Abstract
Introduction
The ultimate goal in the field of drug delivery is to exclusively direct therapeutic agents to pathological tissues in order to increase therapeutic efficacy and eliminate side effects. This goal is challenging due to multiple transport obstacles in the body. Strategies that improve drug transport exploit differences in the characteristics of normal and pathological tissues. Within the field of oncology, these concepts have laid the groundwork for a new discipline termed transport oncophysics.
Areas covered
Efforts to improve drug biodistribution have mainly focused on nanocarriers that enable preferential accumulation of drugs in diseased tissues. A less common approach to enhance drug transport involves priming strategies that modulate the biological environment in ways that favor localized drug delivery. This review discusses a variety of priming and nanoparticle design strategies that have been used for drug delivery.
Expert opinion
Combinations of priming agents and nanocarriers are likely to yield optimal drug distribution profiles. Although priming strategies have yet to be widely implemented, they represent promising solutions for overcoming biological transport barriers. In fact, such strategies are not restricted to priming the tumor microenvironment but can also be directed toward healthy tissue in order to reduce nanoparticle uptake.
Keywords: biodistribution, biological barrier, nanodelivery system, priming agents, transport ocophysics
1. Introduction
Targeted therapy typically refers to drugs that have specific molecular targets that are differentially expressed in healthy and pathological tissues. Modern pharmaceuticals have come a long way since the hallmark discovery of agents such as methotrexate and cisplatin, which proved to be highly beneficial for cancer treatment but showed a broad array of side effects. Researchers have since adopted more focused approaches in terms of drug development, which may have begun with Ahlquist, who identified the alpha and beta subtypes of the adrenergic receptors. Sir James Black then postulated that the beta subtype found in the myocardium can specifically be targeted with an antagonist in order to reduce angina [1], leading to the eventual development of beta-blockers. This methodology encouraged the search for agonist or antagonist agents for a variety of receptors and molecular targets. Since then, numerous receptors and cellular targets have been elucidated and still remain areas of intense research. Sequencing of the human genome and our understanding of biological signaling pathways have brought innumerable new drug targets to the limelight, which has ushered in an era of research on target-specific therapy. For instance, the discovery of small interfering RNA (siRNA) has opened up a new category of therapeutic agents that can be used to suppress specific genes [2].
In addition to viewing targeted therapy from a molecular standpoint, targeting can also take place in the form of localized drug delivery. In parallel with the development of therapies that act on specific molecular targets, increased focus has been placed on achieving specificity in regards to drug distribution within the body. Specifically, with the emergence of the field of nanotechnology, multiple nanocarriers have been developed for localized drug delivery [3–6]. Multiple components and increased functionality can be implemented in nanoparticles, since they have larger dimensions (1–1000 nm) than conventional small molecule drugs (< 1 nm), [7]. Indeed, multifunctionality enables nanoparticles to possess both therapeutic activity and transport-enhancing properties [5, 7]. To date, several nanoparticle-based therapeutics have received clinical approval. For example, liposomal formulations for the treatment of fungal infections, viral infections, and cancer, are currently used in the clinical setting [8, 9]. In addition, several polymeric and metal-based nanoparticles have also entered the market [8, 9]. For a complete list of clinically approved nanoparticles, please refer to reviews by and Anselmo et al. [8] and Bobo et al. [9]. Nanodrugs typically display lower toxicity than their free drug counterparts due to improved biodistribution profiles. For instance, liposomal doxorubicin (Doxil) is associated with a lower incidence of cardiotoxicity compared to the conventional drug formulation [10]. Another example of a clinically approved nanodrug with reduced side effects is Abraxane, consisting of paclitaxel reversibly attached to albumin. Studies have shown that Abraxane displays 33% higher accumulation in tumor tissue in comparison to the cremophor formulation of paclitaxel [11]. This nanoparticle-mediated improvement in tumor-specific transport enables the use of higher doses of paclitaxel. For instance, in a phase III study of metastatic breast cancer, albumin-bound paclitaxel could be administered at a dose that was 49% higher than that used for solvent-based paclitaxel [12]. However, it should be noted that reduced toxicity could also partially stem from not having to use harmful solubilizing agents, which are frequently administered with non-water-soluble cytotoxic agents [13–15]. Various preclinical studies have also demonstrated that nanoparticles can substantially improve the localized delivery of drugs. In certain cases, tumor accumulation of chemotherapeutic agents was shown to increase 16-fold [16] and 100-fold [17] as a result of nanoparticle delivery.
An alternative approach for improving drug delivery involves the use of priming agents that modulate the biological environment. Specifically, priming agents can be used to change the structure or function of tissues in ways that favor localized drug delivery. The majority of priming strategies for localized delivery have focused on changing various components of the tumor microenvironment, including the vasculature and extracellular matrix (ECM) [18]. In addition to using priming agents to modulate the tumor microenvironment, such agents can also be used to prime healthy tissue for decreased drug uptake. For example, pretreatment with agents that affect the function of resident macrophages [19, 20] or other components of the innate immune system [21] is likely to substantially impact the biodistribution of nanoparticles. Indeed, the function of the innate immune system is to recognize and engulf foreign bodies. This engulfment process leads to rapid accumulation of nanoparticle in organs that harbor key functional components of the innate immune system. Therefore, it is probable that a strong correlation exists between the activity of the innate immune system and the distribution of foreign entities within the body. The concept of priming the immune system for improved drug delivery will be discussed in greater detail in later sections of this review.
Strategies for achieving specificity in regards to molecular targets or drug distribution are alternative means of obtaining therapeutic efficacy with minimal side effects. However, these two strategies both display limitations in regards to achieving this ultimate goal. Although therapeutic agents that target specific pathways involved in pathogenesis typically display reduced toxicity, therapeutic efficacy cannot be guaranteed unless the drug is able to reach the intended location in sufficient quantities. For example, systemically administered siRNA molecules rarely reach their intended location. In fact, these molecules are rapidly degraded by enzymes in the circulation and tissue interstitium [4, 22]. Moreover, the bulky dimensions and negative charge of siRNAs substantially hinders cellular uptake [4, 22]. On the contrary, nanoparticles generally ensure efficient transport of cytotoxic drugs to an intended location. However, although nanoparticles dramatically improve drug distribution, the bulk of the injected dose still ends up in healthy tissues. In fact, in most cases, 5% or less of the injected nanoparticle dose accumulates in tumor tissue [23, 24], leading to adverse effects in other organs. A recent study analyzed 117 nanoparticle delivery studies and concluded that 0.7% (median) of the injected dose is delivered to tumor tissue [25]. Therefore, a combination of molecular targeted therapeutics with nanoparticle transport could yield optimal results in treating disease. Although priming agents can be used to improve the biodistribution of small molecules, the implementation of priming strategies for nanoparticle delivery is particularly useful for further improving drug transport.
2. Transport Oncophysics
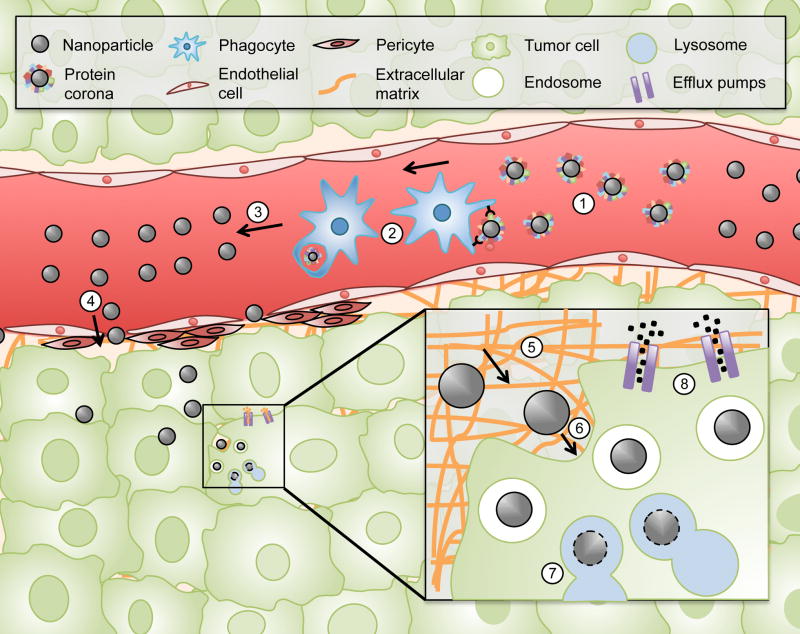
Localized delivery of drugs is especially crucial within the field of oncology, since most cancer deaths occur due to metastatic lesions that require treatment through systemic administration of drugs. The ultimate goal in the field of drug delivery is to exclusively direct and confine therapeutic agents to pathological tissues. If this goal can be achieved, the therapeutic window of drugs would be expanded and the toxic threshold eliminated. These concepts have inspired the emergence of a new field termed transport oncophysics [26–28]. This field views cancer treatment as a transport problem that arises due to biological barriers within the body (Figure 1). These biobarriers include, the protein corona, clearance by the mononuclear phagocyte system (MPS), fluid dynamics, the endothelial surface of blood vessels, the extracellular matrix, the cellular membrane, lysosomal degradation, and efflux pumps such as P-glycoprotein found in multi-drug resistant (MDR) tumors [26]. The multitude and complexity of these transport obstacles necessitates the development of multifunctional nanodelivery systems that can sequentially negotiate different biobarriers. A key concept within transport oncophysics is that tumors display unique physiological features that impact nanoparticle transport. Accordingly, such features can be exploited when designing nanoparticles for localized delivery. If such differences are well understood, nanoparticle properties including size, deformability, charge, and shape can be manipulated to achieve localized delivery [29]. In addition to nanoparticle design approaches, priming strategies can be developed based on differences in the properties of tumors and healthy tissue. Although treatment with priming agents prior to nanoparticle administration is a less commonly utilized approach for drug delivery, such strategies have proven useful in obtaining preferential accumulation of nanoparticles.
