Abstract
Objective
To evaluate the association between brain structural markers and caregiving strain among older informal caregivers.
Design
A secondary data analysis combining data from the Caregiver Health Effects Study (1993-1994) and the Cardiovascular Health Study MRI examination (1992-1994).
Setting
Four United States communities.
Participants
Co-residing spousal caregivers (n=237, mean age=76.2, standard deviation=2.2).
Measurements
Visually-rated ventricular and white matter (WM) grades from MRI, caregiving strain defined as “emotional or physical strain associated with providing care” for any of 12 activities of daily living (ADLs) and instrumental activities of daily living (IADLs), plus measures of caregiving characteristics and caregiver's health.
Results
Overall, 56% of caregivers reported strain. We detected an interaction where strain was very common (>82%) among caregivers who helped with ≥4 IADLs, regardless of WM grades, and among caregivers with the worst WM grades (WM grades ≥4), regardless of the number of IADLs they helped with. Among caregivers helping with <4 IADLs, having WM grades ≥4 was associated with a 55% higher prevalence ratio for reporting strain. This association remained statistically significant but was most markedly attenuated by adjustments for: care recipient's memory and behavioral problems, caregiver's depression symptoms, and caregiver's ADL impairment.
Conclusions
Caregiving strain is very common among older informal caregivers who provide help with many IADLs, and among caregivers who help with fewer IADLs, but have manifest signs of white matter pathology. Modern quantitative-neuroimaging studies are needed to evaluate whether more subtle variability in brain structure confers caregiving strain and the related health consequences.
In the United States alone, there are approximately 14.7 million providers of informal care for older adults with disabilities (1). Prior research from the Caregiver Health Effects Study (CHES) has shown that not all, but some, caregivers experience negative health consequences (2-4). Specifically, the CHES found that caregivers who did not report significant strain related to caregiving (i.e., non-strained caregivers) had similar mortality risk when compared with non-caregivers (2). Non-strained caregivers and non-caregivers (3) also had similar levels of depression symptomology. On the other hand, caregivers experiencing strain had higher mortality risk (2), greater depression symptom levels (3), and larger increases in depression symptoms over time (4).
Depression is consequential among older caregivers and preventing caregiver depression is an important public health priority. Depression negatively impacts quality of life (5) and increases the risk for both dementia (6) and mortality (7). In caregivers, depression can also decrease the quality of care delivered (8). Replacing older adults' informal caregiving services with skilled or unskilled workers, respectively, would cost an estimated 162 or 56 billion dollars per year (9). Since caregiver's depression risk is potentiated by strain (4) and emotional distress (10), caregivers with these characteristics represent those in the greatest need of pathophysiologically informed interventions for depression prevention.
To rationally inform depression prevention approaches for older strained caregivers, there is a need to understand the biological basis shared between caregiving strain and depression. At the core of late-life depression's putative pathophysiological mechanisms is brain structural pathology affecting connectivity (for a recent review, see (11)). In 1997, the ‘vascular depression’ hypothesis was forwarded, positing that cerebrovascular pathology affecting white matter may “predispose, precipitate, or perpetuate a depressive syndrome in many elderly patients” (12). Recently, potential mechanisms linking cerebrovascular pathology to depression have been described (13). Several studies have demonstrated that cerebrovascular disease is associated with: the course of depression symptoms and disorders (14-25), reduced white matter integrity (26, 27), brain hypoperfusion (28, 29), and altered brain function (30, 31), e.g., heightened activation in response to an affective reactivity task (32). In light of these findings, when exposed to caregiving demands, older adults with significant brain structural pathology (e.g., cerebrovascular disease) may be unable to effectively manage the demands, and may be more likely to experience difficulty or distress delivering care (i.e., caregiving strain).
Brain structural pathology may be associated with vulnerability to experience caregiving strain and the associated negative health consequences. Therefore, in the current work, we tested whether brain structural measures are associated with the presence of strain among older caregivers by performing a secondary analysis of data combined from the CHES and Cardiovascular Health Study (CHS) MRI examination. We hypothesized that caregivers with MRI-evidence of brain structural pathology, in particular MRI markers of cerebrovascular disease that have been previously been linked to depression (see above), would be more likely to report strain.
Furthermore, since the negative health consequences of caregiving (including the experience of caregiving strain) vary based on the duration and intensity of the caregiving role (33), we also explored how caregiving characteristics related to the likelihood of caregivers experiencing strain. Brain structure may only be related to strain in certain caregiving contexts. Therefore, we assessed whether associations between brain structural markers and caregiving strain differed depending on caregiving characteristics, including the amount of care being delivered and the extent of behavioral problems exhibited by the care recipient. We also evaluated whether specific characteristics of caregiver's health attenuated associations between brain structural measures and caregiving strain, e.g., as potential mediators or confounders of the relationship between brain structural measures and caregiving strain.
