ABSTRACT
Doublecortin like kinase protein 1 (Dclk1) is a microtubule-associated protein with C-terminal serine/threonine kinase domain. Originally designated Doublecortin and CaM kinase-like 1 protein (Dcamkl1) or KIAA0369, Dclk1 was first described as a marker for radial glia cells in the context of microtubule polymerization and neuronal migration, possibly contributing to early neurogenesis. Additionally, Dclk1 was proposed as a marker of quiescent gastrointestinal and pancreatic stem cells, but in recent years has been recognized as a marker for tuft cells in the gastrointestinal tract. While Dclk1+ tuft cells are now considered as niche or sensory cells in the normal gut, growing evidence supports a role for Dclk1 function in a variety of malignancies, modulating the activity of multiple key pathways, including Kras signaling. Here, we review the recent advances in understanding of the importance of Dclk1 function in tumorigenesis and cancer.
KEYWORDS: cancer, cancer initiating cells, cancer stem cells, Dclk1, inflammation and cancer, Kras, pancreatic cancer
Introduction
Dclk1 was first described as a brain-specific protein in the developing rodent brain, with at least 4 splicing variants located on chromosome 13q13-q14.1 and with close structural relation to doublecortin (DCX). Functionally, Dclk1 was found to be associated with microtubules, and overexpression of the protein stimulated microtubule elongation. Interestingly, this effect was largely independent of the kinase function of Dclk1, as a distinct function of the protein or kinase has not yet been described. Furthermore, multiple groups reported the importance of Dclk1 in the developing brain based on its expression in radial glia cells and neuronal precursors.1-7
Outside of the nervous system, Dclk1 was initially proposed to mark quiescent gastrointestinal stem cells.8 Careful lineage tracing studies, however, led to a more elaborate and nuanced understanding of the properties and function of Dclk1+ cells in the gastrointestinal tract (see below). Most Dclk1+ cells in the gut qualify as typical tuft cells and are predominantly postmitotic.9 Recently, lineage tracing studies demonstrated that gastrointestinal Dclk1+ cells do not serve as a meaningful pool of bona fide stem cells. However, a subset of intestinal and colonic Dclk1+ tuft cells is long-lived, largely quiescent, regulates and contributes to a stem cell niche.
Despite the fact that Dclk1+ tuft cells are not progenitor cells, studies from our group using Dclk1-CreERT-BAC transgenic mice showed that a subset of Dclk1+ colonic and intestinal cells are long-lived and can function as powerful cancer initiating cells in the setting of APC mutation and inflammation.10 Using a knockin construct of Dclk1-CreERT, Chiba and co-workers also investigated the role of Dclk1+ cells in homeostasis and malignancy. The suggested that Dclk1 cells were short lived and did not display progenitor abilities and that Dclk1 might also define cancer stem cells in APCmin adenomas.11 The profound differences between the 2 studies might be explained through the Dclk1 haploinsufficiency in Dclk1-CreERT knockin mice, pointing to an importance of protein function of Dclk1 itself. Indeed, the specific knock out of one copy of Dclk1 in our Dclk1-CreERT-BAC transgenic mice (Dclk1ko/wt) resulted in the loss of the long-lived Dclk1+ population10 (Fig. 1). Such discrepancies in different mouse models need to be investigated further in the future to better understand the specific function of the Dclk1 protein. and its role for quiescence and potentially cancer initiation (see below).
Figure 1.
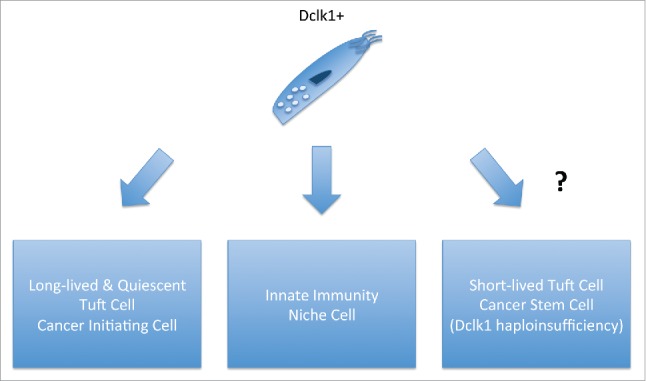
Function of Dclk1 cells in the gastrointestinal tract. (Left) Long-lived Dclk1+ tuft cells can function as potent cancer initiating cells. (Center) Dclk1+ tuft cells are involved in homeostasis, niche formation and innate immunity. (Right) Dclk1+ tuft cells can function as cancer stem cells in the setting of Dclk1 haploinsufficiency.
