Abstract
Inflammatory mediators and metalloproteinases are altered in acute ischemic stroke (AIS) and play a detrimental effect on clinical severity and hemorrhagic transformation of the ischemic brain lesion. Using data from the Italian multicenter observational MAGIC (MArker bioloGici nell’Ictus Cerebrale) Study, we evaluated the effect of inflammatory and metalloproteinases profiles on three-month functional outcome, hemorrhagic transformation and mortality in 327 patients with AIS treated with intravenous thrombolys in according to SITS-MOST (Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy) criteria. Circulating biomarkers were assessed at baseline and 24 h after thrombolysis. Adjusting for age, sex, baseline glycemia and National Institute of Health Stroke Scale, history of atrial fibrillation or congestive heart failure, and of inflammatory diseases or infections, baseline alpha-2macroglobulin (A2M), baseline serum amyloid protein (SAP) and pre-post tissue-plasminogen activator (tPA) variations (Δ) of metalloproteinase 9, remained significantly and independently associated with three-month death [OR (95% CI):A2M:2.99 (1.19–7.53); SAP:5.46 (1.64–18.74); Δmetalloproteinase 9:1.60 (1.12–2.27)]. The addition of baseline A2M and Δmetalloproteinase 9 or baseline SAP and Δmetalloproteinase 9 (model-2 or model-3) to clinical variables (model-1) significantly improved the area under curve for prediction of death [model-2 with A2M: p = 0.0205; model-3 with SAP: p = 0.001]. In conclusion, among AIS patients treated with thrombolysis, circulating A2M, SAP and Δmetalloproteinase 9 are independent markers of poor outcome. These results may prompt controlled clinical research about agents antagonizing their effect.
Keywords: Hemorrhagic transformation, inflammation, ischemic stroke, metalloproteinase, thrombolysis
Introduction
Accumulating evidence suggests that inflammatory mechanisms are consistently involved in acute ischemic stroke (IS), and that high levels of inflammatory molecules are associated with early or late neurological deterioration.1–5 Post-stroke neuro-inflammatory mechanisms are complex cascade phenomena: after the ischemic insult, changes occurring in the different elements of the neurovascular unit and the subsequent release of pro-inflammatory cytokines stimulates the local production of metalloproteinase (MMP) 9 by both resident cells and infiltrating monocytes and neutrophils.6–8 MMP9 contributes to the degradation of neurovascular matrix and the disruption of the tight junction proteins and the cerebrovascular basal lamina protein, further promoting brain injury and favoring, over hours or even days after the initial ischemic event, new leukocyte extravasation, brain edema, and haemorrhagic transformation.9,10 Data about such changes occurring in the clinical setting and studied using circulating biomarkers are still scanty and incomplete.
A growing body of studies is confirming the association between a systemic marker of inflammation, i.e. C-reactive protein (CRP), and evolution of ischemic stroke. In fact, elevations of CRP within 24 to 72 h of admission has been consistently shown to be a valuable independent predictor of poor outcomes, even after adjusting for infection and atherosclerosis.5,11,12
Regarding biomarkers associated with poor prognosis in the setting of stroke patients treated with thrombolysis, evidence exists for few factors only. One is definitely blood glucose: in fact hyperglycemia predicts poor outcome not only among untreated patients but also after thrombolytic treatment, as reviewed recently by Hasan et al.4 A study, recently published by our group, has shown that MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death at three months of follow-up.13
Concerning CRP and other inflammatory markers of ischemic brain lesion after thrombolysis, scarce and contrasting data are available in literature.14–16 No study has addressed hitherto the issue of their potential interaction with MMPs changes.
Using the patients enrolled into MAGIC Study,13 we sought to investigate the association of pre- and post-thrombolysis levels of an extensive array of multiple pro-inflammatory and anti-inflammatory molecules with symptomatic intracerebral hemorrhage (sICH), three-month death and three-month modified Rankin Scale score (mRS). We also studied the interplay between MMPs, tissue inhibitors of metalloproteinase (TIMPs) and the inflammatory markers.
Material and methods
Study population
Eligible were patients admitted for thrombolysis in 14 Italian centers, registered in the Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR), according to SITS-Monitoring Study criteria.17 Study protocol was approved by local Ethical Committee of the Careggi University Hospital (Florence) and all patients gave informed consent.
The study compliance with the Declaration of Helsinki.