Methods
Participants
We performed a secondary data analysis combining data from the CHES (performed in 1993-1994) and the initial Cardiovascular Health Study (or CHS) MRI Study (performed from 1992-1994). Previous publications have described recruitment and inclusion/exclusion criteria for the parent CHS (34, 35) and CHS MRI study (36, 37). Briefly, starting in 1989, the CHS enrolled 5,888 adults aged 65 years and older from four communities (Forsyth County, NC; Pittsburgh, PA; Sacramento County, CA, and Washington County, MD) using Medicare eligibility lists and a stratified random sampling approach. Exclusion criteria were: institutionalization preventive of participation; the presence of terminal illness; the inability to walk, communicate, or give informed consent; plans to leave the study area within three years. An MRI examination was performed on 3,660 (62%) of CHS participants between 1992 and 1994. Participants with contraindications for MRI were excluded.
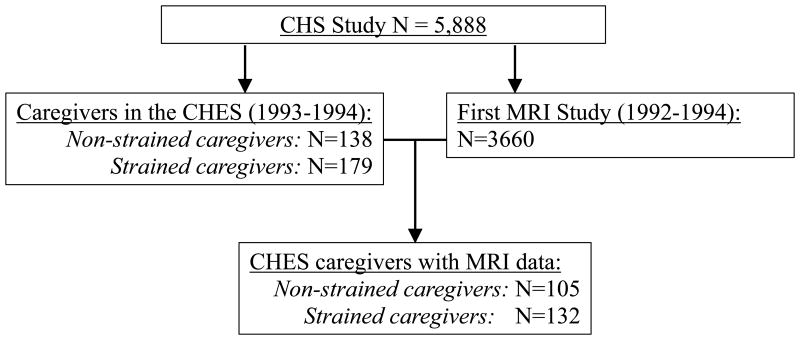
Details of the ancillary CHES and recruitment, which was conducted in 1993-1994, have been described previously (3). Briefly, CHS participants who were married and co-residing with their spouse were eligible for the CHES. Caregivers were defined as those whose spouse had at least one of twelve ADL and IADL difficulties “due to physical or health problems or problems with confusion,” and who provided care to this spouse for at least one of the identified difficulties. The six ADL difficulties were: (1) walking around the home, (2) getting out of bed or a chair, (3) eating, (4), dressing, (5), bathing, (6), toileting. The six IADL difficulties were: (1) heavy house work, (2) light house work, (3) shopping, (4) preparing meals, (5) managing money and bill payments, and (6) using the telephone. Caregivers were classified as those experiencing strain (vs. those not) based on whether they reported any emotional or physical strain associated with providing care. The CHES included 138 non-strained and 179 strained caregivers. The analytic sample utilized in the current report consisted of the subset of these caregivers who had useable MRI data (see Figure 1; n=105 non-strained caregivers and n=132 strained caregivers). Seventy-five percent of caregivers in the CHES contributed an MRI Scan, compared with 62% of CHS participants overall. The proportion of caregivers who reported strain did not differ by MRI participation status (Chi-Square value=0.35, df=1, p=0.56).
Figure 1. Parent study samples related to the current analytic sample.
Measures
Brain structure
MRI collected in the CHS included T1-weighted, T2-weighted, and spin-echo spin-density-weighted sequences. Images were visually rated by board-certified radiologists as described and illustrated previously (36, 37) using a standardized grading system for ventricles (ranging from grade 0, slit-like, to grade 9, markedly enlarged) and white matter (ranging from grade 0, no signal abnormalities, to grade 9, elevated signal intensity in almost all white matter). A previous report from the CHS found this visual-rating protocol yielded inter-reader and intra-rater agreement, respectively, within one grade of 92% and 94% for ventricular grades and 86% and 97% for white matter grades (37). Ventricular and white matter grades were examined both continuously and categorically, as has been done previously, categorizing ventricular grades as ≤2, 3, 4, ≥5, and white matter grades as ≤1, 2, 3, ≥4 (38).
Covariates
We selected covariates based on conceptual reasoning regarding their potential relationships with brain structure and caregiving strain. We considered caregiving characteristics measured in the CHES as potential confounders or moderators of the association between brain structure and caregiving strain, including: the frequency of behavioral problems in care recipients from the Memory and Behavior Problems Checklist (39), and the total number of recipient ADL and IADL (separately) impairments which the caregiver provides help with. Because the association of brain structure and caregiving strain may be apparent only in the context of certain caregiving characteristics, we examined whether any of these caregiving characteristics moderated the associations being studied.
