Our findings show that insurance coverage for CT colonography as an alternative option for colorectal cancer (CRC) screening was associated with a greater likelihood of CRC screening among eligible patients who were due for CRC screening and also was associated with a greater likelihood of being screened with a test that helps both to detect cancer and prevent cancer from developing (CT colonography or colonoscopy).
Abstract
Purpose
To compare overall colorectal cancer (CRC) screening rates for patients who were eligible and due for CRC screening and who were with and without insurance coverage for computed tomographic (CT) colonography for CRC screening.
Materials and Methods
The institutional review board approved this retrospective cohort study, with a waiver of consent. This study used longitudinal electronic health record data from 2005 through 2010 for patients managed by one of the largest multispecialty physician groups in the United States. It included 33 177 patients under age 65 who were eligible and due for CRC screening and managed by the participating health system. Stratified Cox regression models provided propensity-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between CT colonography coverage and CRC screening.
Results
After adjustment, patients who had insurance coverage for CT colonography and were due for CRC screening had a 48% greater likelihood of being screened for CRC by any method compared with those without coverage who were due for CRC screening (HR, 1.48; 95% CI: 1.41, 1.55). Similarly, patients with CT colonography coverage had a greater likelihood of being screened with CT colonography (HR, 8.35; 95% CI: 7.11, 9.82) and with colonoscopy (HR, 1.38; 95% CI: 1.31, 1.45) but not with fecal occult blood test (HR, 1.00; 95% CI: 0.91, 1.10) than those without such insurance coverage.
Conclusion
Insurance coverage of CT colonography for CRC screening was associated with a greater likelihood of a patient being screened and a greater likelihood of being screened with a test that helps both to detect cancer and prevent cancer from developing (CT colonography or colonoscopy).
© RSNA, 2017
Introduction
Despite steady decreases in incidence and mortality from colorectal cancer (CRC), it remains the second leading cause of cancer-related mortality and is responsible for an estimated 50 000 deaths each year (1). CRC should be largely preventable if identified early, yet CRC screening rates remain suboptimal (2,3). Yet, after nearly a decade of continuous improvement (from 54% of the U.S. population up to date in 2002% to 65% in 2010), CRC screening rates have plateaued at 65% from 2010 to 2012 (2).
Greater use of alternative CRC screening methods has been proposed as one strategy to improve screening rates (4,5). It has been suggested that the common practice in the United States of universally recommending colonoscopy may contribute to lower screening rates by not recognizing substantial variation in patient preferences and desire for choice (4–7). Prospective and randomized studies have also suggested that allowing patients to choose between two types of CRC screening tests increases participation in screening (4,7,8) and may increase the use of both tests (8), although these findings are not consistent (9,10).
In the United States, recent guidelines support screening methods that can both help detect cancer and prevent cancer from developing (eg, colonoscopy; computed tomographic [CT] colonography) over methods that depict cancer after it develops (eg, stool-based tests such as fecal occult blood test (FOBT) or stool DNA) (11). CT colonography is relatively new and able to depict both precancerous polyps and cancers, but is not widely covered by insurance for CRC screening, most notably Medicare. Two randomized controlled trials have shown a 55%–90% increase in CRC screening with CT colonography over colonoscopy, although individuals were not given a choice between the two tests (12,13). No studies have compared whether expanding the choice of insurance-covered options for CRC screening to include CT colonography could further increase overall screening rates.
We examined whether insurance coverage of CT colonography as an alternative choice for CRC screening was associated with higher overall CRC screening rates or, alternatively, whether overall screening rates remained stable but only the type of tests used for CRC screening differed. In this study, a substantial proportion of patients eligible for CRC screening had commercial insurance coverage of CT colonography as a CRC screening method (more than 18 000 eligible patients each year). Our goal was to compare overall CRC screening rates for patients who were eligible and due for CRC screening and who were with and without insurance coverage of CT colonography for CRC screening.
