Abstract
Nocturnal human sleep is composed of cycles between rapid eye movement (REM) sleep and non-REM (NREM) sleep. In adults, the structure of ultradian cycles between NREM and REM sleep is well characterized; however, less is known about the developmental trajectories of ultradian sleep cycles across early childhood. Cross-sectional studies indicate that the rapid ultradian cycling of active-quiet sleep in infancy shifts to a more adult-like pattern of NREM-REM sleep cycling by the school-age years, yet longitudinal studies elucidating the details of this transition are scarce. To address this gap, we examined ultradian cycling during nocturnal sleep following 13 h of prior wakefulness in 8 healthy children at 3 longitudinal points: 2Y (2.5-3.0 years of age), 3Y (3.5-4.0 years of age), and 5Y (5.5-6.0 years of age). We found that the length of ultradian cycles increased with age as a result of increased NREM sleep episode duration. In addition, we observed a significant decrease in the number of NREM sleep episodes as well as a nonsignificant trend for a decrease in the number of cycles with increasing age. Together, these findings suggest a concurrent change in which cycle duration increases and the number of cycles decreases across development. We also found that, consistent with data from adolescents and adults, the duration of NREM sleep episodes decreased with time since lights-off whereas the duration of REM sleep episodes increased over this time period. These results indicate the presence of circadian modulation of nocturnal sleep in preschool children. In addition to characterizing changes in ultradian cycling in healthy children ages 2 to 5 years, this work describes a developmental model that may provide insights into the emergence of normal adult REM sleep regulatory circuitry as well as potential trajectories of dysregulated ultradian cycles such as those associated with affective disorders.
Keywords: children, early childhood, sleep, EEG, ultradian, cycle, survival analysis
Mammalian nocturnal sleep is composed of episodes of both rapid eye movement (REM) and non-REM (NREM) sleep. In healthy adults, adolescents, and children, nocturnal sleep architecture typically follows a clearly defined ultradian pattern: Sleep is initiated with NREM sleep and alternates between NREM sleep and REM sleep throughout the night (Carskadon and Dement, 2011). In adults, ultradian cycles of NREM and REM sleep maintain a relatively constant duration of approximately 90 to 110 min across the nocturnal sleep period. However, the relative amounts of NREM and REM sleep within these cycles change over the course of the night, reflecting modulation of sleep by the circadian system (Dijk and Czeisler, 1995). Typically, cycles involving more NREM sleep occur in the early part of the night, and cycles involving more REM sleep occur in the later part of the night (Feinberg, 1974). The production of robust ultradian cycles reflects the dynamics of neurophysiological circuits governing sleep-wake behavior (McCarley and Hobson, 1975; Lu et al., 2006; Luppi et al., 2006; Luppi et al., 2013).
Ultradian cycling has been observed across the lifespan, and cross-sectional studies suggest a transition from the rapid cycling between active and quiet sleep in infancy to an adult pattern of ultradian cycling by the school-age years (Coble et al., 1984; Coble et al., 1987; Jenni et al., 2004). Little is known, however, about the development of ultradian cycling in early childhood, a period of significant change in sleep behavior, including a decline in total sleep duration, the elimination of daytime naps, and the emergence of behavioral sleep problems (Beltramini and Hertzig, 1983; Zuckerman et al., 1987; Iglowstein et al., 2003; Acebo et al., 2005; Crosby et al., 2005; Honaker and Meltzer, 2014). Preschool children may also exhibit REM sleep during midday naps (Kurth et al., 2016), reflecting an absence of the strong circadian gating of REM sleep observed in adults (Czeisler et al., 1980; Dijk and Czeisler, 1995). Furthermore, dysregulation of REM sleep and ultradian cycling may be related to affective disorders in school-age children and adolescents (Ivanenko et al., 2005), which is consistent with observed associations between these measures in adults (Benca et al., 1997). Understanding the normal developmental progression of ultradian cycling may provide insights into the neurophysiology of REM sleep regulation in both healthy children and those with or at risk for affective conditions.
This study extends previous work by examining developmental changes in ultradian cycles across the preschool years. We used longitudinal nocturnal sleep EEG data from healthy children at ages 2, 3, and 5 years to investigate the structure of NREM and REM sleep episodes and NREM/REM ultradian rhythms. In addition to using standard measures, we applied survival analysis techniques to ultradian cycle and NREM/REM sleep episode durations in order to assess potential changes in the entire distribution of these episodes at each age. We also investigated across-the-night changes in sleep architecture in our preschool cohort motivated by data in adults showing circadian modulation of sleep and REM sleep (Czeisler et al., 1980; Dijk et al., 1999) as well as evidence for established circadian rhythmicity in measures such as body temperature and melatonin by age 6 months (Attanasio et al., 1986; Guilleminault et al., 1996). Specifically, we assessed the absolute and relative durations of NREM and REM sleep episodes as a function of time since lights-off or cycle number. Taken together, these analyses provide a detailed characterization of sleep-wake architecture in this longitudinal cohort.
METHODS
Data Collection
Participants
We studied 8 healthy children (3 males; 6 Caucasians) who were reportedly good sleepers at 3 longitudinal time points: 2.8 ± 0.2 (SEM) years (2Y); 3.8 ± 0.2 years (3Y); and 5.9 ± 0.2 (5Y) years. In general, children were excluded for medical, pharmacological, or environmental factors known to affect sleep and circadian timing; additional details about exclusion criteria were reported previously (Kurth et al., 2013). A parent of each child participant signed a consent form approved by the Brown University institutional review board, and the study was performed according to the Declaration of Helsinki. Parents were compensated with $120 cash, and children received small nonmonetary gifts throughout the study.
Experimental design
Children slept in their typical environment (i.e., home, daycare, family care) throughout the study. At each time point, children followed an individualized stable sleep schedule with a minimum sleep opportunity of 12.5 h (2Y and 3Y) or 12 h (5Y) for at least 5 days before the first sleep electroencephalography (EEG) assessment in order to promote sleep optimization and circadian entrainment. The schedule included a nap opportunity of at least 45 min at ages 2Y and 3Y. Sleep schedules were verified with wrist actigraphy, sleep diaries, and daily contact with parents via phone or email. Following the stabilization phase, all-night home EEG recordings during sleep were performed with a portable Vitaport 3 EEG recorder (Temec Instruments; Kerkrade, The Netherlands) following 13 h of prior wakefulness (no nap). Substances (e.g., caffeine, medications) affecting the sleep, circadian, and arousal systems were restricted during the protocol. Children were studied September through May, and data were not collected for at least 12 days following daylight savings time transitions. Children awakened spontaneously in the morning following overnight sleep EEG assessments.