Dclk1 was also discussed to mark pancreatic exocrine stem cells12 and Bailey and colleagues were able to demonstrate that Dclk1+ tuft-like cells are present in murine pancreatic intraepithelial neoplasia and in pancreatic cancer cell lines. Through a series of elegant studies, the authors were able to show that these Dclk1+ cells possess features of cancer stem (initiating) cells.13 Moreover, we recently demonstrated, that Dclk1 marks pancreatic progenitor cells that play a pivotal role in pancreatic regeneration and are a powerful source of cancer initiating cells in the context of pancreatic injury14 (Fig. 2). The role of Dclk1+ cells as progenitor, stem and cancer stem (initiating) cells in the gastrointestinal tract is complex and beyond the scope of this review. Here we would like to focus on the growing body of evidence that Dclk1 is functionally implicated in tumorigenesis and might emerge as promising target in various cancers.
Figure 2.
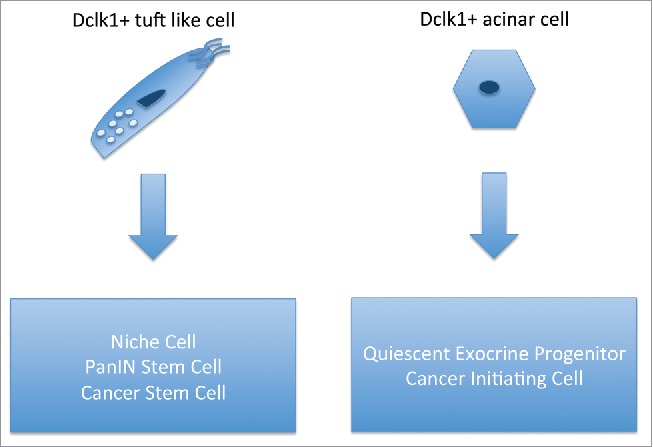
Function of Dclk1 cells in the pancreas. (Left) Dclk1+ tuft-like cells function as niche cells and cancer stem cells in preinvasive and invasive pancreatic cancer. (Right) Dclk1 marks quiescent pancreatic progenitor and potent cancer-initiating cells in the acinar compartment.
Functional relevance of Dclk1 expression in malignancy
Over the past decade, Dclk1 was shown to be expressed in a variety of cancers, and knockdown studies have shown that it is functionally important to tumor growth or progression. In 2009, Houchen and coworkers found increased expression of Dclk1 in human colorectal cancer samples and proceeded to target the gene using siRNA-mediated knock down. Suppressing Dclk1 gene expression caused a growth arrest of colorectal cancer xenografts. Mechanistically, loss of Dclk1 expression led to an upregulation of the miRNA let-7a, a negative regulator of cell cycle progression, and downregulation of the oncogene c-myc,15 pointing to a role in quiescence and malignant transformation. In neuroblastoma cells, silencing of doublecortin-like kinase-1 and doublecortin-like kinase-1 long with Dclk1 siRNA resulted in an increase in apoptotic cell death and profound changes in the gene expression profile.16 Additionally, silencing Dclk1 expression in xenograft models of hepatocellular carcinoma showed a strong growth inhibitory effect.17 Moreover, Dclk1 was found to be upregulated in 93% of clear renal cell carcinoma submitted to The Cancer Genome Atlas (TCGA). Again, siRNA-mediated knockdown of Dclk1 led to a significant inhibition of tumor cell proliferation, focal adhesion, invasion and clonogenic growth.18
However, some of the strongest data on tumor growth can be found in models of pancreatic cancer. Sureban and colleagues extended our knowledge on Dclk1 function in cancer through a series of well-designed experiments in mouse models of pancreatic cancer and pancreatic cancer cell lines. They first demonstrated that Dclk1 expression is increased in early murine PanINs and human pancreatic cancer samples.19 Furthermore, siRNA-mediated knockdown of Dclk1 let to upregulation of miRNA200a, thereby negatively affecting epithelial to mesenchymal transition (EMT). Paralleling the findings in colorectal cancer, loss of Dclk1 led to an increase in let-7a expression and a simultaneous decrease in c-myc expression,15,19 pointing again to a role for Dclk1 in carcinogenesis, as c-myc is an important driver of pancreatic tumorigenesis.20 While a functional connection between Dclk1 and the oncogene c-myc is an intriguing possibility, further studies are needed to determine if the results outlined above are indeed suggestive of a regulatory network.
