Abstract
Atherosclerotic vascular disease results from an imbalance of inflammatory and vascular cell accumulation and removal in the neointimal space. When pathways that promote cell recruitment, survival and proliferation are favored over to those that activate cell death, egress and clearance, the plaque expands. In contrast, programmed cell death and the efficient clearance of apoptotic bodies by efferocytosis reduces lesion cellularity and promotes a reparative environment and lesion stability. However, should these carefully balanced pathways become disturbed, lesions can accumulate cell debris, damaged-associated molecular patterns and arrested macrophages, all contributing to the pro-inflammatory environment and lesion instability. Here, we will review the latest understanding of how cell death in the vessel wall directly coordinates the development of atherosclerosis, and what molecular signals are orchestrating these pathways. We will discuss the necessity of cell death, and the ways in which the execution of different forms of cell death can direct different outcomes in the plaque, and how promoting the effective clearance of dead cells from the lesion is looking like a promising therapeutic path forward.
In the beginning
In the setting of excess cholesterol and fat, inflammatory pathways of the innate immune system are aberrantly activated. It is widely believed that oxidative modifications of the phospholipid and/or protein moieties of low- and very-low density lipoproteins (LDL and VLDL) trigger conserved non-specific scavenging pathways in macrophages and dendritic cells. This initiates what is at first considered a protective pathway to remove unwanted and potentially cytotoxic remnants and debris. However, during the decades of life wherein circulating LDL and VLDL cholesterol are in excess, this protective response turns into a chronic state of inflammation that recruits additional inflammatory cells and activates vascular cells to promote lesion expansion.
During the progression of atherosclerosis, like in many other diseases, there is a constant turnover of cells within and surrounding the plaque. The identification in recent years of the key factors that mediate cell recruitment, proliferation, death and migration out of the plaque has led to an expansion in the understanding of how vascular lesions develop1. With this has come an appreciation of the importance of cell death in maintaining a healthy neointimal space. Indeed, early histopathological studies in humans and mice showed that macrophages, smooth muscle cells (SMC) and endothelial cells all undergo apoptosis, though whether this was a protective or disease-causing mechanism was debated2. In the past decade, direct evidence and mechanistic insight into how cell death contributes to atherosclerosis at all stages has been better elucidated and we will focus on recent evidence connecting the drivers of apoptosis, necrosis and efferocytosis to the progression and advancement of atherosclerotic disease.
The Good
Apoptosis in Early Lesions
Early work by Liu et al3 was the first to show that macrophage apoptosis is necessary to reduce macrophage burden within the developing lesion in Ldlr−/− mice. While initially apoptosis was thought to directly contribute to necrotic core expansion and thus reducing apoptosis might lead to reduced atherosclerosis, reconstituting the bone marrow compartment of Ldlr−/− mice with hematopoietic cells lacking the pro-apoptotic factor Bax led to accelerated lesion development. Similarly, the anti-apoptotic protein AIM promotes macrophage survival and when deleted, accelerates macrophage apoptosis and reduces lesion size in AIM−/−Ldlr−/− mice4. Together this evidence confirmed that lesional macrophage apoptosis is necessary, at least initially, to reduce the pool of macrophages within the expanding lesions.
We now have a better understanding of the signals that promote apoptosis in early atherosclerotic lesions and whose loss may be driving inflammatory lesion development. In response to cholesterol accumulation and ER stress, macrophages activate the unfolded protein response and undergo apoptosis5–7. Within ER-stressed macrophages, the activation of the NFkB signaling cascade by IKKα can promote a pro-survival pathway, and deletion of IKKα in macrophages in Ldlr−/− mice leads to increased early lesional apoptosis and reduced overall atherosclerotic lesion burden8. In contrast, the pro-apoptotic factors Jnk1 and Jnk2 are activated by multiple stress pathways, and their activity is balanced by the pro-survival factors PI3K and Akt9. In mice, deletion of Jnk1 in hematopoietic cells increased lesion area in Ldlr−/− mice, whereas deletion of Jnk2 had no impact on lesion size10. In macrophages, Jnk1 but not Jnk2 controls the activity of Akt, so in the absence of Jnk1 there is enhanced pro-survival Akt signaling, leading to macrophage expansion within the lesion. Together, these studies suggest that in macrophages there is a careful balance between inflammatory and pro-survival pathways that, when activated in favor of pro-apoptotic pathways, contribute directly to macrophage accumulation and inflammation in the vessel wall.