Processing and Analysis
EEG scoring
EEG recordings were visually scored in 30-sec epochs according to standard criteria (Rechtschaffen and Kales, 1968) for sleep stages. We also scored ultradian NREM/REM cycles using the following criteria (Feinberg and Floyd, 1979; Jenni and Carskadon, 2004):
Typical NREM/REM sleep cycles included 1 NREM and 1 REM episode. The first cycle started with sleep onset (Stage 2), and the last cycle ended with the last epoch of REM sleep.
Scored NREM sleep episodes were at least 10 min in duration.
Scored REM sleep episodes, excluding the first REM sleep episode, were at least 5 min in duration.
Cycles were defined to end with REM sleep. If intermittent wake occurred between the end of a REM sleep episode and the beginning of the next NREM sleep episode, then the wake was included in the next cycle.
Wake periods of less than 30 min did not lead to interruption of a cycle. If the intervening behavior lasted longer than 30 min, then a new REM/NREM sleep episode was established.
- In the case of a “skipped” REM sleep episode, cycle boundaries were established using the following criteria:
- There was a trough of slow wave activity (SWA).
- Trough duration lasted at least 10 consecutive minutes (S2, S1) following an slow wave sleep (SWS) episode and was not interrupted by more than 1 min of S3/S4 sleep.
- The end of one cycle and the beginning of the next cycle was defined to be the mid-point between the start and the end of the SWA trough.
When the first REM sleep period was missed and there was a period of wakefulness when REM sleep would be expected, the first NREM sleep episode was defined to end with the start of wake and the second NREM sleep episode was defined to begin with the end of wake.
Statistical Analysis
We analyzed the scored data using a range of statistical techniques to assess changes in sleep architecture with age and over the course of the night. Briefly, we calculated total sleep duration, mean cycle duration, mean NREM sleep, and mean REM sleep episode duration for individual subjects at each age and compared them using 2-way ANOVA and paired t tests. Skipped REM sleep episodes were not included in the calculation of REM sleep episode duration. The 95% confidence intervals for the means are presented. To further explore changes in these measures with age, we computed Kaplan-Meier survival curves (Klein and Moeschberger, 2003) and Cox proportional hazards models (Klein and Moeschberger, 2003). To identify potential differences in the survival of these curves while accounting for intrasubject effects, we used the log-rank test (Neuhaus, 1993; Klein and Moeschberger, 2003). This approach compares the entire distribution of episode durations at each age rather than comparing only the mean durations. We also used linear models to assess changes in NREM and REM sleep episode durations over the course of the night. Since the clock time of bedtime varied according to each child’s habitual sleep schedule, we used “time since lights-off” as a marker of time within the nighttime sleep episode. Specifically, we determined whether NREM and REM sleep episode durations changed with time elapsed since lights-off. We also investigated the structure of cycles in more detail by considering changes in the percentage of NREM sleep per cycle as a function of cycle number, age-related changes in the frequency of cycles, and skipped REM sleep episodes. Details are provided in the Supplementary Online Material.
RESULTS
No Age-related Changes in Sleep Duration following 13 h of Wakefulness
We analyzed sleep architecture during a nighttime sleep episode following 13 h of prior wakefulness in children at ages 2Y, 3Y, and 5Y. Across ages, we did not observe a developmental change in total sleep time (2-way ANOVA, p = 0.52; Fig. 1A) or total NREM sleep (2-way ANOVA, p = 0.42; data not shown). REM sleep time was also stable across development (2-way ANOVA, p = 0.15, respectively; data not shown).
Figure 1.
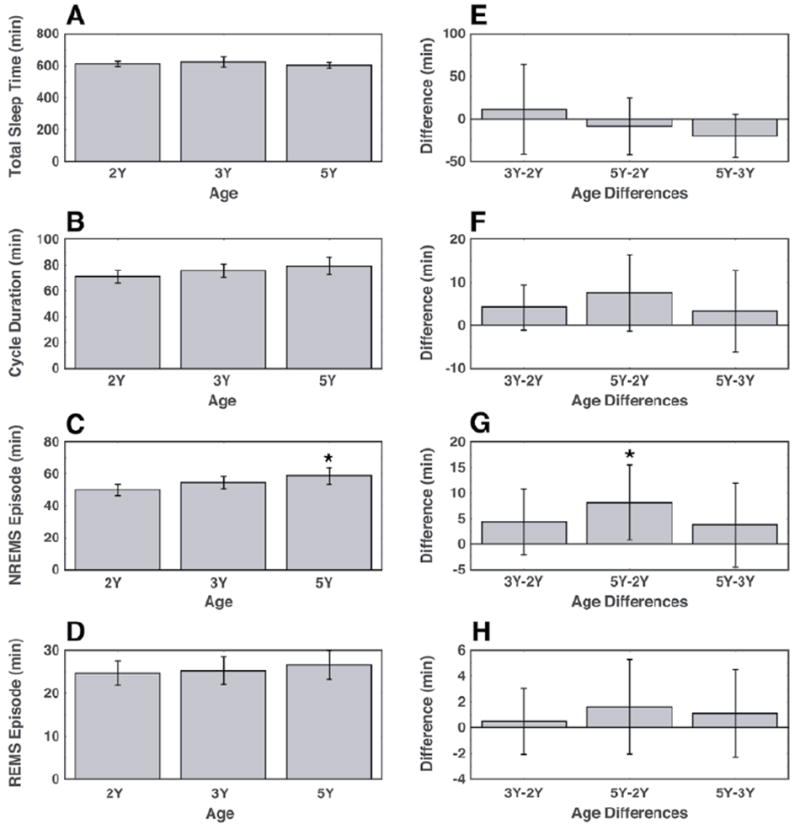
Some standard measures of sleep-wake architecture showed changes with age in early childhood. There was no age-related change in mean total sleep following 13 h of prior wakefulness (A) or mean durations of NREM/REM sleep cycles (B), but NREM sleep episodes (C) increased with age; mean durations of REM sleep episodes showed no age-related change (D). Average changes between ages for each measure except total sleep were positive, suggesting trends for age-related increases (E, F, G, H, respectively). Bars indicate the 95 percent confidence interval of the means (A-D) and paired differences (E-H).
