Abstract
We aim to determine if direct thrombin inhibition by dabigatran will improve long-term brain morphological and neurofunctional outcomes and if potential therapeutic effects are dependent upon reduced PAR-1 stimulation and consequent mTOR activation. Germinal matrix haemorrhage was induced by stereotaxically injecting 0.3 U type VII-S collagenase into the germinal matrix of P7 rat pups. Animals were divided into five groups: sham, vehicle (5% DMSO), dabigatran intraperitoneal, dabigatran intraperitoneal + TFLLR-NH2 (PAR-1 agonist) intranasal, SCH79797 (PAR-1 antagonist) intraperitoneal, and dabigatran intranasal. Neurofunctional outcomes were determined by Morris water maze, rotarod, and foot fault evaluations at three weeks. Brain morphological outcomes were determined by histological Nissl staining at four weeks. Expression levels of p-mTOR/p-p70s6k at three days and vitronectin/fibronectin at 28 days were quantified. Intranasal and intraperitoneal dabigatran promoted long-term neurofunctional recovery, improved brain morphological outcomes, and reduced intracranial pressure at four weeks after GMH. PAR-1 stimulation tended to reverse dabigatran's effects on post-haemorrhagic hydrocephalus development. Dabigatran also reduced expression of short-term p-mTOR and long-term extracellular matrix proteins, which tended to be reversed by PAR-1 agonist co-administration. PAR-1 inhibition alone, however, did not achieve the same therapeutic effects as dabigatran administration.
Keywords: Germinal matrix haemorrhage, mammalian target of rapamycin, post-haemorrhagic hydrocephalus, post-haemorrhagic ventricular dilatation, proteinase-activated receptors
Introduction
Occurring in approximately 3.5 per 1000 live births, germinal matrix haemorrhage (GMH) remains a leading cause of mortality and lifelong morbidity in premature and/or very low birthweight infants.1 The rupturing of immature blood vessels in the subependymal brain tissue is thought to result from deficient autoregulatory mechanisms that inadequately function in response to abnormal cerebral blood flow fluctuations spurred by cardiorespiratory and haemodynamic instability.2 Long-term clinical complications from GMH include developmental delays, learning and psychiatric disorders, cerebral palsy, and post-haemorrhagic hydrocephalus, all of which pose significant economic burdens on both the patients and the US healthcare system.3–5 Prenatal glucocorticoid administration remains the best treatment for preventing GMH in premature infants, yet few clinical approaches exist for GMH clinical management post-ictus.6,7 Minimal advancements have also been made in post-haemorrhagic hydrocephalus clinical management, which is mostly limited to surgical insertion of shunts that drain excess cerebrospinal fluid from the ventricles into the peritoneum.2,8 Thus, a non-invasive, safe therapeutic approach that successfully mitigates post-haemorrhagic hydrocephalus development after GMH would significantly improve the quality of life for this patient population.
Immediately after haemorrhage, the coagulation cascade is triggered, and thrombin, a serine protease, is activated.9 In addition to inducing clot formation by converting fibrinogen into fibrin, thrombin also stimulates proteinase-activated receptors (PARs),10–12 leading to phosphorylation and subsequent activation of mammalian target of rapamycin (mTOR).13 Activated mTOR has been associated with extracellular matrix (ECM) protein proliferation in fibroblasts, which possibly disrupts cerebrospinal fluid (CSF) dynamics in the cerebroventricular system.14–19 In our prior studies, long-term post-GMH ventricular dilation was associated with increased expression of vitronectin and fibronectin,20 combinatorial PAR-1 and PAR-4 inhibitors reduced p-mTOR expression at 72 h after GMH, and rapamycin treatment ameliorated long-term neurocognitive deficits and improved brain morphology at four weeks post-GMH.13 Yet, long-term evaluations for PAR-1 and/or PAR-4 inhibitor administration as well as ECM protein expression levels were not investigated in these studies. Nonetheless, inhibiting the thrombin/PAR/mTOR pathway seems be a promising strategy for ameliorating post-haemorrhagic hydrocephalus formation after GMH.
Dabigatran, an oral anti-coagulant also known as Pradaxa, is a direct thrombin inhibitor clinically approved for preventing deep vein thrombosis, pulmonary embolism, and stroke from atrial fibrillation.21,22 While dabigatran reduced overall major and minor bleeding events compared to warfarin, gastrointestinal bleeds were significantly higher.23 An antidote, Idarucizumab, was developed to reverse dabigatran’s anti-coagulant effects in the event of major, uncontrolled bleeds.24 Although inhibiting thrombin may ameliorate brain injury, dabigatran’s anti-coagulant properties pose a risk of increased bleeding after cerebral haemorrhage. Dabigatran was evaluated in a rodent model of adult intracerebral haemorrhage and was determined to not increase haematoma volume while attenuating brain injury for the doses evaluated,25 but an additional study suggests high doses of dabigatran do increase haematoma volume, which can be controlled by intravenous injection of prothrombin complex concentrate.26 We perform a similar dosing study to evaluate dabigatran’s safety for GMH and to determine the best dose for further investigation.
Herein, we evaluate if direct thrombin inhibition by dabigatran ameliorates long-term neurocognitive deficits, improves brain morphological outcomes, and reduces ECM proliferation. We further investigate if potential therapeutic effects are dependent upon reduced PAR-1 stimulation and subsequent mTOR activation, since PAR-1 inhibition reduced brain injury severity in both adult cerebral ischaemic and haemorrhage models. Given dabigatran’s anti-coagulant effects, inhibiting one of thrombin’s receptors may be a safer approach if it still achieves the same degree of efficacy, and we aim to determine if PAR-1 inhibition alone will potentially achieve the same therapeutic benefits as direct thrombin inhibition. We further evaluate if PAR-1 stimulation will reverse dabigatran’s therapeutic effects to better confirm this pathway’s role in GMH pathophysiology. Additionally, intranasal drug administration has been shown to be an effective, localized delivery route to the brain, and we aimed to determine if this route would be efficacious for dabigatran administration.27,28 We determine the effects of the thrombin/PAR-1 pathway on mTOR phosphorylation in the short term as well as ECM protein expression, brain morphology, neurocognitive and sensorimotor function, and intracranial pressure in the long term.
Materials and methods
GMH surgical procedures
All experiments in this paper were performed according to the ARRIVE guidelines. All procedures were approved by the Loma Linda University Institutional Animal Care and Use Committee in accordance with the National Institute of Health’s guidelines. Animals were housed in bedded cages with littermates and dam until weaned, after which they were housed two to three per cage with same sex littermates. Animals were fed ad libitum and housed in a temperature- and humidity-controlled animal care facility with 12-h light/dark cycles and twice daily welfare assessments. For GMH induction, postnatal day 7, rat pups weighing between 12-15 g (brain development comparable to 30–32-week human gestation) were anesthetized using 2–3% isoflurane delivered in a mixture of medical grade oxygen and air. The scalp was sterilized by topically applying isopropyl alcohol and betadine, and then the heads were fixed onto a stereotaxic frame. An incision was made to expose bregma and a 1 mm burr hole was drilled at 1.6 mm right lateral and 1.5 mm rostral relative to bregma. A 10 µL Hamilton syringe filled with 0.3 U/µL collagenase solution was fixed to an infusion pump (Harvard Apparatus, Holliston, MA) and the needle inserted to a depth of 2.8 mm below the dura with the bevel facing the midline. 0.3 U type VII-S collagenase from Clostridium hystolyticum (Sigma Aldrich, St. Louis, MO) was infused at a rate of 0.1 U/min, and the needle was left in place for 5 min before being removed at a rate of 1 mm/min to reduce backflow. The burr hole was sealed using bone wax and the incision sutured with 5-0 silk. Animals were allowed to recover on a 37℃ heating pad then returned to the dam after awakening from anaesthesia. Sham surgery involved needle insertion without collagenase infusion. The average surgery time was approximately 30 min per animal.