Nevertheless, the most important driver of pancreatic carcinogenesis is Kras, which is mutated in more than 95% of all pancreatic cancer cases, and to date no drugs efficiently targeting mutant Kras have been developed.21,22 The functional importance of mutant Kras, a member of the GTPase enzyme family, in pancreatic cancer, even in the setting of metastatic disease, has been shown in a series of seminal papers.23-25 Consequently, the finding by Sureban and coworkers that siRNA-mediated knockdown of Dclk1 in human pancreatic cancer cells resulted in downregulation of Kras expression was of great interest.19 The authors followed up this study with a more in depth analysis of pancreatic cancer cells with reduced Dclk1 gene expression, and demonstrated that Dclk1 regulated pluripotency and angiogenic programs via distinct miRNAs. Of note, the abovementioned negative effect on Kras was confirmed in this study.26 Given the effect of Dclk1 deficiency on Kras levels, and the functional importance of Dclk1 in various cancers, targeting or inhibiting Dclk1 appeared to be a potential therapeutic option. Consequently, nanoparticle-based delivery of siRNAs against Dclk1, or use of small molecules inhibiting the kinase function of Dclk1, led to marked responses in preclinical models of pancreatic and colorectal cancer.27-29 This needs further investigation in the future to understand the exact mechanism but could be an interesting novel targeted therapy option.
In these lines, work from our group showed that Dclk1 was also a marker for a potential pancreatic cancer-initiating cell. Using genetic lineage tracing, we demonstrated that Dclk1 marked a rare and quiescent progenitor cell type in the murine pancreas, similar to that in the gut. These cells had the ability to contribute substantially to pancreatic regeneration after injury, and were pivotal for recovery after murine pancreatitis14 (Fig. 2). Pancreatitis itself is considered a risk factor for the development of pancreatic cancer.30 Thus, it was not surprising that the combination of targeted mutation of Kras to Dclk1+ cells and pancreatic injury converted Dclk1+ cells into highly potent cancer initiating cells. Importantly, genetic and pharmacological targeting of Dclk1 led to a significant inhibition of pancreatic tumorigenesis in well-established Kras-mutated mouse models of pancreatic cancer.31 Moreover, loss of Dclk1 led to decreased in vivo and in vitro levels of the Kras effector, phospho-ERK, underscoring the importance of the interplay between Dclk1 and mutant Kras. Similarly, pharmacological inhibition and genetic deletion of Dclk1 in Kras mutant organoid cultures led to a significant growth defect, most probably due to reduced signaling downstream of receptor tyrosine kinases. We based this hypothesis on the following observations. In most cases, pancreatic organoids growth depends on the presence of epithelial growth factor (EGF) in the culture medium.32 In contrast, Kras mutant organoids are able to grow in the absence of EGF, but their growth can further be stimulated with exogenous EGF (own unpublished observations). Accordingly, we tried to rescue the effect of Dclk1 deficiency with the addition to EGF to the culture medium. In these experiments, substitution of exogenous EGF had no effect on the growth of Dclk1-deficient Kras mutant organoids, suggesting that the inhibitory effect of Dclk1 deletion was operating downstream of receptor tyrosine kinases such as the EGF-receptor. While Dclk1 deficiency clearly impaired proliferation and p-ERK signaling in Kras mutant pancreatic cells, the explanation for this association emerged from bioinformatic studies of Kras interacting proteins. Computational methods suggested that Dclk1 might be a potential Kras effector and, based on structural similarities between Dclk1 and RalGDS, physically bind directly to Kras. This physically interaction was confirmed using immunoprecipitation assays, opening a novel interaction of these 2 proteins with a functional relevance in cancer. Besides, overexpression of Dclk1 increased proliferation in human pancreatic cancer cell lines,14 and in human pancreatic cancer stem cells conferred a more aggressive phenotype and accelerated tumor invasion and metastasis.33
In addition to phospho-ERK, another downstream effector of Kras is COX-2, an important player in inflammatory carcinogenesis,34,35 DelGiorno and colleagues recently reported that Dclk1+ tuft cells, which are known to express COX-2, contribute substantially to biliary metaplasia present in pancreatic tumors.36 Moreover, recent studies have suggested a direct relationship between Dclk1 and Cox-2 in pancreatic cancer. In murine and human pancreatic cancer, Dclk1, Cox-2 and 5-LOX expression increased in a linear fashion paralleling tumor progression, a process that could be further accelerated through induced pancreatitis. Treatment with the novel dual COX/LOX inhibitor licofelone led to a significant decrease in pancreatic tumors, Dclk1 expression and pancreatic cancer stem cells.37 Thus, Dclk1 expression is closely tied to 2 Kras effectors, p-ERK and COX-2, supporting a concept of Dclk1 being a key regulator of Kras function in pancreatic cancer.
