Abstract
Background and Purpose
While variation in breast cancer quality indicators has been studied, to date there have been no studies examining the degree of surgeon-level variation in patient-reported outcomes. The purpose of this study is to examine surgeon-level variation in patient appraisals of their breast cancer care experiences.
Methods
Survey responses and clinical data from breast cancer patients reported to Detroit and Los Angeles Surveillance, Epidemiology and End Results registries from 6/2005 to 2/2007 were merged with attending surgeon surveys (1,780 patients, 291 surgeons). Primary outcomes were patient reports of access to care, care coordination, and decision satisfaction. Random-effects models examined variation due to individual surgeons for these three outcomes.
Results
Mean values on each patient-reported outcome scale were high. The amount of variation attributable to individual surgeons in the unconditional models was low to modest: 5.4% for access to care, 3.3% for care coordination, and 7.5% for decision satisfaction. Few factors were independently associated with patient reports of better access to or coordination of care, but less-acculturated Latina patients had lower decision satisfaction.
Conclusions
Patients reported generally positive experiences with their breast cancer treatment, though we found disparities in decision satisfaction. Individual surgeons did not substantively explain the variation in any of the patient-reported outcomes.
INTRODUCTION
Concerns about quality of cancer care have persisted for over 10 years, since the 1999 Ensuring Quality Cancer Care report first recommended establishing a cancer quality care reporting system in the USA.1 Since then several initiatives have been developed to measure the quality of cancer care, including the National Initiative on Cancer Care Quality (NICCQ), created by the American Society for Clinical Oncology (ASCO) and released in 2000.2,3 The NICCQ includes a set of seven domains designed to delineate the content of high-quality cancer care delivery. Of the seven measures, four focus on “treatment and follow-up” and three focus on “patient experiences” (referrals and coordination of care, psychosocial support, and patient preferences and inclusion in decision-making).3 The NICCQ measures have been predominantly used to evaluate breast cancer treatment and follow-up. Studies have found that, in general, the quality of breast cancer treatment is high.2,4,5 However, persistent geographic variation in breast cancer care has contributed to lingering concerns about the quality of breast cancer treatment, and specifically whether treatment variation may be attributable to the clinicians involved in care delivery.6–9 A limited number of studies evaluating clinician variation in receipt of breast cancer treatment(s) have found low to moderate variation in surgery and attributable to individual surgeons.9,10 Other studies have found that surgeon characteristics, including gender, years in practice, and surgical volume, have a small but significant association with receipt of breast cancer treatment(s).11–14 These studies suggest that surgeons contribute at least minimally to variation in receipt of breast cancer treatments. Largely missing from the literature on the quality of breast cancer care, however, are studies that assess the degree to which patient-reported experiences may vary according to surgeon. Furthermore, whether patient appraisal of their experiences may be associated with surgeon specialization in breast cancer, and/or delivery system factors (e.g., multidisciplinary care, access to clinical information, and availability of patient decision tools) that have been associated with higher quality care, is not known.7,9,15,16 In general, research on the impact of surgeon characteristics on breast cancer quality of care has been limited by inadequate attention to the patients’ perspectives about their care experiences. To address this gap in the literature we conducted a study with two objectives: (1) to measure patients’ appraisal of their access to care, care coordination, and decision satisfaction in a diverse, population-based sample of recently diagnosed breast cancer patients, and (2) to evaluate the amount of variation in these measures at the surgeon level.
METHODS
Sampling and Data Collection Patient Sample
A detailed description of the sampling and data collection procedures has been published previously.9,17–22 Briefly, from 6/2005 to 2/2007 we accrued a population-based sample of 3,133 women aged 20–79 years and recently diagnosed with primary ductal carcinoma in situ (DCIS) or invasive breast cancer (stage I–III) in the Detroit and Los Angeles (LA) metropolitan Surveillance, Epidemiology and End Results (SEER) sites. Exclusion criteria included stage IV cancer, inability to complete a questionnaire in either English or Spanish, and Asian racial identity due to enrollment in other studies. Latinas in LA and African Americans were oversampled to increase their representation in the dataset.