Experimental groups and treatments
For the dabigatran plasma concentration studies, 72 P7 rat pups were randomly divided into three treatment groups: low dose 3 mg/kg, medium dose 10 mg/kg, or high dose 30 mg/kg administered intraperitoneally. Lyophilized dabigatran (Boehringer Ingelheim, Ingelheim am Rhein, Germany) was dissolved into a 5% dimethyl sulfoxide (DMSO) solution (Sigma Aldrich, St. Louis, MO). Blood was collected by cardiac puncture prior to euthanization at 2, 4, 8, 12, 18, or 24 h after dabigatran administration for all three treatment groups (n = 4/group/endpoint). For the haematoma expansion safety study, 54 P7 rat pups were randomly divided into nine groups (n = 6/group): naïve, sham, vehicle (5% DMSO), 3 mg/kg dabigatran QD, 3 mg/kg dabigatran BID, 10 mg/kg dabigatran QD, 10 mg/kg dabigatran BID, 30 mg/kg dabigatran QD, and 30 mg/kg dabigatran BID. Dabigatran was administered intraperitoneally for this study. For long-term evaluations, 72 P7 rat pups were randomly divided into six groups (n = 10-12/group, split evenly between histology and Western blot/intracranial pressure): sham, vehicle, 3 mg/kg dabigatran intraperitoneally, 3 mg/kg dabigatran intraperitoneally +1 mg/kg TFLLR-NH2 (PAR-1 agonist) intranasally (Tocris, Minneapolis, MN), 10 mg/kg SCH79797 (PAR-1 antagonist) intraperitoneally (Tocris, Minneapolis, MN), and 3 mg/kg dabigatran intranasally. All pups were randomized among treatment groups and all treatments commenced 2 h post-ictus and then twice a day for three days. For time course Western blots, 48 P7 rat pups were randomly divided into eight groups (n = 6/group) and euthanized at the indicated time point after GMH: Naïve, 3, 6, 12 h, 1, 3, 5, and 7 days post-ictus. For short-term evaluations, 48 P7 rat pups were randomly divided into eight groups (n = 6/group): sham, vehicle BID for 3 days, 3 mg/kg dabigatran intraperitoneally; 3 mg/kg dabigatran intraperitoneally + 1 mg/kg TFLLR-NH2 intranasally; 10 mg/kg SCH79797 intraperitoneally; 3 mg/kg dabigatran intranasally; 3 mg/kg dabigatran intranasally + 1 mg/kg TFLLR-NH2 intranasally. The TFLLR-NH2 intranasal dose was derived from a prior in vivo rodent study,29 and the SCH79797 intraperitoneal dose was derived from our prior GMH study.13
Intracranial pressure measurement
Intracranial pressure was measured as previously described.30 At 28 days post-ictus, animals were anesthetized and mounted onto a stereotaxic frame with their heads inclined 30° downward. The atlanto-occipital membrane was exposed by a midline skin excision and the cisterna magna punctured with a 26 G needle connected to a pressure transducer (Digi-Med LPA 400, Micro-Med, Louisville, KY).
Neurobehavioral assessments
A battery of tests was performed, by blinded investigators, to evaluate sensorimotor and cognitive deficits between 21 and 28 days post-ictus, as previously performed.20,31 Foot fault test: Rats were placed on a wire grid (20 × 40 cm) 2 ft above the ground and allowed to walk for 2 min. The left limb faults are reported as a percentage of the total steps taken by the left limbs ((left faults/left steps) × 100). Rotarod: Rats were placed on a rotating cylinder (San Diego Instruments, Columbus, OH), 7 cm diameter, 9.6 cm lanes) accelerating at 2 r/min per 5 s (starting speeds of either 5 or 10 r/min), and a photobeam circuit detected the latency to fall off the cylinder. Morris Water Maze: Briefly, rats were trained using a visible platform (10 cm diameter, Cued test) on day 1. On days 2–5, latency to find a submerged platform was measured (Memory Blocks 1–4, 5 trials each block, 1 min each trial). After completing the memory test, the platform was removed, and the rats were tested to determine the amount of time spent within the platform quadrant (Probe Trial, 1 min trial). An overhead camera with a computerized tracking system (Noldus Ethovision, Tacoma, WA) recorded the swim path and measured swim distance, speed, and time spent in target quadrant.
Perfusions and tissue extraction
Deeply anesthetized (5% isoflurane) animals were euthanized by trans-cardiac perfusion with ice-cold phosphate-buffered saline (PBS) for haemoglobin assay and Western blot samples or with ice-cold PBS followed by 10% formalin for histology samples. Forebrains were either snap-frozen in liquid nitrogen and stored in −80℃ freezer for Western blot and haemoglobin assay or post-fixed in 10% formaldehyde for at least three days then dehydrated with 30% sucrose for at least 3 days (4℃) for histology. Histology forebrains were embedded in Optimal Cutting Temperature compound and stored at −20℃.
Haemoclot assay
Blood was drawn by cardiac puncture and collected in a 0.106 mol/L trisodium citrate solution (1 part anti-coagulant to 9 parts blood). Blood was centrifuged at 2600g for 10 min at 20℃. Platelet-poor plasma was collected and snap frozen in liquid nitrogen and then stored at −80℃ until analysis. Dabigatran plasma concentration was determined by Hemoclot Assay (Aniara, West Chester, OH) as previously described.32 Briefly, a calibration curve was created using control plasma with 0, 1.25, 2.5, 3.75, and 5 µg/mL dabigatran. The hemoclot assay was performed following the manufacturer guidelines. Absorbance was measured on a microplate spectrophotometer (iMark, Bio-Rad, Hercules, CA) at 405 nm against a blank.
Haemoglobin assay
Frozen forebrains were homogenized in 3 mL PBS for at least 60 s then sonicated for at least 60 s. The homogenate was centrifuged at 13,000 r/min, 20℃ for 30 min. 200 mcL of supernatant were combined with 800 µL Drabkin’s reagent (Sigma-Aldrich, St. Louis, MO). After 15 min, absorbance at 450 nm was measured using a spectrophotometer (Genesis 10 UV; Thermo Fisher Scientific, Waltham, MA). Haemorrhage volume was calculated against a standard curve (created using naïve forebrains and known quantities of blood).
Western blot
Forebrains were homogenized in RIPA lysis buffer (Santa Cruz Biotechnology, Dallas, TX) for at least 60 s. The homogenate was centrifuged at 15,000 r/min, 4℃ for 20 min and supernatant collected, aliquoted, then stored at −80℃. Protein concentrations were determined by DC protein assay (Bio-Rad, Hercules, CA). Fifty micrograms of protein per sample were loaded into wells of 10% gels, ran for 30 min at 50 V, then 90 min at 125 V. Proteins were transferred onto a nitrocellulose membrane at 0.3 A for 120 min (Bio-Rad, Hercules, CA). Primary antibodies were applied to the membranes and incubated overnight at 4℃: p-mTOR (1:1000; Cell Signalling Technology, Danvers, MA); p-p70s6k (1:1000; Cell Signalling Technology, Danvers, MA); mTOR (1:1000; Cell Signalling Technology, Danvers, MA); p70s6k (1:1000; Cell Signalling Technology, Danvers, MA); vitronectin (1:1000; Abcam, Cambridge, MA); and Actin (1:1000 Santa Cruz Biotechnology, Dallas, TX). Membranes were washed, then incubated in secondary antibodies (1:2000; Santa Cruz Biotechnology, Dallas, TX) for 2 h at 4℃. Proteins were exposed onto radiography film after applying enhanced chemiluminescent solution (GE Healthcare and Life Science, Piscataway, NJ) onto the membranes. ImageJ software (Media Cybernetics, Silver Spring, MD) was used to analyse relative density. Optical density of target bands was divided by the optical density of their corresponding actin bands to determine relative density. The p-mTOR/mTOR and p-p70s6k/p70s6k short-term Western blots were analysed by dividing p-mTOR or p-70s6k by the relative density of corresponding total mTOR or p70s6k. Time course Western blots were normalized to mean relative density of the naïve group.
