Abstract
ω-Hydroxy polyunsaturated fatty acids (PUFAs), natural metabolites from arachidonic acid (ARA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were prepared via convergent synthesis approach using two key steps: Cu-mediated C-C bond formation to construct methylene skipped poly-ynes and a partial alkyne hydrogenation where the presence of excess 2-methyl-2-butene as an additive that is proven to be critical for the success of partial reduction of the poly-ynes to the corresponding cis-alkenes without over-hydrogenation. The potential biological function of ω-hydroxy PUFAs in pain was evaluated in naive rats. Following intraplantar injection, 20-hydroxyeicosatetraenoic acid (20-HETE, ω-hydroxy ARA) generated an acute decrease in paw withdrawal thresholds in a mechanical nociceptive assay indicating pain, but no change was observed from rats which received either 20-hydroxyeicosapentaenoic acid (20-HEPE, ω-hydroxy EPA) or 22-hydroxydocosahexaenoic acid (22-HDoHE, ω-hydroxy DHA). We also found that both 20-HEPE and 22-HDoHE are more potent than 20-HETE to activate murine transient receptor potential vanilloid receptor1 (mTRPV1).
Keywords: ω-hydroxy PUFA; 20-HETE, 20-HEPE, 22-HDoHE; TRPV1; pain
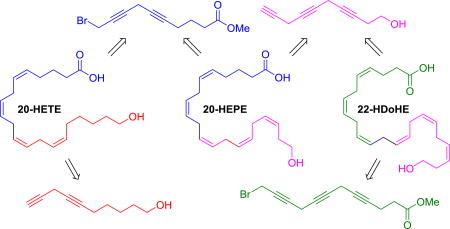
Graphical abstract
Polyunsaturated fatty acids (PUFAs) are mainly converted to oxylipin metabolites by cytochrome P450 (CYP) enzymes that catalyze hydroxylation or epoxidation.1 Arachidonic acid (ARA), an ω-6 PUFA, is metabolized by the CYP enzymes to hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs). While EET regioisomers can be found in roughly similar amounts, 20-HETE is a product of ω-hydroxylation that is a major regioisomer of HETEs derived from ARA. All of these metabolites are important lipid mediators that play critical roles in various diseases.1–2 Interestingly, EETs and HETEs generally have opposing biological functions, e.g., EETs are known to be anti-inflammatory and anti-hypertensive, but 20-HETE shows the opposite effect.2 Among CYP enzymes, isoforms of the CYP 2 family, such as CYP2C and CYP2J, are frequently implicated in production of EETs, but isoforms of the CYP4 family, such as CYP4A and CYP4F, produce 20-HETE. The same CYP isoforms also metabolize both ω-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), to the corresponding epoxy- and hydroxy-PUFAs.3 Recently, beneficial effects from dietary supplements of fish oil including ω-3 fish oil prescriptions have triggered interests in the biological functions of their metabolites.4 For example, epoxides from DHA can reduce pain perception,5 blood pressure,6 and angiogenesis.7 Herein, we are exploring biological roles of ω-hydroxy PUFAs (20-HEPE and 22-HDoHE) derived from EPA and DHA, respectively, compared to the relatively well-studied 20-HETE derived from ARA.
20-HETE has been shown to have detrimental effects in several diseases such as hypertension,8 cancer,9 and cardiovascular and kidney diseases,10 despite its low in vivo concentrations due to reincorporation into membrane phospholipid pools, and plasma protein binding similar to other fatty acids.1 In addition, significantly increased 20-HETE resulting from chronic administration of rofecoxib to mice suggests that it may contribute to the cardiovascular risks associated with coxibs and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs).11 There is also growing evidence that 20-HETE is a potent agonist of a transient receptor potential vanilloid receptor 1 (TRPV1)12 whose activation by endogenous lipid mediators is closely associated with pain.13 However, little is known about the biological roles of ω-hydroxy metabolites derived from ω-3 PUFAs due in part to their difficult synthesis and therefore limited availability. These are 20-hydroxyeicosapentaenoic acid (20-HEPE) and 22-hydroxydocosahexaenoic acid (22-HDoHE) derived from EPA and DHA, respectively. While several methods for the chemical and biosynthesis of 20-HETE have been reported,14 there is only one example for 20-HEPE chemical synthesis.15 To our knowledge, even though bioconversion of 22-HDoHE using fungi or enzymes has been recently reported,14f–h its chemical synthesis, which renders the ease of scale-up compared to its bioconversion, has not yet been reported. These compounds can be also used as standards for expanding metabolite analysis of oxylipins in the ARA cascade to the ω-3 PUFAs such as EPA and DHA. In particular, the chemical synthetic approach to form these molecules will provide a facile route to heavy atom standards.16 Thus, we report here the practical chemical syntheses of all three ω-hydroxy PUFAs and their initial biological evaluation in vitro and in vivo.