NREM/REM Sleep Cycle Duration Increased with Age due to Longer NREM Sleep Episode Duration
A comparison of mean NREM/REM sleep cycle duration among age groups did not reveal a developmental change (2-way ANOVA, p = 0.13) (Fig. 1B). However, the positive average differences between age groups provided evidence for a nonsignificant trend for increasing cycle duration with age (2-way ANOVA, p = 0.12) (Fig. 1F). Mean NREM sleep episode duration increased with age (2-way ANOVA, p = 0.01), with pairwise comparisons showing an increase of 7.5 min on average from 2Y to 5Y (paired t test, p = 0.02) and a nonsignificant trend for increased mean NREM sleep episode duration at 3Y (Fig. 1C). Mean REM sleep episode duration showed no significant change with age (2-way ANOVA, p = 0.76) (Fig. 1D).
To further investigate potential changes in cycle and REM sleep episode durations as well as observed changes in NREM sleep episode durations with age, we computed Kaplan-Meier survival curves to assess the survival of each episode type (Fig. 2). Using survival analysis, we found a significant increase in both cycle duration (p = 0.01) and NREM sleep episode duration (p < 0.01) with increasing age. The survival of REM sleep episode durations did not show an age effect (p = 0.73).
Figure 2.
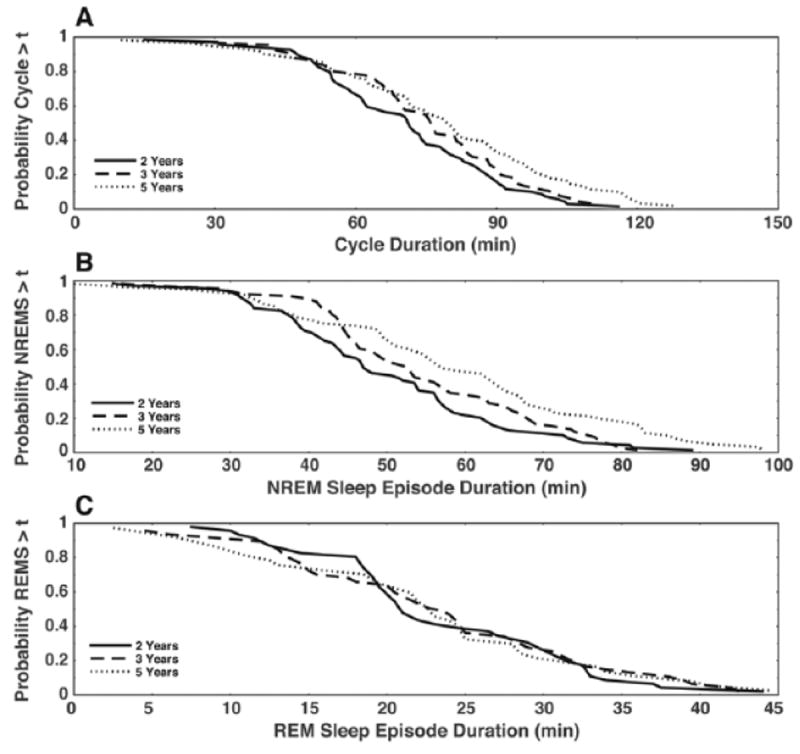
Kaplan-Meier survival curves for cycle, NREM, and REM sleep episode durations. Cycle survival increased with age (A) (bootstrapped log-rank test, p = 0.01) due to increases in NREM sleep episode duration with age (B) (bootstrapped log rank test, p < 0.01), but the survival of REM sleep episodes showed no age-related change (C) (bootstrapped log rank test, p = 0.73).
To explicitly identify the factors contributing to changes in cycle and NREM sleep episode durations, we used Cox proportional hazards models to model these distributions as a function of age, subject, and cycle start time (Table 1). Although no differences were detected for REM sleep episode durations, we also applied Cox proportional hazards models to these distributions for completeness (Table 1). The hazard coefficients describe the difference between the natural log of the baseline hazard (hazard at 2Y) and the log of the hazard for the comparison age group; negative hazards imply longer durations. For example, the significant (p < 0.01) negative hazard coefficient −0.661 for NREM/REM sleep cycle duration at 5Y indicates that a cycle at 5Y was likely to be longer compared with a cycle at 2Y. Cox models showed that both NREM/REM sleep cycle duration and NREM sleep episode duration increased with age, consistent with the analysis using Kaplan-Meier survival curves. These increases in cycle duration and NREM sleep episode duration from the 2Y baseline were significant at 5Y only. Consistent with the previous analyses, Cox models did not detect changes in REM sleep episode duration with age.
Table 1.
Cox proportional hazards models for NREM/REM sleep cycle duration, NREM sleep episode duration, and REM sleep episode duration with covariates age, subject, and time since lights-off.
| 3Y Compared with 2Y | 5Y Compared with 2Y | Time Since Lights-off (h) | ||
|---|---|---|---|---|
| NREM/REM sleep cycle duration | Hazard coefficient | −0.278 | −0.661 | 0.043 |
| Standard error | 0.185 | 0.196 | 0.031 | |
| p value | 0.13 | 0 | 0.17 | |
| NREM sleep episode duration | Hazard coefficient | −0.31 | −0.758 | 0.21 |
| Standard error | 0.177 | 0.188 | 0.026 | |
| p value | 0.08 | <0.01 | <0.01 | |
| REM sleep episode duration | Hazard coefficient | −0.025 | −0.231 | −0.153 |
| Standard error | 0.191 | 0.201 | 0.038 | |
| p value | 0.90 | 0.25 | <0.01 | |
Negative coefficients indicate longer durations.