Materials and Methods
Duality of Interest
P.J.P. is a cofounder of VirtuoCTC; advisor to Bracco and Check-Cap; and a shareholder in Cellectar, Elucent, and SHINE. D.H.K. is a cofounder of VirtuoCTC, a consultant for Viatronix, and a member of the Medical Advisory Board for Digital Artforms. None of these organizations provided support for this study. The remaining authors disclose no conflicts. The data were analyzed and controlled by authors (M.A.S., R.E.G., A.P.) who were not affiliated with nor employed by the above companies in the medical industry.
Population
This retrospective cohort study used longitudinal electronic health record data from a large academic medical center, one of the 10 largest multidisciplinary physician groups in the United States. Patients were included if eligible and due for CRC screening from 2005 through 2010, as defined by (a) at least 50 years of age; (b) not previously screened in the past year with FOBT; last 5 years with CT colonography, flexible sigmoidoscopy, or barium enema; or last 10 years with a colonoscopy; and (c) did not have a total colectomy. To maximize capture of screening results, patients were also included if they had at least (a) two office visits on different dates of service to a primary care provider (PCP) in the prior 36 months and (b) one office visit with a PCP in the most recent 24 months (14). Although we use an accepted approach to identify eligible individuals for CRC screening, we miss patients seen less frequently; these excluded patients represent a small percentage (8%) of the physician group’s eligible primary care patients. We also excluded a small percentage of patients (1%) with a history of metastatic cancer (15). Because Medicare and Medicaid did not cover CT colonography for screening at any time during the study, patients were censored at the time they became eligible for Medicare or Medicaid insurance, if applicable.
The final sample was 33 177 patients eligible and due for CRC screening at some point between 2005 and 2010, assigned to one of 270 PCPs and 30 clinics, and enrolled in any of 158 insurance plans. The Institutional Review Board approved this study with a waiver of consent.
Setting
At the start of our study in 2005, available methods for CRC screening within the health system included colonoscopy, CT colonography, FOBT, flexible sigmoidoscopy, and barium enema. These options did not change during the study time frame. All PCPs in the physician group had equal and open access to all screening methods. All methods were identified as options under the physician group’s CRC screening guidelines and were deemed adequate for CRC screening for the purposes of publicly reporting CRC screening rates (16). All patients were required to visit and receive consultation from their PCP, with a subsequent referral to a gastroenterologist or radiologist prior to scheduling a screening colonoscopy or CT colonography. However, patients differed widely in whether their insurer covered CT colonography as an option for CRC screening, and four large insurers expanded their benefits during the study to include CT colonography as a covered option.
Variables
The first outcome was the time to screening for CRC by any method. Other outcomes included time to screening for CRC with colonoscopy, time to screening for CRC with CT colonography, and time to screening for CRC with FOBT. Screening with barium enema was not separately considered as an outcome since it accounted for less than 0.1% (11 of 17 144) of all screenings. Completion of CRC screening was identified through the presence of a procedure code for one of these methods, including codes for both diagnostic and screening procedures (17). If more than one code was present, the earliest code was used to determine the type (colonoscopy, CT colonography, FOBT, flexible sigmoidoscopy, or barium enema) and timing of screening.
The primary explanatory variable was time-varying by day and indicated whether a patient had insurance coverage for CT colonography as a CRC screening method (yes vs no) on each day that they were in the cohort.
We also accounted for potential residual confounding by variables that are known to affect CRC screening (18,19). We assessed patient characteristics, including age, sex, race (white, nonwhite), marital status (married, other), the percentage of the population in the patient’s zip code with at least a high school education, the percentage of the population in the patient’s zip code below the federal poverty line, rural-urban commuting area code (urban, suburban, large town, and small town/rural) (20), and the Johns Hopkins ACG (Adjusted Clinical Group) health care utilization score (21), a statistically valid tool used to characterize a patient’s prior health resource use while accounting for morbidity or illness burden. We included PCP characteristics that have previously been related to CRC screening such as sex, specialty (internal medicine/geriatrics or family medicine), annual panel size of patients eligible for CRC screening, primary clinic ownership (hospital- or physician-owned), and distance from the primary clinic to the nearest colonoscopy facility (18).
