ABSTRACT
Cell fusion is essential for sexual reproduction and formation of muscles, bones, and placenta. Two families of cell fusion proteins (Syncytins and FFs) have been identified in eukaryotes. Syncytins have been shown to form the giant syncytial trophoblasts in the placenta. The FFs are essential to fuse cells in the skin, reproductive, excretory, digestive and nervous systems in nematodes. EFF-1 (Epithelial Fusion Failure 1), a member of the FF family, is a type I membrane glycoprotein that is essential for most cell fusions in C. elegans. The crystal structure of EFF-1 ectodomain reveals striking structural similarity to class II fusion glycoproteins from enveloped viruses (e.g. dengue and rubella) that mediate virus to cell fusion. We found EFF-1 to be present on the plasma membrane and in RAB-5-positive early endosomes, with EFF-1 recycling between these 2 cell compartments. Only when EFF-1 proteins transiently arrive to the surfaces of 2 adjacent cells do they dynamically interact in trans and mediate membrane fusion. EFF-1 is continuously internalized by receptor-mediated endocytosis via the activity of 2 small GTPases: RAB-5 and Dynamin. Here we propose a model that explains how EFF-1 endocytosis together with interactions in trans can control cell-cell fusion. Kontani et al. showed that vacuolar ATPase (vATPase) mutations result in EFF-1-dependent hyperfusion.1 We propose that vATPase is required for normal degradation of EFF-1. Failure to degrade EFF-1 results in delayed hyperfusion and mislocalization to organelles that appear to be recycling endosomes. EFF-1 is also required to fuse neurons as part of the repair mechanism following injury and to prune dendrites. We speculate that EFF-1 may regulate neuronal tree like structures via endocytosis. Thus, endocytosis of cell-cell fusion proteins functions to prevent merging of cells and to sculpt organs and neurons.
Keywords: AFF-1; C. elegans, cell-cell fusion, Dynamin, EFF-1, endocytosis, RAB-5, vacuolar ATPase
Cell fusion is crucial for sexual reproduction and organ formation throughout development. In mammals, cell-cell fusion is essential for fertilization, placentation, myogenesis, osteogenesis, and inflammation. C. elegans provides one of the best models to study fusion regulation due to extensive cell fusion events during embryogenesis. EFF-1, the first identified eukaryotic fusogen, mediates homotypic fusion of epithelial cells leading to syncytia formation in C. elegans.2-5 Despite the identification and characterization of various transcriptional factors that regulate the expression of EFF-1, the mechanisms of posttranscriptional control of the EFF-1 fusogen are poorly understood.6
We recently studied how EFF-1 intracellular localization is regulated during embryonic syncytia formation in C. elegans.7 Epithelial fusion begins before the embryo starts to elongate and continues throughout embryogenesis and larval development. In the embryo, 23 epidermal cells fuse in an invariant manner within several hours, which makes this system attractive for studying the cell fusion process.8 We recently developed the appropriate tools to follow EFF-1 localization and dynamics. Antibodies against the extracellular domain of EFF-1 were produced and were screened for their specificity.9 For live imaging experiments, we generated a C. elegans transgenic strain in which a mutation in eff-1 was rescued by a fosmid carrying wild-type EFF-1 tagged with GFP.7 Analysis of EFF-1 localization before and during cell fusion showed that EFF-1 mainly localized to intracellular puncta (Fig. 1 A, B, arrowheads, C), and was only transiently transported to the cell surface when cells were ready to fuse.7 EFF-1 showed high degree of colocalization with an early endosome marker RAB-5, but significantly less so with other markers of intracellular organelles (Fig. 2E). EFF-1 was also detected in a low percentage in organelles of the secretory (Fig. 2A) and endocytic (recycling and late endosomes, and lysosomes) pathways. EFF-1 accumulated on the cell membrane after knockdown of RAB-5 and dynamin. Further, these knockdown phenotypes led to increased ectopic fusions. Based on these results, we proposed a model where Dynamin/Rab-5 mediated endocytosis serves as a negative regulator of cell fusion (Fig. 2).7
Figure 1.