Evidence That the Number of Cycles Decreases with Age
Although cycle duration increased with age, we found no significant difference in total sleep time across age groups. Thus, we considered the number of cycles for each child at each age to assess possible compensatory effects (Table 2). Specifically, we focused on the 8th and 9th cycles because these cycles occurred only in a subset of participants. The 7th cycle was observed in more than 75% of the sleep periods studied, and only 1 participant at 1 age experienced 10 NREM/REM cycles. We tested the null hypothesis that an 8th or 9th cycle is equally likely to occur in each of the age groups versus the alternative that the probability of occurrence of an 8th or 9th cycle, respectively, was lower at 5Y. There were four 9th cycles, and none of these 9th cycles occurred at 5Y. The probability, under the null hypothesis, of observing no 9th cycles at 5Y, conditional on the given occurrence of 9th cycles, is p = (2/3)4 = 0.19. There were nine 8th cycles, and one of these 8th cycles occurred at 5Y. The probability that of the nine 8th cycles that occurred, at most one 8th cycle occurred at 5Y is p = (2/3)3(1/3)3 + 3(2/3)2(1/3)4 + 3(2/3)4(1/3)2 = 0.093. These probabilities indicate a nonsignificant trend for decreased numbers of ultradian cycles at 5Y compared with 2Y and 3Y.
Table 2.
Frequency of each cycle at each age shows a trend for a decreased number of cycles at later ages.
| Age | 1st Cycle | 2nd Cycle | 3rd Cycle | 4th Cycle | 5th Cycle | 6th Cycle | 7th Cycle | 8th Cycle | 9th Cycle | 10th Cycle |
|---|---|---|---|---|---|---|---|---|---|---|
| 2Y | 8 (3) | 8 (0) | 8 (0) | 8 (0) | 8 (0) | 8 (0) | 6 (0) | 4 (0) | 3 (0) | 1 (0) |
| 3Y | 8 (4) | 8 (1) | 8 (0) | 8 (0) | 8 (0) | 8 (0) | 7 (0) | 4 (0) | 1 (0) | 0 (0) |
| 5Y | 8 (6) | 8 (2) | 8 (0) | 8 (0) | 8 (0) | 8 (0) | 6 (0) | 1 (0) | 0 (0) | 0 (0) |
The probability of the given distribution across age groups of 9th cycles is p = 0.19; the probability of the given distribution across age groups of 8th cycles is p = 0.09. The occurrence of skipped REM sleep episodes for each cycle is denoted by the number in parentheses and shows skipped REM sleep episodes occurring in the 1st and 2nd cycles.
Because children often awaken from stage 2 sleep, we also tested the null hypothesis that NREM sleep episodes are equally likely to occur in each of the age groups versus the alternative that the probability of occurrence of NREM sleep episodes was lower at 5Y (Table 3). We focused on the 9th NREM sleep episode because these episodes occurred only in a subset of the participants. The sample of NREM sleep episodes included 3 episodes that were not part of a complete NREM/REM sleep cycle. Of the seven 9th NREM sleep episodes observed, 3 participants experienced a 9th NREM sleep episode at 1 age only, and 2 participants experienced a 9th NREM sleep episodes at 2 ages. No 9th NREM sleep episodes were observed at 5Y. The probability, under the null hypothesis, of observing no 9th NREM sleep episodes at 5Y, conditional on the given occurrence of 9th NREM sleep episodes, is p = (1/3)2(2/3)3 = 0.033. Thus, a 9th NREM sleep episode is less likely at 5Y compared with 2Y and 3Y.
Table 3.
Frequency of each NREM sleep episode at each age shows a decreased number of NREM sleep episodes at later ages.
| Age | 1st Episode | 2nd Episode | 3rd Episode | 4th Episode | 5th Episode | 6th Episode | 7th Episode | 8th Episode | 9th Episode | 10th Episode |
|---|---|---|---|---|---|---|---|---|---|---|
| 2Y | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 6 | 3 | 2 |
| 3Y | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 6 | 3 | 1 |
| 5Y | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 6 | 0 | 0 |
The probability of the given distribution across age groups of 9th NREM sleep episodes is p = 0.03.
We also examined the prevalence of skipped REM sleep episodes across ages (Table 2). All of our participants initiated sleep with NREM sleep, and skipped REM sleep episodes were observed in more than half of the first cycles. Skipped REM sleep occurred during the 1st and 2nd cycles only. We did not detect an age effect for the occurrence of skipped REM sleep episodes.
Cycle, NREM Sleep, and REM Sleep Episode Durations Change with Time since Lights-off
To assess the relationship between time since lights-off and cycle, NREM sleep, and REM sleep episode durations, we used Cox proportional hazards models. Because bedtimes were based on each child’s habitual schedule, time since lights-off provided a marker of progression through the sleep episode that reflected circadian variation without imposing an external clock time. Cox models showed that there was no change in cycle duration with time since lights-off. However, NREM sleep episode duration decreased and REM sleep episode duration increased with time since lights-off. To further characterize this relationship, we fit a linear model to NREM and REM sleep episode duration data as a function of time since lights-off (Fig. 3). At each age, NREM sleep episode duration decreased (p ~ 0) and REM sleep episode duration increased (p ~ 0) with time since lights-off. For NREM sleep, the line of best fit had a different intercept at 5Y compared with 2Y (2Y, 62.4 min; 3Y, 66.3 min; 5Y, 70.7 min; 3Y-2Y p = 0.10; 5Y-2Y p = 0.001), but there was no significant age effect on slope (−2.97 min/h, p = 0.12). For REM sleep episode durations, no age effects were observed for the intercepts (2Y, 18.3 min; 3Y, 18.9 min; 5Y, 19.4 min; p > 0.05) or slope (1.27 min/h, p = 0.10) of the best-fit line.
Figure 3.