Eligible patients were identified by the respective SEER registries and mailed an introductory letter, survey materials, and a US $10 cash gift unless their physician objected. Survey materials were sent in both English and Spanish based on hospital records or the 1980 US census list of Spanish surnames.19,23 A modified Dillman survey methodology was used to encourage response.24,25 The study protocol followed established SEER procedure and was approved by the Institutional Review Boards of the University of Michigan, University of Southern California, and Wayne State University. The final response rate was 72.4% (n = 2,268). The mean response time was approximately 9 months postdiagnosis.
Surgeon Sample
An attending surgeon was identified for 98.9% of the patient sample using information from the patient surveys, pathology reports, and SEER records. Approximately 14 months after initiation of the patient survey, surgeons were mailed an introduction letter, survey materials, and a US $40 cash gift. The same methodology was used to increase surgeon response.24,25 Of the 419 surgeons identified, 318 returned a completed survey (response rate, RR = 75.9%).
MEASURES
The patient and surgeon survey records were merged using a unique patient identifier (n = 1,780 patients nested within 291 surgeons).
Dependent Variables
We evaluated three primary outcomes related to patients’ appraisal of their experience with breast cancer treatment, based on the NICCQ domains: (1) access to care, (2) care coordination, and (3) decision satisfaction.3 Each measure was developed based on the existing literature and our prior research. Access to care consisted of three separate questions asking patients to indicate how big of a problem the following logistical issues were: (1) finding a doctor to treat the breast cancer, (2) getting a doctor appointment, and (3) getting to the doctor’s office. Perceived care coordination consisted of five questions that solicited patients’ perceptions of the degree to which their doctors coordinated their breast cancer treatment.17 Decision satisfaction was measured with five questions that reflected patient satisfaction with their breast cancer treatment decisions.18,26
Patient Independent Variables
We evaluated two sets of patient-related independent variables: demographics and clinical factors. Patient demographics included age, education attainment (less than high school, high school graduate, some college or more), marital status (not married, married/partnered), annual family income (less than US $40,000, US $40,000–69,999, US $70,000 or more), and comorbidities (0, 1, 2 or more). Patient race/ethnicity was self-reported and categorized into non-Latina White, African American, Latina, and other. Latinas were further categorized into less versus more acculturated using the Short Acculturation Scale for Hispanics.18,19,27 Patient clinical variables came from the SEER record and included tumor behavior (DCIS, invasive) and tumor stage (0, I, II, III).
Surgeon Independent Variables
We evaluated two sets of surgeon-related independent variables: demographics and practice factors. Demographics included gender and years in practice. Practice factors included degree of breast cancer specialization, practice setting, and practice management factors. Specialization in breast cancer was measured by surgeon-reported percentage of his/her surgical practice devoted specifically to breast cancer (<15, 15–49, ≥50%). Practice setting included being affiliated with a National Cancer Center (NCI) cancer center, an American College of Surgeons (ACoS) cancer program, or neither. Practice management factors included three variables previously developed by our team, designed to capture the surgeon’s perspective of the coordination of cancer care in the surgical practice regarding: (1) multidisciplinary communication, (2) patient decision support, and (3) availability of clinical information.9,22 Each variable was further categorized into three categories (low, moderate, and high). Detail about these scales, including their distribution across the surgeon respondents, has been published.9,22
ANALYSIS
We conducted our analyses on a sample with complete information for all variables (1,495 patients nested within 268 surgeons). There was a median of 6 patients per surgeon with a range of 1–32. We first generated descriptive statistics for the patient and surgeon study samples. The frequencies of patient variables were weighted to account for the differential probability of selection. We evaluated associations for the outcome variables and all patient demographics and clinical factors, using unadjusted ordinary least-squares regression and one-way analysis of variance.
We then evaluated the amount of variation at the surgeon and patient level in each of our three outcomes using random-effects models with the surgeon identifier as the random-effects term.9,10,28 In a stepwise approach, we first generated models with no independent variables, then examined the relative contribution of patient demographics and clinical factors, surgeon demographics, and practice factors in explaining the surgeon-level variation. All models controlled for patient demographics and clinical factors. The two design variables (study site and race/ethnicity) were included in all models to account for the differential probability of subject selection generated by our sampling strategy. We then added surgeon demographics (gender and years in practice), practice setting, and degree of breast cancer specialization to the model, followed by the three practice management variables. In each model, we calculated the amount of surgeon variation in each of our three patient-reported outcome measures, including the proportion of variation explained by patient demographics and clinical factors and the residual surgeon-level variation.28 Finally, we tested for an interaction effect between individual surgeon and our sampling variables (site and race/ethnicity). All analyses were done using Stata version 11.0 and SAS.