Figure 1.
Biological barriers in drug delivery. The protein corona (1), the mononuclear phagocyte system (MPS) (2), fluid dynamics (3), the endothelial surface of blood vessels (4), the extracellular matrix (5), the cellular membrane (6), lysosomal degradation (7), and efflux pumps (8).
3. Strategies for addressing transport obstacles
A wide variety of strategies have been developed to overcome transport obstacles for intravenous delivery of drugs. In this review, such strategies have been divided into categories based on the biological barriers that are addressed. Although multiple transport obstacles exist, this review focuses primarily on the MPS, the vascular endothelium, the tumor microenvironment, and membrane compartments. The majority of efforts within the field of drug delivery have been concentrated on developing nanocarriers that display transport-enhancing properties. However, priming strategies that aim to modulate the biological environment can lead to further improvements in the biodistribution of drugs.
3.1. Strategies for overcoming the mononuclear phagocyte system
Immediately after intravenous injection, nanoparticles are subject to opsonization by plasma proteins, including immunoglobulins and complement proteins [30]. These proteins form a corona around the nanoparticle surface, which promotes nanoparticle recognition and uptake by cells of the MPS [30, 31]. These cells include macrophages as well as other phagocytic cells residing in the spleen, lymph nodes, and the liver [32]. The MPS is sometimes referred to as the reticuloendothelial system (RES), however, this is an outdated term that does not accurately describe the cells that comprise this system [33]. The amount of time that nanoparticles spend in the circulation is largely determined by MPS sequestration rates [29]. Approximately 95% of the injected nanoparticle dose ends up in filtration organs such as the liver and spleen [34], since the MPS system has been fine-tuned by evolution to recognize and engulf foreign bodies.
Several studies have been undertaken to negotiate the MPS transport obstacle. Some of these studies have viewed the protein corona as a beneficial component, which composition can be modulated for targeting purposes [35]. Understanding the interactions that take place between nanoparticles and molecules in biological fluids is a prerequisite for harnessing the protein corona for improved nanoparticle biodistribution. For this purpose, various analytical methods have been developed to characterize the protein corona [36–38]. Using these methods, the absorbed protein profiles of nanoparticles with different characteristics have been compared, revealing that certain particle properties result in unique patterns of protein binding [39–41]. These and other studies have demonstrated that nanoparticle characteristics have a profound impact on the composition of the protein corona [30], which has spurred the notion that nanoparticles could be engineered to favor binding of specific biomolecules. In the context of addressing the MPS barrier, nanoparticles could be designed to bind plasma proteins that reduce interactions with macrophages. Examples of nanoparticle design strategies that have been employed to obtain binding of specific proteins include a study in which carbon nanotube-polymer constructs were designed to display binding pockets for specific proteins, including riboflavin, L-thyroxine, and oestradiol [42]. In addition to geometrical surface properties, other nanoparticle characteristics such as charge can also have a substantial impact on the protein corona [43]. For instance, positively-charged nanoparticles are generally more prone to protein adsorption and MPS sequestration, which leads to reduced circulation times [44]. Moreover, the material composition of nanoparticles has a profound impact on the protein corona. For example, it was show that different lipids attract unique plasma proteins around liposome surfaces. In particular, the presence of cholesterol promoted the binding of opsonins, while other lipids such as 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) preferentially bound apolipoproteins and vitronectin [45]. An important consideration for protein corona studies is that results are usually highly dependent on the biological system that is used. Accordingly, it has been demonstrated that the protein corona of liposomes exposed to mouse plasma is markedly different in terms of surface charge and composition compared to liposomes exposed to human plasma [46]. Therefore, in order to obtain clinically relevant conclusions it is essential to consider the source of the biological fluid that is used for such studies.
An alternative approach to avoid nanoparticle uptake in the MPS is to decrease protein adsorption in the blood circulation. Reduced protein binding can be achieved by the attachment of polyethylene glycol (PEG) to nanoparticles, a process known as pegylation [47]. PEG attracts water molecules on the nanoparticle surface, which form a barrier that prevents protein absorption. This steric barrier reduces macrophage recognition, consequently prolonging the half-life of nanoparticles in circulation [48]. Recently, it was shown that although pegylation reduces overall protein binding, PEG also attracts specific plasma proteins on the nanoparticle surface. In particular, clusterin was present in high amounts on the protein corona of pegylated polystyrene nanoparticles [49]. Studies evaluating the role clusterin on nanoparticle uptake in macrophages found that pre-incubation with this protein substantially reduced the cellular internalization of nanoparticles [49]. These observations suggest that in addition to decreasing overall protein binding, PEG reduces nanoparticle clearance by increasing the binding of dysopsonins. Interestingly, another study demonstrated that while pegylation reduces the total amount of bound opsonins on the surface of cationic liposomes, the relative abundance of opsonins on the protein corona increases [50]. In light of these results, it is important to note that the effect of pegylation on the protein corona composition is likely to be contingent on the type of nanoparticle studied. Despite the fact that pegylation is an effective approach to avoid recognition by the MPS, there are disadvantages of this strategy. For instance, it has been shown that antibodies against PEG can form upon repeated administration of pegylated nanoparticles, leading to rapid particle clearance, a process termed the accelerated blood clearance (ABC) phenomenon [51]. Another drawback of pegylation is that PEG chains can detach from nanoparticle surfaces upon intravenous injection [52]. To overcome this problem, super stealth liposomes have been developed, which display superior stability compared to conventional stealth liposomes [53]. These super stealth liposomes are composed of β-glutamic acid dendron anchors that attach single PEG chains to multiple phosphoethanolamines. These PEG-dendron liposomes differ from conventional stealth liposomes that have a PEG chain attached to a single phospholipid. An increase in the phospholipid/PEG attachment ratio leads to more stable interactions between PEG chains and the vesicular surface. Indeed, super stealth liposomes display prolonged circulation half-life and decreased liver and spleen accumulation [53]. Unfortunately, the PEG-induced stealth effect also pertains to target cells. This phenomenon, referred to as the PEG dilemma, results in reduced interactions between nanoparticles and target tissues, leading to impaired retention and intracellular uptake of nanoparticles [54]. To overcome this dilemma, stimuli-responsive nanodelivery systems with cleavable PEG chains have been developed [54, 55]. In addition to PEG, several other molecules have been employed to reduce the binding of opsonins to nanoparticles. These molecules include polyoxazolines, polyamino acids, N-(2-hydroxypropyl) methacrylamide, polybetaines, polyglycerols, and polysaccharides [56].
