor to accelerated aging
HIV, antiretroviral drugs and substance abuse induce premature senescence
Targeting senescence may be therapeutic for accelerated aging in HIV patients
Acknowledgments
This work was supported by the National Institute of Health (1RO1NS078283, 1RO1NS78283 and R21AG046943). We would like to thank Dr. Sinan F. Tuzer for his suggestions on revising the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared no conflict of interest.
References
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso P, Auclair M, Boccara F, Vantyghem MC, Katlama C, Capeau J, Vigouroux C, Caron-Debarle M. LMNA mutations resulting in lipodystrophy and HIV protease inhibitors trigger vascular smooth muscle cell senescence and calcification: Role of ZMPSTE24 downregulation. Atherosclerosis. 2015;245:200–211. doi: 10.1016/j.atherosclerosis.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita G, Avanesian A, Grimaldi B, Realini N, Justinova Z, Panlilio LV, Basit A, Goldberg SR, Piomelli D. Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PloS one. 2015;10:e0116961. doi: 10.1371/journal.pone.0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badisa RB, Goodman CB. Effects of chronic cocaine in rat C6 astroglial cells. International journal of molecular medicine. 2012;30:687–692. doi: 10.3892/ijmm.2012.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell reports. 2013;3:1164–1174. doi: 10.1016/j.celrep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaupere C, Garcia M, Larghero J, Feve B, Capeau J, Lagathu C. The HIV proteins Tat and Nef promote human bone marrow mesenchymal stem cell senescence and alter osteoblastic differentiation. Aging cell. 2015;14:534–546. doi: 10.1111/acel.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PloS one. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. Journal of neurovirology. 2012;18:247–255. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- Bitto A, Sell C, Crowe E, Lorenzini A, Malaguti M, Hrelia S, Torres C. Stress-induced senescence in human and rodent astrocytes. Experimental cell research. 2010;316:2961–2968. doi: 10.1016/j.yexcr.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Brady HJ, Pennington DJ, Miles CG, Dzierzak EA. CD4 cell surface downregulation in HIV-1 Nef transgenic mice is a consequence of intracellular sequestration. The EMBO journal. 1993;12:4923–4932. doi: 10.1002/j.1460-2075.1993.tb06186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz V, Poveda E, Soriano V. HIV entry inhibitors: mechanisms of action and resistance pathways. The Journal of antimicrobial chemotherapy. 2006;57:619–627. doi: 10.1093/jac/dkl027. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Seminars in cancer biology. 2011;21:354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M, Auclair M, Donadille B, Bereziat V, Guerci B, Laville M, Narbonne H, Bodemer C, Lascols O, Capeau J, Vigouroux C. Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ. 2007;14:1759–1767. doi: 10.1038/sj.cdd.4402197. [DOI] [PubMed] [Google Scholar]
- Caron M, Auclair M, Sterlingot H, Kornprobst M, Capeau J. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. Aids. 2003;17:2437–2444. doi: 10.1097/00002030-200311210-00005. [DOI] [PubMed] [Google Scholar]
- Caron M, Auclairt M, Vissian A, Vigouroux C, Capeau J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antiviral therapy. 2008;13:27–38. [PubMed] [Google Scholar]
- Chang SL, Beltran JA, Swarup S. Expression of the mu opioid receptor in the human immunodeficiency virus type 1 transgenic rat model. J Virol. 2007;81:8406–8411. doi: 10.1128/JVI.00155-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GL, Zeng H, Leung MK, Zhang HJ, Lau BW, Liu YP, Liu GX, Sham PC, Chan CC, So KF, Lee TM. Heroin abuse accelerates biological aging: a novel insight from telomerase and brain imaging interaction. Transl Psychiatry. 2013;3:e260. doi: 10.1038/tp.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Ramirez CM, Wu JE, Effros RB. Accelerated aging in HIV/AIDS: novel biomarkers of senescent human CD8+ T cells. PloS one. 2013;8:e64702. doi: 10.1371/journal.pone.0064702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS biology. 6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R, Bushman FD. HIV DNA integration. Cold Spring Harb Perspect Med. 2012;2:a006890. doi: 10.1101/cshperspect.a006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AT, Harwig A, Berkhout B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J Virol. 2011;85:9506–9516. doi: 10.1128/JVI.00650-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D, group AHs. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. Aids. 2009;23:817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, Nayfield SG, Plaeger SF, Schmader KE, Ashworth JR, Campanelli C, Clayton CP, Rada B, Woolard NF, High KP. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JL, Beland FA. Differential responses of human hepatocytes to the non-nucleoside HIV-1 reverse transcriptase inhibitor nevirapine. J Toxicol Sci. 2013;38:741–752. doi: 10.2131/jts.38.741. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick ME, Singh V, Bertolet M, Lucht L, Kessinger C, Michel J, Logar A, Weinman R, McMahon D, Norris KA, Vallejo AN, Morris A. Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. Aids. 2014;28:2505–2515. doi: 10.1097/QAD.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed EO. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Experimental cell research. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vallejo SJ, Beaupere C, Larghero J, Capeau J, Lagathu C. HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marrow mesenchymal stem cells: beneficial effect of pravastatin. Aging cell. 2013;12:955–965. doi: 10.1111/acel.12119. [DOI] [PubMed] [Google Scholar]
- Jabbari Azad F, Talaei A, Rafatpanah H, Yousefzadeh H, Jafari R, Talaei A, Farid Hosseini R. Association between Cytokine Production and Disease Severity in Alzheimer’s Disease. Iranian journal of allergy, asthma, and immunology. 2014;13:433–439. [PubMed] [Google Scholar]
- Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med. 2012;13:451–460. [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, Sarnak MJ, Newman AB. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67:970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AA, Ray AS, Hanes J, Suo Z, Colacino JM, Anderson KS, Johnson KA. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–40857. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- Junhui Z, Xiaojing H, Binquan Z, Xudong X, Junzhu C, Guosheng F. Nicotine-reduced endothelial progenitor cell senescence through augmentation of telomerase activity via the PI3K/Akt pathway. Cytotherapy. 2009;11:485–491. doi: 10.1080/14653240902887267. [DOI] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nature communications. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SS, Shanahan CM. Vascular calcification and hypertension: cause and effect. Ann Med 44 Suppl. 2012;1:S85–92. doi: 10.3109/07853890.2012.660498. [DOI] [PubMed] [Google Scholar]
- Kanaji N, Basma H, Nelson A, Farid M, Sato T, Nakanishi M, Wang X, Michalski J, Li Y, Gunji Y, Feghali-Bostwick C, Liu X, Rennard SI. Fibroblasts that resist cigarette smoke-induced senescence acquire profibrotic phenotypes. Am J Physiol Lung Cell Mol Physiol. 2014;307:L364–373. doi: 10.1152/ajplung.00041.2014. [DOI] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Awad P, Campisi J, Desprez PY. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5:39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmitt CA, Reimann M. The Myc/macrophage tango: oncogene-induced senescence, Myc style. Seminars in cancer biology. 2011;21:377–384. doi: 10.1016/j.semcancer.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Auclair M, Boccara F, Bastard JP, Capeau J, Vigouroux C, Caron-Debarle M. Premature senescence of vascular cells is induced by HIV protease inhibitors: implication of prelamin A and reversion by statin. Arterioscler Thromb Vasc Biol. 2010;30:2611–2620. doi: 10.1161/ATVBAHA.110.213603. [DOI] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in antiviral medicine. 2011;19:137–142. [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Liu JC, Leung JM, Ngan DA, Nashta NF, Guillemi S, Harris M, Lima VD, Um SJ, Li Y, Tam S, Shaipanich T, Raju R, Hague C, Leipsic JA, Bourbeau J, Tan WC, Harrigan PR, Sin DD, Montaner J, Man SF. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PloS one. 2015a;10:e0124426. doi: 10.1371/journal.pone.0124426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Su W, Yang X, Bai J, Zhong X, He Z. [Cigarette smoke extract induces senescence of murine skeletal muscle cells by oxidative stress-induced down-regulation of histone deacetylase 2] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015b;31:630–633. 638. [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout A, Dechat T, Adam SA, Goldman RD, Gruenbaum Y. Nuclear lamins, diseases and aging. Curr Opin Cell Biol. 2006;18:335–341. doi: 10.1016/j.ceb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messiaen P, Wensing AM, Fun A, Nijhuis M, Brusselaers N, Vandekerckhove L. Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis. PloS one. 2013;8:e52562. doi: 10.1371/journal.pone.0052562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O, Bourdeau V, Roux A, Deschenes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol. 2009;29:4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrill CS, Prevots DR, Miller MS, Linley LA, Royalty JE, Gwinn M, Seroincidence Study G. Incidence of HIV among injection drug users entering drug treatment programs in four US cities. J Urban Health. 2001;78:152–161. doi: 10.1093/jurban/78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Sell C. Mitochondrial stress induces cellular senescence in an mTORC1-dependent manner. Free Radic Biol Med. 2016;95:133–154. doi: 10.1016/j.freeradbiomed.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453–472. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Nolan D. Metabolic complications associated with HIV protease inhibitor therapy. Drugs. 2003;63:2555–2574. doi: 10.2165/00003495-200363230-00001. [DOI] [PubMed] [Google Scholar]