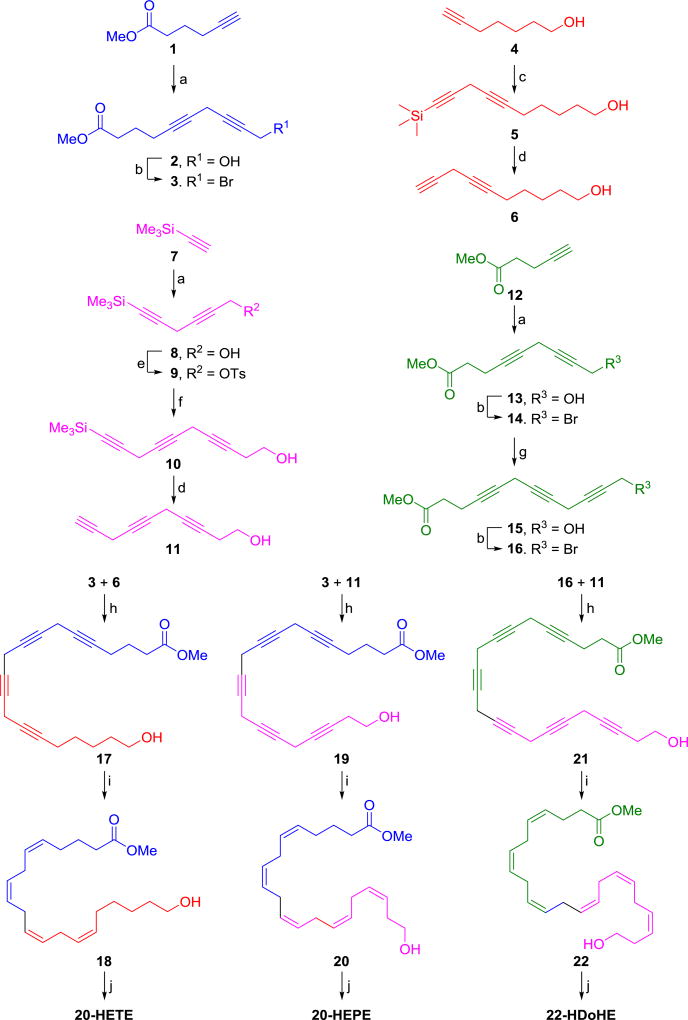
Our convergent synthetic approach of the ω-hydroxy PUFAs, 20-HETE, 20-HEPE, and 22-HDoHE, is summarized in Scheme 1. During their total syntheses, serial Cu-mediated C-C bond formation to construct required methylene skipped poly-ynes and their partial hydrogenation to obtain the desired cis-double bonds of each ω-hydroxy PUFA have been used as two key reaction steps. All of the compounds were prepared by partially sharing common fragments 3, 6, 11, and 16, which were also obtained via serial Cu-mediated C-C bond formation reactions from the corresponding terminal alkynes 1, 4, 7, and 12, respectively. While the preparation of the fragments 3, 6, and 16 was straightforward, the initial attempt of bromination of 8 to prepare 11 in a similar manner to the preparation of 15 from 13 was problematic due to the instability and volatility of the brominated product. Therefore, a tosylate 9, which is solid and is easily separated by column chromatography, was prepared instead. In addition, desilylation of terminal alkynes, as in the compounds 5 and 10, often suffer from isomerization of the desilylated product in a basic condition, but the compounds 6 and 11 were exclusively prepared using Balas’ method where the reaction was performed in neutral condition by adding the same ratio of TBAF:AcOH.17
Scheme 1.
Synthetic routes of compounds 20-HETE, 20-HEPE, and 22-HDoHE.