Laboratory determinations
Whole venous blood was collected in tubes without anticoagulant, before and 24 h after thrombolysis. Tubes were centrifuged at room temperature at 1500 g for 15 min, and the supernatants were stored in aliquots at −80℃ until measurement of inflammatory markers, MMPs and TIMPs. Samples were analyzed in a unique central laboratory. Levels of different inflammatory markers [interleukin (IL) 1β, IL1RA, IL4, IL6, IL8, IL10, IL12, IL17, interferon gamma (IFNγ), IFNγ-induced protein 10 (IP10), monocyte chemo-attractant protein 1 (MCP1), macrophage inflammatory protein 1 beta (MIP1β), tumor necrosis factor alpha (TNFα), CRP, alpha2 macroglobulin (A2M), serum amyloid P(SAP), and haptoglobin] were determined using Bio-Plex suspension array system and Biorad Kits (Bio-Rad Laboratories Inc., Hercules, CA, USA). MMPs (MMP1, MMP2, MMP3, MMP7, MMP8, and MMP9) and TIMPs (TIMP1, TIMP2, and TIMP4) were previously determined in the same patients13 using Bio-Plex suspension array system (Bio-Rad Laboratories Inc., Hercules, CA, USA) and R&D Kits (R&D System, Milan Italy) following manufacturer’s instructions. The coefficient of variation of inflammatory markers, MMPs and TIMPs assays was <6%.
Outcomes
Outcomes were defined as follows: (1) sICH, using the National Institute of Neurological Disorders and Stroke criteria; 18 (2) three-month death; (3) modified Rankin disability score (mRS), dichotomized into good (mRS, 0–2) or poor (mRS, 3–6) outcome.
Statistical analysis
As a main explanatory variable, we used both baseline and single patient’s relative pre- and post-thrombolysis variation (Δ median value) of inflammatory markers, MMPs and TIMPs levels. Pre-post thrombolysis variation was calculated according to the formula: [(24-h post-tPA inflammatory markers, MMPs or TIMPs)–(pre-tPA inflammatory markers, MMPs or TIMPs)/(pre-tPA inflammatory markers, MMPs or TIMPs)]. Differences in these Δ values were analyzed in relation to demographic and clinical features and across subgroups of patients with different outcomes.
We used Pearson χ2 to test for significance while comparing binary variables and ANOVA or Kruskal–Wallis H Test for numeric variables as appropriate. Values are presented as mean and standard deviation or as median and interquartile range if they had a non-Gaussian distribution.
As the parameters investigated had a non-Gaussian distribution, log-transformed pre-tPA values for cyto-chemokines, CRP, haptoglobin, SAP and A2M and cube root-transformed pre-post tPA variations were used.
To analyze differences in biomarkers levels between baseline and 24 h, we choose the Mann–Whitney U Test because of relatively large statistical variations. The net effect of each biomarker’s baseline or variation on outcomes was then estimated by a logistic regression model including as covariates age, sex, baseline blood glucose, baseline NIHSS, history of atrial fibrillation, history of congestive heart failure, time onset-to-treatment, and history of inflammatory disorders or infections, occurred in the last seven days, as the main known outcome determinants. Since there were significant variations in the concentration of studied biomarkers across collaborating centers, in the multivariate analysis we controlled also for center effect. To correct results for multiple comparisons, we used the false discovery rate testing in all the statistical analyses. Furthermore, Spearman correlation test was used to study correlations between each MMP, TIMP and inflammatory marker. Area under the receiver operating characteristic curve (AUC) of different regression models for the detection of bad outcomes was also used.
To quantify how much the addition of a new biomarker correctly increases (upwards movements) or decreases (downwards movements) the risk predicted by the model for events and non-events, we assessed category-free net reclassification improvement (NRI) according to Xanthakis et al.19 To perform NRI calculation, we categorized the continuous variables A2M and SAP into quartiles (A2M:1st quartile <1.28 mg/mL, 2nd quartile 1.28–1.88 mg/mL, 3rd quartile 1.89–2.67 mg/mL, 4th quartile >2.67 mg/mL; SAP 1st quartile <41.5 µg/mL, 2nd quartile 41.5–54.5, 3rd quartile 54.6–70.4, 4th quartile >70.4.
A significant level was defined as P < 0.05.
Integrated discrimination improvement (IDI) analysis was also performed. All analyses were performed with SPSS 20.0 (SPSS Inc, Chicago, Ill) and Stata 13.0 (Lakeway Dr College Station, TX).
Results
We investigated 327 IS patients treated with tPA thrombolysis, enrolled between October 2008 and June 2011 (mean age, 68.9 ± 12.1 years; 58% males). The percentage of patients treated with endovascular procedures after tPA was less than 2% overall. A detailed description of patient cohort is reported in our previous paper.13 Clinical and demographic characteristics are summarized in Table 1.
Table 1.