The parent CHS collected relevant health information at study year 6, which was conducted from 1993-1994, at roughly the same time that the caregiver data was collected. From this data, we selected covariates that we conceptualized as potential confounders between the association of brain structure and caregiving strain. These included: age, gender, race (white vs. non-white), education (number of years), and diabetes status (defined as no diabetes, diabetes with oral hypoglycemic use only, or diabetes with any insulin use). We also selected several covariates which we conceptualized as potential confounders, but could potentially also be on the causal pathway between (mediators), or a downstream of (consequences), any brain structure-caregiving strain associations. These covariates included: current smoking; number of alcohol drinks per week; self-reported hypertension; systolic and diastolic blood pressure (averages of two readings each, with systolic and diastolic treated separately); use of hypertension medication; global cognition (measured with the Modified Mini-Mental State Examination (40)); psychomotor processing and attention (measured with the Digit Symbol Substitution Test (41)); depression symptom severity (measured with the 10-item Center for Epidemiologic Studies Depression Scale (42)); caregivers having at least one ADL or IADL impairment, separately, from the lists above; physical activity (the number of blocks walked per week); and, separately, self-reports of daytime sleepiness, trouble falling asleep, and night-time awakening.
Statistical Analysis
We first assessed whether continuously expressed ventricular or white matter grades differed between strained and non-strained caregivers using independent sample T-tests. We also used Chi-Squared tests to assess whether there were differences in the proportion of caregivers reporting strain across the ventricular and white matter grade categories. Next, we examined whether the identified associations differed based on caregiving characteristics using Poisson regression. Odds ratios do not provide an accurate estimate of the prevalence ratio when the outcome is highly prevalent (43), and since caregiving strain is a common outcome in our sample, we estimated prevalence ratios. We used a Poisson distribution and a robust variance estimator to avoid known convergence issues (44) in modeling the presence of caregiving strain (vs. no caregiving strain) as the dependent variable, entering as independent variables continuous measures of brain structure, caregiving characteristics, and their interactions. To illustrate the interaction detected, we report the percentage of participants with caregiving strain stratifying by both relevant brain structural marker and the moderating caregiving characteristic identified. Finally, we evaluated whether the identified association between brain structure and caregiving strain was attenuated by adjusting for each covariate separately. All models were adjusted for study site.
Results
Descriptive information, stratified by the presence of caregiving strain, is shown in Table 1. Compared with non-strained caregivers, strained caregivers were more often female, had greater levels of depression symptom severity, more often had ≥1 IADL impairment, and more often had complaints of night-time awakening (among other trends observed). Strained caregivers provided for care recipients with more frequent memory and behavioral problems and delivered care for a significantly greater number of ADLs and IADLs on average.