Moreover, phagocytic clearance can be hindered by the use of drug delivery strategies based on biomimicry, a term that refers to the imitation of components or processes in nature in order to solve problems. In the human body, cells, hormones, signaling molecules, metabolites, blood gases, and many other substances successfully reach their targets with near-perfect accuracy, while simultaneously overcoming multiple biobarriers. Therefore, a promising strategy for nanoparticle design involves harnessing the transport properties of naturally occurring biological components. Indeed, studies have demonstrated that liposomes containing proteins from leukocyte cell membranes can evade the MPS. These biomimetic liposomes displayed up to a 2.6-fold decrease in uptake by MPS organs and a fivefold increase in blood concentration 24 h post-injection [57]. The observed reduction in MPS uptake could be partially due to the presence of CD47, which is expressed on cells throughout the body, where it serves as a marker of ‘self’ for the innate immune system [36]. Namely, phagocytosis of cells is inhibited upon binding of CD47 to the signal regulatory protein alpha (SIRPα) receptor that is expressed on macrophages and dendritic cells. In addition to displaying self-tolerance properties, the liposomes preferentially adhered to inflamed vasculature, indicating that the extracted membrane proteins were able to endow the nanoparticles with leukocyte-like properties [57]. These proteolipid vesicles could be utilized for several applications, since inflammation plays a role in multiple diseases, including cancer and autoimmune conditions. For a more detailed discussion of nanoparticle design strategies please refer to a review by Blanco et al. [29].
The aforementioned examples for avoiding phagocytic uptake primarily focus on nanoparticle design. However, the biological environment can also be manipulated in order to reduce immunological clearance of nanoparticles. In particular, pretreatment with inhibitors of the innate immune system could prove useful. Studies have shown that animals administered with clodronate liposomes, which temporarily deplete resident macrophages in the body can substantially increase the plasma and tumor concentration of nanoparticles [19, 20]. Although complete elimination of specific macrophage populations is likely to cause toxic side effects, less radical strategies such as the use of complement inhibitors [21] or agents for reducing macrophage activity could be promising. One study demonstrated that pretreatment with gadolinium chloride (GdCl3), a known inhibitor of Kupffer cell phagocytosis [58], could reduce sequestration of quantum dots in the MPS, thereby prolonging nanoparticle circulation and increasing the fluorescent signal in the tumor [59]. Another approach to reduce macrophage engulfment is to overload the MPS system with decoy nanoparticles prior to administration of therapeutic nanoparticles. For instance, pretreatment with empty liposomes lead to a two-fold increase in the tumor accumulation of iron oxide particles [60]. In essence, the MPS represents one of the most crucial biological barriers that should be addressed in drug delivery. Therefore, nanoparticle design strategies that incorporate components that reduce macrophage interactions and priming strategies that target the innate immune system are promising for substantially improving the biodistribution of drugs.
3.2. Strategies for overcoming the endothelial barrier
Both passive and active targeting strategies have been used to overcome the endothelial barrier. While passive targeting involves optimizing the physical properties of nanoparticles to leverage differences in blood flow, physiology, and morphology between healthy and diseased tissues [26], active targeting relies on the use of targeting ligands [61]. The enhanced permeability and retention (EPR) effect is the most common example of passive targeting [62, 63] and also serves as an exemplar phenomenon in transport oncophysics. The EPR effect is based on the phenomenon that tumor blood vessels have increased permeability due to fenestrations, consequently enabling nanoparticles to preferentially accumulate at the tumor site. Fenestrations arise as a result of angiogenesis, which leads to the formation of immature vasculature, characterized by the lack of pericyte coverage. Although most tumors have vascular fenestrations that allow nanoparticles of various sizes to enter, certain cancer types display smaller fenestrations. For instance, it was demonstrated that the cutoff size for entry into the interstitium of pancreatic tumors is less than 50 nm [64]. However, it is important to remember that particle dimensions do not solely dictate the size of nanoparticles in biological fluids. In fact, plasma proteins can cause nanoparticles to increase or decrease in size. While most nanoparticles undergo a slight increase in size due to the formation of a protein corona [30], liposomes may shrink as a result of osmotic forces [65, 66].
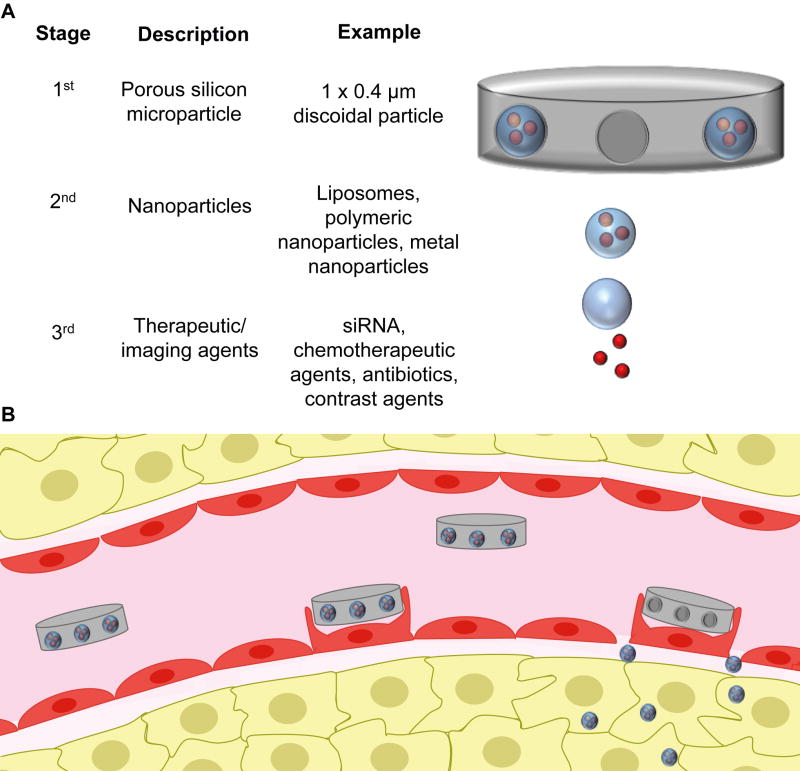
Although all clinically approved nanoparticles and most nanoparticles in preclinical use are spherical, it has been demonstrated that a discoidal shape is the most convenient particle geometry for vascular margination and adhesion [67, 68]. In fact, disc-shaped microparticles (1–2 µm) exhibit a tumbling and rolling flow, facilitating lateral drift [69]. This nanoparticle shape was inspired from the discoid shape of platelets, which marginalize along inflamed blood vessels [70]. The design of discoidal nanoparticles for drug delivery is another example of utilizing the principles of transport oncophysics. Indeed, tumors display abnormal blood flow patterns [71] that result in lower shear rates than in normal vasculature [72, 73]. This difference in shear rate makes it possible for discoidal particles to adhere to the tumor vasculature, while they are rapidly dislodged from the endothelial walls of normal vessels. One example of a disk-shaped particle is the multistage vector (MSV), which consists of three different stages (Figure 2) [26]. The first stage vector is a porous silicon microparticle that preferentially binds to tumor vasculature [74, 75]. Upon adhering to the tumor endothelium, the MSVs form vascular depots that undergo gradual degradation, thereby releasing second stage nanoparticles. The main purpose of the silicon microparticle is to transport nanoparticles to tumor vasculature. The nanoparticles can then enter the tumor interstitium by utilizing the EPR effect. The second stage nanoparticles facilitate the cellular internalization of the third stage vector, which consists of therapeutic or diagnostic agents. In essence, the MSV utilizes both abnormal blood flow patterns and abnormal features of the blood vessel wall to overcome the endothelial barrier in tumors. The MSV has been used for the delivery of siRNA, chemotherapeutic agents, antibiotics, and contrast agents [5]. The most recent development in regards to the MSV involves the delivery of a polymeric nanoparticle that is generated in vivo upon degradation of the silicon material [76]. Notably, as a result of high tumor accumulation, this injectable nanoparticle generator displayed superior therapeutic efficacy in mouse models of metastatic breast cancer [76].
Figure 2.
The multistage vector (MSV) platform. A) The MSV is composed of three different components. The first stage vector is a biodegradable porous silicon microparticle loaded with nanoparticles (second stage vectors). The nanoparticles, in turn, can be loaded with therapeutic or imaging agents. B) Each component of the MSV is designed to overcome a specific set of transport obstacles. The first stage vector preferentially adheres to tumor vasculature, forming vascular depots. As these depots gradually degrade, nanoparticles are released that can enter the tumor intersititum through endothelial fenestrations. The nanoparticles then facilitate cellular internalization of the third stage vectors.