Histology
A cryostat (Leica Microsystems LM3050S, Wetzlar, Germany) was used to cut 10 µm thick coronal sections every 600 µm into the brain. Brain slices were Nissl stained and morphometrically analysed using ImageJ (Media Cybernetics, Silver Spring, MD)-assisted delineation of brain structures, as previously performed in our lab.33 Ventricle volume was calculated as average ventricular area multiplied by the depth of the cerebroventricular system. White matter loss was calculated as the average white matter area multiplied by depth of the cerebroventricular system and expressed as % of the sham group by dividing volumes to the overall average volume of the Sham group. For immunohistochemistry, 10 µm coronal sections were incubated in 0.03% triton solution for 30 min, blocked in donkey serum for 2 h, then incubated in primary antibody overnight at 4℃: NeuN (1:100; Abcam, Cambridge, MA); GFAP (1:100; Abcam, Cambridge, MA); Vimentin (1:50; Abcam, Cambridge, MA); p-mTOR (1:10, Santa Cruz Biotech, Santa Cruz, Ca); Fibronectin (1:100; Abcam, Cambridge, MA); Vitronectin (1:100; Abcam, Cambridge, MA). Slides were washed then incubated in secondary antibody (1:200; Jackson ImmunoResearch Labs, West Grove, PA) for 2 h at room temperature. Slides were then briefly DAPI stained (Vector Laboratories, Burlingame, CA), fixed in paramount, and visualized.
Statistical analysis
Data are expressed as mean ± standard deviation. One-way ANOVA using the Newman-Keuls post-hoc test was used to analyse all data. A p < 0.05 was considered statistically significant in all analyses except neurobehaviour; a p < 0.10 was considered statistically significant for neurobehavioural analyses.
Results
Dabigatran half-life and haematoma volume after GMH
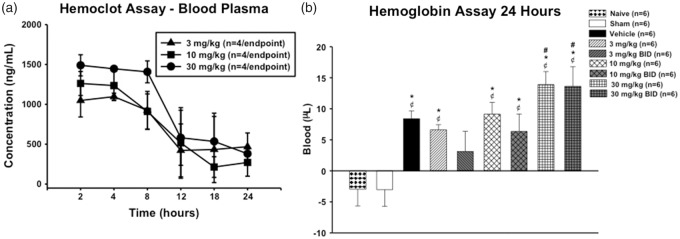
A dosing study was performed to assess the safety and efficacy of dabigatran. Plasma concentrations reached maximum levels 2 h after injection and have an approximate half-life of 8–12 h (Figure 1(a)). Dabigatran (30 mg/kg) administered intraperitoneally QD or BID significantly increased haematoma volume 24 h after GMH. Dabigatran (10 mg/kg) administered intraperitoneally QD or BID had no effect on haematoma volume 24 h after GMH; 3 mg/kg dabigatran QD administered intraperitoneally had no effect on haematoma volume, while dabigatran BID tended to decrease haematoma volume (Figure 1(b)). Excessive bleeding and mortality associated with excessive bleeding, where uncoagulated blood was usually found at the abdomen/intestine, intraperitoneal injection site, and/or surgical suture on the head, were also observed in all groups, particularly the higher dose groups (between 0 and 33% mortality, Table 1).
Figure 1.
Time course of dabigatran plasma concentrations after intraperitoneal injection of either 3 mg/kg, 10 mg/kg, or 30 mg/kg dabigatran (a). Haematoma volumes at 24 h post-GMH (b). ¢P < 0.05 vs. naïve; *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Table 1.
Mortality for all experimental groups in each study subsection.
| Group | Mortality (deaths/total) | Mortality (%) |
|---|---|---|
| Plasma concentration study | ||
| 3 mg/kg dabigatran i.p. | 1/25 | 4 |
| 10 mg/kg dabigatran i.p. | 5/29 | 17 |
| 30 mg/kg dabigatran i.p. | 3/27 | 11 |
| Haematoma volume study | ||
| Naïve | 0/6 | 0 |
| Sham | 0/6 | 0 |
| Vehicle | 0/6 | 0 |
| 3 mg/kg dabigatran i.p. | 0/6 | 0 |
| 3 mg/kg dabigatran i.p. BID | 0/6 | 0 |
| 10 mg/kg dabigatran i.p | 0/6 | 0 |
| 10 mg/kg dabigatran i.p. BID | 3/9 | 33 |
| 30 mg/kg dabigatran i.p. | 0/6 | 0 |
| 30 mg/kg dabigatran i.p. BID | 3/9 | 33 |
| Long-term study | ||
| Sham | 0/12 | 0 |
| Vehicle | 0/12 | 0 |
| 3 mg/kg dabigatran i.p. | 0/12 | 0 |
| 3 mg/kg dabigatran i.p. + 1 mg/kg TFLLR-NH2 i.n. | 2/14 | 14 |
| 10 mg/kg SCH79797 i.p. | 1/12 | 8 |
| 3 mg/kg dabigatran i.n. | 0/10 | 0 |
| Short-term study | ||
| Sham | 0/6 | 0 |
| Vehicle | 0/6 | 0 |
| 3 mg/kg dabigatran i.p. | 1/7 | 14 |
| 3 mg/kg dabigatran i.p. + 1 mg/kg TFLLR-NH2 i.n. | 1/7 | 14 |
| 10 mg/kg SCH79797 i.p. | 0/6 | 0 |
| 3 mg/kg dabigatran i.n. | 0/6 | 0 |
| 3 mg/kg dabigatran i.n. + 1 mg/gk TFLLR-NH2 i.n. | 0/6 | 0 |
Thrombin inhibition improved long-term neurofunctional recovery, which was not reversed by PAR-1 stimulation. PAR-1 inhibition alone was not sufficient to promote recovery
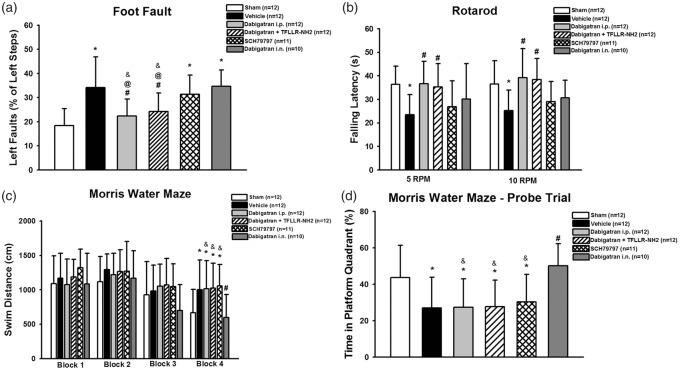
Using the best-tolerated dose, 3 mg/kg dabigatran administered intraperitoneally BID, we evaluated its efficacy on promoting long-term neurofunctional recovery. After observing the bleeding side effects, particularly bleeding near the injection sites, as well as mortality data from systemic dabigatran administration, we decided to investigate if a localized intranasal administration would promote long-term recovery. Dabigatran intranasal administration may increase brain concentration compared to intraperitoneal administration and avoids repeated abdominal injections, which may cause the observed bleeding, although the high bioavailability of small drugs after intranasal delivery may make blood plasma concentrations similar to intraperitoneal delivery and have the same bleeding risk.28 The vehicle group performed significantly worse than sham in the foot fault (Figure 2(a)), rotarod (Figure 2(b)), and Morris water maze tests (Figure 2(c) and (d)). Dabigatran administered intraperitoneally significantly improved performances on the foot fault and rotarod tests, but did not improve performance in the Morris water maze test compared to vehicle. PAR-1 agonist, TFLLR-NH2, did not reverse neurobehavioural outcomes from intraperitoneal dabigatran. PAR-1 antagonist, SCH79797, administration did not improve performances on the foot fault or Morris water maze tests, and only tended to improve performance on the rotarod test compared to vehicle. Intranasal dabigatran did not improve performance on the foot fault test, tended to improve performance on the rotarod test, and significantly improved performance on the Morris water maze test compared to vehicle.