RESULTS
Overall, patients positively appraised their experiences in each domain. The mean value was 4.7 (range 1–5) for access to care, 4.5 (range 1–5) for care coordination, and 4.1 (range 1–5) for decision satisfaction (Figure 1).
FIG. 1.
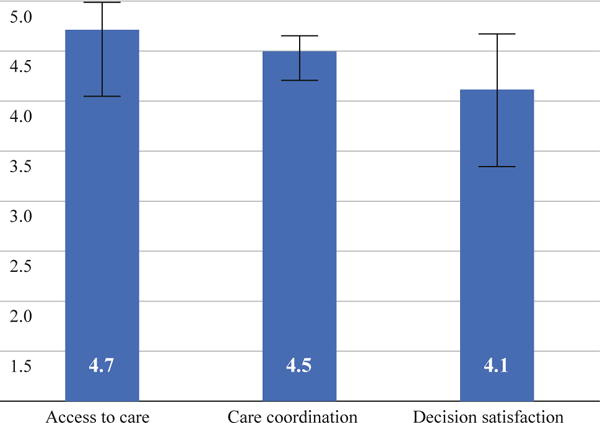
Mean values for patient-reported outcomes (N = 1,495)
Patient Characteristics
Table 1 provides the distribution of patient demographics and clinical factors, and the unadjusted associations between these patient variables and each outcome. Patients had mean age of 56.9 years (range 26–81 years). Using the sampling design weights, 69.5% of patients were White, 14.5% were African American, 7.4% were less-acculturated Latinas, and 7.6% were more-acculturated Latinas. Approximately one-third of the sample fell into each of the educational attainment groups, and the majority (59.0%) were married or partnered. Nearly 80% had invasive breast cancer.
TABLE 1.
Patient characteristics and associations with patient-reported outcomes (N = 1,495)
| Unadjusted regression coefficients
|
|||||
|---|---|---|---|---|---|
| N | %a | Access to care | Care coordination | Decision satisfaction | |
| Age (mean, SD), years | 56.9 (11.3) | 0.0041 | 0.004** | 0.001 | |
| Race | |||||
| White | 681 | 69.5 | Referent | Referent | Referent |
| African American | 398 | 14.5 | 0.055* | −0.041 | −0.285*** |
| Latina-high | 203 | 7.6 | −0.339 | −0.059 | −0.379*** |
| Latina-low | 183 | 7.4 | −0.008 | −0.146** | −0.783*** |
| Other | 30 | 1.1 | −0.095 | −0.047 | −0.458*** |
| F-test/p value | 2.3/0.06 | 2.3/0.06 | 45.2/0.001 | ||
| Education attainment | |||||
| Less than high school | 329 | 31.8 | Referent | Referent | Referent |
| High school graduate | 270 | 36.2 | 0.047 | 0.100* | 0.254*** |
| Some college or more | 896 | 32.0 | 0.131*** | 0.075* | 0.399*** |
| F-test/p value | 12.1/0.001 | 4.2/0.01 | 35.3/0.001 | ||
| Marital status | |||||
| Not married | 598 | 41.0 | Referent | Referent | Referent |
| Married/partnered | 897 | 59.0 | 0.047 | 0.054 | 0.136** |
| Annual family income | |||||
| US $40,000 | 523 | 30.3 | Referent | Referent | Referent |
| US $40,000–69,999 | 299 | 20.9 | −0.055 | 0.108* | 0.358*** |
| US $70,000? | 404 | 30.9 | −0.026 | 0.117** | 0.505*** |
| Don’t know/missing | 250 | 17.7 | −0.031 | 0.041 | 0.185** |
| F-test/p value | 0.5/NS | 4.02/0.007 | 38.6/0.001 | ||
| Number of comorbidities | |||||
| 0 | 643 | 45.7 | Referent | Referent | Referent |
| 1 | 419 | 29.2 | 0.050 | 0.068* | 0.028/NS |
| 2? | 425 | 25.1 | 0.042 | −0.028/NS | −0.161/** |
| F-test/p value | 2.3/NS | 3.08/0.05 | 7.7/0.005 | ||
| Tumor behavior | |||||
| DCIS | 300 | 20.1 | Referent | Referent | Referent |
| Invasive | 1,195 | 79.9 | 0.002 | 0.020 | −0.054 |
| Cancer stage | |||||
| Stage 0 | 299 | 20.4 | Referent | Referent | Referent |
| Stage I | 538 | 39.3 | 0.018 | 0.077 | 0.044 |
| Stage II | 449 | 28.4 | −0.120 | −0.023 | −0.143 |
| Stage III | 164 | 10.0 | −0.020 | −0.014 | −0.050 |
| Unknown/missing | 37 | 1.9 | 0.049 | −0.015 | −0.121 |
| F-test/p value | 0.6/NS | 1.2/NS | 3.9/0.003 | ||
| Site | |||||
| Detroit | 691 | 43.4 | Referent | Referent | Referent |
| LA | 804 | 56.7 | −0.039 | −0.073* | −0.226*** |
NS not significant
p B 0.05,
p B 0.01,
p B 0.001
Percentages weighted to account for sampling design
Unadjusted Correlates of Access to Care, Care Coordination, and Decision Satisfaction