In addition to passive targeting, active targeting approaches can be used to overcome the endothelial barrier. For example, the MSV has been conjugated with surface moeities that are specific to αvβ3 receptors, which are overexpressed on tumor blood vessels [75]. Moreover, an E-selectin thioaptamer on the surface of MSVs was used to achieve enhanced localization of the therapeutic agents in bone marrow vasculature. There are also other examples of active targeting with multistage platforms. For instance, one drug delivery system exploited the coagulation cascade, a naturally occurring process in the circulatory system [77]. The drug delivery process was initiated by injecting first stage components, which consisted of heated gold nanorods or tumor-targeted tissue factors. These first stage components triggered the coagulation cascade in tumor blood vessels, a process that could then be exploited for the delivery of second stage therapeutic liposomes or diagnostic iron oxide particles, which were conjugated with targeting ligands against blood clots. This is an example of a priming process, where the characteristics of tumor vasculature are modified to enable enhanced nanoparticle binding. Ultimately, this approach of amplified drug delivery resulted in a 40-fold increase in drug accumulation at the tumor site compared to a non-amplified approach. However, in the context of active targeting, it should be noted that the formation of a protein corona might hinder recognition and binding of molecular surface moieties, thus affecting the specificity of molecular targeting [78]. Furthermore, ligand binding to the nanoparticle surface also increases nanoparticle size, which could impede diffusion or extravasation. Additionally, surface moieties could make nanoparticles more susceptible to the immune system. An alternative approach for addressing the endothelial barrier is the utilization of endogenous blood components that have increased interactions with tumor vasculature. One example is albumin [79], which binds to the glycoprotein 60 (gp60) receptor typically found on the surface of tumor-associated endothelial cells [80]. Receptor binding initiates endothelial cell transcytosis of albumin, thus facilitating accumulation of this protein in the tumor microenvironment [80]. Albumin-bound paclitaxel nanoparticles can utilize this same transport pathway for increased deposition in tumors [81].
In addition to activation of the coagulation cascade, several other studies have utilized tumor-priming strategies for improved penetration of nanoparticles across the vascular wall. For instance, studies have shown that preheating the tumor environment in can increase the permeability of tumor blood vessels [82, 83]. Other approaches have focused on using angiogenic and anti-angiogenic agents to normalize the tumor vasculature in order to allow sufficient diffusion of nanoparticles into the tumor interstitium [84, 85]. Additionally, metronomic chemotherapy has proven useful for modulating tumor vasculature and improving drug perfusion [86, 87]. Indeed, vascular normalization can restore pressure differences across the vascular wall, since interstitial fluid pressure frequently builds up in the tumor due to poor lymphatic drainage, disrupted blood flow, and fibrosis. In fact, unfavorable pressure gradients represent a major biobarrier that can impede the EPR effect and hinder macromolecules and nanoparticles from entering the tumor interstitium. It is worth noting that the timing and dosing of vascular normalization agents plays a critical role in achieving optimal improvements in nanoparticle transport [88]. Another approach to improve nanoparticle transport across the vasculature wall is to target pericytes, which serve a supporting function for endothelial cells [89]. Strategies that reduce pericyte abundance have been developed, since pericyte coverage is thought to correlate with decreased nanoparticle extravasation [90]. For instance, pH-activated mesoporous silica nanoparticles containing transforming growth factor beta (TGF-β) inhibitors were used to reduce pericyte coverage in tumor vasculature, as this signaling pathway plays an important role in the maintenance and recruitment of these cells [91]. Pretreatment with the silica nanoparticles substantially improved the intratumoral distribution of consecutively administered gemcitabine liposomes [91]. This study is an example of a nanoparticle design strategy with an incorporated priming function.
3.3. Strategies for overcoming the tumor microenvironment
Besides the MPS and the endothelium, the composition of the tumor microenvironment also plays an important role in determining nanoparticle transport. For example, the ratio of tumor-derived matrix metalloproteinase-9 (MMP-9) to tissue inhibitor of metalloproteinase 1 (TIMP-1) [92] was shown to correlate with increased levels of nanoparticle accumulation in tumor tissue. These parameters are also potential candidates for identifying cancer patients that would be particularly amenable to nanoparticle therapy. It is notable that tumors typically have a dense ECM, which can shield cancer cells from therapeutic agents. Accordingly, the distribution of nanoparticles in the intratumoral environment is generally heterogeneous [93]. In addition to hindering the effective transport of drugs throughout the tumor microenvironment, ECM components can also compress the vasculature [94], thereby further interfering with drug delivery.
The majority of approaches designed to overcome the ECM barrier involve the use of priming agents. For instance, injection of proteases in the tumor microenvironment has been shown to improve diffusion of molecules in the tumor interstitium [95]. However, intratumoral injection of priming agents is generally an unsuitable approach for treating metastatic disease. Intravenous injection of hyaluronidase has been shown to degrade the ECM component hyaluronan, resulting in enhanced tumor accumulation of chemotherapeutic agents in a mouse model of pancreatic ductal adenocarcinoma (PDA) [96]. A characteristic feature of PDA is a dense stromal matrix, which makes this disease a suitable candidate for tumor priming strategies. A recent clinical trial demonstrated that administration of pegylated recombinant human hyaluronidase in combination with gemcitabine was well tolerated in patients with advanced pancreatic cancer [97].
Another approach for reducing the density of the ECM involves modulating the activity of cells that are responsible for producing the fibrotic matrix. Recently, it was shown that pretreatment with a systemically administered vitamin D receptor ligand induced stromal remodeling in PDA, leading to increased intratumoral delivery of drugs [98]. This receptor ligand caused pancreatic stellate cells to enter into a quiescent state, leading to reduced production of ECM components. Other studies have devised strategies for inactivating growth factors and peptide hormones that stimulate cancer-associated fibroblasts (CAFs). For instance, anti-TGF-β antibodies [99] and angiotensin inhibitors [94] have been shown to reduce stromal content and improve drug delivery to tumors. Although angiotensin primarily affects the blood pressure by causing vasoconstriction, this peptide hormone has also been shown to downregulate TGF-β signaling, leading to decreased production of ECM proteins [100]. Consequently, inhibition of angiotensin can improve drug delivery by affecting both the vasculature and CAFs [101]. However, clinical effects of angiotensin inhibitors are somewhat contradicting, as retrospective studies in metastatic renal cell carcinoma [102] and advanced non-small-cell lung cancer [103] have shown improved progression free survival (PFS) and/or overall survival, while a recent clinical trial in advanced pancreatic cancer reported adverse effects and failed to show an improvement in PFS [104]. Other strategies to overcome the ECM have focused on eliminating CAFs from the tumor microenvironment. For instance, a recent study utilized peptide-based cytotoxic nanoparticles conjugated to antibodies against human fibroblast activation protein-α (FAP-α), which is selectively expressed on CAFs [105]. Another study designed peptide nanoparticles that disassembled in the presence of FAP-α, thereby exposing CAFs to chemotherapeutic drugs [106]. In this example, drug release was triggered through the cleavage of a peptide substrate against FAP-α.
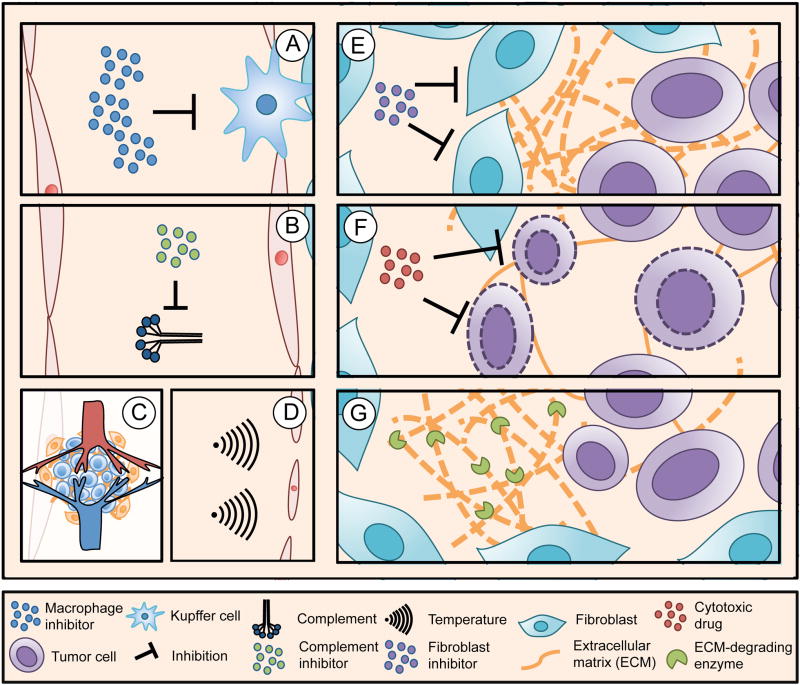
An alternative approach to address the tumor microenvironment involves pretreatment with small molecule cytotoxic agents followed by injection of therapeutic nanoparticles. The idea behind this strategy is to expand the interstitial space by inducing apoptosis in a subset of cancer cells. Specifically, it was shown that tumor-priming with paclitaxel increased the dispersion of nanoparticles in the tumor microenvironment [107]. Although restructuring of the tumor microenvironment presents an effective way to modulate intratumoral drug transport, this approach is likely to have direct effects on tumor progression, due to the proximity of cancer cells to ECM proteins and stromal cells. In this regard, it is worth emphasizing that ECM remodeling could potentially lead to increased cancer cell migration and invasion [108]. Therefore, it is also important to evaluate the impact of priming strategies on cancer cells, a task that can be problematic owing to the complex and dynamic interactions that occur in the tumor microenvironment. A summary of priming strategies in drug delivery is shown in figure 3. These strategies include previously mentioned methods for modulating the innate immune system as well as approaches for modifying the tumor microenvironment, which have been discussed in this section.