Efferocytosis: Apoptosis’ partner in crime
In healthy tissues, apoptotic cells are cleared rapidly by local phagocytes in a process known as efferocytosis. In fact, apoptotic bodies are cleared so rapidly in vivo that they can be difficult to detect using traditional histological methods. During the nascent stages of atherosclerosis, efferocytosis of apoptotic foam cells is mediated largely by the receptor MerTK11 and mutations or deletion of MerTK leads to a dramatic increase in apoptotic cell accumulation within lesions and an increase in lesion size and necrotic core12, 13. These studies positioned MerTK as a critical for the protection from atherosclerosis via efficient clearance of apoptotic bodies. Recently, additional mechanisms for efferocytosis control have been uncovered using both human and mouse genetics. ADAM17 is a metalloproteinase that was identified as a candidate atherosclerotic gene by quantitative trait mapping in mice, and has been found to be elevated in human plaques that had undergone plaque rupture14. MerTK has been shown to be proteolytically cleaved by ADAM1715 and cleavage of MerTK promotes necrotic core expansion and increased atherosclerotic lesion size, presumably due to its inability to effectively clear apoptotic bodies16. Therefore, it was assumed that elevated levels of ADAM17 in the plaque would result in accumulation of apoptotic debris and expansion of atherosclerotic lesions. However, recently Nicolaou et al showed that in Ldlr−/− mice with a hypomorphic mutation that lowers ADAM17 expression, ADAM17-deficient cells had reduced apoptosis and atherosclerotic lesions from these mice had increased numbers of both macrophages and SMCs17. This was attributed to the increased signaling through TNF receptor 2 (TNFR2) and membrane-bound TNFα which promoted cell survival and pro-inflammatory signalling. Although in this study the function of ADAM17 deficiency on efferocytosis and MerTK cleavage was not assessed, clearly the multifactorial role for ADAM17 in atherosclerosis in mice and humans warrants further investigation.
Large genome-wide association studies have revealed novel genomic regions that associate strongly with the risk of coronary artery disease, yet for which no known mechanism exists. For instance, the T-cell immunoglobulin and mucin domain (Tim) proteins have genetic associations with serum triglyceride levels and risk for coronary artery disease18. Because they are expressed by immune cells and have been shown to mediate apoptotic cell clearance19, Foks et al hypothesized that Tim proteins could play a role in the pathogenesis of atherosclerosis. Treatment of Ldlr−/− mice with blocking antibodies directed against Tim-1 or Tim-4 significantly enhanced atherosclerosis compared to control IgG treated animals, largely by preventing the clearance of CD4+ T-cells and apoptotic macrophages20. Another strong genetic risk locus for CAD is found on chromosome 9p21, which contains large stretches of non-coding and coding regions, some of which have been found to mechanistically contribute to atherosclerosis21. Kojima et al found that this locus regulates the expression of the pro-efferocytic molecule calreticulin, and impaired expression of calreticulin reduces efferocytosis and accelerates atherosclerosis22. This prompted the group to further investigate mechanisms of impaired efferocytosis in the vessel wall, and found that the ‘don’t-eat-me’ signal CD47 was paradoxically upregulated in SMC foam cells, and blocking CD47 by a number of approached resolved atherosclerosis via improving efferocytosis capacity23. Together, these studies using human genetics uncovered important and previously unrecognized mechanisms that support the concept that impaired efferocytosis is a key driver of atherosclerotic plaque development.