Of note, Dclk1 is expressed in a number of other tumors, although its role here is less well defined. In a subset of human colorectal cancers and high-grade adenomas strong Dclk1 protein expression is associated with disease recurrence but not with disease stage, suggesting that Dclk1 expression was an independent prognostic factor.38,39 However, Dclk1 gene expression was significantly downregulated in a large number of colorectal cancer samples and cell lines, most likely through hypermethylation of the Dclk1 promoter.40 Recently, Singh and colleagues were able to shed light on the complex epigenetic regulation of different Dclk1 transcripts in colorectal cancer, and showed that expression of a long Dclk1 isoform, transcribed from the 5’ promoter, is silenced in colorectal cancer due to hypermethylation at this site. Nevertheless, the group hypothesized that there could be an alternative promoter site utilized, and the demonstrated that a novel short isoform of Dclk1 was transcribed from another promoter site. Indeed, high levels of expression of this short isoform correlated with a significantly worse prognosis in 92 human colorectal cancers. Thus, O'Connell and colleagues were able to reconcile the apparent contradictory findings of wide-spread epigenetic silencing of the Dclk1+ gene on one hand, and the clear functional importance of the transcript in a broad variety of cancers on the other.41
Accordingly, Dclk1 expression has been observed in neuroendocrine tumors of the mammary gland and the rectum,42,43 consistent with the known expression of Dclk1 in neuronal and enteroendocrine cells, but no specific role for Dclk1 in these tumors has been suggested so far. Finally, the relevance of Dclk1 expression in esophageal cancer is presently unclear,44 but the cells seem to correlate with an increased progression to cancer in a murine model and human patients.45
Dclk1 as a marker of cancer stem and initiating cells
As mentioned above, Dclk1 was proposed as a marker of quiescent gastrointestinal and pancreatic stem cells.8,12 These assumptions were based on the expression pattern of the protein at baseline, and changes in expression after various injurious stimuli. However, in these early studies, evidence from formal genetic lineage tracing supporting any progenitor role for Dclk1+ cells was absent.
In 2013, Chiba and co-workers reported data using a Dclk1-CreERT knockin mouse model. In their study, Dclk1 did not label a significant proportion of active or quiescent stem cells, but did mark most intestinal tuft cells, which were found to turn over quite rapidly within a few weeks (see discussion of discrepancy above). However, in the APCmin mouse model of colorectal cancer, they found that Dclk1+ tumor cells fulfilled all bona fide criteria of tumor stem cells continuously fueling adenoma growth, and also expressed the known intestinal stem cell marker Lgr5. Targeted deletion of Dclk1+ tumor cells using a diphtheria toxin based approach led to a significant regression of established adenomas, without adverse effects on the non-transformed epithelium.46 However, while a potentially important observation, the study must be interpreted in light of the fact that the Dclk1-CreERT2 knockin mice were haploinsufficient for the Dclk1 gene, which seems to be involved in quiescence, self-renewal and longevity10,14,47 (see above).
Our group examined many of the same questions using BAC transgenic mice bearing a Dclk1-CreERT2 transgene and thus retaining both copies of the endogenous Dclk1 gene. We found that a subset of Dclk1+ cells was extremely long-lived (e.g. up to 2 years), but this longevity was lost with deletion of one copy of the Dclk1 gene. Furthermore, in contrast to the results with Dclk1-CreERT2 knockin mice, we found that most (e.g., 60%) of Dclk1+ cells did not take up BrdU after one month of continuous labeling, confirming their relative quiescence. The longevity and quiescence of small intestinal tuft cells has been supported by their genetic signature, which reveals gene signature with enrichment of Bmi1 expression, and hallmarks of quiescence and self-renewal.47 Moreover, in the setting of APC deficiency, Dclk1+ cells remained persistently quiescent, but surprisingly could be converted into potent cancer initiating cells in the setting of an inflammatory stimulus such as DSS colitis.10 While surprising at first, the exquisite role of NF-κB signaling in inflammatory gastrointestinal carcinogenesis is well established,48 and in fact the results are in line with observations from Florian Greten's group.49 In a series of elegant experiments, Schwittala and colleagues were able to demonstrate that intestinal inflammation induced NF-κB signaling, which in turn stimulated Wnt signaling. Most importantly, the authors were able to show that this axis was sufficient to convert non-stem cells into potent cancer initiating cells.49 It is tempting to speculate that at least part of the tumors observed in the Schwittala series emerged from mutated Dclk1+ cells. Furthermore, given the fact that tuft cells express Cox-2,50 an important player in inflammatory colorectal carcinogenesis51 and that Kras signaling can stimulate Cox-2 and colorectal cancer growth,52 it is furthermore possible that the combined presence of Cox-2 and the Kras modulator Dclk1 in tuft cells renders this cell population especially prone to inflammatory induced carcinogenesis. Of note, using the Dclk1-BAC-CreERT2 mice with 2 copies of the endogenous Dclk1 gene, we were not able to confirm the ability of Dclk1 cells to act as tumor stem cells within APCmin adenomas (unpublished observation). Finally, a recent study showed that loss of Elp3 inhibited Wnt-driven intestinal tumorigenesis was at least partly due to decreased expression of Dclk1.53 These results further highlight the significance of Dclk1 expression in intestinal malignancy.