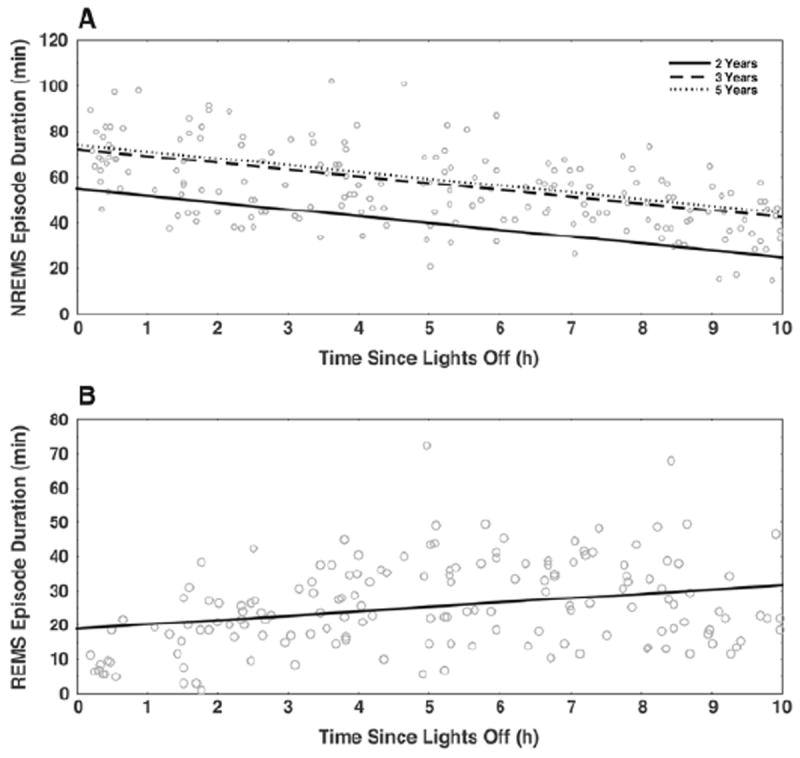
Linear model shows how NREM and REM sleep episode durations change with time since lights-off. At all ages, after accounting for subject effects, NREM sleep episode duration decreased (A) (slope coefficient −2.97 min/h since lights-off, p < 0.01) while REM sleep episode duration increased (B) (slope coefficient 1.28 min/h since lights-off, p < 0.01) with time since lights-off. Ages 2Y, 3Y, and 5Y had statistically different intercepts (2Y, 62.4 min; 3Y, 66.3 min; 5Y, 70.7 min; 3Y-2Y p = 0.10; 5Y-2Y p = 0.001) but not statistically different slope coefficients for NREM sleep episode duration (−2.97 min/h, p = 0.12); for REM sleep episode duration, neither slopes nor intercepts were significantly different between ages (slope for all data, 1.27 min/h; intercept for all data 18.87 min).
Percentage NREM Sleep per Cycle Does Not Decrease Monotonically throughout the Night
To further characterize the decrease in NREM sleep episode duration across the night, we considered the percentage of each cycle spent in NREM sleep as a function of cycle number (Fig. 4). We found that at all ages, the percentage of NREM sleep in each cycle decreased from the 1st to the 2nd cycle (paired t test, p < 0.01 at 2Y; p < 0.01 at 3Y; p = 0.01 at 5Y). For cycles 2 through 7, there was, in general, a nonsignificant trend for cycle-to-cycle decreases in percentage of NREM sleep, with several of these differences reaching significance (3rd to 4th cycle at 2Y; 5th to 6th cycle at 2Y; 2nd to 3rd cycle at 5Y; paired t test, p < 0.05). By contrast, the percentage of NREM sleep in cycle 8 was greater than that in cycle 7 (paired t test, p = 0.01 at all ages). This increase in the percentage of NREM sleep per cycle persisted as a nonsignificant trend for cycles 9 and 10; however, the number of these cycles was small. Although the percentage of NREM sleep in later cycles increased, the mean duration of NREM sleep episodes occurring in cycles 8 through 10 was shorter than that of NREM sleep episodes in cycles 1 through 7 (56.9 min for cycles 1-7, 38.8 min for cycles 8-10; paired t test, p < 0.01). Therefore, this finding represents a change in the relative, but not absolute, amount of NREM sleep in cycles at the end of the sleep period reflecting awakening out of NREM sleep.
Figure 4.
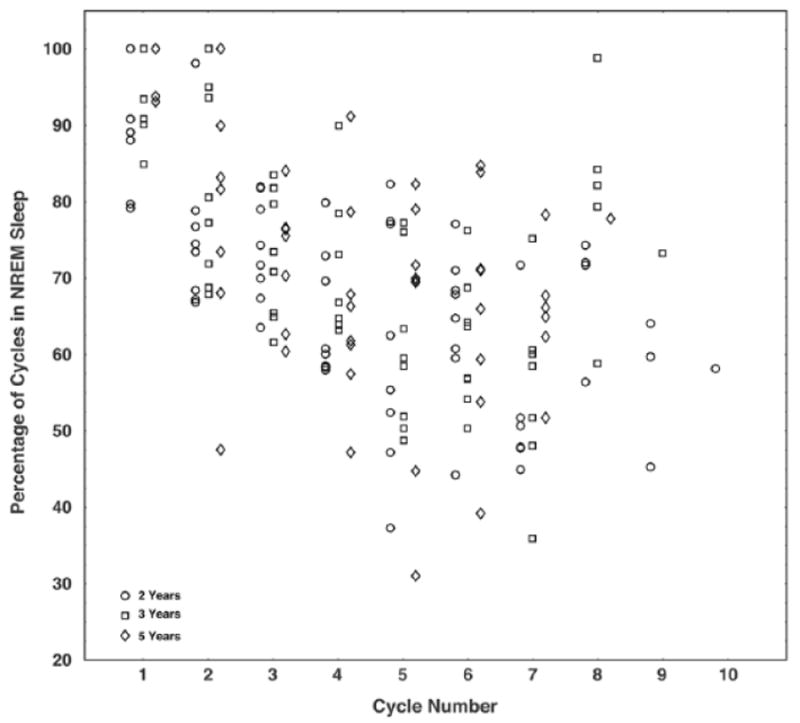
The percentage of NREM sleep in each cycle decreases from the 1st to the 2nd cycle (paired t test, p < 0.01 at 2Y; p < 0.01 at 3Y; p = 0.01 at 5Y) and continues this decreasing trend through cycle 7. The percentage of NREM sleep in cycle 8 was greater than that in cycle 7 (paired t test, p = 0.01 at all ages), and this increasing trend continued in later cycles due to the likelihood of waking up out of NREM sleep.