Statistical Analysis
Stratified Cox regression models provided propensity-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between CT colonography coverage and CRC screening (22). Insurance coverage for CT colonography was included as a time-varying covariate for each patient. Patients entered the cohort on the date they became eligible and due for CRC screening and left if they were screened or became ineligible, resulting in 58 461 patient-years. To avoid overcounting screening methods that are recommended more frequently, only the first period of eligibility was included (eg, for patients with FOBT screening who became eligible for CRC screening 1 year later, subsequent periods of eligibility were not included). As a result, patients were only in the cohort during the time that they were eligible and due for screening. We examined time to screening for several dependent variables, including screening by means of any method, colonoscopy, CT colonography, and FOBT. As is typical for Cox regression, patients were censored at the time of the first test even if they went on to have other tests (eg, a CT colonography was followed by a colonoscopy).
To construct propensity scores, a logistic regression predicting insurance coverage for CT colonography was fit by using all covariates as predictors, as well as indicator variables for each year. Specifically, insurance coverage was set to “yes” in a given calendar year if the patient had an insurer who covered CT colonography during the entire year. The regression produced a propensity score for each person-year. The propensity score was categorized into bins of length of 0.05, for a total of 20 equally spaced bins between 0 and 1. These propensity score categories were treated as strata in the Cox model, thereby allowing each stratum to have its own baseline hazard. Percentages and means in Table 1 were adjusted by including indicators for the propensity score categories as control variables in a series of logistic regression models predicting CT colonography coverage. All regressions were estimated by using Stata version 13.1 (Stata, College Station, Tex), conservatively accounting for correlation by using robust estimates of the variance clustered by clinic.
Table 1.
Patient, PCP, and Clinic Characteristics
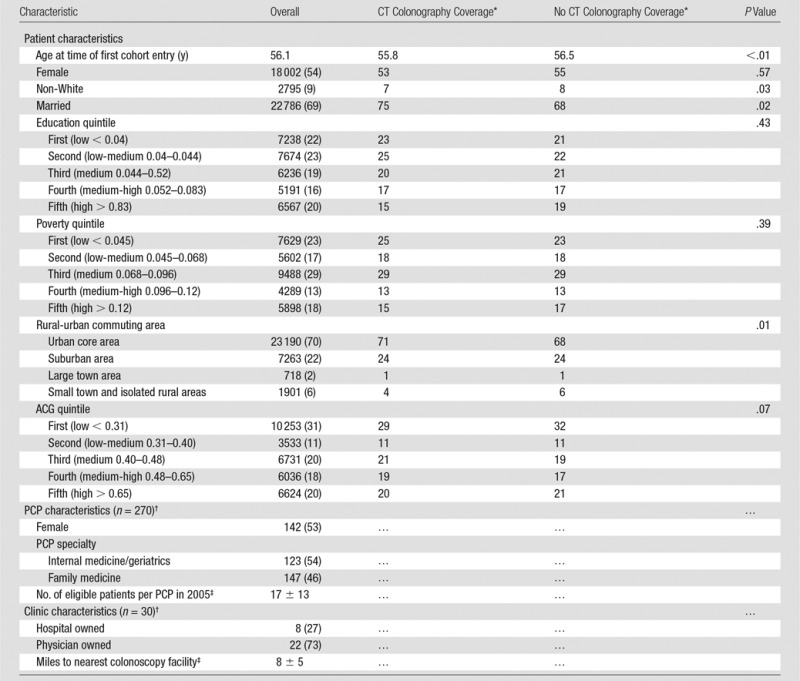
Note.— Unless otherwise indicated, data are the number of patients and numbers in parentheses are percentages. ACG = Johns Hopkins ACG healthcare utilization score.
*Values are adjusted for propensity score categories.
†PCPs and clinics treat patients with and patients without CT colonography coverage; therefore, their characteristics do not differ, and they are not presented separately.