Characteristics of the 327 patients enrolled in the study.
| Age, years, mean and SD | 68.9 ± 12 |
| Sex (male), n (%) | 190/327 (58.1%) |
| Onset to treatment time, minutes, mean and SD | 163.5 ± 75.7 |
| Baseline National Institute of Health Stroke Scale, mean and SD | 1.9 ± 6.0 |
| Baseline systolic blood pressure, mmHg mean and SD | 148.2 ± 21.7 |
| Baseline diastolic blood pressure, mmHg mean and SD | 80.1 ± 12.7 |
| Blood glucose, mg/dl mean and SD | 130.2 ± 47.9 |
| Risk Factors | |
| Hypertension, n (%) | 197/327 (61.0) |
| Diabetes, n (%) | 50/327 (15.4) |
| Hyperlipidemia, n (%) | 81/327 (25.8) |
| Current smoking, n (%) | 51/327 (15.9) |
| Atrial fibrillation, n (%) | 73/327 (22.7) |
| Congestive heart failure, n (%) | 35/327 (10.9) |
SD: standard deviation.
Pre-, post-thrombolysis levels and pre-/post-thrombolysis variations of inflammatory markers according to the main outcomes are reported in the on-line-only Data Supplement (see Tables 1, 2 and 3 in the Supplement).
Correlation between inflammatory markers and MMPs, TIMPs
Baseline A2M significantly correlated with baseline TIMP1 (r = 0.128), TIMP2 (r = 0.191), TIMP4 (r =0.298), MMP1 (r = 0.111) and MMP2 (r = 0.176) (p < 0.05). Baseline SAP levels were also significantly related with baseline MMP3 (r = 0.156), MMP7 (r = 0.160) and MMP8 (r = 0.111), (p < 0.05). The other inflammatory markers were not significantly correlated with TIMPs or MMPs (data not shown).
Logistic regression analyses
At multivariate logistic regression analyses, after adjusting for clinical and demographic determinants of unfavorable outcome, patients with high pre-tPA levels of A2M and SAP (Table 2) and patients with higher variation in A2M, IL1Ra, IL8, IL10, and TNFα had significantly higher risk of death (Table 3).
Table 2.
Effect of baseline inflammatory marker circulating level on sICH, three-month death, three-month mRS 3 to 6, adjusting *for major determinants of the outcomes.
| sICH |
Death 3 month |
mRS 3–6 |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P | OR (95% CI) | P | |
| Ln (C reactive protein-CRP) (mg/L) | 0.94 (0.68–1.29) | .70 | 1.23 (0.89–1.69) | .21 | 1.01 (0.99–1.02) | .17 |
| Ln (haptoglobin) (mg/mL) | 0.99 (0.74–1.32) | .98 | 1.29 (0.94–1.76) | .11 | 0.99 (0.98–1.02) | .79 |
| Ln (serum amyloid P-SAP) (µg/mL) | 0.89 (0.35–2.28) | .86 | 5.08 (1.58–16.29) | .006 | 1.01 (0.99–1.02) | .10 |
| Ln (alpha2 macroglobulin-A2M) (mg/mL) | 1.14 (0.53–2.45) | .74 | 2.85 (1.19–3.87) | .009 | 1.12 (0.91–1.28) | .30 |
| Ln (interleukin 1Beta-IL1Beta) (pg/ml) | 1.09 (060–1.96) | .78 | 0.57 (0.30–1.09) | .09 | 1.03 (0.86–1.23) | .76 |
| Ln (interleukin 1 receptor antagonist-IL-1Ra) (pg/mL) | 0.68 (0.49–0.93) | .02 | 0.82 (0.60–1.12) | .20 | 1.00 (0.99–1.01) | .84 |
| Ln (interleukin 4-IL4) (pg/mL) | 0.74 (0.46–1.21) | .23 | 0.70 (0.39–1.25) | .23 | 0.99 (0.91–1.07) | .72 |
| Ln (interleukin 6-IL6) (pg/mL) | 0.81 (0.54–1.22) | .30 | 1.28 (0.87–1.89) | .21 | 0.99 (0.98–1.06) | .45 |
| Ln (interleukin 8-IL8) (pg/mL) | 0.68 (0.40–1.16) | .16 | 1.00 (0.63–1.60) | .97 | 1.00 (0.99–1.01) | .29 |
| Ln (interleukin 10-IL10) (pg/mL) | 0.77 (0.60–0.98) | .03 | 0.79 (0.61–1.02) | .07 | 0.99 (0.98–1.01) | .50 |
| Ln (interleukin 12-IL12) (pg/mL) | 0.79 (0.55–1.13) | .20 | 0.64 (0.43–0.95) | .03 | 0.99 (0.98–1.01) | .83 |
| Ln (interleukin 17-IL17) (pg/mL) | 0.85 (0.58–1.24) | .39 | 0.76 (0.52–1.09) | .13 | 0.99 (0.94–1.01) | .67 |
| Ln (interferon gamma-IFN gamma) (pg/mL) | 1.21 (0.67–2.20) | .53 | 0.60 (0.37–1.08) | .09 | 1.00 (0.99–1.01) | .97 |
| Ln (interferon gamma inducing protein-10-IP10) (pg/ml) | 0.84 (0.46–1.54) | .66 | 0.74 (0.41–1.31) | .30 | 1.0 (0.99–1.01) | .08 |
| Ln (macrophage inflammatory protein 1Beta-MIP1Beta) (pg/ml) | 0.51 (0.20–1.32) | .17 | 1.00 (0.42–2.40) | .99 | 1.0 (0.99–1.01) | .90 |
| Ln (monocyte chemotactic protein 1-MCP1) (pg/mL) | 0.60 (0.35–1.05) | .07 | 0.88 (0.51–1.50) | .63 | 1.0 (0.99–1.01) | .85 |
| Ln (tumor necrosis factor alpha-TNFalpha) (pg/ml) | 0.79 (0.63–1.01) | .05 | 0.80 (0.63–1.02) | .07 | 1.0 (0.99–1.01) | .95 |
*Binary logistic regression analyses adjustment for age, sex, onset-to-treatment time, baseline blood glucose level, baseline NIHSS, history of infections, history of atrial fibrillation, history of congestive heart failure, and for center effect. P value was adjusted for multiple comparisons using the Benjamini and Hochberg False Discovery Rate.