Figure 2.
Neurobehavioral assessment using the foot fault (a), rotarod (b), and Morris water maze tests for swim distance (c) and percentage of time spent in platform quadrant (d). *P < 0.10 vs. sham; #P < 0.10 vs. vehicle; $P < 0.10 vs. dabigatran i.p.; &P < 0.10 vs. dabigatran i.n.; @P < 0.10 vs. SCH79797 i.p.
Thrombin inhibition improved long-term brain morphological outcomes, which tended to be reversed by PAR-1 stimulation. PAR-1 inhibition alone was not sufficient to promote recovery
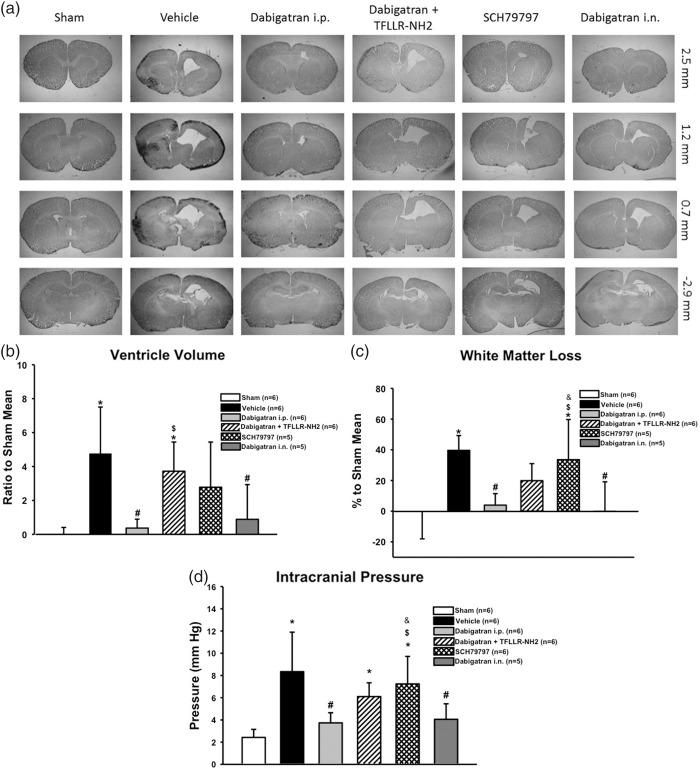
To determine the treatment effects on long-term post-haemorrhagic hydrocephalus outcomes, intracranial pressure was measured and brain morphological outcomes determined by histological analysis at four weeks post-GMH (Figure 3). Dabigatran administered either intraperitoneally or intranasally had significantly diminished post-haemorrhagic ventricular dilation (Figure 3(b)) and white matter loss (Figure 3(c)) compared to vehicle. PAR-1 agonist co-administration significantly reversed intraperitoneal dabigatran’s effect on reduced post-haemorrhagic ventricular dilation and tended to reverse intraperitoneal dabigatran’s effect on ameliorating white matter loss. PAR-1 antagonist administration did not significantly diminish post-haemorrhagic ventricular dilation or white matter loss compared to vehicle. Additionally, the vehicle group had significantly increased intracranial pressure compared to sham, which was prevented by dabigatran (intraperitoneal and intranasal) but not PAR-1 antagonism (Figure 3(d)). PAR-1 agonist co-administration significantly reversed the intraperitoneal Dabiagatran’s effects on diminishing intracranial pressure.
Figure 3.
Long-term brain morphological outcomes at four weeks’ post-GMH. Representative photomicrographs (a), ventricular volumes (ratio to sham mean) (b), and white matter loss (percentage to sham mean) (c). Intracranial pressure at four weeks’ post-GMH (d). *P < .05 vs. Sham; *P < .05 vs. sham; #P < .05 vs. vehicle; $P < .05 vs. dabigatran i.p.; &P < .05 vs. dabigatran i.n.
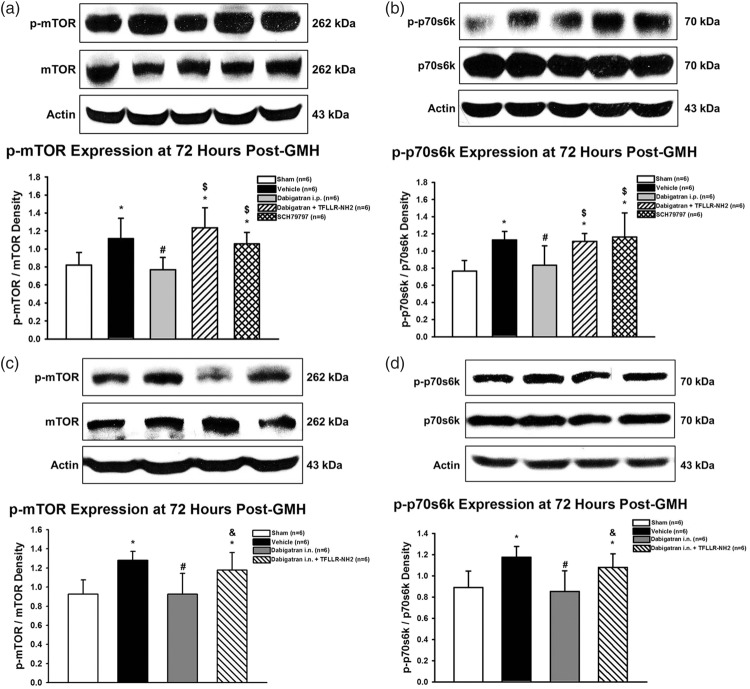
Short-term p-mTOR and downstream p-p70s6k expression levels are significantly increased after GMH
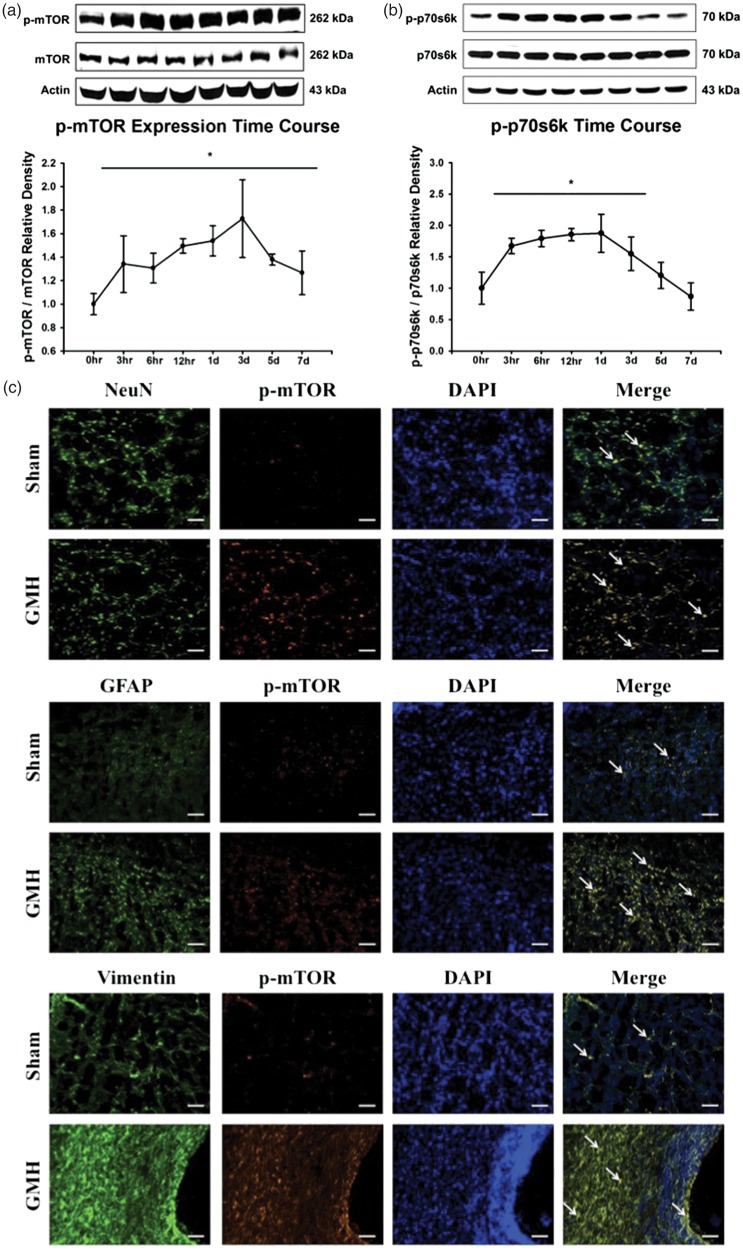
Activated mTOR has been implicated as a potential downstream effector of thrombin-induced PAR stimulation, leading to consequent ECM protein proliferation. We evaluated the expression time course of p-mTOR (Figure 4(a)) and its downstream effector, p-p70s6k (Figure 4(b)), after GMH. Activated mTOR (p-mTOR) expression levels were significantly increased 3 h and remained elevated up to seven days post-ictus. Activated p70s6k (p-p70s6k) expression levels were significantly increased 3 h and remained increased at three days post-ictus, before returning to levels indistinguishable from that of sham at five days post-ictus. Immunohistochemical co-localization of activated mTOR with neurons (NeuN), astrocytes (GFAP), and fibroblasts (Vimentin) in sham and GMH brains at 72 h post-ictus determined profound mTOR activation in all three cell types after GMH in the subventricular region (Figure 4(c)) as well as significant proliferation of astrocytes and fibroblasts.