DISCUSSION
Using data from longitudinal sleep EEG assessments, we found that preschoolers demonstrate developmental changes in some features of sleep architecture during a nighttime sleep episode following 13 h of prior wakefulness. Specifically, although we observed no significant change in total sleep time, the duration of ultradian NREM/REM cycles and NREM episodes increased as children aged from 2Y to 5Y. This increase in ultradian cycle duration was not detected when mean cycle durations were compared across ages, but analysis of Kaplan-Meier survival curves demonstrated that increases in cycle duration were present across the entire distribution of cycles and NREM sleep episodes. In addition, our data suggest that the circadian modulation of ultradian cycles that has been observed in adolescents and adults over the course of the night may be present in preschool-age children. Comparisons within subjects were particularly salient in this longitudinal study, which is consistent with previous work showing that preschool and school-aged children generally maintain individual standing in sleep duration over the course of development (Jenni and Carskadon, 2012).
Age-related Changes in Ultradian Cycles in Early Childhood
Earlier cross-sectional studies have examined various aspects of sleep during childhood (Coble et al., 1984), and previous longitudinal studies have assessed changes in sleep architecture across infancy (Jenni et al., 2004) and adolescence (Feinberg et al., 2012). Data from older children suggest that the duration of ultradian cycles increases during development while the frequency of these cycles decreases (Coble et al., 1984; Feinberg et al., 2012). In infants, ultradian NREM/REM sleep cycles typically have a period of approximately 55 min, and 7 to 13 cycles may occur during nocturnal sleep (Jenni et al., 2004). By school age, mean ultradian cycle length is approximately 85 to 90 min, with 4 to 6 cycles occurring across the night (Coble et al., 1984). Together, these findings suggest a concurrent change in which cycle duration increases and the number of cycles decreases across development.
In our cohort of preschool children, we found evidence for gradual changes in cycle duration and frequency that bridges findings from studies of younger and older children. Using survival analysis and Cox proportional hazards models, we identified an increase in ultradian cycle duration. Given a mean cycle duration of 57.5 ± 2.4 min at 9 months (Jenni et al., 2004) and approximately 87 min in 6- and 7-year-olds (Coble et al.,1984), our longitudinal results showing mean cycle durations of 71 ± 2.5 min at 2Y and 79 ± 3.5 min at 5Y support a continuous increase in mean cycle duration across early childhood. The probability of a 9th NREM sleep episode was decreased at 5Y, consistent with prior reports of decreasing cycle frequency with age (Coble et al., 1984). Although a decrease in cycle frequency with age (assessed as a decreased probability of an 8th or 9th cycle at 5Y compared with 2Y or 3Y) did not reach significance in our data, our results may have occurred due to sleep episodes terminating following stage 2 sleep or protocol effects. Additional work considering ultradian cycles in preschoolers on habitual sleep-wakefulness schedules is needed to determine cycle frequency across development in more detail.
In previous work by our group and others, survival analysis techniques have been applied to detect subtle differences in sleep-wake architecture under different disease conditions including obstructive sleep apnea (Norman et al., 2006; Chervin et al., 2009) and orexin/hypocretin dysregulation (Diniz Behn et al., 2010; Branch et al., 2016). These techniques have also been used to assess sleep continuity in aging adults (Klerman et al., 2013) and to compare sleep features across species (Lo et al., 2004). In this study, we quantified sleep-wake behavior using survival analysis of cycle/episode durations in order to consider the entire distribution of cycles/episodes in addition to analyzing means of cycle/episode durations. Survival analysis of cycle duration identified an age-related increase that was supported by the Cox proportional hazards model but was not detected with an analysis of average cycle duration. In addition, survival analysis of cycle and NREM sleep episode durations showed that the age-related increases in these measures occurred across the entire distribution of cycles and provide evidence for a developmentally mediated change in the mechanisms governing ultradian cycling. Furthermore, this analysis suggests that changes in sleep cycle patterns in early childhood are not limited to changes in the first cycle only, despite evidence that the first cycle dominates age-related changes in ultradian cycles later in life (Feinberg, 1974).
Changes in NREM and REM Sleep Episodes with Age and Time Since Lights-off
Consistent with previous observations in adults and adolescents (Feinberg, 1974; Carskadon and Dement, 2011; Feinbergavis et al., 2012), we found that regular ultradian cycling occurred throughout the nocturnal sleep period, and the duty cycle of NREM and REM episodes within ultradian cycles changed over the course of the night. Specifically, following lights-off time, the duration of NREM sleep episodes decreased, while the duration of REM sleep episodes increased. This finding reveals the presence of time-of-day features of sleep architecture in our preschool cohort and provides preliminary evidence for circadian modulation of sleep in young children consistent with results established in adults using forced desynchrony protocols (Dijk and Czeisler, 1995). Our results are also congruent with previously reported sleep-dependent disinhibition of REM sleep (Dijk and Czeisler, 1995).
Despite the consistent decrease in NREM sleep episode duration and increase in REM sleep episode duration with time since lights-off, our analysis also identified a relative increase in the occurrence of NREM sleep near the end of the sleep period. This was manifested as an increase in the percentage of NREM sleep in cycles 8 through 10 as well as in the occurrence of final episodes of sleep that contained NREM sleep only. In our sample of young children, these findings may be related to the developmental changes that alter sleep architecture while maintaining relatively stable total sleep durations. Our data suggest that an age-related increase in cycle durations earlier in the nighttime sleep period is compensated by a decrease in the duration of the final NREM/REM cycle with a preferential loss of REM sleep. If the amount of REM sleep in the final cycle decreases below 5 min (the threshold required to score a cycle under standard cycle scoring criteria), awakening occurs from a final NREM sleep episode rather than a cycle. As cycle durations earlier in the nighttime sleep period continue to increase with age, the duration of the final NREM sleep episode decreases until the final NREM sleep episode is lost, resulting in a reduced number of NREM sleep episodes as children approach adolescence. Alternatively, the relative increase in NREM sleep in later cycles could result from developmental differences in sleep need arising from the 13 h of prior wakefulness protocol, napping status, or circadian gating of REM sleep. It is possible that circadian modulation of REM sleep propensity may be different in preschoolers, or, depending on the relationship between the timing of the sleep episode and the internal clock, the end of a nocturnal sleep period may coincide with a decrease in REM sleep propensity. Additional work is needed at these and other developmental stages to investigate the mechanisms underlying these observations.