The importance of Dclk1+ cells in tumorigenesis was further underscored by a study by Leach and colleagues in which the authors investigated the appearance of Dclk1+ cells in preinvasive and established pancreatic cancer. The authors found that Dclk1+ cells were present at the earliest stages of pancreatic tumorigenesis, but could also be found in more advanced pancreatic cancers and pancreatic cancer cell lines. These cells grouped as a functionally and morphologically distinct subpopulation. In murine PanINs, Dclk1+ cells readily gave rise to pancreatic spheres, a surrogate for stemness. Dclk1+ cells isolated from pancreatic cancer cells lines were found to be highly tumorigenic.13 Finally, our group recently reported on the ability of pancreatic Dclk1+ cells to serve as a potent source of preinvasive pancreatic cancer initiating cells in the setting of Kras mutation and pancreatic injury. Overall, growing evidence from in vivo and in vitro studies suggests that Dclk1+ cells can function as cancer stem and cancer initiating cells in colorectal and pancreatic cancer.14 Further studies are needed for other cancers with Dclk1 expression in order to extrapolate a general function of the Dclk1 protein or the expressing cell type in carcinogenesis.
Dclk1 as a potential tumor marker
As Dclk1 is broadly expressed in a range of different cancers, it is not surprising that some investigators proposed to use Dclk1 as a tumor marker in cancer bearing individuals. In Barrett's esophagus and esophageal cancer, similar to the findings in IL1-β mouse model of Barrett's esophagus,45 Dclk1 protein was detectable by ELISA in all patients studied. In contrast, the protein was not detectable in all but one control patient.44 Using free circulating tumor DNA, Milanowski and coworkers were able to analyze the promoter region of Dclk1 with regards to its methylation status. In this study, 65 patients with lung cancer and 95 healthy controls were analyzed. Dclk1 promoter methylation was found in less then 10% of healthy individuals while almost 50% of the patients diagnosed with lung cancer had detectable levels of the methylated Dclk1 promoter in their plasma, and the methylation status was a predictor of poor survival in these patients.54 Houchen and colleagues analyzed both circulating tumor cells and the serum of patients diagnosed with pancreatic cancer for Dclk1 expression. In a representative genetically engineered mouse model of pancreatic cancer, greater than 50% of all circulating tumor cells expressed Dclk1. Furthermore, serum Dclk1 levels were also elevated in this model. In humans, serum Dclk1 levels were elevated in stage I/II when compared to healthy controls. Interestingly, there was no difference between stage III/IV patients and healthy controls, suggesting a change in the expression pattern or shedding in late stage pancreatic cancer.55 Finally, Dclk1 protein was also detectable in the plasma of patient with diagnosed with hepatocellular carcinoma.17 Overall, Dclk1 protein levels can be measured in the blood of patients with a number of different malignancies. However, the clinical significance and the potential diagnostic value of these findings need to be rigorously validated in future prospective studies with large cohorts of patients and controls.
Concluding remarks
After the initial description of Dclk1 in the context of neuronal development, recent advances have almost exclusively focused on the role of Dclk1+ tuft cells in the gastrointestinal tract and Dclk1+ cells in a variety of cancers. In this review, we have focused primarily on the role of Dclk1 in cancer, and have not been able to review the exciting studies focused linking tuft cells to type 2 immunity and ILC2 cells in the gut.56-58 While published data consistently show that targeting Dclk1 has negative effects on cancer growth, the underlying mechanisms of these findings are less clear. Only recently, work from many laboratories has shed some light on the mechanisms by which Dclk1 promotes tumorigenesis. However, the mode of action remains to be defined. As Dclk1 has a kinase domain and targeting this domain inhibits tumorigenesis in some models, it is tempting to speculate that the kinase activity of Dclk1 is partly responsible for its effects in malignancy, nevertheless the function of this kinase needs to be determined as well. As the targets of Dclk1 are unknown to date, future studies are needed to confirm the outlined concepts. Others and we have recently shown that Dclk1 functionally and physically engages with Kras, one of the most powerful oncogenes. While major efforts have so far failed to therapeutically target Kras, small molecules targeting Dclk1 are available and might have a role in the treatment of Kras-driven cancers. Moreover, the epigenetic regulation of the short and long isoforms, creating a “cancer-specific” isoform of Dclk1 warrants further investigation, potentially opening the window for isoform specific drugs. As some groups have proposed to use the detection of Dclk1 in the blood of patients as a biomarker, future studies are needed to understand an than validate this concept and its prognostic and clinical relevance in prospective cohorts. Finally, as Dclk1 marks cancer stem and initiating cells in some cancers, early diagnosis and preventive approaches based on this finding might be a viable option in the future.