‡Data are mean ± standard deviation.
To test the robustness of our results, we also performed several sensitivity analyses. We re-estimated models restricting our sample to patients who live within the largest central county to account for any missing data in the electronic health record. Second, we censored individuals who underwent a diagnostic procedure, as these procedures are unlikely to be affected by the availability of insurance coverage. Finally, we adjusted for the potential for bias due to self-selection into different insurance plans by limiting our sample to patients who were eligible and due for CRC screening and who were enrolled in one of four large insurance plans that expanded their coverage between 2005 and 2010 to include CT colonography for CRC screening. This approach allowed us to compare patients who were members of those four plans before the expanded coverage to patients who were members of those same plans after the expanded coverage.
Results
Population Characteristics
Patients were 54% female (18 002 of 33 177), 9% nonwhite (2795 of 32 651), and 69% were married (22 786 of 33 130) (Table 1). About half of PCPs were female (142 of 270), and about half were internal medicine physicians (147 of 270). PCPs had an average of 17 CRC-eligible patients in the first year of the study cohort (2005). Clinics were primarily physician-owned (73%; 22 of 30) and averaged 8 miles to the nearest facility that conducted colonoscopy. Patients with CT colonography coverage were slightly more likely to be younger, white, married, and urban.
Screening Characteristics
About one-quarter of patients eligible and due for CRC screening in any given year were screened during that year (Table 2), with about half (52%; 17 144 of 33 177) of eligible patients screened across all study years. Among all patients who were eligible and due for CRC screening (including those who did and those who did not have insurance coverage for CT colonography), most screenings were with colonoscopy (73%; 12 599 of 17 144), followed by FOBT (13%; 2214 of 17 144) and CT colonography (11%; 1945 of 17 144). Flexible sigmoidoscopy (2%; 375 of 17 144) and barium enema (< 0.1%; 11 of 17 144) were used rarely (not shown). The percentage of screenings that were with CT colonography was relatively stable over time. In contrast, the percentage of screenings with colonoscopy increased substantially (from 61% to 86%; from 2442 of 4035 to 2058 of 2393), and the percentage of screenings with FOBT declined substantially (from 19% to 4%; from 783 of 4035 to 95 of 2393).
Table 2.
Unadjusted Screening Rates by Year
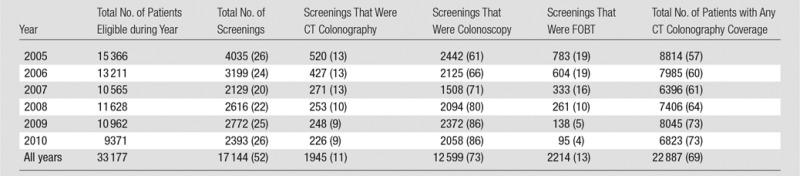
Note.— Data in parentheses are percentages.
Insurance Coverage and CRC Screening
At any given time, among eligible patients who became due for CRC screening, there was a 48% greater likelihood of CRC screening by any method for patients who had insurance coverage for CT colonography when compared with those who did not have insurance coverage (HR, 1.48; 95% CI: 1.41, 1.55) (Table 3). As expected, patients who had insurance coverage for CT colonography were more likely to be screened by using CT colonography (HR, 8.35; 95% CI; 7.11, 9.82). Notably, there was also a greater likelihood of screening by using colonoscopy; patients with insurance coverage had a 38% greater likelihood of being screened by using colonoscopy as the screening method (HR, 1.38; 95% CI: 1.31, 1.45). There was no significant difference in the likelihood of being screened by using FOBT (HR, 1.00; 95% CI: 0.91, 1.10).
Table 3.
Relationship between CT Colonography Coverage and CRC Screening

Note.—Models adjusted for sex, race, marital status, education status quintile, poverty status quintile, rural-urban commuting area code, Johns Hopkins ACG score quintile, age, PCP gender, PCP practice, PCP panel size at baseline, clinic ownership, and distance from clinic to nearest colonoscopy facility.