Table 1. Characteristics of strained and non-strained caregivers.
| Strained caregivers (n=1321, 55.7%) | Non-strained caregivers (n=1051, 44.3%) | Test-statistic | DF | p-value | |
|---|---|---|---|---|---|
|
|
|||||
| Age at CHS visit 6 | 76.2 (5.0) | 75.6 (5.0) | -1.03 | 232 | 0.30 |
| Female gender, % (n) | 57 (76) | 39 (41) | 8 | 1 | 0.005 |
| Race (white vs. non-white), % (n) | 92.4 (122) | 93.3 (98) | 0.7 | 1 | 0.79 |
| Years of education | 14.6 (4.7) | 13.8 (4.8) | -1.37 | 235 | 0.17 |
| Diabetes Status, % (n): | |||||
| None | 93.3 (112) | 92 (88) | 0.043 | - | 0.40 |
| Diabetes (oral hypoglycemic use only) | 6.7 (8) | 6.3 (6) | |||
| Diabetes (any insulin use) | 0 (0) | 2.1 (2) | |||
| Smoking, % (n): | |||||
| Never | 44.1 (56) | 43.4 (43) | 0.08 | 2 | 0.96 |
| Former | 44.9 (57) | 46.5 (46) | |||
| Current | 11.0 (14) | 10.1 (10) | |||
| Alcohol drinks per week, % (n)**: | |||||
| 0 | 52 (68) | 47.6 (49) | 1.23 | 2 | 0.54 |
| 1-7 | 39 (51) | 39.8 (41) | |||
| 7+ | 8.5 (11) | 12.6 (13) | |||
| Self-reported hypertension, % (n) | 45.7 (59) | 39.8 (41) | 0.82 | 1 | 0.36 |
| Systolic blood pressure | 133 (20.5) | 131 (19.4) | -0.76 | 226 | 0.45 |
| Diastolic blood pressure | 69.3 (11.1) | 68.4 (9.2) | -0.67 | 226 | 0.51 |
| Use of hypertension medication, % (n) | 49.6 (64) | 46.6 (48) | 0.21 | 1 | 0.65 |
| Global cognition (MMSE) | 93.0 (6.3) | 93.1 (5.6) | -0.284 | - | 0.78 |
| Psychomotor processing/attention (DSST) | 41.1 (13.3) | 41.3 (13.0) | 0.11 | 220 | 0.91 |
| Depression symptoms (CES-D 10 score) | 6.9 (5.9) | 4.0 (3.6) | -4.322 | 210.3 | <0.0001 |
| ≥1 ADL impairment, % (n) | 19.5 (24) | 11.0 (11) | 3.02 | 1 | 0.08 |
| ≥1 IADL impairment, % (n) | 29.9 (38) | 11.9 (12) | 10.7 | 1 | 0.001 |
| Physical activity (blocks per week) | 36.2 (64.0) | 46.3 (49.7) | 1.31 | 230 | 0.19 |
| Self-reported sleep disturbance, % (n): | |||||
| Daytime sleepiness | 23.6 (30) | 15.8 (16) | 2.11 | 1 | 0.15 |
| Trouble falling asleep | 17.5 (22) | 13.9 (14) | 0.54 | 1 | 0.46 |
| Night-time awakenings | 72.2 (91) | 60.4 (61) | 3.54 | 1 | 0.06 |
| Caregiving Characteristics: | |||||
| Frequency of memory and behavior aaproblems in care recipients | 20.2 (17.0) | 8.7 (10.1) | -5.404 | - | <0.0001 |
| Number of ADLs cared for | 1.4 (1.7) | 0.7 (1.2) | -3.694 | - | 0.0002 |
| Number of IADLs cared for | 3.0 (1.7) | 1.8 (1.4) | -5.634 | - | <0.0001 |
Means (standard deviations) shown unless otherwise specified; Test-statistics are t-values for differences in means and chi-square values for differences in proportions;
Each row total differs from overall total due to missing covariate data (all ≤15% missing except the frequency of memory and behavior problems in care recipients which was measured among n=181 or 76%);
Satterthwaite test for unequal variances used;
Fisher's exact test used.
Wilcoxon Two-Sample Test used;
Crude differences in brain structural measures between strained and non-strained caregivers
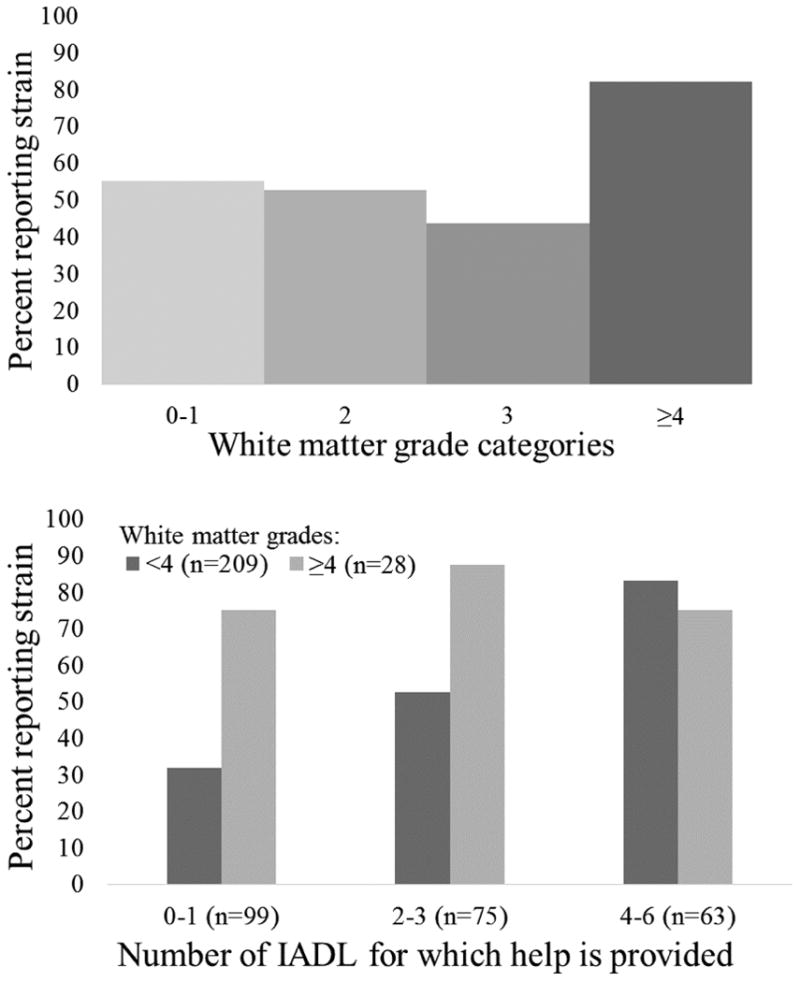
Strained caregivers tended to have higher white matter grades when compared with non-strained caregivers (Table 2), and there were significant differences in the proportion of caregivers reporting strain by white matter category (p=0.01, Chi-Sq=10.52, df=3). As shown in Figure 2A, this difference was driven by caregivers with white matter grades ≥4, among whom caregiving strain was more common. Ventricular grades were not associated with caregiver strain when expressed continuously (as shown in Table 2) or categorically (p=0.38, Chi-Sq=3.06, df=3).