Reagents and conditions: (a) CuI, NaI, Cs2CO3, 4-chloro-2-butyn-1-ol, DMF, rt, 12h; (b) PPh3, CBr4, DCM, 0 °C, 2h; (c) CuI, NaI, Cs2CO3, 3-bromo-1-(trimethylsilyl)-1-propyne, DMF, rt, 12h; (d) 1M TBAF in THF, AcOH, THF, rt, 12h; (e) Et3N, DMAP, p-TsCl, DCM, 0 °C to rt, 12h; (f) CuI, NaI, Cs2CO3, 3-butyn-1-OH, DMF, rt, 12h; (g) CuI, NaI, Cs2CO3, propargyl alcohol, DMF, rt, 12h; (h) CuI, NaI, Cs2CO3, DMF, rt, 12h; (i) Lindlar catalyst, 2-methyl-2-butene:MeOH:pyridine (4:4:1), H2, rt, 18h-2d (note: 1:1 MeOH/EtOAc was used instead of MeOH for both 20 and 22); (j) 1N NaOH, MeOH, rt, 5h.
With the required fragments 3, 6, 11, and 16 for the total syntheses of all three ω-hydroxy PUFAs in hand, their convergent syntheses by cross-mixing the fragments were performed to prepare 20-HETE (a combination of 3 and 6), 20-HEPE (a combination of 3 and 11), and 22-HDoHE (a combination of 11 and 16) as in Scheme 1. The most challenging step during the synthesis was the difficulty in controlling the partial hydrogenation of multiple triple bonds in 17, 19, and 21 to convert them into the corresponding desired cis-double bonds without over-hydrogenation in compounds 18, 20, and 22. Partial hydrogenation of the triple bonds using the Lindlar catalyst is a well-known approach to stop the reaction of alkynes at the stage of desired alkenes without over-hydrogenation to alkanes.18 Even though palladium metal itself inside the Lindlar catalyst is intentionally deactivated with various forms of lead, further deactivation by adding an additive such as quinoline or pyridine is often required to prevent over-hydrogenation to alkanes. Other methods such as Ni/B reduction have also been used for the partial hydrogenation of alkynes. Therefore, these conventional methods were applied to synthesize 20-HETE and other PUFAs such as DHA. Interestingly, none of the reported procedures, however, described the exact same conditions such as amounts of reagents, temperature, reaction times, or additives.14b,19 This is likely because the reaction is hard to control and variable depending on nature of starting materials. Due to this reason, we were unable to stop the partial hydrogenation reaction of the alkyne 17 to obtain the desired alkene 18 using such conventional partial hydrogenation conditions, which yielded significant amounts of over-hydrogenated alkanes. To overcome this, we tested whether an additional additive, 2-methyl-2-buene (bp 39 °C), which is easily removable to due to its lower boiling point, could prevent undesired over-hydrogenation of 17 based on Ho’s finding where an extra additive, cyclohexene (bp 83 °C), which possesses a double-bond, prevents over-hydrogenation of the desired partially hydrogenated product.20 Therefore 17 was subjected to partial hydrogenation with the Lindlar catalyst in MeOH in the presence of both additives ― pyridine and 2-methyl-2-butene — to obtain the desired 20-HETE methyl ester 18 with none or minimum amounts of the corresponding over-hydrogenated alkanes.
To test if this method is repeatable and scalable, the reaction condition was confirmed by performing the reaction in duplicate (entry I, Table 1) and in a 5-fold scale (entry II, Table 1). With these results in hand, the partial hydrogenation of both 19 and 21 was successfully applied to reduce their multiple triple bonds to the desired cis-double bonds of 20, and 22, respectively. Finally, hydrolysis of the esters 18, 20, and 22 gave the desired free acids, 20-HETE, 20-HEPE, and 22-HDoHE, respectively (synthetic procedure, characterization and purity data of 20-HETE, 20-HEPE, and 22-HDoHE are available in supporting information).
Table 1.
Partial hydrogenation of poly-yne compound 17 to the corresponding alkene methyl ester 18.
reaction condition: Lindlar catalyst, 2-methyl-2-butene:MeOH:pyridine (4:4:1), room temperature, H2 gas (1 atm),
average yield in duplicates
Several studies suggest that 20-HETE has effects in multiple cardiovascular disease states, including ischemic disease, hypertension, and stroke and that this activity is dependent on its ability to activate TRPV1.12 TRPV1 channels are also expressed in peripheral sensory neurons which mediate pain sensation activated by inflammation.21 Therefore TRPV1 has been a therapeutic target for pain management.22 Repeated sensitization of TRPV1, however, induces desensitization to the stimuli, and thus both TRPV1 agonists such as capsaicin and TRPV1 antagonists such as capsazepine have been used to treat pain.23 Several TRPV1 antagonists have been developed for pain treatment and have reached clinical trials.24 However, several of these compounds failed due to adverse effects or poor efficacy.24,25 Given that TRPV1 activation by capsaicin induces pain26 and the 20-HETE is known to be an agonist of TRPV1 which modulates nociception, we tested the three ω-hydroxy PUFAs for their pain induction in vivo and activation of TRPV1 in vitro.