The Bad
Apoptosis in late lesions
The understanding that apoptosis is necessary to combat early lesion expansion led to the question whether apoptosis had a similar function at later stages of atherosclerosis. Cell-specific and temporal control of cell death can be achieved using exogenous overexpression of the diphtheria toxin receptor (which is not normally active in mice) in specific cell types to induce rapid apoptosis in response to administration of diphtheria toxin. Using this model, apoptosis of macrophages and monocytes (i.e. expressing DTR in CD11b+ cells) was induced in Apoe−/− mice either during initial lesion development (during 12 weeks of high-fat diet feeding) or after lesions were established (after 22 weeks of high-fat diet feeding)24. As expected, induction of apoptosis in macrophages during atherogenesis reduced lesion and necrotic core size. Unexpectedly, there was no impact of late apoptosis of macrophages on lesion characteristics, including necrotic core formation and total macrophage content. Using another strategy, apoptosis was inhibited in macrophages by the macrophage-specific over-expression of Bcl-2, which similarly exacerbated lesion size in early lesions (i.e. 5 weeks) but reduced lesion size at later stages and protected against the development of advanced plaques25. These two similar yet complementary approaches established that apoptosis induction at different stages of lesion development could have dramatically different outcomes.
As these pathways continue to be explored, new mechanisms governing macrophage function during stages of development are being revealed. microRNA-155 has been shown in many disease states to act as a pro-inflammatory cue by fine-tuning the expression of negative regulators of inflammation26. In the atherosclerotic plaque, however, blocking miR-155 using either genetic deletion or anti-sense oligonucleotides led to reduced lesion sizes in some instances27, 28 whereas in others it exacerbated lesion development29. The reason behind these divergent findings was revealed in a recent study in miR-155−/− Apoe−/− mice after 12 and 24 weeks of high-fat feeding30. In early lesions, miR-155 serves to limit macrophage proliferation via targeting the M-CSF receptor Csfr1, which is preferentially active during early stages of plaque development31. In contrast, in advanced atherosclerotic plaques, miR-155 dampens the expression of Bcl6, which is highly expressed in late but not early atherosclerotic lesions. Bcl6 acts as an inhibitor of RhoA, which blocks efferocytosis32 thus when miR-155 levels are reduced as seen in miR-155−/−Apoe−/− mice, Bcl6 expression is elevated and efferocytosis is enhanced, reducing lesion burden overall. These data offer new insights into the stage-specific macrophage signaling pathways that are at play during early and late lesion formation, and provide clues that microRNAs and their target-to-miRNA ratio may be a under-appreciated factor in disease progression.
Defective efferocytosis
Despite the accumulation of free cholesterol and the detectable activation of the unfolded protein response in macrophages in early lesions, efferocytosis occurs so rapidly that apoptotic cells are difficult to find6. However, as lesions progress the accumulation of apoptotic cells becomes apparent in the advanced plaque33. The hypothesis that defective efferocytosis contributed to this was first confirmed by Schrijvers et al34 who found direct evidence of impaired phagocytosis of apoptotic cells in human and rabbit arteries. Subsequently, this hypothesis was solidified by various studies demonstrating that rendering efferocytosis defective can directly contribute to advanced lesion development12, 13, 16.