Taken together, Dclk1 plays a role in a variety of different cancer and is functionally important in these malignancies. Further research efforts are needed to clarify and manipulate the underlying mechanisms before Dclk1 can be used as a meaningful therapeutic target, although it appears to be a promising approach for the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Walker TL, Yasuda T, Adams DJ, Bartlett PF. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci 2007; 27(14):3734-42; PMID:17409237; https://doi.org/ 10.1523/JNEUROSCI.5060-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vreugdenhil E, Kolk SM, Boekhoorn K, Fitzsimons CP, Schaaf M, Schouten T, Sarabdjitsingh A, Sibug R, Lucassen PJ. Doublecortin-like, a microtubule-associated protein expressed in radial glia, is crucial for neuronal precursor division and radial process stability. Eur J Neurosci 2007; 25(3):635-48; PMID:17313568; https://doi.org/ 10.1111/j.1460-9568.2007.05318.x [DOI] [PubMed] [Google Scholar]
- [3].Shu T, Tseng HC, Sapir T, Stern P, Zhou Y, Sanada K, Fischer A, Coquelle FM, Reiner O, Tsai LH. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and m phase progression. Neuron 2006; 49(1):25-39; PMID:16387637; https://doi.org/ 10.1016/j.neuron.2005.10.039 [DOI] [PubMed] [Google Scholar]
- [4].Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron 2006; 49(1):55-66; PMID:16387639; https://doi.org/ 10.1016/j.neuron.2005.10.040 [DOI] [PubMed] [Google Scholar]
- [5].Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA. Dcamkl1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci 2000; 20(24):9152-61; PMID:11124993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sossey-Alaoui K, Srivastava AK. Dcamkl1, a brain-specific transmembrane protein on 13q12.3 that is similar to doublecortin (dcx). Genomics 1999; 56(1):121-6; PMID:10036192 [DOI] [PubMed] [Google Scholar]
- [7].Omori Y, Suzuki M, Ozaki K, Harada Y, Nakamura Y, Takahashi E, Fujiwara T. Expression and chromosomal localization of kiaa0369, a putative kinase structurally related to doublecortin. J Hum Genetics 1998; 43(3):169-77; PMID:9747029 [DOI] [PubMed] [Google Scholar]
- [8].May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and cam kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem cells (Dayton, Ohio) 2008; 26(3):630-7; PMID:18055444; https://doi.org/ 10.1634/stemcells.2007-0621 [DOI] [PubMed] [Google Scholar]
- [9].Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. Dcamkl-1 expression identifies tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterol 2009; 137(6):2179-80; author reply 2180-E2171; PMID:19879217; https://doi.org/ 10.1053/j.gastro.2009.06.072 [DOI] [PubMed] [Google Scholar]
- [10].Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, et al.. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 2014; 124(3):1283-95; PMID:24487592; https://doi.org/ 10.1172/JCI73434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et al.. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genetics 2012; 98-103; PMID:23202126 [DOI] [PubMed] [Google Scholar]
- [12].May R, Sureban SM, Lightfoot SA, Hoskins AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant S, et al.. Identification of a novel putative pancreatic stem/progenitor cell marker dcamkl-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol 2010; 299(2):G303-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al.. Dclk1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterol 2014; 146(1):245-56; PMID:24096005; https://doi.org/ 10.1053/j.gastro.2013.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y, et al.. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell 2016; 18(4):441-55; PMID:27058937; https://doi.org/ 10.1016/j.stem.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, Houchen CW. Selective blockade of dcamkl-1 results in tumor growth arrest by a let-7a microrna-dependent mechanism. Gastroenterol 2009; 137(2):649-59, 659.e641-642; PMID:19445940; https://doi.org/ 10.1053/j.gastro.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Verissimo CS, Molenaar JJ, Meerman J, Puigvert JC, Lamers F, Koster J, Danen EH, van de Water B, Versteeg R, Fitzsimons CP, et al.. Silencing of the microtubule-associated proteins doublecortin-like and doublecortin-like kinase-long induces apoptosis in neuroblastoma cells. Endocrine-Related Cancer 2010; 17(2):399-414; PMID:20228126; https://doi.org/ 10.1677/ERC-09-0301 [DOI] [PubMed] [Google Scholar]