Figure 3.
Priming strategies for drug delivery. A) Suppression of resident macrophages in the liver and spleen reduces nanoparticle uptake in these organs. B) Inhibition of the complement system may reduce nanoparticle recognition and clearance by the mononuclear phagocyte system (MPS). C) Vascular normalization agents improve nanoparticle extravasation. D) Mild hypothermia increases vascular permeability. E) Suppression of fibroblasts reduces the production of extracellular matrix (ECM) components. F) Tumor cell apoptosis expands the interstitial space, leading to improved intratumoral distribution of nanoparticles. G) Degradation of ECM components improves the diffusion of nanoparticles in the interstitium.
3.4. Strategies for overcoming membrane compartments
The final set of transport obstacles that will be discussed in this review is membrane compartments. The majority of drugs are required to cross the cellular membrane in order to exert therapeutic activity. One approach to obtain enhanced levels of nanoparticle internalization in cells is through ligand-receptor binding. For example, many tumor cells overexpress transferrin membrane receptors [109], which can be harnessed by coating nanoparticles with transferrin [110]. Moreover, a frequently used strategy in molecular targeting involves the use of surface moieties against prostate membrane antigen, which is highly expressed in prostate cancer and breast cancer [111]. However, in the context of active targeting, the binding-site-barrier poses a challenge. This term refers to a phenomenon in which targeting ligands bind too tightly to target receptors, thereby preventing widespread distribution of nanoparticles throughout the tissue [112]. Computational studies have proposed that the binding-site-barrier could be overcome by the use nanoparticles with targeting ligands that are unveiled after nanoparticles have diffused in the entire tissue [113]. For example, pH and enzymatic gradients in the tissue environment could serve as triggers for gradually unveiling targeting moieties, which would serve to increase cellular internalization of nanoparticles at different penetration depths. A practical example of this concept was illustrated by Harris et al, who devised an iron oxide-based nanoparticle with a sheddable polymeric coating [114]. Upon exposure to proteases in the tumor microenvironment, the polymeric coating was removed, revealing targeting ligands on the nanoparticle surface. In addition to the use of conjugated ligands to obtain increased intracellular uptake, nanoparticles have been designed to bind specific plasma proteins that can interact with receptors on pathological cells. For instance, one study demonstrated that DOTAP/DNA lipoplexes acquire a coating of vitronectin, which facilitates nanoparticle uptake by cancer cells that express ανβ3 integrin receptors on the cell surface [115].
Besides the cell membrane, intracellular membrane compartments also pose a challenge for successful drug delivery. One of the main mechanisms of nanoparticle entry in tumor cells is clathrin-mediated endocytosis [116]. This uptake pathway can be detrimental as it eventually delivers nanoparticles to the acidic environment of lysosomes, which contains pH-sensitive enzymes that rapidly breakdown organic components [117, 118]. In particular, such enzymes should be avoided in the case of RNA and DNA delivery, since nucleic acids are sensitive to degradation [22]. On the contrary to clathrin-mediated pathways of uptake, caveolae-dependent endocytosis typically delivers nanocarriers to the neutral pH environment of caveosomes, thereby bypassing the lysosome. A potential way to avoid lysosomal degradation is to harness caveolae-dependent endocytosis. Since albumin and folic acid are predominantly internalized through the caveolae pathway, these biomolecules can be utilized for avoiding nanoparticle deposition in lysosomes [119, 120].
A different approach to the predicament of endocytosis and lysosomes is endosomal escape. It has been shown that cationic nanoparticles facilitate breakdown of endosomal membranes, thus releasing nanocarriers into the cytoplasm [4, 121]. One possible explanation for this phenomenon is the proton sponge effect [122]. The proton sponge effect is thought to arise due to proton sequestering by amine groups, consequently causing water to enter the endosome and eventual rupture of this compartment [123]. However, evidence suggests that proton sequestering and osmotic swelling are not sufficient to cause lysosomal escape. In fact, the maximum protein absorbing capacity of polycations would result in a 2.3% expansion of the membrane area, which would not be enough to affect the integrity of the lipid bilayer [124]. Therefore, it is possible that other polycation-mediated mechanisms facilitate endosomal escape. For instance, it has been suggested that polyethylenimine chains become embedded inside the lipid membrane, where they cause destabilization of the endosome [125]. Other strategies to promote endosomal escape include the use of various membrane-penetrating peptides. For example, the cationic peptide melittin, which forms pores in the endosomal membrane [126] has been utilized for effective DNA transfection [127].
An obstacle for retaining drugs in the intracellular environment is efflux pumps such a P-glycoprotein [128], which is overexpressed in multidrug resistant cancer cells [129]. Efflux pumps efficiently expel foreign compounds from the cell interior, causing drug doses to drop below the threshold for therapeutic efficacy. In general, efflux pumps do not represent a problem for nanoparticle delivery. On the contrary, nanocarriers are frequently able to overcome multidrug resistance, since the pathways of cellular internalization differ from those used by small molecule drugs [76, 130].
4. Conclusion
In this review, we have discussed drug delivery strategies for overcoming transport obstacles. Particular emphasis has been placed on improving nanoparticle transport through addressing the MPS, the endothelium, the tumor microenvironment, and membrane compartments. Although most drug delivery approaches described to date involve the design of nanocarriers, strategies for priming the biological environment are beginning to emerge. In regards to the MPS, nanoparticle design strategies that have been utilized to enhance drug transport include pegylation and biomimetic nanoparticles. Additionally, proposed approaches for priming the biological environment involve modulating the innate immune system through e.g. agents that reduce macrophage activity. In regards to the vascular wall, nanoparticle transport can be improved by optimizing nanoparticle properties (size, shape, charge) and by utilizing targeting ligands. Furthermore, priming strategies for overcoming the endothelial barrier have focused on mild hyperthermia and vascular normalization. Moreover, the ECM is a transport obstacle that has largely been tackled with priming agents. For example, pretreatment with enzymes that degrade ECM components can improve drug and nanoparticle diffusion. Additionally, agents that remodel the interstitial space or the stromal environment can improve drug penetration. Finally, approaches for addressing membrane compartments, such as the cell membrane and the lysosomal membrane, involve the use of targeting ligands, nanoparticle design strategies that modulate the composition of the protein corona, and agents for disrupting the lysosome.
In conclusion, although targeted therapy is generally viewed from a molecular standpoint, specific cell populations can be targeted through the manipulation of drug transport. In fact, the field of transport oncophysics is driven by the idea that tumors and healthy tissue display different characteristics that affect the movement and location of atoms, molecules, macromolecules, and cells. Since healthy and pathological tissues display differences in transport properties, it is possible to design drug delivery strategies that make use of disease-specific transport phenomena. Specifically, such transport phenomena are usually expressed in the form of biological barriers. Both nanocarriers and priming strategies represent effective approaches for improving drug distribution. It is likely that a combination of strategies that target multiple transport obstacles will prove most beneficial for drug delivery.
5. Expert Opinion
An important notion in the field of drug delivery is that control over drug distribution leads to control over therapeutic efficacy. Thus, the ultimate goal in this field is to exclusively direct and confine drugs to pathological tissues. However, even with the use of nanodelivery vehicles, the bulk of the injected dose ends up in healthy tissue. Therefore, a promising approach to achieve high therapeutic efficacy with minimal side effects is to combine drugs that have disease-specific molecular targets with nanoparticles and priming strategies that achieve improved accumulation of drugs in pathological tissues.