But what are the mechanisms by which efferocytosis can become defective? One mechanism recently identified involves the resolvin family of lipid mediators. During the inflammatory response, fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are processed by the lipoxygenase enzymes 5-LOX and 12/15-LOX, respectively, to generate the resolvins E and D (RvE and RvD). Resolvins serve to limit inflammation via blocking the production of cytokines and limiting neutrophil infiltration35 but importantly, also promote efferocytosis during the resolution of inflammation36. To test the contribution of resolvins to the development of atherosclerosis, Hasturk et al administered RvE1 to rabbits while simultaneously feeding a high-cholesterol diet, and found a significant attenuation of plaque formation, inflammation and necrotic core area in RvE1-treated animals37. Although efferocytosis was not directly evaluated in this study, the premise that resolvins could serve to limit lesion progression was established. Indeed, a defect in resolvin synthesis was found in advanced human plaques, and replacement of RvD1 in established lesions in mice reduced necrotic core size by enhancing lesional efferocytosis38. Other pro-resolving mediators RvD2 and Maresin-1 prevented atheroprogression in mice when delivered in conjuntion with a high-fat diet, restoring these mediators to levels seen in stable plaques39. These studies provide compelling evidence that defective efferocytosis is a consequence of defective production of pro-resolving lipid mediators, and can be used as an intervention in established atherosclerosis.
Additional pathways may also underlie defective efferocytosis in the advanced atherosclerotic plaque. For example, Annexin A1 is a ligand that is important for the clearance of apoptotic cells 40. De Jong et al recently found that Annexin A1 expression in the vessel wall and in the circulation negatively correlated with neointima size, suggesting it may serve to protect from lesion development by enhancing efferocytosis41. In addition, the endogenous expression of miR-155 is found to be elevated during the progression of atherosclerosis in mice coincident with the downregulation of Bcl6 and defective effeoctyosis. Accordingly, when miR-155 is blocked, efferocytosis is restored. Together, these mechanisms offer multiple possible ways by which efferocytosis becomes defective as lesions progress, and further studies are needed to understand how these factors become dysregulated in the plaque.
The Ugly
Pyroptosis & Necroptosis
In addition to apoptosis, other emerging forms of programmed cell death have been found to be active in the vessel wall and in some cases contribute to the progression of atherosclerosis. Among the most well-defined are pyroptosis and necroptosis. Pyroptosis is a caspase-dependent form of lytic cell death that shares features with both apoptosis and necrosis. When the inflammasome is triggered via innate immune toll-like and nod-like receptors (TLR and NLRs), this induces the aggregation and activation of the multi-protein inflammasome complex which, in atherosclerotic macrophages, includes NLRP3, ASC and caspase-1. Caspase-1 cleaves pro-IL-1β into its mature form for secretion, but in some cases can form a pore in the plasma membrane allowing release of cellular contents and cell death42. Whether caspase-1 induces inflammation or cell death depends critically on the levels of caspase-1 in the cell43. In atherosclerotic plaques that have undergone rupture, dead or dying macrophages have greater caspase-1 expression than what is seen in stable plaques44. Recently, Emrich et al found that SMC apoptosis and caspase-1 activity were elevated in aortic aneurysm disease in a mouse model of Marfan’s syndrome, and treatment of these mice with an inhibitor of caspases reduced SMC apoptosis and overall disease burden45. Similarly, excess circulating cholesterol induces the activation of caspase-1 in the endothelium of Apoe−/− mice, and in vitro treatment of ECs with oxidized lipids induces pyroptosis46. Endothelial activation, inflammation and early atherosclerosis in Apoe−/− is subsequently inhibited by the deletion of caspase-1, which confirms earlier reports that caspase-1 plays a key role in vascular disease progression47–49. In human macrophages, p38δ-MAPK is necessary for the activation of caspase-1 via the NLRP3 inflammasome and is upregulated in advanced but not early human atherosclerotic plaques50. Although many of these studies do not dissect the precise contribution of the pro-death versus pro-inflammatory roles for caspase-1, they underscore the potential importance of caspase-1 regulation as a key determining factor in the inflammation that drives atherosclerosis.