Figure 4.
Time course of p-mTOR (a) and p-p70s6k (b) protein expression in the brain after GMH. Representative immunohistochemical co-localization stains (c) of p-mTOR with NeuN, GFAP, or Vimentin in the ipsilateral subventricular region of sham and GMH brains at 72 h post-ictus. *P < .05 vs. Sham. Scale bar = 20 µm.
Thrombin inhibition reduced p-mTOR and p-p70s5k expression levels at three days post-ictus, which were reversed by PAR-1 stimulation. PAR-1 inhibition alone did not significantly reduce p-mTOR and p-p70s6k expression
Three days post-GMH, the effect of treatment on p-mTOR expression was evaluated (Figure 5). Dabigatran (intraperitoneal and intranasal) significantly decreased p-mTOR and p-p70s6k expression levels compared to vehicle, which were reversed by PAR-1 agonist co-administration. Intraperitoneal PAR-1 antagonist administration did not significantly reduce p-mTOR or p-p70s6k expression levels compared to vehicle (Figure 5(a) and (b)).
Figure 5.
Expression of p-mTOR and p-p70s6k protein expression in the brain at three days post-GMH in rats treated with dabigatran administered intraperitoneally (a and b) or intranasally (c and d). *P < .05 vs. sham; #P < .05 vs. vehicle; $P < .05 vs. dabigatran i.p.; &P < .05 vs. dabigatran i.n.
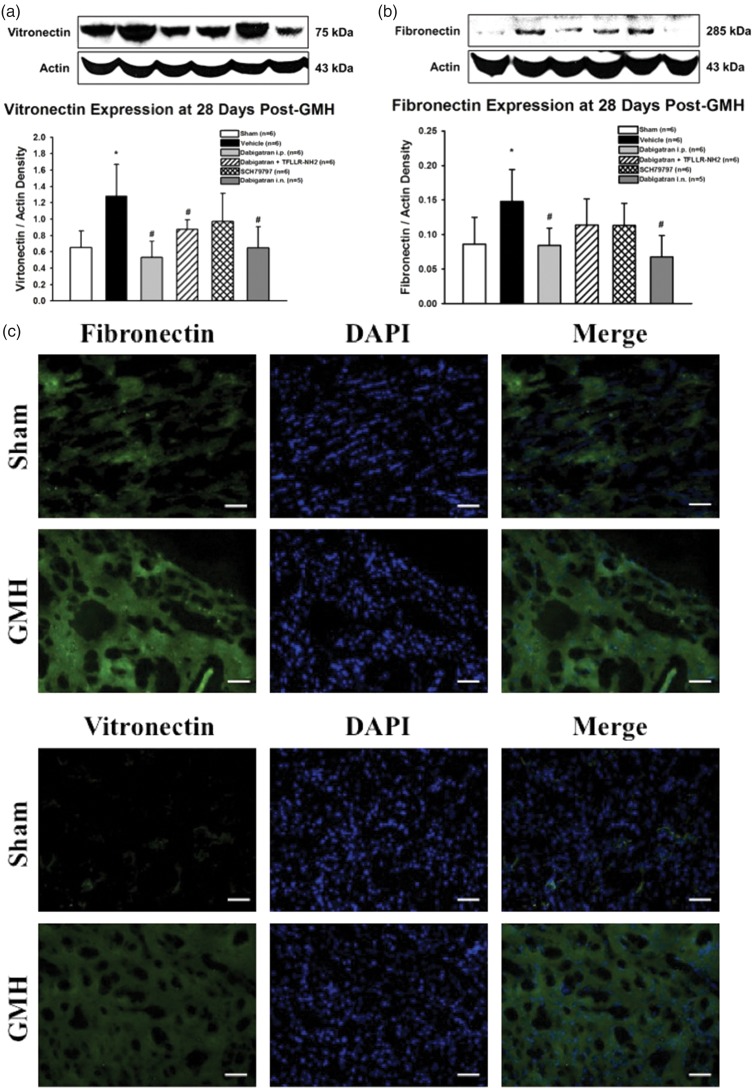
Thrombin inhibition significantly reduced long-term ECM protein proliferation after GMH, which was not reversed by PAR-1 stimulation. PAR-1 inhibition alone tended to reduce ECM protein proliferation
ECM protein proliferation has been associated with post-haemorrhagic hydrocephalus after GMH, thus we evaluated the treatment effects on vitronectin (Figure 6(a)) and fibronectin (Figure 6(b)) expression levels four weeks’ post-ictus. The vehicle group had significantly increased the expression of both vitronectin and fibronectin compared to sham, which was prevented by dabigatran (intraperitoneal and intranasal) and tended to decrease by PAR-1 antagonism. PAR-1 agonist co-administration did not significantly reverse intraperitoneal dabigatran effects on reducing vitronectin expression and only tended to reverse intraperitoneal dabigatran effects on reducing fibronectin expression. Immunohistochemical staining for fibronectin and vitronectin in sham and GMH brains at four weeks’ post-ictus indicated slightly increased ipsilateral periventricular ECM expression in GMH brains (Figure 6(c)).