Table 1 also presents the unadjusted patient-level correlates of the three patient-reported outcomes. Reports of better access to care were significantly associated (p ≤ 0.001) with having some college or more (versus less than high school), and minorities were somewhat less likely to report better access to care than Whites (p = 0.06). Better care coordination was significantly associated (p = 0.01) with some college or more (versus less than high school), higher income (p = 0.007), and increasing age (p = 0.004). Less-acculturated Latinas less often reported better care coordination (p = 0.06), but multidisciplinary communication (67.6%) and availability of clinical information (69.8%), however most reported low levels of patient decision support (63.8%).
Adjusted Correlates of Patient-Reported Outcomes
In addition to evaluating the surgeon-level variation in each outcome, our multilevel models assessed patient-level correlates of these outcomes, controlling for all factors in the model. These results are similar to the unadjusted findings presented in Table 1, and are briefly summarized here (data not shown in table).
We found that reporting better access to care among patients was significantly (p ≤ 0.05) associated with older age, some college or more (versus high school or less), and two or more comorbid conditions (versus none). Reports of better access to care was associated with a higher level of clinical information (p ≤ 0.05), but no other surgeon-level factors. Reporting better care coordination was significantly (p ≤ 0.05) associated with older age, and two or more comorbid conditions (versus none). Reports of better care coordination were also associated (p ≤ 0.05) with having a female surgeon and a higher level of multidisciplinary communication. Greater decision satisfaction was associated (p ≤ 0.05) with older age, some college or more (versus high school or less), two or more comorbid conditions (versus none), and a greater degree of breast cancer specialization by the surgeon. All racial/ethnic groups were significantly (p ≤ 0.001) less likely than White patients to report high levels of decision satisfaction. We did not find any significant interactions between patient- and surgeon-level factors in any of the models.
Surgeon-Level Variation in Patient-Reported Outcomes
We found relatively low variation in each of the patient-reported outcomes attributable to individual surgeons obtained from the unconditional (i.e., “surgeon only”) model (5.4% for access to care, 3.3% for care coordination), and moderate variation (7.5%) for decision satisfaction (Table 3). Table 3 presents the results of the random-effects models for each patient-reported outcome. Column A shows the total variation in each of the three outcomes (e.g., R2) due to the addition of sets of variables (patient clinical factors and demographics, surgeon demographics, and surgeon practice factors). The independent variables explained a small amount of total variation in each outcome; patient clinical factors and demographics explained 2.4% of access to care variation, surgeon demographics 2.6%, and surgeon practice factors 3.3%. A similar pattern was displayed in the amount of variation that these three independent variables explained in care coordination and decision satisfaction (1.3, 1.7, 2.0, 11.5, 11.7, 12.9%, respectively).
TABLE 3.