sCI: confidence interval; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; sICH: symptomatic intracerebral hemorrhage; TIMP: tissue inhibitors of metalloproteinases; Ln: natural logarithm.
Table 3.
Effect of Pre-Post-tPA variations of inflammatory marker circulating level on sICH, 3-month Death, 3-month mRS 3 to 6, adjusting * for major determinants of the outcomes.
| sICH |
Death 3 month |
mRS 3–6 |
||||
|---|---|---|---|---|---|---|
| Pre – Post-tPA variations (**) | OR (95% CI) | P | OR (95% CI) | P Value | OR (95% CI) | P |
| C reactive protein (CRP) (mg/L) | 1.64 (1.15–2.34) | .006 | 1.36 (0.94–1.95) | .10 | 1.33 (1.03–1.72) | .03 |
| Haptoglobin (mg/mL) | 0.96 (0.71–139) | .96 | 1.21 (0.89–1.64) | .22 | 1.02 (0.80–1.28) | .92 |
| Serum amyloid P (µg/mL) | 1.57 (0.78–3.17) | .20 | 1.44 (0.72–2.91) | .31 | 1.07 (0.68–1.68) | .78 |
| Alpha2 macroglobulin (A2M) (mg/mL) | 1.36 (0.69–2.69) | .37 | 2.32 (1.13–4.76) | .02 | 1.15 (0.72–1.82) | .56 |
| Interleukin 1Beta (IL1Beta) (pg/ml) | 1.42 (0.82–2.46) | .21 | 1.91 (0.96–3.78) | .06 | 2.45 (1.36–4.39) | .003 |
| Interleukin 1 receptor antagonist (IL-1Ra) (pg/mL) | 1.16 (0.86–1.57) | .32 | 1.58 (1.13–2.19) | .007 | 1.53 (1.10–2.12) | .012 |
| Interleukin 4 (IL4) (pg/mL) | 1.73 (0.91–3.30) | .09 | 1.57 (0.77–3.29) | .21 | 1.26 (0.77–2.05) | .36 |
| Interleukin 6 (IL6) (pg/mL) | 1.11 (0.88–1.41) | .38 | 1.64 (1.09–2.47) | .01 | 1.49 (1.10–2.12) | .01 |
| Interleukin 8 (IL8) (pg/mL) | 1.32 (0.97–1.81) | .08 | 1.52 (1.02–2.26) | .04 | 1.88 (1.32–2.69) | <.001 |
| Interleukin 10 (IL10) (pg/mL) | 2.02 (1.31–3.12) | .002 | 2.02 (1.30–3.12) | .002 | 1.66 (1.17–2.35) | .004 |
| Interleukin 12 (IL12) (pg/mL) | 1.06 (0.55–2.03) | .86 | 1.34 (0.66–2.73) | .42 | 1.33 (1.03–1.72) | .03 |
| Interleukin 17 (IL17) (pg/mL) | 1.18 (0.72–1.92) | .51 | 1.40 (0.84–2.34) | .20 | 1.02 (0.80–1.28) | .92 |
| Interferon gamma (IFN gamma) (pg/mL) | 1.51 (0.83–2.75) | .18 | 1.97 (0.99–3.92) | .05 | 1.07 (0.68–1.68) | .78 |
| Interferon gamma inducing Protein-10 (IP10) (pg/ml) | 0.42 (0.17–1.05) | .06 | 1.64 (0.87–3.11) | .13 | 1.15 (0.72–1.82) | .56 |
| Macrophage inflammatory protein 1Beta (MIP1Beta) (pg/ml) | 1.25 (0.61–2.53) | .54 | 2.16 (0.98–4.77) | .06 | ||
| Monocyte chemotactic protein 1 (MCP1) (pg/mL) | 0.51 (0.25–1.03) | .06 | 1.39 (0.78–2.47) | .26 | 2.45 (1.36–4.39) | .003 |
| Tumor necrosis factor alpha (TNFalpha) (pg/ml) | 1.23 (0.87––1.74) | .25 | 2.15 (1.28–3.61) | .004 | 1.53 (1.10–2.12) | .01 |
(**)Cube root-transformed values of Pre-Post tPA variations were used.