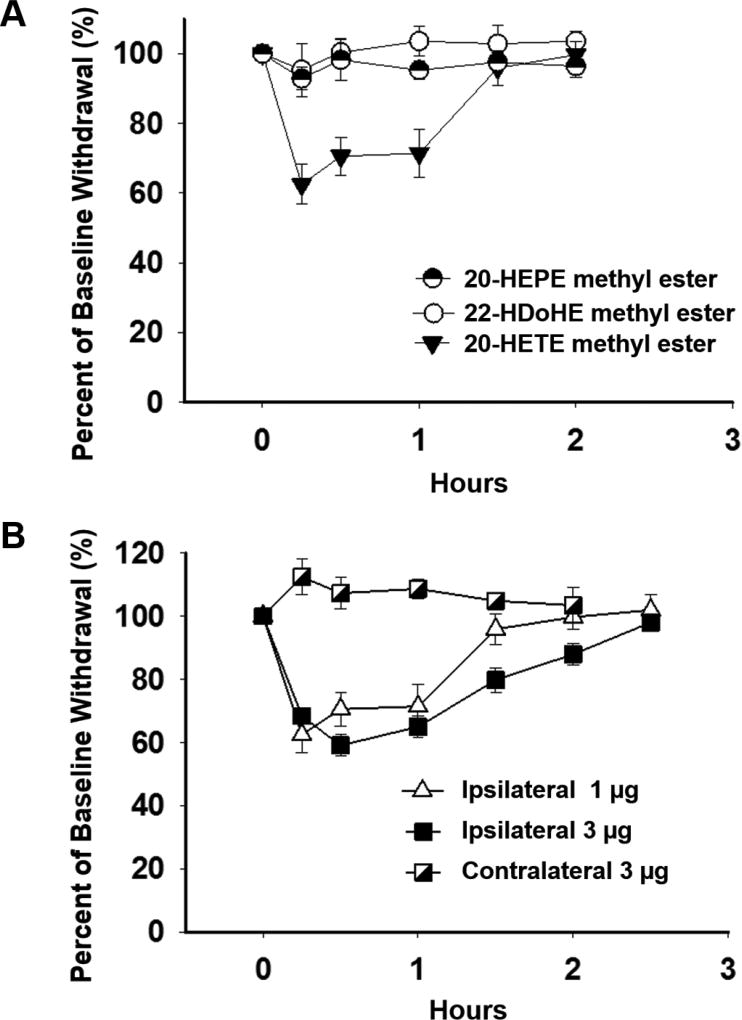
We conducted an in vivo investigation in naïve rats to better observe the individual action of the directly applied metabolites and avoid complications of inflammation induced activation and upregulation of the TRP channels or their sensitization. The methyl esters 18, 20, and 22 of 20-HETE, 20-HEPE and 22-HDoHE, respectively, were tested for their ability to induce mechanical allodynia in vivo. The methyl esters of the metabolites were used to enhance absorption. For the assay each ester (1 µg) was administered by intraplantar injection in naïve rats. Pain response was assessed using an electronic von Frey aesthesiometer over a time course (Figures 1A and S1). While 20-HETE acutely decreased nociceptive thresholds suggesting that 20-HETE induced pain in the rats, 20-HEPE and 22-HDoHE did not elicit any change. 20-HETE also decreased the thresholds in a dose-dependent manner (ipsilateral, Figure 1B) and the effects were local in that there was no change in the contralateral paw even at a higher dose.
Figure 1.
Comparison of pain induction by ω-hydroxy PUFAs, 20-HETE, 20-HEPE, and 22-HDoHE, in naïve rats. (A) Intraplantar injection of the corresponding methyl esters (1 µg in 1:9 EtOH/saline) revealed ω-6 hydroxy PUFA (20-HETE), but not ω-3 hydroxy PUFAs (20-HEPE and 22-HDoHE), changed mechanical withdrawal sensitivity in the von Frey assay (Two Way Analysis of Variance, Holm-Sidak post hoc p < 0.001, n = 6, male Sprague Dawley rats). (B) Intraplantar injection of 20-HETE (1 µg of 20-HETE methyl ester in 1:9 EtOH/saline) decreased mechanical withdrawal thresholds in a dose-dependent manner indicating that pain behavior was localized to the injected (ipsilateral) hind paw (Two Way Analysis of Variance, Holm-Sidak post hoc p = 0.005, n = 6, male Sprague Dawley rats).