*Data in parentheses are 95% CIs.
Sensitivity Analysis
To test the robustness of our results, we performed several sensitivity analyses (Table 3). First, as not all colonoscopies are documented in the electronic health record, it is possible that missing data on colonoscopies would affect our conclusions. We re-estimated models restricting our sample to 65% (21 452 of 33 177) of patients who live within the largest central county. In this county, the physician group has confirmed that 98.5% of patients who underwent colonoscopies did so within an outpatient clinic that uses the same electronic health record. Limiting to this subsample, our results did not differ significantly. Second, many screened patients (39%; 6735 of 17 144) underwent procedures (colonoscopies and flexible sigmoidoscopies) for diagnostic purposes, and these procedures were unlikely to be affected by the availability of insurance coverage for CT colonography. We included these diagnostic procedures as they are included in well-established and standardized national quality metrics (17). However, removing these diagnostic procedures from consideration by censoring these individuals at the time of their diagnostic procedure also did not change our conclusions.
We also conducted a sensitivity analysis to address the potential for bias due to patient self-selection into different insurance plans (data not shown) (23,24). Our conclusions did not change. Specifically, we limited our sample to 6669 patients who were eligible and due for CRC screening and who were enrolled in one of four large insurance plans that expanded their coverage between 2005 and 2010 to include CT colonography for CRC screening. After adjustment, patients whose options expanded to include CT colonography had an 18% greater likelihood of being screened by any method compared with those without coverage (HR, 1.18; 95% CI: 1.06, 1.32). Patients with CT colonography coverage had a greater likelihood of being screened by CT colonography (HR, 4.11; 95% CI: 2.70, 6.23) and by colonoscopy (HR, 1.23; 95% CI: 1.09, 1.38) but not by FOBT (HR, 0.69; 95% CI: 0.40, 1.18).
Discussion
Our findings show that insurance coverage for CT colonography as an alternative option for CRC screening was associated with a greater likelihood of CRC screening among eligible patients who were due for CRC screening and was also associated with a greater likelihood of being screened with a test that helps both to detect cancer and prevent cancer from developing (CT colonography or colonoscopy). Specifically, insurance coverage of CT colonography was associated with an increase in overall CRC screening rates, increase in screening by CT colonography, increase in screening by colonoscopy, and no change in screening by FOBT. A comparable study of expanding Medicare insurance coverage in the United States in 2001 to cover screening colonoscopy also showed increased overall CRC screening rates and increased use of colonoscopy for screening, as well as decreased use of flexible sigmoidoscopy and FOBT (25).
Although higher rates of CRC screening with CT colonography were expected for those with insurance coverage for CT colonography, our results are also consistent with evidence that patients who are offered a choice of CRC screening tests are more likely to complete screening than patients offered either type of test alone (4,7,8). In one of these prior studies, patients were randomized to (a) a choice between one-time colonoscopy and fecal immunochemical test (FIT) annually for up to 3 years, (b) one colonoscopy, or (c) FIT annually for up to 3 years. The rate of overall screening was higher among those who had a choice (86% vs 70%) (8). Similar to our study, this was not solely due to additional individuals receiving screening by a second test. The rates of both FIT and colonoscopy were higher among those who had a choice. The group that chose colonoscopy over FIT was more likely to complete colonoscopy than the group that was assigned colonoscopy (96% vs 91%), and the group that chose FIT over colonoscopy was more likely to complete FIT than the group that was assigned FIT (73% vs 63%).
A choice of screening technologies might serve to increase participation in CRC screening due to greater engagement in screening decisions (26). Nearly all individuals (96%) prefer to be offered treatment choices, discuss options, and share their opinions about possible approaches (27). Consistent with this, the physician group required that all patients receive consultation from their PCP prior to scheduling a CRC screening test during the years of this study. PCPs and patients with an additional option to colonoscopy may have been more likely to engage in “choice talk” (ie, notification that reasonable options or choices are available) (28). The availability of choice itself may serve to engage the patient and increase participation (29). This may explain our finding that patients with insurance coverage for both colonoscopy and CT colonography had increased overall CRC screening rates, with increases in both colonoscopy and CT colonography. Because PCPs in the United States overwhelmingly recommend colonoscopy as the preferred option (4,5,30,31), discussions prompted by the availability of additional options may have resulted in a decision to screen by colonoscopy.