*Binary logistic regression analyses adjustment for age, sex, onset-to-treatment time, baseline blood glucose level, baseline NIHSS, history of infections, history of atrial fibrillation, history of congestive heart failure, and for center effect. P value was adjusted for multiple comparisons using the Benjamini and Hochberg False Discovery Rate.
sCI: confidence interval; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; sICH: symptomatic intracerebral hemorrhage; TIMP: tissue inhibitors of metalloproteinases.
Among the inflammatory markers, only CRP, IL1β, IL1Ra, IL6, IL8 and IL10 variations were associated with three-month mRS 3–6 (Table 3).
None of the pre-tPA inflammatory markers was significantly related with hemorrhagic transformation (Tables 2). Only pre-post-tPA variation of IL10 was a significant predictor of the hemorrhagic transformation (Table 3).
When in the logistic regression model containing as independent variables age, sex, glycemia, baseline NIHSS, history of atrial fibrillation, or congestive heart failure, history of inflammatory diseases or infections occurred within the last seven days before stroke onset, the inflammatory markers (each one at a time), we added ΔMMP9 or ΔTIMP1 only pre-tPA A2M, SAP and ΔMMP9 remained significantly and independently associated with three-month death [OR (95% CI): baseline A2M: 2.99 (1.19–7.53), p = 0.020; baseline SAP: 5.46 (1.64–18.74); p < 0.01; ΔMMP9: 1.60(1.12–2.27), p < 0.01].
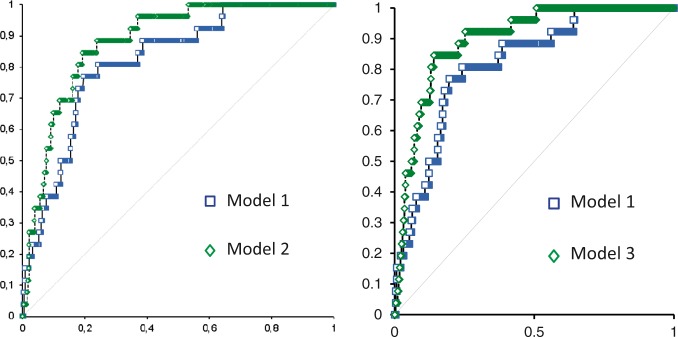
ROC analysis demonstrated that the addition of baseline A2M and ΔMMP9 (model 2) or baseline SAP and ΔMMP9 (model 3) to a model that included factors known to affect the outcome (model 1) significantly improved the area under the curve for the prediction of mortality in IS patients [model 1:AUC = 0.82 (95% CI 0.74–0.90); model 2:AUC = 0.88 (95% CI 0.83–0.94), p = 0.0205; model 3: AUC = 0.90 (95% CI 0.84–0.95), p = 0.001] (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curves for death of the two models of logistic regression. (model 1: age, gender, glycemia, baseline NIHSS, atrial fibrillation, heart failure, recent infections or inflammations; model 2: model 1 + baseline α-2-macroglobulin + delta MMP9; model 3: model 1 + baseline SAP + delta MMP9).
The addition of A2M as categorical variable (4th quartile vs. others quartiles) led to a significant change in the prediction of death (NRI = 0.469 ±0.197; p = 0.0177) by the model 1 plus ΔMMP-9. Changes in the risk of death estimated by the model were also significant when SAP was added to the model with traditional risk factors and ΔMMP9 (NRI =0.493 ± 0.198; p = 0.0126).
The IDI for pre-tPA SAP was 0.0467 ± 0.0187, p = 0.0307, whereas for A2M was not significant (IDI = 00118 ± 0185, p = 0.2617).
No statistical interaction was found between inflammatory markers and MMPs/TIMPs.
Discussion
In this large series of patients treated with i.v. thrombolysis we found that: (1)baseline A2M and SAP levels independently predict three-month death, (2)pre-post tPA variations of CRP, IL1β, and IL6 are associated with worse clinical outcome, (3)pre-tPA values of any of the tested inflammatory markers are related with SICH, whereas only pre-post tPA variation of IL-10 was a significant predictor of hemorrhagic transformation and (4)after adjustment for several potential confounders and MMP9 and TIMP1, only baseline A2M, baseline SAP and ΔMMP9 (reported in our previous study on the same series of patients) remain significantly and independently associated with three-month death.