Table 2. MRI characteristics of strained and non-strained caregivers.
| Strained caregivers (n=132) | Non-strained caregivers (n=105) | Test-statistic | DF | p-value | |
|---|---|---|---|---|---|
|
|
|||||
| White matter grades (0-9), mean (SD) | 2.2 (1.5) | 1.8 (1.0) | -2.02* | 230.6 | 0.04 |
| Ventricular grades (0-9), mean (SD) | 3.5 (3.3) | 3.5 (3.2) | -0.38 | 235 | 0.70 |
Means (standard deviations) shown unless otherwise specified; Test-statistics are t-values for differences in means and chi-square values for differences in proportions;
Satterthwaite test for unequal variances used.
Figure 2. The percentage of participants reporting strain by white matter grade category (a) overall and (b) stratified by the number of IADLs for which caregivers provide help.
Interactions between caregiving characteristics and white matter grades in association with caregiving strain
No significant interactions were detected between white matter grades and either the number of ADLs for which care was delivered or the frequency of care recipient's memory and behavioral problems (respectively, interaction p=0.16 (Z=-1.4) and p=0.16 (Z=-1.4)).
There was a significant interaction between continuously expressed white matter grades and the number of IADLs for which care was delivered (p=0.015, Z=-2.4). Since the association between white matter grades and strain was driven by caregivers with white matter grades ≥4 (see Figure 2A), we illustrate this interaction by showing the percentage of caregivers who reported strain stratified by this white matter grade level (grades ≥4 vs. not) and the level of IADL burden (see Figure 2B). We categorized the number of IADL impairments for which care was provided as minimal (0-1 IADLs cared for), moderate (2-3 IADLs cared for), or high levels (4-6) to help illustrate the interaction, and because the distribution of IADL impairments for which caregivers helped was skewed right, with few participants in the tail categories. While providing care for a greater number of IADLs was associated with a greater proportion of caregivers having strain, the proportion with strain was high among caregivers with white matter grades ≥4, regardless of the number of IADLs for which they provided care.
Covariate attenuation of the association identified between white matter grades and caregiving strain
As described above, the association of high white matter grades with caregiving strain was driven by caregivers with the highest white matter grades, and was only apparent among participants that provided care for <4 IADLs (i.e., caregivers helping with ≥4 IADLs had a high level of strain regardless of white matter grades, see Figure 2). Therefore, we evaluated how adjustments for each covariate separately attenuated this specific association, i.e., the relationship of white matter grades ≥4 (vs. <4) with the likelihood of having strain among caregivers who provided care for <4 IADLs (Table 3). This association retained statistical significance and was attenuated most markedly by adjustment for depression symptoms (20% attenuation), the frequency of care recipient's memory and behavioral problems (18% attenuation), and whether the caregiver themselves had any ADL impairments (15% attenuation).
Table 3. Attenuation of the association between having white matter grades ≥4 with caregiving strain among participants providing care for <4 IADLs.
| n | Prevalence ratio* (95% confidence interval) WM grades ≥4 vs. not | Percent attenuation (from base model) | |
|---|---|---|---|
|
|
|||
| Base model | 174 | 1.55 (1.30, 1.83) | |
| Base model + Age at CHS visit 6 | 174 | 1.54 (1.30, 1.84) | 2 |
| Base model + Female gender | 174 | 1.52 (1.28, 1.82) | 5 |
| Base model + Race (white vs. non-white) | 174 | 1.56 (1.31, 1.86) | -2 |
| Base model + Years of education | 174 | 1.54 (1.30, 1.83) | 2 |
| Base model + Diabetes Status** | 167 | 1.55 (1.30, 1.86) | 0 |
| Base model + Smoking | 165 | 1.52 (1.27, 1.81) | 5 |
| Base model + Alcohol drinks per week | 170 | 1.50 (1.26, 1.80) | 11 |
| Base model + Self-reported hypertension | 169 | 1.52 (1.28, 1.82) | 5 |
| Base model + Systolic blood pressure | 167 | 1.51 (1.25, 1.81) | 7 |
| Base model + Diastolic blood pressure | 167 | 1.51 (1.25, 1.81) | 7 |
| Base model + Use of hypertension medication | 170 | 1.53 (1.28, 1.82) | 4 |
| Base model + Global cognition (MMSE score) | 164 | 1.52 (1.25, 1.84) | 7 |
| Base model + Psychomotor processing/attention (DSST) | 162 | 1.55 (1.29, 1.87) | 0 |
| Base model + Depression symptoms (CES-D 10 score) | 165 | 1.44 (1.17, 1.78) | 20 |
| Base model + Caregiver has ≥1 ADL impairment | 165 | 1.47 (1.21, 1.79) | 15 |
| Base model + Caregiver has ≥1 IADL impairment | 167 | 1.49 (1.23, 1.80) | 11 |
| Base model + Physical activity (blocks per week) | 169 | 1.53 (1.27, 1.83) | 4 |
| Base model + Self-reported daytime sleepiness | 167 | 1.49 (1.23, 1.79) | 11 |
| Base model + Self-reported trouble falling asleep | 166 | 1.52 (1.26, 1.82) | 5 |
| Base model + Self-reported night-time awakenings | 167 | 1.52 (1.26, 1.83) | 5 |
| Base model + Frequency of recipient's memory and behavior problems | 129 | 1.45 (1.16, 1.81) | 18 |
| Base model + Number of ADLs care is given for | 174 | 1.54 (1.30, 1.83) | 2 |
| Base model + Number of IADLs care is given for | 174 | 1.50 (1.25, 1.79) | 9 |
Base model includes adjustment for study site;
Prevalence ratio for being a strained vs. non-strained caregiver;
Diabetes status was expressed as present vs. absent; N varies due to missing covariate data.