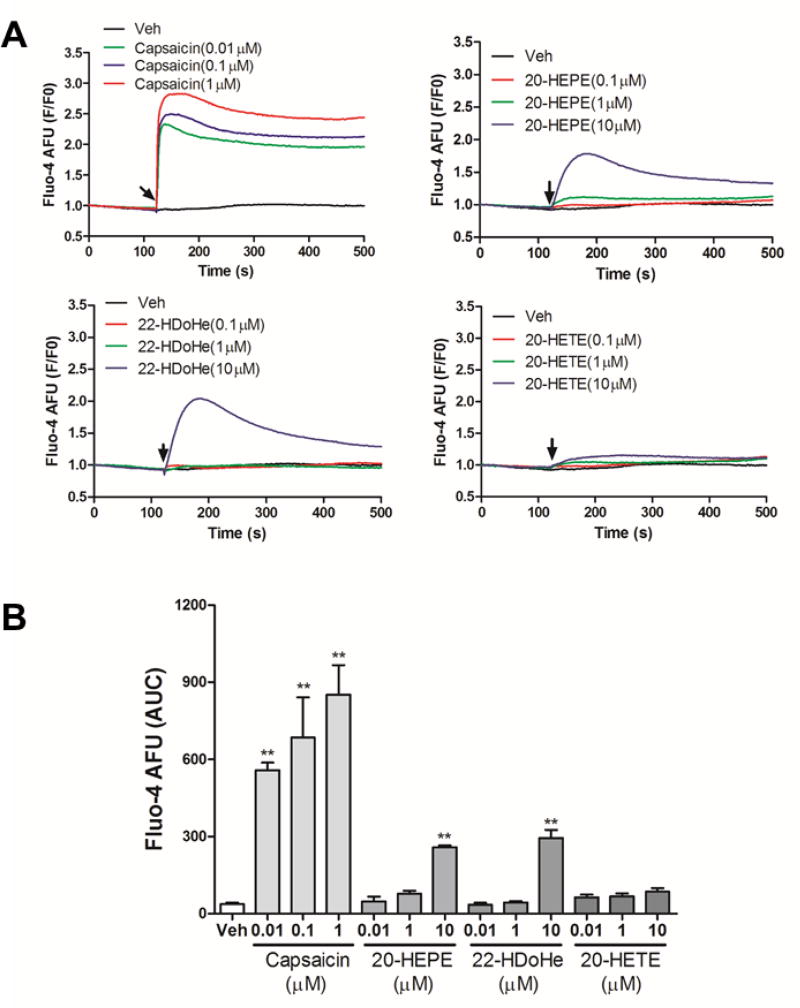
To study whether the in vivo pain data are associated with TRPV1 activation by these ω-hydroxy PUFAs, the influence of 20-HETE, 20-HEPE, and 22-HDoHE on mTRPV1 were measured using a calcium influx assay in HEK293 cells that heterologously expressed mTRPV1. Capsaicin was applied to stimulate the Ca2+ influx in mTRPV1 expressed HEK293 cells as a positive control (Figure 2). We found that 20-HEPE and 22-HDoHE produced significant Ca2+ influx at 10 µM concentration, even though their activity was less than that of the classical agonist capsaicin. However, 20-HETE produced marginal Ca2+ response at the concentrations examined. (Figure 2). It is worth noting that 20-HETE at 10 µM concentration activated hTRPV1 in a previous study.27 The discrepancy between two studies could be derived from the difference in species (human (hTRPV1) versus murine (mTRPV1) receptors). Nevertheless, the data presented here demonstrate that both 20-HEPE and 22-HDoHE are more potent (or more efficacious) in stimulating mTRPV1 than 20-HETE.
Figure 2.
Calcium influx assay. (A) Capsaicin, 20-HETE, 20-HEPE and 22-HDoHE-induced Ca2+ influx in mTRPV1-transfected HEK293 cells as a function of time. (B) Quantification of the Ca2+ responses (area under curve, AUC) induced by capsaicin, 20-HETE, 20-HEPE and 22-HDoHE. The experiments were repeated twice each in triplicates with similar results.