Impact of patient and surgeon factors on surgeon-level variation in patient-reported outcomes
| A: Total variation (%)
|
B: Surgeon variation (%)
|
C: % between-surgeon variation explaineda
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AC | CC | Dsat | AC | CC | Dsat | AC | CC | Dsat | |
| Surgeon only | 5.4 | 3.3 | 7.5 | ||||||
| Patient clinical factors and demographics | 2.4 | 1.3 | 11.5 | 5.0 | 2.7 | 2.0 | – | – | – |
| Surgeon demographics | 2.6 | 1.7 | 11.7 | 4.7 | 2.1 | 1.3 | 6.7 | 24.3 | 35.0 |
| Surgeon practice factors | 3.3 | 2.0 | 12.9 | 3.8 | 2.5 | 1.2 | 23.9 | 43.4 | 40.0 |
AC access to care, CC care coordination, Dsat decision satisfaction
Surgeon effect calculated after controlling for patient-level factors
In addition to showing the surgeon variation from the unconditional model, column B of Table 3 also presents the proportion of total variation attributable to individual surgeons. Overall, the proportion of variance at the surgeon level was low, and was only slightly reduced as sets of variables were added; For instance, patient clinical factors and demographics explained 5.0% of surgeon-level variation in access to care, 2.7% of variation in care coordination, and 2.0% of variation in decision satisfaction. Surgeon demographics explained 4.7, 2.1, and 1.3% of these outcome variables (respectively) and surgeon practice factors explained 3.8, 2.5, and 1.2% (respectively).
Finally, column C of Table 3 presents the amount of between-surgeon variation explained by the addition of surgeon demographics and surgeon practice factors, after controlling for patient demographics and clinical factors. Although the percent of overall variation attributable to individual surgeons was low (see column B) for each patient-reported outcome, column 3 shows that the amount of total between-surgeon variation in each of the models increased as surgeon demographics and practice factors were added. For patient-reported access to care and care coordination, the addition of the surgeon practice factors increased the explained proportion of between-surgeon variation by a considerable amount, when compared with surgeon demographics alone (from 6.7 to 23.9% for access and from 23.4 to 43.4% for care coordination). For decision satisfaction, the added contribution of surgeon practice factors was smaller, increasing from 35.0 to 40.0%.
DISCUSSION
These results provide evidence of “good news” regarding patients’ appraisals of their breast cancer care experiences. Specifically, in this large racially/ethnically diverse sample of women with breast cancer, patients reported positive experiences with access to and coordination of their care, and were generally very satisfied with their treatment decisions. We observed few substantial disparities in patient perceptions of access to or coordination of care in our multivariable models. These results are consistent with prior work documenting positive experiences with care coordination in breast cancer patients, but are in contrast to some studies indicating that minorities report greater difficulty accessing breast cancer treatment.17,29,30 In this analysis, we found disparities in decision satisfaction, in less-acculturated Latinas, as well as those with lower education, reporting lower levels of decision satisfaction than their counterparts. Our result is also in line with research suggesting that racial/ethnic minorities, especially those who are non-English speaking, may be most vulnerable to poor decision outcomes.18,31,32
Overall, we found that individual surgeons did not explain observable differences in patient appraisal of their care experiences. The variation observed at the surgeon level was largely explained by patient factors, rather than by surgeon demographics or practice factors. However, among the three patient-reported outcomes evaluated in this analysis, we found that the greatest amount of surgeon-level variation was observed for decision satisfaction, and that the addition of surgeon factors (specifically breast cancer specialization) somewhat reduced this variation. Prior work has also found an association between greater surgeon specialization in breast cancer and increased treatment decision satisfaction in patients, and that higher-volume breast surgeons more often initiate communication about treatment options which, in turn, positively impacts patient satisfaction.33,34
One important gap in the breast cancer quality care literature is whether patient-reported outcomes are observed uniformly across clinician practices or are associated with clinic attributes such as breast cancer specialization. This has important implications for whether interventions should be aimed broadly across clinician practices or more narrowly targeted at practices with certain attributes. Our results suggest that interpersonal processes across surgeons are more important in explaining variation in patient-reported outcomes than systematic differences between surgeon practices.
Several limitations to our study deserve mentioning. The generalizability of our findings is limited to groups included in our sample from two geographic locations; we cannot extend our findings to rural areas or Asian Americans. However, the large population-based sample, diverse race/ethnicity of patients, and high response rates from both patients and surgeons suggest that our sample is representative of breast cancer patients in the USA. In addition, self-reported variables, such as breast cancer surgery volume, may be inaccurate or subject to recall bias. Finally, our outcome variables designed to measure patient attitudes and perceptions about access to care and care coordination may not reflect the actual experiences of the respondents.