EFF-1 localizes to intracellular organelles in wild-type embryos. (A) Immunofluorescence using anti-EFF-1 (endogenous EFF-1, green) and anti-DLG-1 (apical junction, red) in wild-type embryo. (B) The magnification of the boxed area from (A) is showing 2 cells in the process of fusion. Endogenous EFF-1 localizes to intracellular puncta (arrowheads) and only partially on the cell membrane prior to fusion (not shown). (C) Localization of EFF-1 (green) in cells before the fusion of plasma membranes (red). (D) Anti-GFP (EFF-1*::GFP mutant, green) and anti-DLG-1 (apical junction, red) in an embryo carrying eff-1*::gfp mutant transgene.11 Scale bar, 20 μm. (E) The magnification of the boxed area from (D). EFF-1*::GFP shows plasma membrane mislocalization in the interface between 2 cells expressing EFF-1::GFP (arrow) and partial intracellular localization (arrowheads). (F) Localization of EFF-1*::GFP (green) in cells before fusion of plasma membranes (red).
Figure 2.

Model of epidermal cell fusion regulation in C. elegans. Cytoplasms and plasma membranes of 2 cells that are ready to fuse are colored in light and dark orange and blue. (A) EFF-1 monomers produced by an orange cell are orange, and EFF-1 monomers synthesized by a blue cell are blue. EFF-1 is delivered to the plasma membranes from both fusion-competent cells in a monomeric state. (B) Fusion is initiated by EFF-1 trans-oligomerization (hetero-dimers). (C) Formation of EFF-1 trans-oligomers (hetero-trimers). (D) Conformational change of EFF-1 trimers induces fusion of opposing membranes and mixing of the cytoplasms. (E) EFF-1 in a mixture of monomers and oligomers in a postfusion state is actively removed from the cell surface via receptor-mediated endocytosis in a dynamin- and RAB-5-dependent mechanism and EFF-1 accumulates in early endosomes. RAB-5 is represented by green, and dynamin in magenta.
Mutated EFF-1*::GFP accumulates at the plasma membrane
As previously described, EFF-1* (a mutated form of EFF-1) fused to GFP can be seen accumulating in the fusion-fated borders through interactions between cells expressing EFF-1 (Fig. 1D, E, arrow, F).7,10 However, our results indicate that this EFF-1*::GFP was unable to rescue eff-1 fusion failure phenotype.11 Moreover, 2 mutations (T173A and N529D) were identified in the coding region of EFF-1*::GFP possibly inactivating fusion potency and localization of the transgenic protein (Fig. 3).10 Based on the crystal structure of EFF-1 ectodomain, the T173A mutation resides in domain II, and N529D localizes to the stem region of the EFF-1 protein (Fig. 3).12 According to the model of membrane fusion proposed by Perez-Vargas et al., fusion is initiated by trans-oligomerization of EFF-1 monomers arriving from opposing plasma membranes and is followed by conformational changes of EFF-1 trimers from a prefusion to a postfusion state (Fig. 2A–D).12 We speculate that EFF-1*::GFP accumulation at the plasma membrane is caused by the docking of EFF-1*::GFP trans-oligomers in a prefusion state, probably due to the lost flexibility in the stem region (Fig. 2B, C). This hypothesis is supported by our finding that active EFF-1::GFP is equally transported to all apical plasma membrane domains, including the membranes that will not fuse.7 By contrast, EFF-1*::GFP was only observed at the interface of 2 cells that express EFF-1*::GFP (Fig. 1E, arrow). We suggest that EFF-1*::GFP protein trans-oligomers on the plasma membrane are docked in a prefusion conformation and fail to undergo efficient endocytosis (Fig. 2C). The endocytosis-defective EFF-1*::GFP then accumulates on the junctions between cells expressing the mutant proteins (Fig. 1E, F).
Figure 3.