While the 20-HETE was able to induce pain in naïve rats, the effect was minimal compared to a potent algogen (pain-producing agent), prostaglandin E2 (PGE2), which is an arachidonic acid metabolite formed by cyclooxygenases (COXs). In a previous study,28 PGE2 (100 ng) induced a 60% decrease in withdrawal thresholds indicating pain. However, only 30% decrease in withdrawal thresholds (indicating less pain) was observed with 20-HETE methyl ester (1 µg) (Figure 1A). TRPV1 channels are subject to both desensitization, where agonists can induce a conformational change and acutely block the opening of the channel, and tachyphylaxis, where the channel can be stimulated by an agonist into a reoccurring intermediate state and become refractory to further agonists and other types of nociceptive stimuli.13,23 Therefore it is possible that if, per the in vitro assays, the ω-3 hydroxy metabolites are more potent agonists of the channels, they could act to block further signal transduction when administered in vivo. However, it should be noted that the signal transduction involving TRP channels in vivo is a complex regulatory system and there are several putative TRP channels involved in the sensation of mechanical pain. We focused our initial in vitro investigation on the TRPV1 channel activity because it is the most well-studied channel related to nociception and 20-HETE was previously shown to activate the channel.12 Nevertheless, there are reports of ten other possible TRP receptors that participate in nociception, all of which are present in primary sensory neurons.29 More specifically, the canonical TRP channel TRPC6 is involved in mechanical nociception30 and 20-HETE is a known ligand of this channel.31 The outcome of these experiments is unique as the biological role of the ω-hydroxy metabolites derived from ω-3 PUFAs has not been previously investigated. Thus, this marked difference in both in vitro and in vivo outcomes between ω-3 and ω-6 hydroxy metabolites is novel and will require further exploration to determine the mechanisms of action in pain biology.
In summary, three ω-hydroxy PUFAs, 20-HETE, 20-HEPE, 22-HDoHE, have been prepared through practical convergent synthesis where the addition of an extra additive, 2-methyl-2-butene, during partial hydrogenation with compounds 18, 20, and 22 is critical to success and safe to obtain the required cis-double bond in 20-HETE, 20-HEPE, and 22-HDoHE. In naïve rats, withdrawal thresholds were decreased by 20-HETE, which indicates pain was induced, but not with either 20-HEPE or 22-HDoHE. Therefore, there was a distinct difference between the effects of the ω-6 versus ω-3 derived metabolites in the nociceptive assay with 20-HETE alone demonstrating painful effects. In contrast to the in vivo results, our in vitro data showed that 20-HEPE and 22-HDoHE activate mTRPV1 more than 20-HETE does. We cannot rule out that 20-HEPE and 22-HDoHE may bind a distinct TRPV1 agonistbinding site from 20-HETE, which may contribute to the different outcome.32 We also tested their COX inhibition, but no COX activity changes were found up to 100 µM concentrations (see the supporting information). Taken together, ω-3 hydroxy metabolites derived from EPA and DHA may be beneficial, unlike 20-HETE. In addition, we found that both 20-HEPE and 22-HDoHE are potent TRPV1 agonist but do not induce pain like 20-HETE. These compounds may have therapeutic property where TRPV1 activation is beneficial.24 Overall, this current practical synthesis of these ω-hydroxy PUFAs can be a useful tool to further investigate their roles in biological systems.
Supplementary Material
Acknowledgments
This work was supported in part by NIEHS grant R01 ES02710, NIEHS Superfund Basic Research Program grant P42 ES04699, and NIHLB grant HL059699, National Institute of Neurological Disorders and Stroke (NINDS) U54 NS079202. NIH 5T32DC008072-05 and 5T32HL086350-08 (to K.W.). K.S.S.L. has been partially supported by the NIH Pathway to Independence Award from NIH/NIEHS (1K99ES024806-01).
Abbreviations
- ARA
arachidonic acid
- COX
cyclooxygenase
- CYP 450
cytochrome P450
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FLIPR
fluorescent imaging plate reader
- 22-HDoHE
22-hydroxydocosahexaenoic acid
- 20-HEPE
20-hydroxyeicosapentaenoic acid
- 20-HETE
20-hydroxyeicosatetranoic acid
- PUFA
polyunsaturated fatty acids
- TRPV1
transient receptor potential vanilloid 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Experimental details for synthetic procedures and characterization data of compounds 20-HETE, 20-HEPE, and 22-HDoHE. Experimental procedures of von Frey mechanical nociceptive, COX inhibition, and FLIPR calcium assays can be found, in the online version, at http://dx.doi.org/xx.xxx/j.bmcl.xxxx.xx.xxx.