IMPLICATIONS
Our optimistic findings indicate that patients appraise all three quality measures positively, and we observed little disparity in these appraisals across a highly racially/ethnically diverse urban population. Our finding that surgeon specialization (rather than practice factors) explained some of the variation in decision satisfaction suggests that surgeons’ communication experience may result in more satisfied patients, and that interventions for improving decision satisfaction may be more effective if they are directed at interpersonal communication as opposed to practice-level factors. Further research to evaluate the impact of clinicians and practice settings on all patient quality measures, including patient-reported outcomes, is needed to inform interventions to achieve high-quality breast cancer care across the care continuum.
TABLE 2.
Surgeon characteristics (N = 268)
| N | % | |
|---|---|---|
| Years in practice (mean, SD) | 18.5 (10.7) | |
| Gender | ||
| Male | 220 | 82.1 |
| Female | 48 | 17.9 |
| Breast cancer specialization (%) | ||
| \15 | 95 | 35.6 |
| 15–49 | 129 | 48.3 |
| [50 | 44 | 16.1 |
| Practice setting | ||
| NCI cancer center | 76 | 28.4 |
| ACoS cancer program | 103 | 38.4 |
| Neither | 89 | 33.2 |
| Multidisciplinary communication | ||
| Low | 87 | 32.5 |
| Moderate | 124 | 46.3 |
| High | 57 | 21.3 |
| Availability of clinical information | ||
| Low | 81 | 30.2 |
| Moderate | 115 | 42.9 |
| High | 72 | 26.9 |
| Patient decision support | ||
| Low | 171 | 63.8 |
| Moderate | 76 | 28.4 |
| High | 21 | 7.8 |
| Study site | ||
| Detroit | 101 | 37.7 |
| Los Angeles | 167 | 62.3 |
Acknowledgments
Funding: Funding for this research comes from R01CA109696 and R01CA08870 to the University of Michigan.
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.Institute of Medicine, Commission on life Sciences National Research Council. Ensuring quality cancer care. National Academy Press; Washington DC: 1999. [Google Scholar]
- 2.Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel E, Kahn KL. Results of the national initiative for cancer care quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24(4):626–34. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- 3.Schneider EC, Malin JL, Kahn KL, Emanuel EJ, Epstein AM. Developing a system to assess the quality of cancer care: ASCO’s national initiative on cancer care quality. J Clin Oncol. 2004;22(15):2985–91. doi: 10.1200/JCO.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 4.Malin JL, Diamant AL, Leake B, et al. Quality of care for breast cancer for uninsured women in California under the breast and cervical cancer prevention treatment act. J Clin Oncol. 2010;28(21):3479–8434. doi: 10.1200/JCO.2009.27.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117(15):3311–21. doi: 10.1002/cncr.25915. [DOI] [PubMed] [Google Scholar]
- 6.Pieters HC, Heilemann MV, Grant M, Maly RC. Older women’s reflections on accessing care across their breast cancer trajectory: navigating beyond the triple barriers. Oncol Nurs Forum. 2011;38(2):175–84. doi: 10.1188/11.ONF.175-184. [DOI] [PubMed] [Google Scholar]
- 7.McCahill LE, Privette A, James T, et al. Quality measures for breast cancer surgery: initial validation of feasibility and assessment of variation among surgeons. Arch Surg. 2009;144(5):455–62. doi: 10.1001/archsurg.2009.56. [DOI] [PubMed] [Google Scholar]
- 8.Katz SJ, Hawley ST. From policy to patients and back: surgical treatment decision making for patients with breast cancer. Health Affairs. 2007;26(3):761–9. doi: 10.1377/hlthaff.26.3.761. [DOI] [PubMed] [Google Scholar]
- 9.Katz SJ, Hawley ST, Abrahamse P, et al. Does it matter where you go for breast surgery: attending surgeon’s influence on variation in receipt of mastectomy for breast cancer. Med Care. 2010;48(10):892–9. doi: 10.1097/MLR.0b013e3181ef97df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley ST, Hofer TP, Janz NK, et al. Correlates of between surgeon variation in breast cancer treatments. Med Care. 2006;44(7):609–16. doi: 10.1097/01.mlr.0000215893.01968.f1. [DOI] [PubMed] [Google Scholar]