Discussion
We found that MRI-measured white matter pathology is associated with a greater likelihood that caregivers report strain. Interestingly, this association was apparent only in caregivers who provided help with <4 IADLs. In caregivers providing care for ≥4 IADLs, caregiving strain was highly prevalent, regardless of white matter measures. Thus, caregiving strain is common among older adults with intensive IADL caregiving demands, as well as in caregivers who provide care for lower-levels of IADL demands, but have white matter disease that is clearly manifest on MRI.
Histopathological studies have found that MRI-assessed white mater hyperintensities reflect myelin damage and fluid accumulation (45, 46). White matter hyperintensites have been previously associated with mobility (47) and cognitive impairment (48), depression (14, 18, 21-25), and mortality (49) in older adults. This includes past literature that has linked visually-rated white matter grades to physical functioning (50), dementia (51), depression (17), and mortality (52). As such, it is not surprising that we have now added, for the first time, that caregiving strain belongs among the health problems associated with white matter hyperintensities. Producing empirical evidence of this association is an important step towards understanding the biological basis of caregiving strain, which could lead to pathophysiologically-informed early detection and prevention approaches addressing the burden of caregiving strain and associated consequences. In other words, these findings have clinical implications regarding why, and for whom, caregiving is associated with strain and the related sequelae which include depression (4) and early death (2).
The design of the current study prohibits us from establishing temporality in the association between white matter changes and caregiving strain. That said, we suspect the extensive white matter pathology associated with caregiving strain (white matter grades ≥4) developed over a longer time-frame than the caregiving exposure. While recognizing that it is premature to rule-out caregiving strain as a toxic exposure affecting white matter, the white matter-caregiving strain association we observed is likely predominately due to an effect of pre-existing white matter pathology impacting older adult's ability to manage caregiving demands without difficulty. It is plausible that the white matter hyperintensities observed indicate that white matter connectivity is compromised, with resulting difficulty processing, for example, the cognitive and affective information required to complete caregiving tasks for a disabled spouse. Interestingly, ventricular grades were not associated with caregiving strain. These findings suggest that white matter damage may be more relevant than ventricular enlargement (a proxy of global atrophy) to caregiving strain. However, it is also possible that nuanced aspects of cortical morphology, which are not captured with the proxy measure of visually-rated ventricular grades, are relevant to outcomes among informal caregivers.
We also noted that the white matter-strain association was independent of, but partially attenuated by, several covariates. Again, we cannot empirically ascertain whether these characteristics preceded or resulted from white matter pathology or caregiving strain. Nevertheless, our findings (regarding the factors that attenuated the white matter-caregiving strain association) can be interpreted within the context of existing literature and current conceptual frameworks. Depression, a known consequence of strain in caregivers (4, 10), is often conceptualized as a consequence of white matter disease (11-13). Depression symptoms attenuated the association of interest, potentially because depression is an intermediate (mediator) between white matter pathology and caregiving strain, or a downstream consequence of caregiving strain. Similarly, we observed attenuation when adjusting for caregiver's own ADL impairment. Because ADL impairment in caregivers is more likely to result from, rather than cause, white matter pathology, it again seems likely that caregiver's ADL impairment is an intermediate between, or consequence of, the white matter-caregiving strain relationship.
Finally, we also observed the white matter-strain association was attenuated by the frequency of memory and behavioral problems among the care recipient. Since these memory and behavioral problems were in the care recipient, it is unlikely that they are the result of white matter pathology in the caregiver. Rather, we interpret these findings to indicate that the white matter pathology associated caregiving strain likely occurs in the context of dementia caregiving. Since these were co-residing spousal caregivers, shared environmental or selection factors could plausibly lead to care recipient's memory and behavioral problems (e.g., due to dementia), white matter pathology in caregivers, and the related caregiving strain.