Programmed necrosis, or necroptosis, is a more recently defined form of programmed cell death. Necroptosis differs from both apoptosis and pyroptosis mainly because it is not dependent on caspase activation. When a death receptor is triggered, RIP kinase 1 (RIPK1) becomes activated together with other death effector proteins TRADD, FADD and caspase-8 to promote apoptosis51. When caspase-8 is inactive or overwhelmed, RIP1 interacts with and phosphorylates RIP kinase 3 (RIP3) to form the oligomeric necrosome. This phospho-RIP1/phospho-RIP3 complex then recruits and phosphorylates the multi-lineage kinase-like domain MLKL, which further oligmerizes and facilitates membrane rupture via pore formation and ion release. The result is a cell that shares morphological features with necrosis, and the release of ATP, mitochondria and other damage-associated molecular patterns. Until recently, it was thought that the necrosis observed in advanced atherosclerotic lesions, particularly in the large necrotic cores found in ruptured human plaques, was a consequence of secondary necrosis due to failed efferocytosis. Lin et al demonstrated that RIP3 could directly contribute to atherogenesis, and that deletion of RIP3 in either Ldlr−/− or Apoe−/− mice reduced necrotic core size52. Our group demonstrated that in the setting of established atherosclerosis in Apoe−/− mice, inhibition of the RIP1 activation of RIP3 via the small molecule Necrotstatin-1 reduced lesion size and, importantly, necrotic core formation53. We also found evidence of active necroptosis in advanced but not early coronary atherosclerotic lesions in humans, suggesting this process may be contributing to human disease. Although the activators of active necroptosis in the lesion are continuing to be established, it is conceivable that pathways that exacerbate or protect from necrosis in advanced lesions could be doing so through a necroptosis mechanisms. For example, activation of the necrosis-sensing receptor Cecl4e in atherosclerotic plaques, which has been shown to propagate atherosclerosis via the ER-stress response, could be achieved through the release of damage-associated molecular patterns from a necroptotic cell54. Interestingly, atherosclerotic ligands can activate the glycolytic pathway in macrophages via the glucose transporter PFKFB3 which potentiates their inflammatory activation. Blocking PFKFB3 accelerates the rates of apoptotic and necrotic death in macrophages, possibly due to the high glycolytic rate needed to maintain their viability55. Although RIP kinases and necroptosis were not directly evaluated in this study, it is plausible that the balance of apoptosis and necroptosis in atherogenic macrophages is carefully controlled by the energetic needs of the cell.
Emerging concepts
In more recent years, our global understanding of how atherosclerotic lesions develop and progress has changed. In particular, the origin of the macrophage foam cells found within advanced lesions has been challenged, and has now been shown by multiple approaches that many of these macrophages are in fact derived from the SMC lineage.56–59 With this new evidence, what can be said about the role of cell death in the atherosclerotic plaque? In studies examining the mass apoptosis of SMCs using the DTR approach as described above, it was found that SMC apoptosis in established lesions induced the destruction of the fibrous plaque and induction of inflammation with the necrotic core60, which is similar to what is observed for macrophages. But what about the capacity of SMC-derived macrophages to execute efferocytosis? SMCs loaded with cholesterol acquire macrophage markers, but Vengrenyuk and colleagues showed that they are less efficient than monocyte-derived macrophages at efferocytosis in vitro61. The contribution of SMC-derived macrophage apoptosis and defective efferocytosis has not be directly investigated in vivo, but could have a profound impact on the understanding of the mehcnaisms that promote lesion vulnerability.
In addition to the ability of SMCs to transdifferentiate into macrophages, there is a clear need for the recruitment of monocytes into the intimal space and differentiate into monocyte-derived macrophages. Indeed, hypercholesterolemia induces monocytosis, which promotes excessive recruitment of pro-inflammatory Ly6Chi monocytes into the vessel wall62. However, compared to macrophages, the role for apoptosis of monocytes in the development of atherosclerosis is less well understood. A recent report revealed that in circulating pro-atherogenic monocytes, TLR signaling via the kinase Map3k8 renders monocytes resistant to apoptosis and when Map3k8 is deleted in hemotapoietic cells, atherosclerotic lesions are small due to high rates of monocyte apoptosis63. Although the endogenous regulators of monocyte apoptosis during atherosclerotic lesion formation have not been fully elucidated, this study suggests that monocyte-targeting therapies may be of therapeutic use.