In the context of nanoparticle design strategies, biomimetic approaches have proven especially useful in improving drug delivery. In this review, we have discussed three different approaches to biomimicry. Firstly, the geometry of particles can be modeled after endogenous blood components that have unique transport properties in the circulation. For example, the MSV mimics the size and shape of platelets, which leads to increased adhesion of this delivery system to inflamed vasculature [5]. Secondly, interactions between biological components can be harnessed for improved drug delivery. For instance, liposomes with leukocyte proteins exploit biological surface moieties on leukocyte membranes in order to reduce MPS uptake and increase accumulation in inflamed vasculature [57]. Thirdly, innate biological cascades can be taken advantage of in order to amplify nanoparticle transport. In particular, one drug delivery strategy relied on tumor-specific activation of the coagulation cascade, which was then exploited for accumulation of targeted nanoparticles [77]. Although these approaches represent important advances in biomimetic drug delivery, there is a multitude of biological phenomena that have not yet been harnessed for drug delivery purposes. It is likely that biomimicry will become an increasingly important component in the design of drug delivery vehicles.
In addition to the design of nanocarriers, priming strategies represent a promising approach for improving drug delivery. The vast majority of priming strategies used for localized delivery have focused on modulating the tumor microenvironment. Such strategies provide ways to increase the tumor accumulation of small molecule drugs, antibodies, and nanoparticles. However, an important consideration is whether priming strategies designed to temporarily change the biological environment of healthy tissues would be equally or more beneficial. Conceivably, such strategies would involve using priming agents to reduce nanoparticle interactions with normal cells. Less than 5% of the injected nanoparticle dose is usually found in tumors [23, 24], while up to 95% accumulates in the MPS [34]. In a hypothetical example, a priming agent has the ability to cause a 10% change in tissue accumulation. If this priming agent is directed at the tumor environment, the tumor deposition of nanoparticles would increase from 5% to 5.5%. On the contrary, if this priming agent is directed at reducing liver and spleen uptake, nanoparticle accumulation in the MPS would decrease from 95% to 85.5%. This 9.5% reduction in MPS deposition is likely to have a greater impact on nanoparticle biodistribution than the 0.5% increase in tumor accumulation. In fact, nanoparticles that are prevented from going to MPS organs are likely to end up in the tumor due to the EPR effect. In the best-case scenario, this reduction in MPS uptake would cause tumor accumulation to increase from 5% to 14.5% (5% + 9.5%). Ultimately, this argument comes down to the fact that the MPS usually has a larger mass of biological components that can be manipulated compared to the tumor. Therefore, the priming of healthy tissues has the potential to cause a greater overall change in nanoparticle biodistribution.
In addition, it may also be easier for priming agents to initially reach the liver and spleen as opposed to the tumor microenvironment, which usually has an inadequate and/or abnormal vascular network. Although localized injection of priming agents in tumors represents an efficient approach to change the tumor microenvironment, this method cannot be utilized for the treatment of metastatic disease. Notably, it is probable that the concurrent use of tumor-priming strategies with agents that modulate the innate immune system could result in superior drug delivery. However, it is worth emphasizing that priming agents directed at healthy tissue could pose a concern for normal physiological functions. Therefore, it is important that such strategies have temporary effects, which can easily be reversed in the absence of priming agents. Notably, since most nanodrugs are administered on a monthly basis, exposure to priming agents would follow a similar schedule, thereby enabling the body to recover between priming treatments. A temporary suppression of MPS function would most likely entail a weakened response to foreign pathogens. Moreover, prevention of nanoparticle accumulation in the MPS does not necessarily imply that nanoparticles would deposit in diseased tissues. It is possible that the majority of the nanoparticles would remain in circulation until the function of the MPS is restored. Alternatively, healthy organs that would normally be spared from exposure to large numbers of nanoparticles may be subjected to toxic doses. In conclusion, it will be important for future studies to determine the biodistribution of nanoparticles under conditions in which the MPS is compromised. Furthermore, it would be interesting to assess the specific contribution of macrophages to the deposition of nanoparticles in the liver and spleen, since other factors such as the presence of specialized endothelial cells and the organization of the vasculature network in these organs may play a key role in nanoparticle sequestration.
Ultimately, it is only recently that we are beginning to understand and utilize transport phenomena for treatment of disease. Since the study of molecular and macromolecular transport in the body extends far beyond the field of biology, the design of nanocarriers and priming strategies is likely to greatly benefit from interdisciplinary approaches. Specifically, the fields of physics and mathematics can be applied for tracking and optimizing the movement of drugs in the body. Taken together, the scientific community should embrace and implement the mantra of “location, location, location” for treatment of disease.
Article highlights box.
Localized drug delivery is an active area of research that aims to increase therapeutic efficacy and reduce side effects.
Lesion-specific drug delivery is hampered by biological transport barriers.
Solutions to improve drug delivery take advantage of differences between normal and pathological tissues.
Localized drug delivery has been attempted with nanocarriers designed to overcome biological barriers.
A less common strategy to improve drug delivery involves the use of priming agents that modulate the host environment in ways that favor lesion-specific drug accumulation.
Acknowledgments
Funding
This work was funded by the Houston Methodist Research Institute. Partial funds were acquired from: the Ernest Cockrell Jr. Distinguished Endowed Chair (M. Ferrari), the U.S. Department of Defense (W81XWH-09-1-0212, W81XWH-12-1-0414) (M Ferrari), the National Institutes of Health (U54CA143837, U54CA151668; M Ferrari), Nylands nation Finland (J Wolfram), Victoriastiftelsen Finland (J Wolfram), and the Cancer Prevention Research Institute of Texas (RP121071) (M Ferrari and H Shen).
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Looking back on the millennium in medicine. N Engl J Med. 2000;342(1):42–9. doi: 10.1056/NEJM200001063420108. [DOI] [PubMed] [Google Scholar]
- 2.Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;5:329–40. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentile E, Cilurzo F, Di Marzio L, Carafa M, Ventura CA, Wolfram J, et al. Liposomal chemotherapeutics. Future Oncol. 2013;9:1849–59. doi: 10.2217/fon.13.146. [DOI] [PubMed] [Google Scholar]
- 4.Molinaro R, Wolfram J, Federico C, Cilurzo F, Di Marzio L, Ventura CA, et al. Polyethylenimine and chitosan carriers for the delivery of RNA interference effectors. Expert Opin Drug Deliv. 2013;10(12):1653–68. doi: 10.1517/17425247.2013.840286. [DOI] [PubMed] [Google Scholar]
- 5•.Wolfram J, Shen H, Ferrari M. Multistage vector (MSV) therapeutics. J Control Release. 2015;219:406–15. doi: 10.1016/j.jconrel.2015.08.010. Example of a multifunctional drug delivery platform for overcoming biological barriers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, et al. Safety of Nanoparticles in Medicine. Curr Drug Targets. 2015;16(14):1671–81. doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 8.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & Translational Medicine. 2016;1(1):10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res. 2016;33(10):2373–87. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 10.Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 13.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–8. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 14.Cho HJ, Park JW, Yoon IS, Kim DD. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. Int J Nanomedicine. 2014;9:495–504. doi: 10.2147/IJN.S56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis P, Schneider J, Hann L, Balmaceda C, Barakat R, Phillips M, et al. Phase II trial of docetaxel in patients with platinum-refractory advanced ovarian cancer. J Clin Oncol. 1994;12(11):2301–8. doi: 10.1200/JCO.1994.12.11.2301. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki R, Takizawa T, Kuwata Y, Mutoh M, Ishiguro N, Utoguchi N, et al. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome. Int J Pharm. 2008;346(1–2):143–50. doi: 10.1016/j.ijpharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Chau Y, Dang NM, Tan FE, Langer R. Investigation of targeting mechanism of new dextran-peptide-methotrexate conjugates using biodistribution study in matrix-metalloproteinase-overexpressing tumor xenograft model. J Pharm Sci. 2006;95(3):542–51. doi: 10.1002/jps.20548. [DOI] [PubMed] [Google Scholar]
- 18.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Ohara Y, Oda T, Yamada K, Hashimoto S, Akashi Y, Miyamoto R, et al. Effective delivery of chemotherapeutic nanoparticles by depleting host Kupffer cells. Int J Cancer. 2012;131(10):2402–10. doi: 10.1002/ijc.27502. [DOI] [PubMed] [Google Scholar]