Several limitations are important to note. Because our study included only older, co-residing, spousal caregivers, our findings may not generalize to younger caregivers who do not live with, or who do not have as long of a history sharing an environment with, their care recipient. White matter hyperintensities are more common in older age groups (37, 53), therefore, their relationship with caregiving strain may be limited to aged individuals. As mentioned above, our study cannot establish temporality in the relations between brain structure, caregiving strain, aspects of the caregiving role, and caregivers' own health characteristics like depression symptomology. With regard to our measures, the MRI assessments utilized are crude compared with the neuroimaging measures available today (e.g., which quantify specific vascular markers, distributions of cortical thickness, and brain function). We searched for other potential datasets to perform similar analyses, but found none that had adequate caregiving assessments with more modern neuroimaging measures. There are also some relevant factors that were not measured in the current study. These include potential residual confounders (e.g., personality and other psychosocial factors) and known consequences of white matter disease (e.g., executive dyfunction) that could explain why caregivers with high white matter grades were more likely to experience strain.
In conclusion, we have provided novel evidence suggesting that white matter health may serve as a shared biological basis related to both caregiving strain and its consequence on mood. Our study also revealed that older adults who provide care for a large number of IADL impairments are very likely to experience strain while doing so. However, not all of the caregivers in our study who provided care for a large number of IADLs reported strain. This raises the question of what, biologically, explains why some older adults can manage a high level of caregiving demands without experiencing strain and the associated negative health consequences. As mentioned in our limitations section, we only assessed crude, whole brain measures of brain structure. It remains to be seen whether subtle neurobiological variability in key circuits determines who is able to manage a high level of caregiving demands without difficulty and detriment to health. For example, preservation of structure or reserve in cognitive and emotional control circuits could explain why some older adults manage a high level of caregiving demands without experiencing strain. Alternatively, compromised structure in circuits responsible for cognitive and emotional functions may explain the effects of white matter observed, and why so many older caregivers experience strain that places them at elevated risk for depression. Future longitudinal studies using modern neuroimaging approaches will be needed to establish the extent to which variability in brain structure determines health risk and resilience in older caregivers.
Acknowledgments
Funding Support: This research was supported by contracts N01HC15103, HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Mental Health grants R01 MH46015 and P30 MH052247. Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. SFS has been supported by T32 MH019986 and T32 HL082610 in completing this work.
Footnotes
There are no disclosures to report.
References
- 1.Wolff JL, Spillman BC, Freedman VA, et al. A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Internal Medicine. 2016;176:372–379. doi: 10.1001/jamainternmed.2015.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Newsom J, Mittelmark M, et al. Health effects of caregiving: the caregiver health effects study: an ancillary study of the Cardiovascular Health Study. Ann Behav Med. 1997;19:110–116. doi: 10.1007/BF02883327. [DOI] [PubMed] [Google Scholar]
- 4.Beach SR, Schulz R, Yee JL, et al. Negative and positive health effects of caring for a disabled spouse: longitudinal findings from the caregiver health effects study. Psychol Aging. 2000;15:259–271. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, McAvay GJ, Chang SS, et al. Contribution of multiple chronic conditions to universal health outcomes. J Am Geriatr Soc. 2011;59:1686–1691. doi: 10.1111/j.1532-5415.2011.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuijpers P, Vogelzangs N, Twisk J, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. American Journal of Psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 8.Smith GR, Williamson GM, Miller LS, et al. Depression and Quality of Informal Care: A Longitudinal Investigation of Caregiving Stressors. Psychology and aging. 2011;26:584–591. doi: 10.1037/a0022263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chari AV, Engberg J, Ray KN, et al. The opportunity costs of informal elder-care in the United States: new estimates from the American Time Use Survey. Health Serv Res. 2015;50:871–882. doi: 10.1111/1475-6773.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco V, Rohde P, Vazquez FL, et al. Identification of caregivers at greatest risk of major depression in two prevention studies. Psychother Res. 2014;24:578–593. doi: 10.1080/10503307.2013.847989. [DOI] [PubMed] [Google Scholar]
- 11.Smagula SF, Aizenstein HJ. Brain Structural Connectivity in Late-Life Major Depressive Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1:271–277. doi: 10.1016/j.bpsc.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Archives of general psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 13.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiden A, Kettenbach J, Fischer P, et al. White matter hyperintensities and chronicity of depression. Journal of psychiatric research. 2005;39:285–293. doi: 10.1016/j.jpsychires.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Grool AM, Gerritsen L, Zuithoff NPA, et al. Lacunar Infarcts in Deep White Matter Are Associated with Higher and More Fluctuating Depressive Symptoms During Three Years Follow-up. Biological Psychiatry. 2013;73:169–176. doi: 10.1016/j.biopsych.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Saavedra Perez HC, Direk N, Hofman A, et al. Silent brain infarcts: a cause of depression in the elderly? Psychiatry Res. 2013;211:180–182. doi: 10.1016/j.pscychresns.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Steffens DC, Krishnan KR, Crump C, et al. Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke. 2002;33:1636–1644. doi: 10.1161/01.str.0000018405.59799.d5. [DOI] [PubMed] [Google Scholar]