- Nolan D, Mallal S. Complications associated with NRTI therapy: update on clinical features and possible pathogenic mechanisms. Antiviral therapy. 2004;9:849–863. [PubMed] [Google Scholar]
- Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35:681–688. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, Port J, Christ T, Barclay K, Wood R, Bekker LG, Shiels PG. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. Aids. 2013a;27:2375–2384. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Lawn SD, Shiels PG, Weiss HA, Cook C, Wood R, Gilbert CE. Corneal endothelial cells provide evidence of accelerated cellular senescence associated with HIV infection: a case-control study. PloS one. 2013b;8:e57422. doi: 10.1371/journal.pone.0057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard VW, Malim MH. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS. Evidence of accelerated ageing in clinical drug addiction from immune, hepatic and metabolic biomarkers. Immun Ageing. 2007;4:6. doi: 10.1186/1742-4933-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. Journal of neurovirology. 2012;18:388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. The Journal of cell biology. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Russo G, Puca A, La Montagna R, Caputo M, Mattioli E, Lopez M, Giordano A, Pentimalli F. The antiretroviral nucleoside analogue Abacavir reduces cell growth and promotes differentiation of human medulloblastoma cells. Int J Cancer. 2009;125:235–243. doi: 10.1002/ijc.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Simon V, Ho DD, Abdool Karim Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet. 2006;368:489–504. doi: 10.1016/S0140-6736(06)69157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluis-Cremer N, Tachedjian G. Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors. Virus Res. 2008;134:147–156. doi: 10.1016/j.virusres.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H. Premature and accelerated aging: HIV or HAART? Frontiers in genetics. 2012;3:328. doi: 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis K, Loutradis D, Vassiliou LV, Anastasiadou V, Kiapekou E, Nikas V, Patris G, Vlachos G, Rodolakis A, Antsaklis A. Nevirapine induces growth arrest and premature senescence in human cervical carcinoma cells. Gynecol Oncol. 2008;111:344–349. doi: 10.1016/j.ygyno.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Sued O, Figueroa MI, Cahn P. Clinical challenges in HIV/AIDS: Hints for advancing prevention and patient management strategies. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Nair MP, Saxena SK. Latest trends in drugs of abuse - HIV infection and neuroAIDS. Future virology. 2013;8:121–127. doi: 10.2217/fvl.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde MC, Flynn JM, Day NU, Melov S, Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY) 2012;4:3–12. doi: 10.18632/aging.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, Tang C, Liang L, Xu G. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PloS one. 2014a;9:e89811. doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Benkdane M, Alons E, Lotersztajn S, Pavoine C. M2 kupffer cells promote hepatocyte senescence: an IL-6-dependent protective mechanism against alcoholic liver disease. Am J Pathol. 2014b;184:1763–1772. doi: 10.1016/j.ajpath.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Fu Q, Cheng L, Zhai M, Wu W, Huang L, Du G. Inflammatory response in Parkinson’s disease (Review) Molecular medicine reports. 2014;10:2223–2233. doi: 10.3892/mmr.2014.2563. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yao H, Lu Y, Wang C, Buch S. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PloS one. 2010;5:e13427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SG, Fong LG, Michaelis S. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria--new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res. 2005;46:2531–2558. doi: 10.1194/jlr.R500011-JLR200. [DOI] [PubMed] [Google Scholar]
- Zhao M, Chen L, Qu H. CSGene: a literature-based database for cell senescence genes and its application to identify critical cell aging pathways and associated diseases. Cell Death Dis. 2016;7:e2053. doi: 10.1038/cddis.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]