Concluding remarks & outlook
The importance of cell death throughout all stages of atherosclerosis is clear, yet the type, timing and response to cell death is critical in determining the pro- or anti-atherogenic outcome. If cell death is to be targeted by novel therapeutics, consideration of other normal or pathological process that depend on cell death is essential, especially the reparative pro-angiogenic pathways that may be active following plaque rupture and myocardial infarction47. As additional concepts about the main drivers of atherosclerosis emerge, this may require re-evaluation of the factors that control apoptosis, pyroptosis and necroptosis, and importantly, how each of these drivers of cell death may be mitigated by the activity of efferocytosis. The balance and counterbalance of cell death in the inflammatory milieu within the plaque is fertile ground for the development of targeted therapies aimed at reducing advanced atherosclerotic lesion vulnerability.
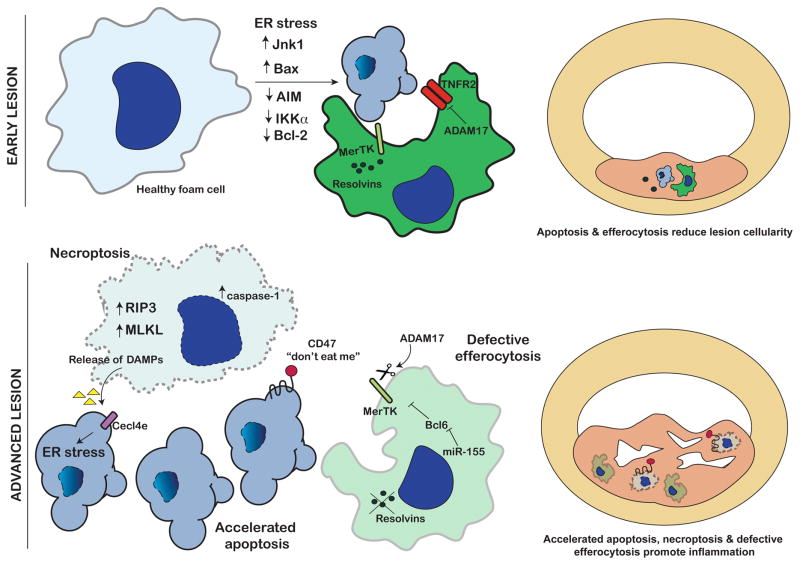
Figure 1. Cell death during early and advanced atherosclerosis.
In early atherosclerosis (top), lesion cellularity is limited via apoptosis through activation of ER stress and/or pro-apoptotic factors (i.e. Jnk1, Bax-1) or through downregulation of anti-apoptotic factors (Bcl-2, AIM, IKKα). Efficient efferocytosis via MerTK and adequate Resolvin synthesis clears apoptotic body accumulation. Other anti-inflammatory and anti-apoptotic factors (i.e. ADAM17 and TNFR2) also reduce lesion expansion. In advanced lesions (bottom), excessive apoptosis and activation of necroptosis (i.e. RIP3, MLKL) can cause release of necrotic material that may be recognized by receptors like Cecl4e and activate further apoptosis and/or inflammation. “Don’t eat me” signals presented on dying atherosclerotic cells prevent efferocytosis clearance. Defective efferocytosis via cleavage of MerTK, reduced Resolvin synthesis and/or miR-155-dependent inhibition of Bcl6 also prevent clearance of apoptotic and necrotic cells. Cell debris accumulates and necrotic core expands in advanced atherosclerosis.
Acknowledgments
Sources of funding
KJR is supported by the Canadian Institutes for Health Research (MOP130365) and the National Institutes of Health (R01HL119047).