Figure 6.
Expression of vitronectin (a) and fibronectin (b) proteins in the brain at four weeks’ post-GMH. Representative immunohistochemical stains (c) of fibronectin and vitronectin in the ipsilateral subventricular region of sham and GMH brains at four weeks’ post-ictus. *P < .05 vs. sham; #P < .05 vs. vehicle. Scale bar = 10 µm.
Discussion
Post-haemorrhagic hydrocephalus is a common neurological sequelae afflicting severe grade GMH patients, and a non-invasive therapeutic approach would significantly improve the quality of life for this patient population. Elucidating clinically translatable pathophysiological mechanisms contributing towards post-haemorrhagic hydrocephalus development will hopefully yield novel therapeutic modalities to accomplish this aim. Thrombin has been identified as a potentially critical player, since intraventricular thrombin injection results in brain tissue damage and ventricular dilation in adult rats, which was reversed by intraventricular PAR-1 antagonist injection.34 Thrombin activity is significantly upregulated 24 h after GMH and tends to remain elevated up to 10 days post-ictus.13 Additionally, stimulation of thrombin’s receptors, PARs-1, -3, and -4, upregulates downstream proliferative pathways associated with brain injury, including PI3K/Akt, mTOR, and MAPK.10–12 Our prior studies determined that active p-mTOR is significantly upregulated in GMH rats at 72 h post-ictus, and co-administration of PAR-1 and PAR-4 inhibitors reduced the p-mTOR expression levels. Additionally, mTOR inhibition by rapamycin treatment improved long-term neurofunctional and brain morphological outcomes after GMH.13 Thus, this pathway warrants further investigation.
Dabigatran is a novel direct thrombin inhibitor clinically approved for treating deep vein thrombosis and preventing stroke from atrial fibrillation.21,22 Similar to argatroban,35 dabigatran is a competitive inhibitor that reversibly binds to thrombin’s active site. We aimed to evaluate this clinically available drug in our neonatal rat GMH model. We first performed a dosing study to (1) identify the half-life of dabigatran and (2) determine the best treatment regimen to prevent haematoma expansion and other adverse effects. Plasma dabigatran concentrations after intraperitoneal administration was measured at 2, 4, 8, 12, 18, and 24 h after injection using three doses: 3 mg/kg, 10 mg/kg, and 30 mg/kg. Plasma concentrations for all three doses had peaked by 2 h and the half-life was 8–12 h in neonatal rats (Figure 1(a)).
While thrombin inhibition has yielded positive results in intracerebral haemorrhage models, evidence suggests that high doses of thrombin inhibitors result in increased haematoma volumes due to their anti-coagulant effects.25,26 Indeed, direct thrombin inhibition risks increased haemorrhaging after GMH, which likely may augment GMH pathophysiology rather than ameliorate it. Any harmful downstream factors attenuated from direct thrombin inhibition will be mitigated by an enlarged haematoma, which will further damage tissue and disrupt the cerebroventricular system. Thus, determining the safest dabigatran dose and probing downstream thrombin pathways that will not disrupt coagulation is imperative. Given dabigatran’s 8–12-h half-life, dabigatran was given QD and BID. Dabigatran (30 mg/kg) QD and BID resulted in significantly greater haematoma volumes compared to that of the vehicle. Dabigatran (10 mg/kg) QD and BID and dabigatran (3 mg/kg) QD had haematoma volumes comparable to that of the vehicle, whereas dabigatran (3 mg/kg) BID tended to decrease haematoma volume (Figure 1(b)). We speculate dabigatran’s anti-coagulant properties at 3 mg/kg are potent enough to attenuate the accumulation of blood in the brain and cerebroventricular system while preventing excess bleeding and consequent haematoma expansion. These findings are analogous to studies investigating fibrinolytic agents to breakdown cerebroventricular blood clots after intraventricular haemorrhage.36
During the dosing study, some mortality was observed in the medium and high-dose groups (Table 1), but many of the animals in those groups suffered from adverse bleeding side effects, particularly in the abdomen. We were concerned systemic administration of dabigatran may be risky, so we decided to investigate if intranasal administration, which is more localized to the brain, could be therapeutically beneficial. In addition to being convenient, easy, and relatively painless, the intranasal administration route has several key attributes. The nasal cavity’s rich vascular plexus directly absorbs small molecular drugs into the bloodstream, with rates of absorption and plasma concentrations being similar to intravenous administration.37 Intraperitoneal administration is comparatively slower since the drugs are primarily absorbed in mesenteric vessels, which drains into the portal vein and passes through the liver.38 Thus, intraperitoneally administered drugs may exhibit first-pass hepatic metabolism before entering the systemic circulation and often have pharmacokinetics similar to orally administered drugs.39 Intranasal administration avoids the first-pass effect, since the drugs are absorbed directly into the bloodstream.40 Additionally, intranasal administration may achieve higher CSF concentrations than plasma concentration, given the route’s close proximity to the brain.41–43 More research is warranted to determine the differences in bioavailability and biodistribution between dabigatran administration routes. An intranasal administration group was added to the long-term study, and only 3 mg/kg dabigatran BID administered either intraperitoneally or intranasally was used, since it was best tolerated. Recognizing that direct thrombin inhibitors have an inherent bleeding risk, we investigated if (1) inhibiting PAR-1, a downstream thrombin receptor, will achieve the same therapeutic results and (2) stimulating PAR-1 will reverse dabigatran’s therapeutic effects.
Dabigatran treatment ameliorated long-term neurofunctional deficits. Interestingly, intraperitoneal dabigatran only improved locomotor and sensorimotor outcomes, evidenced by better foot fault and rotarod performances, while intranasal dabigatran administration only improved neurocognitive function, evidenced by better Morris water maze performances (Figure 2). PAR-1 stimulation did not reverse intraperitoneal dabigatran’s therapeutic effects on foot fault and rotarod performance, and PAR-1 inhibition did not significantly attenuate any neurofunctional deficits, although it tended to improve performance on the rotarod test. While both administration routes had identical effects in every aspect of this study with neurobehavioral outcomes being the only exception, these results may partially be explained by the inherent error and variability within the tests, but the potential differential effects from the given administration routes cannot be ignored. Regardless, both administration routes showed significant neurofunctional recovery after GMH.
Both dabigatran administration routes improved long-term brain morphological outcomes by reducing post-haemorrhagic ventricular dilation and white matter loss compared to vehicle, and both routes reduced long-term intracranial pressure compared to vehicle (Figure 3). The mechanism of post-haemorrhagic hydrocephalus attenuation by dabigatran remains unknown. Hydrocephalus may be caused by a number of mechanisms: hypersecretion or reduced absorption of CSF (communicating, non-obstructive hydrocephalus), non-communicating obstructive hydrocephalus and hydrocephalus ex vacuo.44 Hydrocephalus ex vacuo refers to enlargement of the ventricles due to brain tissue loss in the absence of increased CSF pressure. In the rat model of GMH, the post-haemorrhagic hydrocephalus mechanism(s) is not yet known. The observed ventricle enlargement coupled with increased ICP suggests that the mechanism is related to hypersecretion or reduced absorption of CSF, or obstructive hydrocephalus. However, we cannot discount hydrocephalus ex vacuo because we also observed significant ipsilateral white matter loss. Although brain tissue loss occurs in response to increased CSF pressures, the brain tissue loss should be symmetrical, and since the contralateral hemispheric tissue loss is negligible, the mechanism of post-haemorrhagic hydrocephalus in the rat GMH model may be caused by a combination of hydrocephalus mechanisms: hypersecretion of CSF, reduced CSF absorption, obstructive hydrocephalus, and hydrocephalus ex vacuo. To uncouple the progression of hydrocephalus, and understand its mechanisms, following GMH in our rat model, future studies will be undertaken.