Inflammation is increasingly recognized to be the key element in pathological progression of IS. After cerebral ischemia, an inflammatory response mediated by the activation of nuclear factor Kappa-B occurs, and cytokines production by microglia and neurons may significantly increase.20 A recent review of clinical studies aimed to assess biomarkers related to poor stroke outcomes showed that none of the inflammatory molecules are associated with death risk.4
By using an extensive array of circulating cytokines and other inflammatory markers, we identified, for the first time, A2M and SAP as factors selectively associated with three-month death.
Human A2M is a broad-spectrum proteinase inhibitor, and a cargo protein for growth factors and cytokines in the blood and other extracellular spaces.21 A2M enhances prothrombotic properties by neutralizing plasmin, plasminogen activators and activated protein C.22–24 and acts as proteinase inhibitor through steric shielding and rapid clearance of the bound proteinases. This binding causes change in A2M conformation to an activated form, which recognizes the low-density lipoprotein receptor-related protein 1 (LRP1).25
In a case-control study,26 A2M levels were significantly higher in patients with acute IS (especially those with lacunar stroke) than in controls and they were associated with older age and white matter lesion severity.30 Interestingly, in our study, we found significant correlations between A2M and age (r = 0.27, p < 0.001), and between A2M and NIHSS score at admission (r = 0.21, p < 0.001). In addition, A2M levels turned out to be independently associated with the total anterior circulation syndrome (TACS) (p = 0.034).
SAP is a member of pentraxin family and plays a key role in innate immunity and inflammation. SAP activates the classical complement pathway, has opsonin activity and is a component of extracellular matrices, and is present in elastic fibers in blood vessels.27,28
In our study, pre-post tPA variations of CRP, IL1β and IL6 were associated with it three-month mRS 3–6, after adjusting for potential confounders.
In humans, peripheral white blood cell count, CRP level and cytokines are increased within 24 h after the onset of stroke,7 and a prolonged activity of the post-ischemic inflammatory response even three months after the onset of stroke29 was demonstrated.
Our data showed that the patients with higher pre-post tPA increase in CRP, IL1β, IL6 and SAP were those patients with higher risk of poor outcome (mRS 3–6), confirming the role of CRP in influencing the risk of poor outcome in IS and extend to other inflammatory markers such as IL1β and IL6.
Inflammation may underpin multiple factors known to contribute to the hemorrhagic transformation after thrombolysis such as vascular injury, reperfusion and altered permeability. However, in our study, we did not found any significant association between inflammatory markers and hemorrhagic transformation.
Our data on pre- and post-tPA CRP are consistent with those obtained by Karlinski et al.,16 showing that patients treated with thrombolysis and with high 24 h-serum CRP experienced more frequently sICH than patients with normal CRP level, but after the adjustment for clinical predictors of hemorrhagic transformation, this association did not remain, as in the present study. An unexpected result of our study was the significant association between pre-post tPA variations of IL10 and sICH: patients who experienced the hemorrhagic transformation had higher pre-post tPA variations of the anti-inflammatory cytokine IL-10, suggesting that in these patients, the increased pro-inflammatory response observed after tPA administration was counterbalanced by a higher anti-inflammatory response.
Among different definitions available for ICH occurring after thrombolysis, we choose NINDS criteria as the most sensitive one. In our cohort, the number of patients with symptomatic ICH defined using NINDS criteria was 8.3%, whereas it was 3.7% using ECASS criteria, and only 0.3% using SITS-MOST criteria. The choice of NINDS criteria might affect the results of our study as a recent paper evidenced that the NINDS criteria had the lowest clinical/functional effect.31 However, in our study, functional outcome was anyhow determined since we had the three months Rankin’s score assessed and analyzed for all patients.
In analyzing the interplay between MMPs and the inflammatory markers in determining poor outcomes, we demonstrated that ΔMMP9, baseline A2M and SAP are the only factors independently associated with three-month death, suggesting that both A2M, SAP and MMP9 contributed to the cerebral damage after IS and thrombolysis. A2M, in fact, determines a reduction in the fibrinolytic potential. Furthermore, A2M and LRP1 can regulate matrix MMP,30 and A2M could be crucial in the progression of atherosclerotic lesions through interaction with LRP1.
The results of our study evidenced that A2M and SAP may be clinically useful as they significantly and correctly increases the risk predicted by the model including traditional risk factors and ΔMMP-9 for death, suggesting that high pre-tPA levels of A2M and SAP may be useful to identify those patients with and increase probability of death after the administration of tPA.