References
- 1.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–71. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovascular research. 2000;45:736–46. doi: 10.1016/s0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–9. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–13. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–72. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–21. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 7.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 8.Babaev VR, Ding L, Zhang Y, May JM, Lin PC, Fazio S, Linton MF. Macrophage IKKalpha Deficiency Suppresses Akt Phosphorylation, Reduces Cell Survival, and Decreases Early Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:598–607. doi: 10.1161/ATVBAHA.115.306931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 10.Babaev VR, Yeung M, Erbay E, Ding L, Zhang Y, May JM, Fazio S, Hotamisligil GS, Linton MF. Jnk1 Deficiency in Hematopoietic Cells Suppresses Macrophage Apoptosis and Increases Atherosclerosis in Low-Density Lipoprotein Receptor Null Mice. Arterioscler Thromb Vasc Biol. 2016;36:1122–31. doi: 10.1161/ATVBAHA.116.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–17. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 12.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–31. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 13.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–8. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh M, Ishikawa Y, Itoh T, Minami Y, Takahashi Y, Nakamura M. The expression of TNF-alpha converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur J Clin Invest. 2008;38:97–105. doi: 10.1111/j.1365-2362.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 15.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK) J Biol Chem. 2011;286:33335–44. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, Tabas I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. The Journal of clinical investigation. 2017;127:564–568. doi: 10.1172/JCI90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolaou A, Zhao Z, Northoff BH, Sass K, Herbst A, Kohlmaier A, Chalaris A, Wolfrum C, Weber C, Steffens S, Rose-John S, Teupser D, Holdt LM. Adam17 Deficiency Promotes Atherosclerosis by Enhanced TNFR2 Signaling in Mice. Arterioscler Thromb Vasc Biol. 2017;37:247–257. doi: 10.1161/ATVBAHA.116.308682. [DOI] [PubMed] [Google Scholar]
- 18.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T, Lin SY, Lindstrom J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Muller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancakova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrieres J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Jarvelin MR, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, Marz W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–52. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–89. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foks AC, Engelbertsen D, Kuperwaser F, Alberts-Grill N, Gonen A, Witztum JL, Lederer J, Jarolim P, DeKruyff RH, Freeman GJ, Lichtman AH. Blockade of Tim-1 and Tim-4 Enhances Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2016;36:456–65. doi: 10.1161/ATVBAHA.115.306860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CL, Pjanic M, Quertermous T. From Locus Association to Mechanism of Gene Causality: The Devil Is in the Details. Arterioscler Thromb Vasc Biol. 2015;35:2079–80. doi: 10.1161/ATVBAHA.115.306366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. The Journal of clinical investigation. 2014;124:1083–97. doi: 10.1172/JCI70391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–93. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–75. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 27.Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:759–67. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. The Journal of clinical investigation. 2012;122:4190–202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donners MM, Wolfs IM, Stoger LJ, van der Vorst EP, Pottgens CC, Heymans S, Schroen B, Gijbels MJ, de Winther MP. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Zhu M, Corbalan-Campos J, Heyll K, Weber C, Schober A. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:796–803. doi: 10.1161/ATVBAHA.114.304723. [DOI] [PubMed] [Google Scholar]
- 31.Di Gregoli K, Johnson JL. Role of colony-stimulating factors in atherosclerosis. Curr Opin Lipidol. 2012;23:412–21. doi: 10.1097/MOL.0b013e328357ca6e. [DOI] [PubMed] [Google Scholar]
- 32.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–80. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolodgie FD, Petrov A, Virmani R, Narula N, Verjans JW, Weber DK, Hartung D, Steinmetz N, Vanderheyden JL, Vannan MA, Gold HK, Reutelingsperger CP, Hofstra L, Narula J. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108:3134–9. doi: 10.1161/01.CIR.0000105761.00573.50. [DOI] [PubMed] [Google Scholar]