Limitations
Several limitations of this study should be noted. First, these results reflect data from 8 healthy children. Additional data from larger and more diverse cohorts are needed to establish the generalizability of our findings. Second, our observations may reflect differential effects of the protocol at different ages. The 13 h of prior wakefulness protocol was selected to normalize the duration of the waking period; however, this approach likely generated higher sleep propensity at ages 2Y than 5Y. Based upon data from sleep deprivation studies in adults (Moses et al., 1975; Borbely et al., 1981) indicating competitive recovery of NREM sleep compared with REM sleep, our protocol may have affected our observed NREM and REM sleep amounts at different ages. Differential effects of the protocol may also explain the absence of age-related changes in total sleep time or REM sleep time in our data in contrast to other studies reporting reductions in these measures across childhood (Iglowstein et al., 2003; Ohayon et al., 2004; Acebo et al., 2005; Jenni and Carskadon, 2012). Third, differences in napping status across ages may have influenced our results. In our study, all children napped regularly at 2Y; 2 children had ceased napping at 3Y; and none of the children were napping at 5Y. The confounding role of napping status is a difficult factor to control during the stabilization segment of the study, and this may be particularly relevant for children at the 3Y time point when napping behavior was less stable. Furthermore, the 13 h of prior wakefulness protocol represents a significant sleep deprivation challenge for children who are regularly napping (Lassonde et al., 2016), suggesting that the protocol is differentially nonphysiologic for children with different napping statuses. Relatedly, our previous work and that of others show that toddlers who nap have later circadian phases and decreased nighttime sleep compared with nonnappers (Koch et al., 1984; Tikotzky and Sadeh, 2001; Acebo et al., 2005; Akacem et al., 2015). These limitations support the need for additional studies to examine sleep-wakefulness behavior in preschoolers on their habitual sleep schedules.
CONCLUSIONS AND IMPLICATIONS
This study provides preliminary evidence for developmental changes in ultradian sleep cycles across early childhood and contributes to establishing developmental trajectories for ultradian cycles. Studies in adolescents have revealed different maturational trajectories for NREM and REM sleep with shortening NREM sleep episode durations and lengthening REM sleep episode durations across adolescence (Feinberg et al., 2012). Our study also shows differential changes in NREM and REM sleep with increases in NREM but not REM sleep episode durations during early childhood. Taken together, these findings suggest that changes in sleep architecture reflect distinct maturational processes at different developmental stages associated with changes in synaptic density and functional brain connectivity (Huttenlocher and Dabholkar, 1997; Kurth et al., 2013). Relatedly, investigation of the physiological mechanisms underlying the generation of ultradian cycles represents an area of active research, and these mechanisms have been widely debated in recent years (McCarley and Hobson, 1975; Lu et al., 2006; Luppi et al., 2006; Luppi et al., 2013). Previous work has promoted developmental analyses for elucidating sleep-wake circuitry in rodents (Blumberg et al., 2014), and our developmental findings in ultradian cycling may provide a novel perspective for addressing these questions in humans.
Furthermore, understanding typical development of ultradian cycling may provide new insights into the genesis and mechanisms of dysregulation of ultradian NREM/REM cycles, as observed in affective disorders (McCarley, 1982; Ivanenko et al., 2005). In adult patients with affective disorders, dysregulation of ultradian cycles may include reduced latency to REM sleep, increased REM density, and higher numbers of REM episodes (Kupfer et al., 1977; Kupfer et al., 1978; Benca et al., 1997). Studies of ultradian cycles in children and adolescents with affective disorders have been less conclusive, but many features of ultradian cycle dysregulation identified in adults have also been observed in children and adolescents with major depression (Ivanenko et al., 2005). In addition, antidepressants have been shown to exert similar effects on sleep in children and adults with depression, suggesting that childhood depression and adult depression share similar biological features manifested at different maturational levels (Kupfer et al., 1979; Shain et al., 1990). Some key features of ultradian dysregulation such as reduced latency to REM sleep are difficult to assess in early childhood when, as observed in our cohort, skipped REM episodes are common. However, improved understanding of developmental trajectories for ultradian cycles may contribute to the identification of new markers for ultradian dysregulation during the sensitive period of early childhood and reveal implications for future trajectories of affective disorder risk later in life.
Supplementary Material
Acknowledgments
We thank the children and families for their generosity, time, and effort in making this study possible. We also thank the Brown University undergraduate students who assisted with data collection. This study was supported by the National Science Foundation grant DMS 1412571 (C.D.B.); a Colorado School of Mines Undergraduate Research Fellowship (S.L.); the Swiss National Science Foundation grant 32003B_146643 (P.A.); and the National Institute of Mental Health grants K01-MH074643 and R01-MH086566 (M.L.). This work was performed at Brown University and Colorado School of Mines.
Footnotes
NOTE
Supplementary material is available on the journal’s website at http://journals.sagepub.com/home/jbr/supplemental.