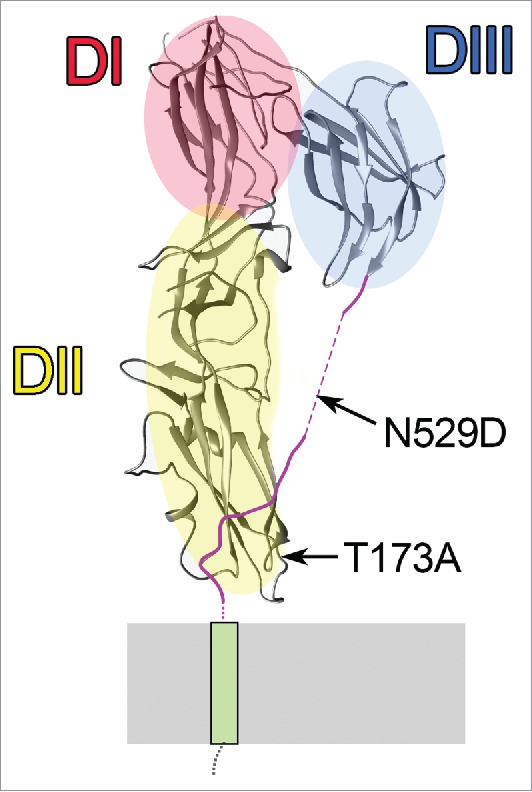
EFF-1 protomer showing the location of the 2 point mutations in EFF-1*. EFF-1 structural domains DI (red), DII (yellow), and DIII (blue) and the positions of T173A and N529D mutations on the protomer of the EFF-1 trimer.12 Stem region (magenta) connects DIII to the transmembrane domain (green). N529D is located in a region of the stem that is unstructured in the crystal. T173A is in the CD loop.12
Vacuolar ATPase mutations induce retarded hyperfusion and change EFF-1 localization
We performed candidate gene screens looking for trafficking mutants with characteristic cell fusion abnormalities and found genes involved in cell fusion regulation.7 Mutations in the vacuolar ATPase complex proteins encoded by vha-17 (fus-1) and vha-5 genes caused hypefusion, as shown before.1 Acidification of organelles by the vATPase complex or its sorting activities are crucial for intracellular trafficking.13,14 Hyperfusion caused by vha-17 happened 5-20 hours later than in rab-5 homozygous embryos.1,7 In the vATPase mutants, EFF-1 was targeted to a smaller but more dense set of organelles and did not colocalize with cell junctions.7 These organelles were probably recycling endosomes that cannot mature or fuse with lysosomes, as suggested by our observation of enrichment of the recycling endosome pool in vha-17 embryos (Smurova and Podbilewicz, unpublished). Because mutations in the vATPase complex affect all the endocytic trafficking pathways, it is difficult to distinguish which part of the pathway was involved in EFF-1 trafficking and hyperfusion. We suggest that VHA-17 and VHA-5 act in the EFF-1 degradation pathway.
EFF-1 helps to sculpt neurons and repair neurites following injuries
EFF-1 has functions unrelated to cell fusion, such as maintenance of neuronal shape and fusion of regenerating axons.15-17 EFF-1 preserves dendritic trees of the PVD neuron, and eff-1 depletion results in disorganized and hyperbranched dendrites. We speculate that the targeted transport to particular domains of the plasma membrane and fast membrane uptake induced by EFF-1 endocytosis might be a clue to the mechanism to reduce PVD branching.
In the nervous system of C. elegans, EFF-1 was able to mediate the fusion of injured axons after laser ablation.16,17 In the context of axonal injuries, a dynamic pattern of EFF-1::GFP localization on the axonal membrane has been observed, with enrichment on the regenerating growth cone.16 This example shows, that the dynamic cellular transport may regulate EFF-1 presence on the plasma membrane of neuronal cells. In general, the dynamic EFF-1 localization to specific membrane domains may be used to avoid developmental or repair errors.
The other C. elegans fusogen, AFF-1, was found to be localized mainly at the plasma membrane and partially in internal organelles of AFF-1 expressing cells in C. elegans (Gattegno, unpublished).18 This difference in localization between AFF-1 and endogenous EFF-1 in the epithelia may point to the various fusion capabilities and different fusion mechanism of EFF-1 and AFF-1 proteins.
In summary, we found that the small GTPase RAB-5 that is essential for endocytosis, sorting and intracellular fusion of endocytic organelles, controls EFF-1 transient localization to the surface of cells destined to fuse and prevents excess fusion by internalizing and sequestering this intercellular fusion protein. Keeping EFF-1 fusion protein inside early endosomes protects the cells from excessive fusion, thus RAB-5-mediated endocytosis protects the embryos from cell-cell fusion-induced lethality.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dan Cassel for critically reading the manuscript; K. S. was supported by the Ministry of Absorption, Israel (N061486).
Funding
The work was funded by European Research Council (ERC) Advanced grant 268843, GIF German-Israeli Foundation for Scientific Research and Development (grant 937/2006), US-Israel Binational Science Foundation (grant 2013151) and the Israel Science Foundation grant 443/12.