- 11.Hershman DL, Buone D, McBride RB, Tsai WY, Neugut AI. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115(17):3848–57. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershman DL, Buone D, Jacobson JS, McBride RB, Tsai WY, Joseph KA, Neugut AI. Surgeon characteristics and use of breast conservation surgery in women with early stage breast cancer. Ann Surg. 2009;249(5):828–33. doi: 10.1097/SLA.0b013e3181a38f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating NL, Kouri E, He Y, Weeks JC, Winer EC. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47(7):765–73. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]
- 14.Spinks T, Albright HW, Feely R, et al. Ensuring quality cancer care: a follow up review of the Institute of Medicine’s 10 recommendation for improving the quality of cancer care in America. Cancer. 2011 doi: 10.1002/cncr.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillner BE, Smith RJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18(11):2327–40. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 16.Dilts DM. Practice variation: the Achilles’ heel in quality cancer care. J Clin Oncol. 2005;23(25):5881–2. doi: 10.1200/JCO.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Hawley ST, Janz NK, Lillie SE. Perceptions of care coordination in a population-based sample of diverse breast cancer patients. Patient Educ Counsel. 2010;81(Suppl):S34–40. doi: 10.1016/j.pec.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley ST, Janz NK, Hamilton A, Griggs JJ, Alderman AK, Mujahid M, et al. Latina patient perspectives about informed treatment decision making for breast cancer. Patient Educ Counsel. 2008;73(2):363–70. doi: 10.1016/j.pec.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2022–9. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–6. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337–47. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz SJ, Hawley ST, Morrow MM, et al. Coordinating cancer care: patient and practice management processes among surgeons who treat breast cancer. Med Care. 2010;48(1):45–51. doi: 10.1097/MLR.0b013e3181bd49ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Word D, Perkins JR. Building a Spanish Surname List for the 1990’s—a new approach to an old problem. 1996. (U.S. Census Bureau, Technical Working Paper No. 13). Surgeon Variation in Patient Care Experiences 13. [Google Scholar]
- 24.Dillman DA, Smyth DA, Christian LM. Internet, mail and mixedmode surveys: the tailored design method. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 25.Anema MG, Brown BE. Increasing survey responses using the total design method. J Continuing Educ Nurs. 1995;26(3):109–4. doi: 10.3928/0022-0124-19950501-06. [DOI] [PubMed] [Google Scholar]
- 26.Holmes-Rovner M, Kroll J, Schmitt DR, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making. 1996;16(1):58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 27.Marin G, Sabogal BV, Marin R, Otero-Sabogal R, Perez-Stable E. Development of a short acculturation scale for hispanics. Hispanic J Behav Sci. 1987;9(2):183–205. [Google Scholar]
- 28.Snijders TAB, Boster RJ. Multilevel Analysis. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- 29.Tariman JD, Berry B, Cochrane A, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):45–51. doi: 10.1093/annonc/mdp534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008;3(2):426–33. doi: 10.1002/cncr.23547. [DOI] [PubMed] [Google Scholar]
- 31.Na´poles-Springer AM, Livaudais JC, Bloom J, Hwang S, Kaplan CP. Information exchange and decision making in the treatment of Latina and White women with ductal carcinoma in situ. J Psychosocial Oncol. 2007;25(4):19–36. doi: 10.1300/J077v25n04_02. [DOI] [PubMed] [Google Scholar]
- 32.Polacek GN, Ramos MC, Ferrer RL. Breast cancer disparities and decision-making among U.S. women. Patient Educ Counsel. 2007;65(2):158–65. doi: 10.1016/j.pec.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Waljee JF, Hawley ST, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;24(24):3694–8. doi: 10.1200/JCO.2007.10.9272. [DOI] [PubMed] [Google Scholar]
- 34.Liang W, Burnett CB, Rowland JH, et al. Communication between physicians and older women with localized breast cancer: implications for treatment and patient satisfaction. J Clin Oncol. 2002;20(4):1008–16. doi: 10.1200/JCO.2002.20.4.1008. [DOI] [PubMed] [Google Scholar]