From a translational, clinical practice point of view, the determination of pre-tPA levels of A2M and SAP, easily and quickly assessed by immune-nephelometric assay already available in general laboratories, could help identifying very early, i.e. soon after the treatment, patients at higher risk of worse outcome to be eventually subjected to a more intensive surveillance and care.
Further studies are needed to confirm the role of biomarkers also in ischemic stroke patients treated with endovascular thrombectomy.
The specific role of rtPA in stroke-related inflammation remains largely unknown since most of the side effects could be due to reperfusion injury itself. Recent experimental data show an effect on rtPA in enhancing microglia cell recruitment after transient middle cerebral artery occlusion (MCAO) in mice.32 In another study, protein levels of IL6, TNF alpha, MMP9 were attenuated after MCAO in those animals where the thrombolysis was successful.33
One limitation of our study is the lack of a control group of patients with stroke not treated with tPA. A further limitation rests upon the lack of further blood sampling to determine the inflammatory response during the first days after stroke, as different time-courses are characteristic feature of different inflammatory markers.
In conclusion, our study documented that inflammation and particularly A2M and SAP influence, the adverse outcomes in IS patients treated with tPA, and suggests the usefulness of determining pre-tPA A2M and SAP as surrogate predictor of unfavorable stroke outcomes.
By exploring the complex interaction among several inflammatory markers and MMPs, and by using an extensive array of cytokine/chemokines and MMPs, our results provide further insights into the pathophysiological mechanisms underlying the unfavorable outcomes after thrombolysis.
Present findings encourage controlled clinical trials to test the effectiveness of MMP antagonists and anti-inflammatory therapy administered together with tPA.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Biological Markers Associated with Acute Ischemic Stroke (MAGIC) Study was funded by grants from Italian Ministry of Health, 2006 Finalized Research Programmes (RFPS-2006-1-336520) and Ente Cassa di Risparmio di Firenze (2010.06.03).
Supplementary Material
Acknowledgments
We thank the hospital staff for data collection: N Micheletti, Verona; F Muscia, Como; A De Vita, Ferrara; F Galati, Vibo Valentia; S Marcheselli, Pavia; M Acampa, Siena; A Chiti, Pisa; M Bacigaluppi, Milano; A De Boni, Vicenza; F Carinati, Varese; P Palumbo, Prato.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Study concept and design: BP, VP, MN, PN, GP, DT, AN, RA, and DI. Acquisition of data: BP, VP, MN,PN, GP, DT, AN, RA, DI, PT, EI, AS, ES, PB, MG, MRT, DC, GM, RT, GO, FP, FM, MLD, and MS. Statistical analysis: AMG, BP, VP, MN, PN, BG, GP, DT, AN, RA, and DI. Analysis and interpretation of data: AMG, BP, VP, MN, PN, BG, GP, DT, AN, RA, DI, PT, EI, AS, ES, PB, MG, MRT, DC, GM, RT, GO, FP, FM, MLD, MLZ and MS. Drafting and critical revision of manuscript: AMG, BP, VP, MN, PN, BG, GP, DT, AN, RA, and DI. Study supervision: AMG, BP, VP, MN, PN, BG, GP, DT, AN, RA, and DI.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: A statement for health care professionals from the CRP Pooling Project members. Stroke 2005; 36: 1316–1329. [DOI] [PubMed] [Google Scholar]
- 2.Kang DW, Yoo SH, Chun S, et al. Inflammatory and hemostatic biomarkers associated with early recurrent ischemic lesions in acute ischemic stroke. Stroke 2009; 40: 1653–1658. [DOI] [PubMed] [Google Scholar]
- 3.Di Napoli M, Elkind MS, Godoy DA, et al. Role of C-reactive protein in cerebrovascular disease: A critical review. Expert Rev Cardiovasc Ther 2011; 9: 1565–1584. [DOI] [PubMed] [Google Scholar]
- 4.Hasan N, McColgan P, Bentley P, et al. Towards the identification of blood biomarkers for acute stroke in humans: A comprehensive systematic review. Br J Clin Pharmacol 2012; 74: 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerging Risk Factors Collaboration, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J Transl Med 2009; 7: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilupul Perera M, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci 2006; 13: 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Emsley HC, Smith CJ, Tyrrell PJ, et al. Inflammation in acute ischemic stroke and its relevance to stroke critical care. Neurocrit Care 2008; 9: 125–138. [DOI] [PubMed] [Google Scholar]
- 9.Romanic AM, White RF, Arleth AJ, et al. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998; 29: 1020–1030. [DOI] [PubMed] [Google Scholar]