- 20.Kennel SJ, Woodward JD, Rondinone AJ, Wall J, Huang Y, Mirzadeh S. The fate of MAb-targeted Cd(125m)Te/ZnS nanoparticles in vivo. Nucl Med Biol. 2008;35(4):501–14. doi: 10.1016/j.nucmedbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Moghimi SM, Farhangrazi ZS. Nanomedicine and the complement paradigm. Nanomedicine. 2013;9(4):458–60. doi: 10.1016/j.nano.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Sun T, Ferrari M. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther. 2012;19(6):367–73. doi: 10.1038/cgt.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florence AT. “Targeting” nanoparticles: the constraints of physical laws and physical barriers. J Control Release. 2012;164(2):115–24. doi: 10.1016/j.jconrel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumors. Nature Reviews Materials. 2016;1:16014. [Google Scholar]
- 26.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010;28(4):181–8. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michor F, Liphardt J, Ferrari M, Widom J. What does physics have to do with cancer? Nat Rev Cancer. 2011;11(9):657–70. doi: 10.1038/nrc3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koay EJ, Ferrari M. Transport Oncophysics in silico, in vitro, and in vivo. Preface. Phys Biol. 2014;11(6):060201. doi: 10.1088/1478-3975/11/6/060201. [DOI] [PubMed] [Google Scholar]
- 29•.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015 Sep 8;33(9):941–51. doi: 10.1038/nbt.3330. Review of biological barriers and nanoparticle design strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfram J, Yang Y, Shen J, Moten A, Chen C, Shen H, et al. The nano-plasma interface: Implications of the protein corona. Colloids Surf B Biointerfaces. 2014;124:17–24. doi: 10.1016/j.colsurfb.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–81. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 32.Patel HM, Moghimi SM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system - The concept of tissue specificity. Adv Drug Deliv Rev. 1998;32(1–2):45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 33.Yona S, Gordon S. From the Reticuloendothelial to Mononuclear Phagocyte System - The Unaccounted Years. Front Immunol. 2015;6:328. doi: 10.3389/fimmu.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today. 2015;10(4):487–510. doi: 10.1016/j.nantod.2015.06.006. Review of the mononuclear phagocyte system as a major barrier for drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahon E, Salvati A, Baldelli Bombelli F, Lynch I, Dawson KA. Designing the nanoparticle-biomolecule interface for “targeting and therapeutic delivery”. J Control Release. 2012;161(2):164–74. doi: 10.1016/j.jconrel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Capriotti AL, Caracciolo G, Cavaliere C, Colapicchioni V, Piovesana S, Pozzi D, et al. Analytical Methods for Characterizing the Nanoparticle–Protein Corona. Chromatographia. 2014;77(11):755–69. [Google Scholar]
- 37.Capriotti AL, Caracciolo G, Caruso G, Cavaliere C, Pozzi D, Samperi R, et al. Analysis of plasma protein adsorption onto DC-Chol-DOPE cationic liposomes by HPLC-CHIP coupled to a Q-TOF mass spectrometer. Anal Bioanal Chem. 2010;398(7–8):2895–903. doi: 10.1007/s00216-010-4104-y. [DOI] [PubMed] [Google Scholar]
- 38.Kelly PM, Aberg C, Polo E, O’Connell A, Cookman J, Fallon J, et al. Mapping protein binding sites on the biomolecular corona of nanoparticles. Nat Nanotechnol. 2015;10(5):472–9. doi: 10.1038/nnano.2015.47. [DOI] [PubMed] [Google Scholar]
- 39.Capriotti AL, Caracciolo G, Caruso G, Foglia P, Pozzi D, Samperi R, et al. Differential analysis of “protein corona” profile adsorbed onto different nonviral gene delivery systems. Anal Biochem. 2011;419(2):180–9. doi: 10.1016/j.ab.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Capriotti AL, Caracciolo G, Caruso G, Cavaliere C, Pozzi D, Samperi R, et al. Label-free quantitative analysis for studying the interactions between nanoparticles and plasma proteins. Anal Bioanal Chem. 2013;405(2–3):635–45. doi: 10.1007/s00216-011-5691-y. [DOI] [PubMed] [Google Scholar]
- 41.O’Connell DJ, Bombelli FB, Pitek AS, Monopoli MP, Cahill DJ, Dawson KA. Characterization of the bionano interface and mapping extrinsic interactions of the corona of nanomaterials. Nanoscale. 2015;7(37):15268–76. doi: 10.1039/c5nr01970b. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Landry MP, Barone PW, Kim JH, Lin S, Ulissi ZW, et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat Nanotechnol. 2013;8(12):959–68. doi: 10.1038/nnano.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capriotti AL, Caracciolo G, Cavaliere C, Crescenzi C, Pozzi D, Lagana A. Shotgun proteomic analytical approach for studying proteins adsorbed onto liposome surface. Anal Bioanal Chem. 2011;401(4):1195–202. doi: 10.1007/s00216-011-5188-8. [DOI] [PubMed] [Google Scholar]
- 44.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PloS One. 2011;6(9):e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Piovesana S, Amenitsch H, et al. Lipid composition: a “key factor” for the rational manipulation of the liposome-protein corona by liposome design. RSC Advances. 2015;5(8):5967–75. [Google Scholar]
- 46.Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Piovesana S, La Barbera G, et al. The liposome-protein corona in mice and humans and its implications for in vivo delivery. J Mater Chem B. 2014;2(42):7419–28. doi: 10.1039/c4tb01316f. [DOI] [PubMed] [Google Scholar]
- 47.Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–81. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton A, Biganzoli L, Coleman R, Mauriac L, Hennebert P, Awada A, et al. EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx, Doxil) at a 6-week interval in patients with metastatic breast cancer. Ann Oncol. 2002;13(6):910–8. doi: 10.1093/annonc/mdf157. [DOI] [PubMed] [Google Scholar]
- 49•.Schottler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11(4):372–7. doi: 10.1038/nnano.2015.330. Study showing a new mechanism of PEG. [DOI] [PubMed] [Google Scholar]
- 50.Pozzi D, Colapicchioni V, Caracciolo G, Piovesana S, Capriotti AL, Palchetti S, et al. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale. 2014;6(5):2782–92. doi: 10.1039/c3nr05559k. [DOI] [PubMed] [Google Scholar]
- 51.Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J Control Release. 2013;172(1):38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Parr MJ, Ansell SM, Choi LS, Cullis PR. Factors influencing the retention and chemical stability of poly(ethylene glycol)-lipid conjugates incorporated into large unilamellar vesicles. Biochim Biophys Acta. 1994;1195(1):21–30. doi: 10.1016/0005-2736(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 53.Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, Cosco D, et al. PEG-dendron phospholioids as innovative biomaterials for the preparation of super stealth liposomes for anticancer therapy. J Control Release. 2015;199:106–13. doi: 10.1016/j.jconrel.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Hatakeyama H, Akita H, Harashima H. The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull. 2013;36(6):892–9. doi: 10.1248/bpb.b13-00059. [DOI] [PubMed] [Google Scholar]
- 55.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25(1):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(2):219–33. doi: 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15(9):1037–46. doi: 10.1038/nmat4644. Biomimetic study utilizing leukocyte membrane proteins for drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Husztik E, Lazar G, Parducz A. Electron microscopic study of Kupffer-cell phagocytosis blockade induced by gadolinium chloride. Br J Exp Pathol. 1980;61(6):624–30. [PMC free article] [PubMed] [Google Scholar]
- 59.Diagaradjane P, Deorukhkar A, Gelovani JG, Maru DM, Krishnan S. Gadolinium chloride augments tumor-specific imaging of targeted quantum dots in vivo. ACS Nano. 2010;4(7):4131–41. doi: 10.1021/nn901919w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu T, Choi H, Zhou R, Chen IW. RES blockade: A strategy for boosting efficiency of nanoparticle drug. Nano Today. 2015;10(1):11–21. [Google Scholar]
- 61.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–26. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164(2):138–44. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 63.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 64.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–23. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 65.Wolfram J, Suri K, Yang Y, Shen J, Celia C, Fresta M, et al. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf B Biointerfaces. 2014;114C:294–300. doi: 10.1016/j.colsurfb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hadjidemetriou M, Al-Ahmady Z, Mazza M, Collins RF, Dawson K, Kostarelos K. In Vivo Biomolecule Corona around Blood-Circulating, Clinically Used and Antibody-Targeted Lipid Bilayer Nanoscale Vesicles. ACS Nano. 2015;9(8):8142–56. doi: 10.1021/acsnano.5b03300. [DOI] [PubMed] [Google Scholar]
- 67.Lee SY, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20(49):495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 68.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27(30):5307–14. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 69.Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM, et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41(10):2312–8. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Kuwahara M, Sugimoto M, Tsuji S, Matsui H, Mizuno T, Miyata S, et al. Platelet shape changes and adhesion under high shear flow. Arterioscler Thromb Vasc Biol. 2002;22(2):329–34. doi: 10.1161/hq0202.104122. [DOI] [PubMed] [Google Scholar]