References and notes
- 1.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 2.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J. Biol. Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Russell FD, Burgin-Maunder CS. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar. drugs. 2012;10:2535–2559. doi: 10.3390/md10112535. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad. Med. 2014;126:7–18. doi: 10.3810/pgm.2014.11.2828. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other. Lipid Mediat. 2014;113–115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wagner K, Lee KSS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur. J. Pain. doi: 10.1002/ejp.939. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulu A, Stephen Lee KS, Miyabe C, Yang J, Hammock BG, Dong H, Hammock BD. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 2014;64:87–99. doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. U.S.A. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J. Cardiovasc. Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bubb KJ, Wen H, Panayiotou CM, Finsterbusch M, Khan FJ, Chan MV, Priestley JV, Baker MD, Ahluwalia A. Activation of neuronal transient receptor potential vanilloid 1 channel underlies 20-hydroxyeicosatetraenoic acid-induced vasoactivity: role for protein kinase A. Hypertension. 2013;62:426–433. doi: 10.1161/HYPERTENSIONAHA.111.00942. [DOI] [PubMed] [Google Scholar]
- 9.(a) Borin TF, Zuccari DA, Jardim-Perassi BV, Ferreira LC, Iskander AS, Varma NR, Shankar A, Guo AM, Scicli G, Arbab AS. HET0016, a selective inhibitor of 20-HETE synthesis, decreases pro-angiogenic factors and inhibits growth of triple negative breast cancer in mice. PLoS One. 2014;9:e116247. doi: 10.1371/journal.pone.0116247. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Alexanian A, Rufanova VA, Miller B, Flasch A, Roman RJ, Sorokin A. Down-regulation of 20-HETE synthesis and signaling inhibits renal adenocarcinoma cell proliferation and tumor growth. Anticancer Res. 2009;29:3819–3824. [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Sporkova A, Kopkan L, Varcabova S, Huskova Z, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Cervenka L. Role of cytochrome P-450 metabolites in the regulation of renal function and blood pressure in 2-kidney 1-clip hypertensive rats. Am. J. Physiol-Reg. I. 2011;300:R1468–R1475. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hoff U, Lukitsch I, Chaykovska L, Ladwig M, Arnold C, Manthati VL, Fuller TF, Schneider W, Gollasch M, Muller DN, Flemming B, Seeliger E, Luft FC, Falck JR, Dragun D, Schunck WH. Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 2011;79:57–65. doi: 10.1038/ki.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JY, Li N, Yang J, Li N, Qiu H, Ai D, Chiamvimonvat N, Zhu Y, Hammock BD. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17017–17022. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen H, Ostman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J. Biol. Chem. 2012;287:13868–13876. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales-Lazaro SL, Simon SA, Rosenbaum T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1) J. Physiol. 2013;591:3109–3121. doi: 10.1113/jphysiol.2013.251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Gopal VR, Jagadeesh SG, Reddy YK, Bandyopadhyay A, Capdevila JH, Falck JR. A practical, stereospecific route to 18-, 19-, and 20-hydroxyeicosa-5(Z),8(Z),11(Z),14(Z)-tetraenoic acids (18-, 19-, and 20-HETEs) Tetrahedron Lett. 2004;45:2563–2565. [Google Scholar]; (b) Proteau-Gagne A, St-Jean F, Morin C, Gendron L, Rousseau E, Dory YL. Synthesis and Functional Pharmacological Effects on Human Bronchi of 20-Hydroxyeicosatetraenoic Acid. Chem. Nat. Compd+ 2011;46:841–847. [Google Scholar]; (c) Li C, Xu W, Vadivel SK, Fan P, Makriyannis A. High Affinity Electrophilic and Photoactivatable Covalent Endocannabinoid Probes for the CB1 Receptor. J. Med. Chem. 2005;48:6423–6429. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]; (d) Van Bogaert I, Zhang G, Yang J, Liu JY, Ye Y, Soetaert W, Hammock BD. Preparation of 20-HETE using multifunctional enzyme type 2-negative Starmerella bombicola. J. Lipid Res. 2013;54:3215–3219. doi: 10.1194/jlr.D042226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yu M, Alonso-Galicia M, Sun C-W, J Roman R, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR, Yu J, Takhi M, Falck JR. 20-Hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg. Med. Chem. 2003;11:2803–2821. doi: 10.1016/s0968-0896(03)00192-5. [DOI] [PubMed] [Google Scholar]; (f) Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe W-H. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J. Biol. Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Derogis PBMC, Freitas FP, Marques ASF, Cunha D, Appolinário PP, de Paula F, Lourenço TC, Murgu M, Di Mascio P, Medeiros MHG, Miyamoto S. The development of a specific and sensitive LC-MS-based method for the detection and quantification of hydroperoxy- and hydroxydocosahexaenoic acids as a tool for lipidomic analysis. PLoS One. 2013;8:e77561. doi: 10.1371/journal.pone.0077561. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Sanaki T, Inaba Y, Fujiwara T, Yoshioka T, Matsushima K, Minagawa K, Higshino K, Nakano T, Numata Y. A hybrid strategy using global analysis of oxidized fatty acids and bioconversion by Bacillus circulans. Rapid. Commun. Mass Spectrom. 2016;30:751–762. doi: 10.1002/rcm.7504. [DOI] [PubMed] [Google Scholar]
- 15.Harmon SD, Fang X, Kaduce TL, Hu S, Raj Gopal V, Falck JR, Spector AA. Oxygenation of omega-3 fatty acids by human cytochrome P450 4F3B: effect on 20-hydroxyeicosatetraenoic acid production. Prostag. Leukotr. Ess. 2006;75:169–177. doi: 10.1016/j.plefa.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Dong H, Hammock BD. Profiling the regulatory lipids: another systemic way to unveil the biological mystery. Curr. Opin. Lipidol. 2011;22:197–203. doi: 10.1097/MOL.0b013e3283468c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balas L, Durand T, Saha S, Johnson I, Mukhopadhyay S. Total synthesis of photoactivatable or fluorescent anandamide probes: novel bioactive compounds with angiogenic activity. J. Med. Chem. 2009;52:1005–1017. doi: 10.1021/jm8011382. [DOI] [PubMed] [Google Scholar]
- 18.Molnar A, Sarkany A, Varga M. Hydrogenation of carbon-carbon multiple bonds: chemo-, region- and stereo-selectivity. J. Mol. Catal. A-Chem. 2001;173:185–221. [Google Scholar]
- 19.(a) Khan, M. A. J. O. D., Morgan Hill, CA, 95037, US), WOOD, Paul L. (515 Shawanee Road, Harrogate, TN, 37752, US) Method for the synthesis of DHA. 2012. [Google Scholar]; (b) Khan Amin L (US), W. P. L. U., Goodenowe Dayan (CA) Methods for the synthesis of 13C labeled DHA and use as a reference standard. 2013. [Google Scholar]; (c) Qi LW, Meijler MM, Lee SH, Sun CZ, Janda KD. Solid-phase synthesis of anandamide analogues. Org. Lett. 2004;6:1673–1675. doi: 10.1021/ol049474j. [DOI] [PubMed] [Google Scholar]
- 20.Ho T-L, Liu S-H. Semihydrogenation of triple bonds in 1-alkene solutions. Synthetic Comm. 1987;17:969–973. [Google Scholar]
- 21.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol. Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y. The functional regulation of TRPV1 and its role in pain sensitization. Neurochem. Res. 2008;33(10):2008–12. doi: 10.1007/s11064-008-9750-5. [DOI] [PubMed] [Google Scholar]
- 23.(a) Jara-Oseguera A, Simon SA, Rosenbaum T. TRPV1: on the road to pain relief. Curr. Mol. Pharmacol. 2008;1:255–269. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 2014;171:2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Morales-Lázaro SL, Simon SA, Rosenbaum T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1) J. Physiol. 2013;591:3109–3121. doi: 10.1113/jphysiol.2013.251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 2014;171:2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov. Today. 2009;14:56–67. doi: 10.1016/j.drudis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Yee JR, Kenkel W, Caccaviello JC, Gamber K, Simmons P, Nedelman M, Kulkarni P, Ferris CF. Identifying the integrated neural networks involved in capsaicin-induced pain using fMRI in awake TRPV1 knockout and wild-type rats. Front. Syst. Neurosci. 2015;9:1–13. doi: 10.3389/fnsys.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen H, Oestman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic Acid (20-HETE) Is a Novel Activator of Transient Receptor Potential Vanilloid 1 (TRPV1) Channel. J. Biol. Chem. 2012;287:13868–13876. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa-Valente J, Andreou AP, Urban L, Nagy I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 2014;171(10):2508–27. doi: 10.1111/bph.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J. Biol. Chem. 2003;278:31709–31716. doi: 10.1074/jbc.M304437200. [DOI] [PubMed] [Google Scholar]
- 32.Conway SJ. TRPing the switch on pain: an introduction to the chemistry and biology of capsaicin and TRPV1. Chem. Soc. Rev. 2008;37:1530–1545. doi: 10.1039/b610226n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.