- 10.Heo JH, Lucero J, Abumiya T, et al. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 624–633. [DOI] [PubMed] [Google Scholar]
- 11.Winbeck K, Poppert H, Etgen T, et al. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke 2002; 33: 2459–2464. [DOI] [PubMed] [Google Scholar]
- 12.Elkind MS, Luna JM, McClure LA, et al. C-reactive protein as a prognostic marker after lacunar stroke: Levels of inflammatory markers in the treatment of stroke study. Stroke 2014; 45: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inzitari D, Giusti B, Nencini P, et al. MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke 2013; 44: 2901–2903. [DOI] [PubMed] [Google Scholar]
- 14.Audebert HJ, Rott MM, Eck T, et al. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke 2004; 35: 2128–2133. [DOI] [PubMed] [Google Scholar]
- 15.Topakian R, Strasak AM, Nussbaumer K, et al. Prognostic value of admission C-reactive protein in stroke patients undergoing iv thrombolysis. J Neurol 2008; 255: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 16.Karlinski M, Bembenek J, Grabska K, et al. Routine serum C-reactive protein and stroke outcome after intravenous thrombolysis. Acta Neurol Scand 2014; 130: 305–311. [DOI] [PubMed] [Google Scholar]
- 17.Wahlgren N, Ahmed N, Dàvalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITSMOST): An observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 18.Larrue V, von Kummer RR, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001; 32: 438–441. [DOI] [PubMed] [Google Scholar]
- 19.Xanthakis V, Sullivan LM, Vasan RS, et al. Assessing the incremental predictive performance of novel biomarkers over standard predictors. Stat Med 2014; 33: 2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denes A, Thormton P, Rothwell NJ, et al. Inflammation and brain injury: Acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun 2010; 24: 708–723. [DOI] [PubMed] [Google Scholar]
- 21.LaMarre J, Wollenberg GK, Gauldie J, et al. Alpha 2-macroglobulin and serum preferentially counteract the inhibitory effect of transforming growth factor-beta 2 in rat hepatocytes. Lab Invest 1990; 62: 545–551. [PubMed] [Google Scholar]
- 22.Hoogendoorn H, Toh CH, Nesheim ME, et al. Alpha 2-macroglobulin binds and inhibits activated protein C. Blood 1991; 78: 2283–2290. [PubMed] [Google Scholar]
- 23.Cvirn G, Gallistl S, Koestenberger M, et al. Alpha 2-macroglobulin enhances prothrombin activation and thrombin potential by inhibiting the anticoagulant protein C/protein S system in cord and adult plasma. Thromb Res 2002; 105: 433–439. [DOI] [PubMed] [Google Scholar]
- 24.Cvirn G, Gallistl S, Muntean W. Effects of alpha(2)- macroglobulin and antithrombin on thrombin generation and inhibition in cord and adult plasma. Thromb Res 2001; 101: 183–191. [DOI] [PubMed] [Google Scholar]
- 25.Herz J, Kowal RC, Ho YK, et al. Low density lipoprotein receptor-related protein mediates endocytosis of monoclonal antibodies in cultured cells and rabbit liver. J Biol Chem 1990; 265: 21355–21362. [PubMed] [Google Scholar]
- 26.Nezu T, Hosomi N, Aoki S, et al. Alpha2-macroglobulin as a promising biomarker for cerebral small vessel disease in acute ischemic stroke patients. J Neurol 2013; 260: 2642–2649. [DOI] [PubMed] [Google Scholar]
- 27.Bottazzi B, Doni A, Garlanda C, et al. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu Rev Immunol 2010; 28: 157–183. [DOI] [PubMed] [Google Scholar]
- 28.Inforzato A, Doni A, Barajon I, et al. PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin Immunol 2013; 25: 79–85. [DOI] [PubMed] [Google Scholar]
- 29.Emsley HC, Smith CJ, Gavin CM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J Neuroimmunol 2003; 139: 93–101. [DOI] [PubMed] [Google Scholar]
- 30.Cáceres LC, Bonacci GR, Sánchez MC, et al. Activated α(2)macroglobulin induces matrix metalloproteinase 9 expression by low-density lipoprotein receptor-related protein 1 through MAPK-ERK1/2 and NF-κB activation in macrophage-derived cell lines. J Cell Biochem 2010; 111: 607–617. [DOI] [PubMed] [Google Scholar]
- 31.Rao NM, Levine SR, Gornbein JA, et al. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: Analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke 2014; 45: 2728–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenglet S, Montecucco F, Denes A, et al. Recombinant tissue plasminogen activator enhances microglial cell recruitment after stroke in mice. J Cereb Blood Flow Metab 2014; 34: 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansar S, Chatzikonstantinou E, Thiagarajah R, et al. Pro-inflammatory mediators and apoptosis correlate to rt-PA response in a novel mouse model of thromboembolic stroke. PLoS One 2014; 9: e85849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.