- 71.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adriani G, de Tullio MD, Ferrari M, Hussain F, Pascazio G, Liu X, et al. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials. 2012;33(22):5504–13. doi: 10.1016/j.biomaterials.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sevick EM, Jain RK. Viscous resistance to blood flow in solid tumors: effect of hematocrit on intratumor blood viscosity. Cancer Res. 1989;49(13):3513–9. [PubMed] [Google Scholar]
- 74.Godin B, Chiappini C, Srinivasan S, Alexander JF, Yokoi K, Ferrari M, et al. Discoidal Porous Silicon Particles: Fabrication and Biodistribution in Breast Cancer Bearing Mice. Adv Funct Mater. 2012;22(20):4225–35. doi: 10.1002/adfm.201200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.van de Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2012;158(1):148–55. doi: 10.1016/j.jconrel.2011.10.021. Study using platelet-like particles for drug delivery to tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34(4):414–18. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.von Maltzahn G, Park JH, Lin KY, Singh N, Schwoppe C, Mesters R, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10(7):545–52. doi: 10.1038/nmat3049. Priming strategy involving the induction of the coagulation cascade in tumor tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137–43. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 79.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60(8):876–85. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 80.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7(8):1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 81.Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS, et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother Pharmacol. 2015;76(4):699–712. doi: 10.1007/s00280-015-2833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirui DK, Celia C, Molinaro R, Bansal SS, Cosco D, Fresta M, et al. Mild hyperthermia enhances transport of liposomal gemcitabine and improves in vivo therapeutic response. Adv Healthc Mater. 2015;4(7):1092–103. doi: 10.1002/adhm.201400738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li L, ten Hagen TL, Bolkestein M, Gasselhuber A, Yatvin J, van Rhoon GC, et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release. 2013;167(2):130–7. doi: 10.1016/j.jconrel.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 84.Jiang W, Huang Y, An Y, Kim BY. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano. 2015;9(9):8689–96. doi: 10.1021/acsnano.5b02028. [DOI] [PubMed] [Google Scholar]
- 85.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59(16):4129–35. [PubMed] [Google Scholar]
- 86.Cham KK, Baker JH, Takhar KS, Flexman JA, Wong MQ, Owen DA, et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer. 2010;103(1):52–60. doi: 10.1038/sj.bjc.6605727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luan X, Guan YY, Lovell JF, Zhao M, Lu Q, Liu YR, et al. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials. 2016;95:60–73. doi: 10.1016/j.biomaterials.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Huang Y, Stylianopoulos T, Duda DG, Fukumura D, Jain RK. Benefits of vascular normalization are dose and time dependent--letter. Cancer Res. 2013;73(23):7144–6. doi: 10.1158/0008-5472.CAN-13-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Nishihara H, Kano MR. Pericyte-coverage of human tumor vasculature and nanoparticle permeability. Biol Pharm Bull. 2012;35(5):761–6. doi: 10.1248/bpb.35.761. [DOI] [PubMed] [Google Scholar]
- 91.Meng H, Zhao Y, Dong J, Xue M, Lin YS, Ji Z, et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7(11):10048–65. doi: 10.1021/nn404083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yokoi K, Tanei T, Godin B, van de Ven AL, Hanibuchi M, Matsunoki A, et al. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Lett. 2014;345(1):48–55. doi: 10.1016/j.canlet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54(13):3352–6. [PubMed] [Google Scholar]
- 94.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eikenes L, Tufto I, Schnell EA, Bjorkoy A, De Lange Davies C. Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer Res. 2010;30(2):359–68. [PubMed] [Google Scholar]
- 96.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62(1):112–20. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2016;22(12):2848–54. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98•.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. doi: 10.1016/j.cell.2014.08.007. Study showing that stromal restructuring improves drug delivery to tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, et al. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2012;109(41):16618–23. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–14. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci U S A. 2013;110(46):18632–7. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, et al. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur J Cancer. 2011;47(13):1955–61. doi: 10.1016/j.ejca.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilop S, von Hobe S, Crysandt M, Esser A, Osieka R, Jost E. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135(10):1429–35. doi: 10.1007/s00432-009-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakai Y, Isayama H, Ijichi H, Sasaki T, Takahara N, Ito Y, et al. A multicenter phase II trial of gemcitabine and candesartan combination therapy in patients with advanced pancreatic cancer: GECA2. Invest New Drugs. 2013;31(5):1294–9. doi: 10.1007/s10637-013-9972-5. [DOI] [PubMed] [Google Scholar]
- 105.Ji T, Ding Y, Zhao Y, Wang J, Qin H, Liu X, et al. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv Mater. 2015;27(11):1865–73. doi: 10.1002/adma.201404715. [DOI] [PubMed] [Google Scholar]
- 106.Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew Chem Int Ed Engl. 2016;55(3):1050–5. doi: 10.1002/anie.201506262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther. 2007;322(1):80–8. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 108.Kovar JL, Johnson MA, Volcheck WM, Chen J, Simpson MA. Hyaluronidase expression induces prostate tumor metastasis in an orthotopic mouse model. Am J Pathol. 2006;169(4):1415–26. doi: 10.2353/ajpath.2006.060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, et al. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820(3):291–317. doi: 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 112.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–63. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 113.Hauert S, Berman S, Nagpal R, Bhatia SN. A computational framework for identifying design guidelines to increase the penetration of targeted nanoparticles into tumors. Nano Today. 2013;8(6):566–76. doi: 10.1016/j.nantod.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harris TJ, von Maltzahn G, Lord ME, Park JH, Agrawal A, Min DH, et al. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4(9):1307–12. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Caracciolo G, Cardarelli F, Pozzi D, Salomone F, Maccari G, Bardi G, et al. Selective targeting capability acquired with a protein corona adsorbed on the surface of 1,2-dioleoyl-3-trimethylammonium propane/DNA nanoparticles. ACS Appl Mater Interfaces. 2013;5(24):13171–9. doi: 10.1021/am404171h. [DOI] [PubMed] [Google Scholar]
- 116.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weissmann G. Lysosome. N Engl J Med. 1965 Nov 11;273(20) doi: 10.1056/NEJM196511112732006. 1084-90 contd. [DOI] [PubMed] [Google Scholar]
- 118.Sun-Wada GH, Wada Y, Futai M. Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct. 2003;28(5):455–63. doi: 10.1247/csf.28.455. [DOI] [PubMed] [Google Scholar]
- 119.Botos E, Klumperman J, Oorschot V, Igyarto B, Magyar A, Olah M, et al. Caveolin-1 is transported to multi-vesicular bodies after albumin-induced endocytosis of caveolae in HepG2 cells. J Cell Mol Med. 2008;12(5A):1632–9. doi: 10.1111/j.1582-4934.2007.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gabrielson NP, Pack DW. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J Control Release. 2009;136(1):54–61. doi: 10.1016/j.jconrel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Cheng Q, Jiang Q, Huang Y, Liu H, Zhao Y, et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J Control Release. 2014;176:104–14. doi: 10.1016/j.jconrel.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 122.Behr J. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia. 1997;51:34–36. [Google Scholar]
- 123.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40(1):233–45. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 124.Won YY, Sharma R, Konieczny SF. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. J Control Release. 2009;139(2):88–93. doi: 10.1016/j.jconrel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yue Y, Jin F, Deng R, Cai J, Chen Y, Lin MC, et al. Revisit complexation between DNA and polyethylenimine - Effect of uncomplexed chains free in the solution mixture on gene transfection. J Control Release. 2011;155(1):67–76. doi: 10.1016/j.jconrel.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 126.Allende D, Simon SA, McIntosh TJ. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys J. 2005;88(3):1828–37. doi: 10.1529/biophysj.104.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ogris M, Carlisle RC, Bettinger T, Seymour LW. Melittin enables efficient vesicular escape and enhanced nuclear access of nonviral gene delivery vectors. J Biol Chem. 2001;276(50):47550–5. doi: 10.1074/jbc.M108331200. [DOI] [PubMed] [Google Scholar]
- 128.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10(3):218–27. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mamot C, Drummond DC, Hong K, Kirpotin DB, Park JW. Liposome-based approaches to overcome anticancer drug resistance. Drug Resist Updat. 2003;6(5):271–9. doi: 10.1016/s1368-7646(03)00082-7. [DOI] [PubMed] [Google Scholar]