- 34.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 35.Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 2011;437:185–97. doi: 10.1042/BJ20110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–27. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–33. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res. 2016;119:1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- 40.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–98. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 41.de Jong RJ, Paulin N, Lemnitzer P, Viola JR, Winter C, Ferraro B, Grommes J, Weber C, Reutelingsperger C, Drechsler M, Soehnlein O. Protective Aptitude of Annexin A1 in Arterial Neointima Formation in Atherosclerosis-Prone Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:312–315. doi: 10.1161/ATVBAHA.116.308744. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y, Gardner SE, Clarke MC. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:2781–6. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- 43.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolodgie FD, Narula J, Burke AP, Haider N, Farb A, Hui-Liang Y, Smialek J, Virmani R. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000;157:1259–68. doi: 10.1016/S0002-9440(10)64641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emrich FC, Okamura H, Dalal AR, Penov K, Merk DR, Raaz U, Hennigs JK, Chin JT, Miller MO, Pedroza AJ, Craig JK, Koyano TK, Blankenberg FG, Connolly AJ, Mohr FW, Alvira CM, Rabinovitch M, Fischbein MP. Enhanced caspase activity contributes to aortic wall remodeling and early aneurysm development in a murine model of Marfan syndrome. Arterioscler Thromb Vasc Biol. 2015;35:146–54. doi: 10.1161/ATVBAHA.114.304364. [DOI] [PubMed] [Google Scholar]
- 46.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, Tilley DG, Monroy MA, Choi ET, Thomas CJ, Jiang X, Wang H, Yang XF. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–16. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1805–14. doi: 10.1161/ATVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]
- 48.Gage J, Hasu M, Thabet M, Whitman SC. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol. 2012;28:222–9. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2012;425:162–8. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 50.Rajamaki K, Mayranpaa MI, Risco A, Tuimala J, Nurmi K, Cuenda A, Eklund KK, Oorni K, Kovanen PT. p38delta MAPK: A Novel Regulator of NLRP3 Inflammasome Activation With Increased Expression in Coronary Atherogenesis. Arterioscler Thromb Vasc Biol. 2016;36:1937–46. doi: 10.1161/ATVBAHA.115.307312. [DOI] [PubMed] [Google Scholar]
- 51.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nature immunology. 2015;16:689–97. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 52.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–10. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, Hedin U, Sad S, Guo L, Kolodgie FD, Virmani R, Ruddy T, Rayner KJ. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016;2:e1600224. doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clement M, Basatemur G, Masters L, Baker L, Bruneval P, Iwawaki T, Kneilling M, Yamasaki S, Goodall J, Mallat Z. Necrotic Cell Sensor Clec4e Promotes a Proatherogenic Macrophage Phenotype Through Activation of the Unfolded Protein Response. Circulation. 2016;134:1039–1051. doi: 10.1161/CIRCULATIONAHA.116.022668. [DOI] [PubMed] [Google Scholar]
- 55.Tawakol A, Singh P, Mojena M, Pimentel-Santillana M, Emami H, MacNabb M, Rudd JH, Narula J, Enriquez JA, Traves PG, Fernandez-Velasco M, Bartrons R, Martin-Sanz P, Fayad ZA, Tejedor A, Bosca L. HIF-1alpha and PFKFB3 Mediate a Tight Relationship Between Proinflammatory Activation and Anerobic Metabolism in Atherosclerotic Macrophages. Arterioscler Thromb Vasc Biol. 2015;35:1463–71. doi: 10.1161/ATVBAHA.115.305551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–9. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 57.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–7. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 58.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nature medicine. 2015;21:628–37. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA. 2003;100:13531–6. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nature medicine. 2006;12:1075–80. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 61.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–46. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of clinical investigation. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanz-Garcia C, Sanchez A, Contreras-Jurado C, Cales C, Barranquero C, Munoz M, Merino R, Escudero P, Sanz MJ, Osada J, Aranda A, Alemany S. Map3k8 Modulates Monocyte State and Atherogenesis in ApoE−/− Mice. Arterioscler Thromb Vasc Biol. 2017;37:237–246. doi: 10.1161/ATVBAHA.116.308528. [DOI] [PubMed] [Google Scholar]