PAR-1 stimulation showed a strong tendency to reverse intraperitoneal dabigatran’s therapeutic effects on brain morphological and intracranial pressure outcomes since this group did not achieve a statistically significant difference from the vehicle group. PAR-1 inhibition alone failed to ameliorate post-haemorrhagic ventricular dilation, white matter loss, and elevated intracranial pressure. These results suggest that thrombin activity is associated with long-term post-haemorrhagic hydrocephalus development, but post-haemorrhagic hydrocephalus development is not exclusively dependent upon thrombin-induced PAR-1 stimulation, although it may play a minor role in conjunction with other pathways. Indeed, activated thrombin also stimulates PAR-3 and PAR-4 as well, and their potential roles in GMH pathophysiology have not been elucidated. PAR-1 inhibition alone also failed to ameliorate short-term subventricular zone damage in a neonatal mouse GMH model.45 which is consistent with our results showing PAR-1 inhibition failed to ameliorate long-term white matter loss. Future investigations should rule out PAR-1 inhibition alone and focus on PAR-3, PAR-4, or a combinatorial treatment approach.
Active thrombin is hypothesized to contribute towards fibrosis and consequent ECM protein proliferation, which disrupt cerebrospinal fluid dynamics in the cerebroventricular system, potentially causing post-haemorrhagic hydrocephalus. PAR stimulation leads to activation of multiple proliferative signalling pathways, including PI3K/Akt, MAPK, and mTOR.10–12 Previously, mTOR inhibition improved long-term brain morphological and neurofunctional outcomes after GMH, and combinatorial PAR-1,-4 inhibition reduced mTOR activation at 72 h post-GMH. Yet, neither mTOR inhibition, PAR-1,-4 inhibition, nor ECM protein proliferation have been evaluated for long-term outcomes after GMH.13 Herein, p-mTOR expression was increased immediately following GMH and remained elevated for seven days, and a similar trend was observed for p-p70s6k (Figure 4(a) and (b)). Additionally, increased activated mTOR was observed in neurons, astrocytes, and fibroblasts of GMH brains compared to sham at three days post-ictus (Figure 4(c)), indicating that GMH-induced mTOR activation is complex with a diverse range of potential effects. Dabigatran attenuated the elevated expressions of p-mTOR and p-p70s6k, which was reversed by PAR-1 stimulation. Yet, PAR-1 inhibition alone did not significantly decrease p-mTOR or p-p70s6k (Figure 5). Our results suggest that thrombin inhibition decreases mTOR activation, yet it seems that stimulation of any of thrombin’s downstream PARs is sufficient to increase mTOR activation. When thrombin is inhibited, it is not able to stimulate PARs inducing mTOR activation, yet stimulation of one PAR, PAR-1 here, was capable of activating mTOR. This is supported by the current work that inhibition of only PAR-1 was unable to prevent mTOR activation. Additionally, this is supported the work of Lekic et al. that combinational PAR-1,-4 inhibition reduced p-mTOR expression 72 h after GMH, indicating that combinatorial PAR inhibition may be necessary to completely target this pathway.
Activated mTOR is hypothesized to contribute towards long-term ECM protein proliferation, which was observed in this work. Dabigatran (intraperitoneally and intranasally) significantly decreased vitronectin and fibronectin, and PAR-1 agonist co-administration did not reverse dabigatran’s effects on vitronectin expression but tended to reverse fibronectin expression. PAR-1 inhibition alone tended to reduce vitronectin and fibronectin (Figure 6(a) and (b)). Immunohistochemical staining indicated a slightly increased expression of both fibronectin and vitronectin in the ipsilateral periventricular region of GMH animals compared to sham at four weeks post-ictus (Figure 6(c)), which may partially disrupt cerebroventricular dynamics and contribute to post-haemorrhagic hydrocephalus development. Significant visual differences in fibronectin and vitronectin immunostaining were not detected in other brain regions, indicating ECM proliferation is either localized to the injured region or too diffuse to visualize by immunohistochemistry. Long-term ECM protein proliferation weakly correlates with short-term p-mTOR expression levels, suggesting that PAR-1/mTOR plays a small role, but other signalling pathways must be involved as well. Most importantly, ECM protein proliferation does not strongly correlate with brain morphological and intracranial pressure outcomes, but p-mTOR expression levels do correlate, suggesting future investigations into post-GMH hydrocephalus development should focus less on ECM protein proliferation and more on alternative p-mTOR-related pathways, particularly active mTOR’s relationship with immunomodulatory and cell survival pathways.
Our study has some limitations that need to be considered. First, neither the time course of dabigatran plasma concentrations after intranasal injection, nor the time course of dabigatran brain levels after intraperitoneal or intranasal administration. Second, the effects of intranasal dabigatran on haematoma volume were also not examined, nor were a dosing study on intranasal dabigatran performed. Third, thrombin has a plethora of downstream targets in addition to PAR/mTOR-induced ECM proliferation, including immunomodulatory and cell survival pathways.46 The scope of this study was on PAR-1/mTOR to isolate a potentially effective pathway downstream of thrombin which may play an important role in post-haemorrhagic hydrocephalus development. PAR-1 agonist and antagonist effects should also be interpreted cautiously, since PAR-1 exhibits biased agonism and responds to various doses of drugs and stimuli differently.47 Fourth, some evidence suggests plasma fibronectin and vitronectin leak into the brain tissue following haemorrhagic transformation after ischaemia in adult mice, indicating another source of ECM proteins in addition to those produced from fibrosis.48 Increased long-term ECM protein expression after GMH, however, may depend more on chronic fibrosis than acute vascular extravasation. Fifth, the therapeutic window for post-GMH treatments is poorly defined due to lack of clinically approved or tested therapies, and the initial 2 h post-ictus treatment time point examined may be too narrow for clinical translation. Finally, although our model produces consistent haemorrhages with consequent gliosis, neurological deficits, white matter loss, brain atrophy, and post-haemorrhagic hydrocephalus consistent with human neonatal brain haemorrhage, vascular integrity is disrupted by direct protease infusion,33 which does not perfectly model GMH pathophysiology caused by haemodynamic, microvascular, and cardiorespiratory instability emulated in other animal models.49,50 In addition to not perfectly emulating GMH pathophysiology, the injected collagenase also exacerbates inflammation, producing a robust, enduring inflammatory response after the initial haemorrhage.51 Augmented inflammation may further damage tissue as well as stimulate both proliferative and apoptotic pathways, potentially confounding some of our findings. These limitations will be the focus of future studies.
Conclusions
Direct thrombin inhibition by dabigatran decreased acute mTOR activation, reduced long-term ECM protein proliferation, ameliorated post-haemorrhagic hydrocephalus development, and improved long-term neurofunctional outcomes. Inhibition of PAR-1, a thrombin downstream receptor, was not sufficient to achieve the same observed therapeutic effectiveness as direct thrombin inhibition by dabigatran. Yet PAR-1 stimulation tended to reverse dabigatran’s therapeutic effects on long-term post-haemorrhagic hydrocephalus development and short-term mTOR activation, suggesting a role for PAR-1 in GMH sequelae. Future investigations should employ combinatorial PAR-1, -3, and/or -4 inhibition treatment modalities and further investigate additional pathways related to thrombin and active mTOR, including inflammatory and cell survival pathways.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the NIH grant R01 NS078755 to Dr. John H Zhang.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
DK, WBR, PRK, and TL conceived and designed the study. DK, JJF, and DWM collected and analysed the data. DK created the figures and drafted the article. JHZ supervised and quality controlled the overall project.