Increased choice of screening technologies may have also allowed patients to choose an option that corresponded best with their preferences (6,7). Patients vary in their preference for colonoscopy or CT colonography for CRC screening (6,32,33), and some patients may not participate in screening if only a single option is offered with no alternative. Two separate studies found that up to one-third of individuals undergoing CT colonography screening may not have been screened if CT colonography were not available (34,35), and a Dutch randomized controlled trial showed a 55% increase in screening participation among those offered CT colonography (compared with those offered colonoscopy) (13). PCP recommendations for CRC screening tests should be based at least partly on patient preferences and likely adherence (36).
Only a minority of patients (11%) used CT colonography for screening. CT colonography is a relatively new screening test and was not yet recommended by the U.S. Preventive Services Task Force (USPSTF) during the study timeframe. As such, substantial variation in PCP adoption of CT colonography existed at our institution (37). Uptake in other centers throughout the United States is currently much lower. There are several possible approaches to increase use, including recent inclusion in guidelines (eg, USPSTF), coverage by additional insurers such as the Center for Medicare and Medicaid Services for Medicare beneficiaries, and broader acceptance by PCPs (37). Primarily, due to lack of coverage for Medicare beneficiaries, utilization at the study center has not yet increased since 2010.
There are strengths and limitations to this study. First, we report findings from a single large multispecialty physician group, although it is the only center in the United States for whom a large proportion of patients eligible for CRC screening have had insurance coverage for CT colonography as a screening method over an extended period of time (38). Second, despite propensity score adjustment, there may still be unobserved variables associated with CT colonography coverage and CRC screening rates. For example, patients may self-select into different insurance plans by age, sex, educational attainment, household income, employment, and health status/utilization (23,24). To assess this bias, we conducted a sensitivity analysis that limited our sample to patients who were enrolled in one of four large insurance plans that expanded their coverage during the study years to include CT colonography for CRC screening. This approach allowed us to compare patients who were members of those four plans before the expanded coverage to patients who were members of those same plans after the expanded coverage, thereby reducing bias due to patient self-selection into insurance plans. Our conclusions did not change with this analysis. In addition, our use of Cox regression does account for temporal trends such as an increasing trend over time in the rate of CRC screening. Finally, we examined guaiac-based FOBT as the only stool-based CRC screening option; both fecal immunochemical and stool DNA tests were not introduced to this physician group until after this study.
We conclude that insurance coverage of CT colonography as an additional option for CRC screening is associated with greater likelihood of a patient being screened and a greater likelihood of being screened using a preventive test (CT colonography or colonoscopy). Policymakers should consider additional options for CRC screening when considering strategies to increase overall screening rates.
Advances in Knowledge
■ Among eligible patients who became due for colorectal cancer (CRC) screening, there was a 48% greater likelihood of CRC screening by any method for patients who had insurance coverage for CT colonography when compared with those who did not have coverage (hazard ratio [HR], 1.48; 95% confidence interval [CI]: 1.41, 1.55).
■ Patients who had insurance coverage for CT colonography and were due for CRC screening were substantially more likely to be screened with CT colonography than those without such insurance coverage who were due for CRC screening (HR, 8.35; 95% CI: 7.11, 9.82).
■ Patients who had insurance coverage for CT colonography and were due for CRC screening also had a 38% greater likelihood of being screened with colonoscopy as the screening method than those without such insurance coverage who were due for CRC screening (HR, 1.38; 95% CI: 1.31, 1.45).
■ CT colonography screening accounted for 11% (1945 of 17 144) of all screening studies performed over the 6-year study period.