References
- [1].Kontani K, Moskowitz IPG, Rothman JH. Repression of cell-cell fusion by components of the C. elegans vacuolar ATPase complex. Dev Cell 2005; 8:787-94; PMID:15866168; https://doi.org/ 10.1016/j.devcel.2005.02.018 [DOI] [PubMed] [Google Scholar]
- [2].Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG, Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell 2002; 2:355-62; PMID:11879640; https://doi.org/ 10.1016/S1534-5807(02)00129-6 [DOI] [PubMed] [Google Scholar]
- [3].Gattegno T, Mittal A, Valansi C, Nguyen KCQ, Hall DH, Chernomordik LV, Podbilewicz B. Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans. Mol Biol Cell 2007; 18:1153-1166; PMID:17229888; https://doi.org/ 10.1091/mbc.E06-09-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, Chernomordik LV. The C. elegans Developmental Fusogen EFF-1 Mediates Homotypic Fusion in Heterologous Cells and In Vivo. Dev Cell 2006; 11:471-81; PMID:17011487; https://doi.org/ 10.1016/j.devcel.2006.09.004 [DOI] [PubMed] [Google Scholar]
- [5].Shinn-Thomas JH, Del Campo JJ, Wang J, Mohler WA. The EFF-1A Cytoplasmic Domain Influences Hypodermal Cell Fusions in C. elegans But Is Not Dependent on 14-3-3 Proteins. PLoS One 2016; 11(1):e0146874; PMID:26800457; https://doi.org/ 10.1371/journal.pone.0146874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Podbilewicz B. Virus and Cell Fusion Mechanisms. Annu Rev Cell Dev Biol 2014; 30:111-139; PMID:25000995; https://doi.org/ 10.1146/annurev-cellbio-101512-122422 [DOI] [PubMed] [Google Scholar]
- [7].Smurova K, Podbilewicz B. RAB-5- and DYNAMIN-1-Mediated Endocytosis of EFF-1 Fusogen Controls Cell-Cell Fusion. Cell Rep 2016; 14:1517-27; PMID:26854231; https://doi.org/ 10.1016/j.celrep.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Podbilewicz B, White JG. Cell fusions in the developing epithelial of C. elegans. Dev Biol 1994; 161:408-24; PMID:8313992; https://doi.org/ 10.1006/dbio.1994.1041 [DOI] [PubMed] [Google Scholar]
- [9].Fridman K. Ultrastructure and function of AFF-1 and EFF-1 in membrane remodeling. 2012; MSc thesis, Haifa, Technion [Google Scholar]
- [10].Avinoam O, Podbilewicz B. Eukaryotic Cell-Cell Fusion Families. Curr Top Membr 2011; 68:209-234; PMID:21771501; https://doi.org/ 10.1016/B978-0-12-385891-7.00009-X [DOI] [PubMed] [Google Scholar]
- [11].del Campo JJ, Opoku-Serebuoh E, Isaacson AB, Scranton VL, Tucker M, Han M, Mohler WA. Fusogenic activity of EFF-1 is regulated via dynamic localization in fusing somatic cells of C. elegans. Curr Biol 2005; 15:413-23; PMID:15753035; https://doi.org/ 10.1016/j.cub.2005.01.054 [DOI] [PubMed] [Google Scholar]
- [12].Pérez-Vargas J, Krey T, Valansi C, Avinoam O, Haouz A, Jamin M, Raveh-Barak H, Podbilewicz B, Rey FA. Structural basis of eukaryotic cell-cell fusion. Cell 2014; 157:407-19; https://doi.org/ 10.1016/j.cell.2014.02.020 [DOI] [PubMed] [Google Scholar]
- [13].Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada G-H, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, et al.. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 2006; 8:124-36; PMID:16415858; https://doi.org/ 10.1038/ncb1348 [DOI] [PubMed] [Google Scholar]
- [14].Liégeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 2006; 173:949-61; https://doi.org/ 10.1083/jcb.200511072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oren-Suissa M, Hall D, Treinin M, Shemer G, Podbilewicz B. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science 2010; 328:1285-88; PMID:20448153; https://doi.org/ 10.1126/science.1189095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Neumann B, Coakley S, Giordano-Santini R, Linton C, Lee ES, Nakagawa A, Xue D, Hilliard MA. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature 2015; 517:219-22; PMID:25567286; https://doi.org/ 10.1038/nature14102 [DOI] [PubMed] [Google Scholar]
- [17].Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 2010; 30:3175-83; PMID:20203177; https://doi.org/ 10.1523/JNEUROSCI.5464-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sapir A, Choi J, Leikina E, Avinoam O, Valansi C, Chernomordik LV, Newman AP, Podbilewicz B. AFF-1, a FOS-1-Regulated Fusogen, Mediates Fusion of the Anchor Cell in C. elegans. Dev Cell 2007; 12:683-98; PMID:17488621; https://doi.org/ 10.1016/j.devcel.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