References
- 1.Heron M, Sutton PD, Xu J, et al. Annual summary of vital statistics: 2007. Pediatrics 2010; 125: 4–15. [DOI] [PubMed] [Google Scholar]
- 2.Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol 2014; 41: 47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 2000; 105: 1216–1226. [DOI] [PubMed] [Google Scholar]
- 4.Murphy BP, Inder TE, Rooks V, et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Childhood Fetal Neonatal ed 2002; 87: F37–F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Vermont-Oxford Trials Network: very low birth weight outcomes for 1990. Investigators of the Vermont-Oxford Trials Network Database Project. Pediatrics 1993; 91: 540–545. [PubMed] [Google Scholar]
- 6.Vinukonda G, Dummula K, Malik S, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke 2010; 41: 1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leviton A, Kuban KC, Pagano M, et al. Antenatal corticosteroids appear to reduce the risk of postnatal germinal matrix hemorrhage in intubated low birth weight newborns. Pediatrics 1993; 91: 1083–1088. [PubMed] [Google Scholar]
- 8.Whitelaw A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol 2001; 6: 135–146. [DOI] [PubMed] [Google Scholar]
- 9.Babu R, Bagley JH, Di C, et al. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg Focus 2012; 32: E8. [DOI] [PubMed] [Google Scholar]
- 10.Steinhoff M, Buddenkotte J, Shpacovitch V, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev 2005; 26: 1–43. [DOI] [PubMed] [Google Scholar]
- 11.Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev 2007; 56: 331–345. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka H, Hamilton JR, McKemy DD, et al. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 2003; 102: 3224–3231. [DOI] [PubMed] [Google Scholar]
- 13.Lekic T, Klebe D, McBride DW, et al. Protease-activated receptor 1 and 4 signal inhibition reduces preterm neonatal hemorrhagic brain injury. Stroke 2015; 46: 1710–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews L, Wyss-Coray T, Masliah E. Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain Pathol 2004; 14: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol 2004; 14: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul DA, Leef KH, Stefano JL. Increased leukocytes in infants with intraventricular hemorrhage. Pediatr Neurol 2000; 22: 194–199. [DOI] [PubMed] [Google Scholar]
- 17.Xue M, Del Bigio MR. Immune pre-activation exacerbates hemorrhagic brain injury in immature mouse brain. J Neuroimmunol 2005; 165: 75–82. [DOI] [PubMed] [Google Scholar]
- 18.Ballabh P, Xu H, Hu F, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nature Med 2007; 13: 477–485. [DOI] [PubMed] [Google Scholar]
- 19.Dummula K, Vinukonda G, Xu H, et al. Development of integrins in the vasculature of germinal matrix, cerebral cortex, and white matter of fetuses and premature infants. J Neurosci Res 2010; 88: 1193–1204. [DOI] [PubMed] [Google Scholar]
- 20.Klebe D, Krafft PR, Hoffmann C, et al. Acute and delayed deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke 2014; 45: 2475–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 22.Eisert WG, Hauel N, Stangier J, et al. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30: 1885–1889. [DOI] [PubMed] [Google Scholar]
- 23.Blommel ML, Blommel AL. Dabigatran etexilate: a novel oral direct thrombin inhibitor. Am J Health Syst Pharm 2011; 68: 1506–19. [DOI] [PubMed] [Google Scholar]
- 24.Starke RM, Komotar RJ, Connolly ES. A prospective cohort study of idarucizumab for reversal of dabigatran-associated hemorrhage. Neurosurgery 2015; 77: N11–N13. [DOI] [PubMed] [Google Scholar]
- 25.Lauer A, Cianchetti FA, Van Cott EM, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation 2011; 124: 1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 2011; 42: 3594–3599. [DOI] [PubMed] [Google Scholar]
- 27.Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv 2014; 5: 709–733. [DOI] [PubMed] [Google Scholar]
- 28.McMartin C, Hutchinson LE, Hyde R, et al. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci 1987; 76: 535–540. [DOI] [PubMed] [Google Scholar]
- 29.Aerts L, Hamelin ME, Rheaume C, et al. Modulation of protease activated receptor 1 influences human metapneumovirus disease severity in a mouse model. PloS One 2013; 8: e72529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lackner P, Vahmjanin A, Hu Q, et al. Chronic hydrocephalus after experimental subarachnoid hemorrhage. PloS One 2013; 8: e69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flores JJ, Klebe D, Rolland WB, et al. PPARgamma-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol Dis 2016; 87: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23: 138–143. [DOI] [PubMed] [Google Scholar]
- 33.Lekic T, Manaenko A, Rolland W, et al. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol 2012; 236: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao F, Liu F, Chen Z, et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 2014; 34: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JL. Argatroban: a direct thrombin inhibitor for heparin-induced thrombocytopenia and other clinical applications. Heart Dis 2001; 3: 189–198. [DOI] [PubMed] [Google Scholar]
- 36.Staykov D, Huttner HB, Struffert T, et al. Intraventricular fibrinolysis and lumbar drainage for ventricular hemorrhage. Stroke 2009; 40: 3275–3280. [DOI] [PubMed] [Google Scholar]
- 37.Turker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci 2004; 26: 137–142. [DOI] [PubMed] [Google Scholar]
- 38.Turner PV, Brabb T, Pekow C, et al. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Animal Sci 2011; 50: 600–613. [PMC free article] [PubMed] [Google Scholar]
- 39.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 1971; 178: 562–564. [PubMed] [Google Scholar]
- 40.Grassin-Delyle S, Buenestado A, Naline E, et al. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther 2012; 134: 366–379. [DOI] [PubMed] [Google Scholar]
- 41.Sakane T, Akizuki M, Yoshida M, et al. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol 1991; 43: 449–451. [DOI] [PubMed] [Google Scholar]
- 42.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. J Pharmacol Exp Ther 2004; 309: 469–475. [DOI] [PubMed] [Google Scholar]
- 43.Westin UE, Bostrom E, Grasjo J, et al. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm Res 2006; 23: 565–572. [DOI] [PubMed] [Google Scholar]
- 44.Rekate HL. A contemporary definition and classification of hydrocephalus. Semin Pediatr Neurol 2009; 16: 9–15. [DOI] [PubMed] [Google Scholar]
- 45.Mao X, Del Bigio MR. Interference with protease-activated receptor 1 does not reduce damage to subventricular zone cells of immature rodent brain following exposure to blood or blood plasma. J Negat Results Biomed 2015; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krenzlin H, Lorenz V, Danckwardt S, et al. The importance of thrombin in cerebral injury and disease. Int J Mol Sci 2016; 17 pii: E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosnier LO, Sinha RK, Burnier L, et al. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 2012; 120: 5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.del Zoppo GJ, Frankowski H, Gu YH, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab 2012; 32: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chua CO, Chahboune H, Braun A, et al. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke 2009; 40: 3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D, Baumann JM, Sun YY, et al. Overexpression of vascular endothelial growth factor in the germinal matrix induces neurovascular proteases and intraventricular hemorrhage. Sci Transl Med 2013; 5: 193ra90. [DOI] [PubMed] [Google Scholar]
- 51.Manaenko A, Chen H, Zhang JH, et al. Comparison of different preclinical models of intracerebral hemorrhage. Acta Neurochir Suppl 2011; 111: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]