- 18.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Archives of general psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 19.Lavretsky H, Lesser IM, Wohl M, et al. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- 20.Pavlovic AM, Pekmezovic T, Zidverc Trajkovic J, et al. Baseline characteristic of patients presenting with lacunar stroke and cerebral small vessel disease may predict future development of depression. Int J Geriatr Psychiatry. 2016;31:58–65. doi: 10.1002/gps.4289. [DOI] [PubMed] [Google Scholar]
- 21.Godin O, Dufouil C, Maillard P, et al. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon study. Biol Psychiatry. 2008;63:663–669. doi: 10.1016/j.biopsych.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Olesen PJ, Gustafson DR, Simoni M, et al. Temporal Lobe Atrophy and White Matter Lesions are Related to Major Depression over 5 years in the Elderly. Neuropsychopharmacology. 2010;35:2638–2645. doi: 10.1038/npp.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Lee SB, Lee JJ, et al. Epidemiology of MRI-defined vascular depression: A longitudinal, community-based study in Korean elders. J Affect Disord. 2015;180:200–206. doi: 10.1016/j.jad.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Firbank MJ, Teodorczuk A, van der Flier WM, et al. Relationship between progression of brain white matter changes and late-life depression: 3-year results from the LADIS study. The British Journal of Psychiatry. 2012;201:40–45. doi: 10.1192/bjp.bp.111.098897. [DOI] [PubMed] [Google Scholar]
- 25.van Sloten TT, Sigurdsson S, van Buchem MA, et al. Cerebral Small Vessel Disease and Association With Higher Incidence of Depressive Symptoms in a General Elderly Population: The AGES-Reykjavik Study. American Journal of Psychiatry. 2015;172:570–578. doi: 10.1176/appi.ajp.2014.14050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor WD, Bae JN, MacFall JR, et al. Widespread effects of hyperintense lesions on cerebral white matter structure. AJR Am J Roentgenol. 2007;188:1695–1704. doi: 10.2214/AJR.06.1163. [DOI] [PubMed] [Google Scholar]
- 27.Taylor WD, Payne ME, Krishnan KR, et al. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry. 2001;50:179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- 28.Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry research. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Veen PH, Muller M, Vincken KL, et al. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke. 2015;46:1233–1238. doi: 10.1161/STROKEAHA.114.008030. [DOI] [PubMed] [Google Scholar]
- 30.Patel MJ, Boada FE, Price JC, et al. Association of small vessel ischemic white matter changes with BOLD fMRI imaging in the elderly. Psychiatry research. 2012;204:117–122. doi: 10.1016/j.pscychresns.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu M, Andreescu C, Butters MA, et al. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aizenstein HJ, Andreescu C, Edelman KL, et al. fMRI correlates of white matter hyperintensities in late-life depression. The American journal of psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Academies of Sciences Engineering and Medicine. Families Caring for an Aging America. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 34.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Annals of epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 35.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Annals of epidemiology. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 36.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke; a journal of cerebral circulation. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 37.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 38.Mukamal KJ, Longstreth WT, Jr, Mittleman MA, et al. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke. 2001;32:1939–1946. doi: 10.1161/hs0901.095723. [DOI] [PubMed] [Google Scholar]
- 39.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. The Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 40.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 41.Psychological Corporation. Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: 1981. [Google Scholar]
- 42.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 43.Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. International journal of epidemiology. 1997;26:220–223. doi: 10.1093/ije/26.1.220. [DOI] [PubMed] [Google Scholar]
- 44.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 45.Braffman BH, Zimmerman RA, Trojanowski JQ, et al. Brain MR: pathologic correlation with gross and histopathology. 2 Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- 46.Murray ME, Vemuri P, Preboske GM, et al. A Quantitative Postmortem MRI Design Sensitive to White Matter Hyperintensity Differences and their Relationship with Underlying Pathology. Journal of neuropathology and experimental neurology. 2012;71:1113–1122. doi: 10.1097/NEN.0b013e318277387e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolandzadeh N, Liu-Ambrose T, Aizenstein H, et al. Pathways linking regional hyperintensities in the brain and slower gait. NeuroImage. 2014;99:7–13. doi: 10.1016/j.neuroimage.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke; a journal of cerebral circulation. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabayan B, van der Grond J, Westendorp RG, et al. Accelerated progression of white matter hyperintensities and subsequent risk of mortality: a 12-year follow-up study. Neurobiology of aging. 2015;36:2130–2135. doi: 10.1016/j.neurobiolaging.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. Journal of neurology, neurosurgery, and psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 52.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiology of aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]