Implication for Patient Care
■ Patients whose insurance plans cover the option of CT colonography for CRC screening may have higher CRC screening rates with a test that helps both detect cancer and prevent cancer from developing (CT colonography or colonoscopy).
Received April 20, 2017; revision requested May 4; final revision received May 17; accepted May 22; final version accepted June 2.
Study supported by the National Cancer Institute (P30 CA014520, R01 CA144835), the Health Innovation Program, the UW School of Medicine and Public Health from the Wisconsin Partnership Program, the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research through the National Center for Advancing Translational Sciences (UL1TR000427), the University of Wisconsin Carbone Cancer Center, and the Mentored Research Scholar Grant in Applied and Clinical Research from the American Cancer Society (MSRG-13-144-01-CPHPS).
The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of Conflicts of Interest: M.A.S. disclosed no relevant relationships. J.M.W. disclosed no relevant relationships. A.P. disclosed no relevant relationships. J.R.S. disclosed no relevant relationships. R.E.G. disclosed no relevant relationships. D.H.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: co-founder of VirtuoCTC and on medical advisory board of Digital Artforms. Other relationships: disclosed no relevant relationships. L.A.W.F. disclosed no relevant relationships. P.J.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received consultancy fees from Bracco and Check-Cap, co-founder of VirtuoCTC, and received stock/stock options from Cellectar Biosciences, SHINE, and Elucent. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- CRC
- colorectal cancer
- HR
- hazard ratio
- FOBT
- fecal occult blood test
- PCP
- primary care provider
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) . Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 3.McLachlan SA, Clements A, Austoker J. Patients’ experiences and reported barriers to colonoscopy in the screening context: a systematic review of the literature. Patient Educ Couns 2012;86(2):137–146. [DOI] [PubMed] [Google Scholar]
- 4.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172(7):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQueen A, Bartholomew LK, Greisinger AJ, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med 2009;24(11):1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health 2007;10(5):415–430. [DOI] [PubMed] [Google Scholar]
- 7.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med 2008;23(2):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong MC, Ching JY, Chan VC, et al. Informed choice vs. no choice in colorectal cancer screening tests: a prospective cohort study in real-life screening practice. Am J Gastroenterol 2014;109(7):1072–1079. [DOI] [PubMed] [Google Scholar]
- 9.Scott RG, Edwards JT, Fritschi L, Foster NM, Mendelson RM, Forbes GM. Community-based screening by colonoscopy or computed tomographic colonography in asymptomatic average-risk subjects. Am J Gastroenterol 2004;99(6):1145–1151. [DOI] [PubMed] [Google Scholar]
- 10.Segnan N, Senore C, Andreoni B, et al. Randomized trial of different screening strategies for colorectal cancer: patient response and detection rates. J Natl Cancer Inst 2005;97(5):347–357. [DOI] [PubMed] [Google Scholar]
- 11.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58(3):130–160. [DOI] [PubMed] [Google Scholar]
- 12.Sali L, Mascalchi M, Falchini M, et al. Reduced and full-preparation CT colonography, fecal immunochemical test, and colonoscopy for population screening of colorectal cancer: a randomized trial. J Natl Cancer Inst 2015;108(2):108. [DOI] [PubMed] [Google Scholar]
- 13.Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012;13(1):55–64. [DOI] [PubMed] [Google Scholar]
- 14.Wisconsin Collaborative for Healthcare Quality . WCHQ ambulatory measure specification: colorectal cancer screening performance measure. Middleton, Wis: Wisconsin Collaborative for Healthcare Quality, 2011. [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 16.Hatahet MA, Bowhan J, Clough EA. Wisconsin Collaborative for Healthcare Quality (WCHQ): lessons learned. WMJ 2004;103(3):45–48. [PubMed] [Google Scholar]