CONFLICT OF INTEREST STATEMENT
The authors have declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: Dr. Diniz Behn has received consulting fees from Merck Pharmaceuticals.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28(12):1568–1577. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Akacem LD, Simpkin CT, Carskadon MA, Wright KP, Jr, Jenni OG, Achermann P, LeBourgeois MK. The timing of the circadian clock and sleep differ between napping and non-napping toddlers. PLoS One. 2015;10(4):e0125181. doi: 10.1371/journal.pone.0125181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio A, Rager K, Gupta D. Ontogeny of circadian rhythmicity for melatonin, serotonin, and N-acetylserotonin in humans. J Pineal Res. 1986;3(3):251–256. doi: 10.1111/j.1600-079x.1986.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Beltramini AU, Hertzig ME. Sleep and bedtime behavior in preschool-aged children. Pediatrics. 1983;71(2):153–158. [PubMed] [Google Scholar]
- Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, Obermeyer WH. Sleep and mood disorders. Sleep Med Rev. 1997;1(1):45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Gall AJ, Todd WD. The development of sleep-wake rhythms and the search for elemental circuits in the infant brain. Behav Neurosci. 2014;128(3):250–263. doi: 10.1037/a0035891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–495. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- Branch AF, Navidi W, Tabuchi S, Terao A, Yamanaka A, Scammell TE, Diniz Behn C. Progressive loss of the orexin neurons reveals dual effects on wakefulness. Sleep. 2016;39(2):369–377. doi: 10.5665/sleep.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. St. Louis (MO): Elsevier Saunders; 2011. [Google Scholar]
- Chervin RD, Fetterolf JL, Ruzicka DL, Thelen BJ, Burns JW. Sleep stage dynamics differ between children with and without obstructive sleep apnea. Sleep. 2009;32(10):1325–1332. doi: 10.1093/sleep/32.10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: Findings using standard measurement methods. Sleep. 1984;7(4):289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- Coble PA, Reynolds CF, 3rd, Kupfer DJ, Houck P. Electroencephalographic sleep of healthy children. Part II: Findings using automated delta and REM sleep measurement methods. Sleep. 1987;10(6):551–562. [PubMed] [Google Scholar]
- Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115(1 Suppl):225–232. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC, Weitzman ED. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2(3):329–346. [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33(3):297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10(3-4):283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R533–R540. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16(3):283–291. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Leger D, Pelayo R, Gould S, Hayes B, Miles L. Development of circadian rhythmicity of temperature in full-term normal infants. Neurophysiol Clin. 1996;26(1):21–29. doi: 10.1016/0987-7053(96)81531-0. [DOI] [PubMed] [Google Scholar]
- Honaker SM, Meltzer LJ. Bedtime problems and night wakings in young children: an update of the evidence. Paediatr Respir Rev. 2014;15(4):333–339. doi: 10.1016/j.prrv.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Ivanenko A, Crabtree VM, Gozal D. Sleep and depression in children and adolescents. Sleep Med Rev. 2005;9(2):115–129. doi: 10.1016/j.smrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Borbely AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R528–R538. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;15(4):774–783. [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Sleep behavior and sleep regulation from infancy through adolescence: normative aspects. Sleep Med Clin. 2012;7:529–538. [Google Scholar]
- Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer; 2003. [Google Scholar]
- Klerman EB, Wang W, Duffy JF, Dijk DJ, Czeisler CA, Kronauer RE. Survival analysis indicates that age-related decline in sleep continuity occurs exclusively during NREM sleep. Neurobiol Aging. 2013;34(1):309–318. doi: 10.1016/j.neurobiolaging.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P, Soussignan R, Montagner H. New data on the wake-sleep rhythm of children aged from 2 1/2 to 4 1/2 years. Acta Paediatr Scand. 1984;73(5):667–673. doi: 10.1111/j.1651-2227.1984.tb09993.x. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Coble P, Kane J, Petti T, Conners CK. Imipramine and EEG sleep in children with depressive symptoms. Psychopharmacology (Berl) 1979;60(2):117–123. doi: 10.1007/BF00432281. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG, Coble P, McPartland RJ, Ulrich RF. The application of EEG sleep for the differential diagnosis of affective disorders. Am J Psychiatry. 1978;135(1):69–74. doi: 10.1176/ajp.135.1.69. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG, Coble PA, McPartland RJ. EEG sleep parameters for the classification and treatment of affective disorders [proceedings] Psychopharmacol Bull. 1977;13(2):57–58. [PubMed] [Google Scholar]
- Kurth S, Achermann P, Rusterholz T, Lebourgeois MK. Development of brain EEG connectivity across early childhood: does sleep play a role? Brain Sci. 2013;3(4):1445–1460. doi: 10.3390/brainsci3041445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Lassonde JM, Pierpoint LA, Rusterholz T, Jenni OG, McClain IJ, Achermann P, LeBourgeois MK. Development of nap neurophysiology: preliminary insights into sleep regulation in early childhood. J Sleep Res. 2016 doi: 10.1111/jsr.12427. published online June 2, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassonde JM, Rusterholz T, Kurth S, Schumacher A, Achermann P, LeBourgeois MK. Sleep physiology in toddlers: effects of missing a nap on subsequent night sleep. Neurobiol Sleep Circadian Rhythms. 2016;1(1):19–26. doi: 10.1016/j.nbscr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov P. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci U S A. 2004;101(50):17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Clement O, Fort P. Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Curr Opin Neurobiol. 2013;23(5):786–792. doi: 10.1016/j.conb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100(5-6):271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- McCarley RW. REM sleep and depression: common neurobiological control mechanisms. Am J Psychiatry. 1982;139(5):565–570. doi: 10.1176/ajp.139.5.565. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189(4196):58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- Moses JM, Johnson LC, Naitoh P, Lubin A. Sleep stage deprivation and total sleep loss: effects on sleep behavior. Psychophysiology. 1975;12(2):141–146. doi: 10.1111/j.1469-8986.1975.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Neuhaus G. Conditional rank tests for the two-sample problem under random censorship. Ann Stat. 1993;21(4):1760–1779. [Google Scholar]
- Norman RG, Scott MA, Ayappa I, Walsleben JA, Rapoport DM. Sleep continuity measured by survival curve analysis. Sleep. 2006;29(12):1625–1631. doi: 10.1093/sleep/29.12.1625. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles (CA): UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Shain BN, Naylor M, Shipley JE, Alessi N. Imipramine effects on sleep in depressed adolescents: a preliminary report. Biol Psychiatry. 1990;28(5):459–462. doi: 10.1016/0006-3223(90)90414-w. [DOI] [PubMed] [Google Scholar]
- Tikotzky L, Sadeh A. Sleep patterns and sleep disruptions in kindergarten children. J Clin Child Psychol. 2001;30(4):581–591. doi: 10.1207/S15374424JCCP3004_13. [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Stevenson J, Bailey V. Sleep problems in early childhood: continuities, predictive factors, and behavioral correlates. Pediatrics. 1987;80(5):664–671. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