- 17.National Committee for Quality Assurance . HEDIS 2014 Measures: Summary Table of Measures, Product Lines and Changes. National Committee for Quality Assurance. https://www.ncqa.org/Portals/0/HEDISQM/HEDIS2014/List_of_HEDIS_2014_Measures.pdf. Published September 30, 2013. Accessed March 27, 2015. [Google Scholar]
- 18.Weiss JM, Smith MA, Pickhardt PJ, et al. Predictors of colorectal cancer screening variation among primary-care providers and clinics. Am J Gastroenterol 2013;108(7):1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control 2008;19(4):339–359. [DOI] [PubMed] [Google Scholar]
- 20.Rural Health Research Center . Rural-Urban Commuting Area Codes (RUCAs). University of Washington. http://depts.washington.edu/uwruca/index.php. Published July 2005. Accessed April 6, 2017. [Google Scholar]
- 21.Johns Hopkins Univerity Health Services Research & Development Center . The Johns Hopkins ACG System reference manual. Version 8.2. Baltimore, Md: Johns Hopkins University, Bloomberg School of Public Health, 2008. [Google Scholar]
- 22.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33(7):1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanlon DP, Chernew M, Lave JR. Consumer health plan choice: current knowledge and future directions. Annu Rev Public Health 1997;18:507–528. [DOI] [PubMed] [Google Scholar]
- 24.Naessens JM, Khan M, Shah ND, Wagie A, Pautz RA, Campbell CR. Effect of premium, copayments, and health status on the choice of health plans. Med Care 2008;46(10):1033–1040. [DOI] [PubMed] [Google Scholar]
- 25.Fenton JJ, Cai Y, Green P, Beckett LA, Franks P, Baldwin LM. Trends in colorectal cancer testing among Medicare subpopulations. Am J Prev Med 2008;35(3):194–202. [DOI] [PubMed] [Google Scholar]
- 26.Barry MJ, Edgman-Levitan S. Shared decision making: pinnacle of patient-centered care. N Engl J Med 2012;366(9):780–781. [DOI] [PubMed] [Google Scholar]
- 27.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med 2005;20(6):531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dwight-Johnson M, Unutzer J, Sherbourne C, Tang L, Wells KB. Can quality improvement programs for depression in primary care address patient preferences for treatment? Med Care 2001;39(9):934–944. [DOI] [PubMed] [Google Scholar]
- 30.Zapka J, Klabunde CN, Taplin S, Yuan G, Ransohoff D, Kobrin S. Screening colonoscopy in the US: attitudes and practices of primary care physicians. J Gen Intern Med 2012;27(9):1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med 2009;37(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin OS, Kozarek RA, Gluck M, et al. Preference for colonoscopy versus computerized tomographic colonography: a systematic review and meta-analysis of observational studies. J Gen Intern Med 2012;27(10):1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghanouni A, Smith SG, Halligan S, et al. Public perceptions and preferences for CT colonography or colonoscopy in colorectal cancer screening. Patient Educ Couns 2012;89(1):116–121. [DOI] [PubMed] [Google Scholar]
- 34.Moawad FJ, Maydonovitch CL, Cullen PA, Barlow DS, Jenson DW, Cash BD. CT colonography may improve colorectal cancer screening compliance. AJR Am J Roentgenol 2010;195(5):1118–1123. [DOI] [PubMed] [Google Scholar]
- 35.Pooler BD, Baumel MJ, Cash BD, et al. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. AJR Am J Roentgenol 2012;198(6):1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zapka JM, Klabunde CN, Arora NK, Yuan G, Smith JL, Kobrin SC. Physicians’ colorectal cancer screening discussion and recommendation patterns. Cancer Epidemiol Biomarkers Prev 2011;20(3):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss JM, Kim DH, Smith MA, et al. Predictors of primary care provider adoption of CT colonography for colorectal cancer screening. Abdom Radiol (NY) 2017;42(4):1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickhardt PJ, Taylor AJ, Kim DH, Reichelderfer M, Gopal DV, Pfau PR. Screening for colorectal neoplasia with CT colonography: initial experience from the 1st year of coverage by third-party payers. Radiology 2006;241(2):417–425. [DOI] [PubMed] [Google Scholar]