- [17].Sureban SM, Madhoun MF, May R, Qu D, Ali N, Fazili J, Weygant N, Chandrakesan P, Ding K, Lightfoot SA, Houchen CW. Plasma dclk1 is a marker of hepatocellular carcinoma (hcc): Targeting dclk1 prevents hcc tumor xenograft growth via a microrna-dependent mechanism. Oncotarget 2015; 6(35):37200-15; PMID:26468984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weygant N, Qu D, May R, Tierney RM, Berry WL, Zhao L, Agarwal S, Chandrakesan P, Chinthalapally HR, Murphy NT, et al.. Dclk1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget 2015; 6(4):2193-205; PMID:25605241; https://doi.org/ 10.18632/oncotarget.3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV, et al.. Dcamkl-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a mir-200a-dependent mechanism. Cancer Res 2011; 71(6):2328-38; PMID:21285251; https://doi.org/ 10.1158/0008-5472.CAN-10-2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Skoudy A, Hernández-Muñoz I, Navarro P. Pancreatic ductal adenocarcinoma and transcription factors: Role of c-myc. J Gastrointestinal Cancer 2011; 42(2):76-84; PMID:21279552; https://doi.org/ 10.1007/s12029-011-9258-0 [DOI] [PubMed] [Google Scholar]
- [21].di Magliano MP, Logsdon CD. Roles for kras in pancreatic tumor development and progression. Gastroenterol 2013; 144(6):1220-9; PMID:23622131; https://doi.org/ 10.1053/j.gastro.2013.01.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deramaudt T, Rustgi AK. Mutant kras in the initiation of pancreatic cancer. Biochimica Et Biophysica Acta 2005; 1756(2):97-101; PMID:16169155 [DOI] [PubMed] [Google Scholar]
- [23].Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, et al.. Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012; 149(3):656-70; PMID:22541435; https://doi.org/ 10.1016/j.cell.2012.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Collins MA, Brisset JC, Zhang Y, Bednar F, Pierre J, Heist KA, Galbán CJ, Galbán S, di Magliano MP. Metastatic pancreatic cancer is dependent on oncogenic kras in mice. PLos One 2012; 7(12):e49707; PMID:23226501; https://doi.org/ 10.1371/journal.pone.0049707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, di Magliano MP. Oncogenic kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest 2012; 122(2):639-53; PMID:22232209; https://doi.org/ 10.1172/JCI59227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG. Dclk1 regulates pluripotency and angiogenic factors via microrna-dependent mechanisms in pancreatic cancer. PLoS One 2013; 8(9):e73940; PMID:24040120; https://doi.org/ 10.1371/journal.pone.0073940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weygant N, Qu D, Berry WL, May R, Chandrakesan P, Owen DB, Sureban SM, Ali N, Janknecht R, Houchen CW. Small molecule kinase inhibitor lrrk2-in-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1. Mol Cancer 2014; 13(1):103; PMID:24885928; https://doi.org/ 10.1186/1476-4598-13-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC, et al.. Xmd8-92 inhibits pancreatic tumor xenograft growth via dclk1-dependent mechanism. Cancer Letters 2014; 351(1):151-61; PMID:24880079; https://doi.org/ 10.1016/j.canlet.2014.05.011 [DOI] [PubMed] [Google Scholar]
- [29].Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, Anant S, Ramanujam RP, Houchen CW. Nanoparticle-based delivery of sidcamkl-1 increases microrna-144 and inhibits colorectal cancer tumor growth via a notch-1 dependent mechanism. J Nanobiotechnol 2011; 9(1):40; PMID:21929751; https://doi.org/ 10.1186/1477-3155-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Practice Amp; Res Clin Gastroenterol 2006; 20(2):197-209; https://doi.org/ 10.1016/j.bpg.2005.10.001 [DOI] [PubMed] [Google Scholar]
- [31].Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA. et al.. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4(6):437-50; PMID:14706336 [DOI] [PubMed] [Google Scholar]
- [32].Schreiber FS, Deramaudt TB, Brunner TB, Boretti MI, Gooch KJ, Stoffers DA, Bernhard EJ, Rustgi AK. Successful growth and characterization of mouse pancreatic ductal cells: Functional properties of the ki-ras(g12v) oncogene. Gastroenterol 2004; 127(1):250-60; PMID:15236190; https://doi.org/ 10.1053/j.gastro.2004.03.058 [DOI] [PubMed] [Google Scholar]
- [33].Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, Matsumura S, Aihara A, Mitsunori Y, Ban D, Ochiai T. et al.. Dominant expression of dclk1 in human pancreatic cancer stem cells accelerates tumor invasion and metastasis. PLoS One 2016; 11(1):e0146564; PMID:26764906; https://doi.org/ 10.1371/journal.pone.0146564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mutoh H, Sashikawa M, Sakamoto H, Tateno T. Cyclooxygenase 2 in gastric carcinoma is expressed in doublecortin- and cam kinase-like-1-positive tuft cells. Gut Liver 2014; 8(5):508-18; PMID:25228975; https://doi.org/ 10.5009/gnl13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, Ji B, Huang H, Wang H, Fleming JB, et al.. A high-fat diet activates oncogenic kras and cox2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterol 2013; 145(6):1449-58; PMID:23958541; https://doi.org/ 10.1053/j.gastro.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].DelGiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CV, et al.. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterol 2014; 146(1):233-244.e235; PMID:23999170; https://doi.org/ 10.1053/j.gastro.2013.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mohammed A, Janakiram NB, Madka V, Brewer M, Ritchie RL, Lightfoot S, Kumar G, Sadeghi M, Patlolla JM, Yamada HY, et al.. Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression. Oncotarget 2015; 6(17):15524-39; PMID:25906749; https://doi.org/ 10.18632/oncotarget.3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gagliardi G, Goswami M, Passera R, Bellows CF. Dclk1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol 2012; 5:35-42; PMID:22557932; https://doi.org/ 10.2147/CEG.S30281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bellows CF. Dclk1 expression in gastrointestinal stem cells and neoplasia. Journal of Cancer Therapeutics & Research 2012; 1-10. [Google Scholar]
- [40].Vedeld HM, Skotheim RI, Lothe RA, Lind GE. The recently suggested intestinal cancer stem cell marker dclk1 is an epigenetic biomarker for colorectal cancer. Epigenetics 2014; 9(3):346-50; PMID:24384857; https://doi.org/ 10.4161/epi.27582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O'Connell MR, Sarkar S, Luthra GK, Okugawa Y, Toiyama Y, Gajjar AH, Qiu S, Goel A, Singh P. Epigenetic changes and alternate promoter usage by human colon cancers for expressing dclk1-isoforms: Clinical implications. Sci Rep 2015; 5:14983; PMID:26447334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ikezono YU, Koga H, Abe M, Akiba J, Kawahara A, Yoshida T, Nakamura T, Iwamoto H, Yano H, Kage M, et al.. High expression of the putative cancer stem cell marker, dclk1, in rectal neuroendocrine tumors. Oncol Letters 2015; 10(4):2015-20; PMID:26622789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu YH, Tsang JY, Ni YB, Hlaing T, Chan SK, Chan KF, Ko CW, Mujtaba SS, Tse GM. Doublecortin-like kinase 1 expression associates with breast cancer with neuroendocrine differentiation. Oncotarget 2016; 7(2):1464-76; PMID:26621833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Whorton J, Sureban SM, May R, Qu D, Lightfoot SA, Madhoun M, Johnson M, Tierney WM, Maple JT, Vega KJ, et al.. Dclk1 is detectable in plasma of patients with barrett's esophagus and esophageal adenocarcinoma. Dig Dis Sci 2015; 60(2):509-13; PMID:25283374; https://doi.org/ 10.1007/s10620-014-3347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, et al.. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of barrett-like metaplasia. Cancer Cell 2012; 21(1):36-51; PMID:22264787; https://doi.org/ 10.1016/j.ccr.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et al.. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2013; 45(1):98-103; PMID:23202126 [DOI] [PubMed] [Google Scholar]
- [47].Chandrakesan P, May R, Qu D, Weygant N, Taylor VE, Li JD, Ali N, Sureban SM, Qante M, Wang TC, et al.. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget 2015; 6(31):30876-86; PMID:26362399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fang HY, Greten FR. Cell autonomous and non-autonomous functions of ikkbeta and nf-kappab during the pathogenesis of gastrointestinal tumors. Cancers (Basel) 2011; 3(2):2214-22; PMID:24212805; https://doi.org/ 10.3390/cancers3022214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al.. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013; 152(1-2):25-38; PMID:23273993; https://doi.org/ 10.1016/j.cell.2012.12.012 [DOI] [PubMed] [Google Scholar]
- [50].Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: Specification and function. Cell Mole Life Sci 2012; 69(17):2907-17; PMID:22527717; https://doi.org/ 10.1007/s00018-012-0984-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tsujii M. Cyclooxygenase, cancer stem cells and DNA methylation play important roles in colorectal carcinogenesis. Digestion 2013; 87(1):12-6; PMID:23343963; https://doi.org/ 10.1159/000343898 [DOI] [PubMed] [Google Scholar]
- [52].Smakman N, Kranenburg O, Vogten JM, Bloemendaal AL, van Diest P, Borel Rinkes IH. Cyclooxygenase-2 is a target of krasd12, which facilitates the outgrowth of murine c26 colorectal liver metastases. Clin Cancer Res 2005; 11(1):41-8; PMID:15671526 [PubMed] [Google Scholar]
- [53].Ladang A, Rapino F, Heukamp LC, Tharun L, Shostak K, Hermand D, Delaunay S, Klevernic I, Jiang Z, Jacques N, et al.. Elp3 drives wnt-dependent tumor initiation and regeneration in the intestine. J Exp Med 2015; 212(12):2057-75; PMID:26527802; https://doi.org/ 10.1084/jem.20142288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Powrozek T, Krawczyk P, Nicos M, Kuznar-Kaminska B, Batura-Gabryel H, Milanowski J. Methylation of the dclk1 promoter region in circulating free DNA and its prognostic value in lung cancer patients. Clin Translational Oncol 2016; 18(4):398-404; https://doi.org/ 10.1007/s12094-015-1382-z [DOI] [PubMed] [Google Scholar]
- [55].Qu D, Johnson J, Chandrakesan P, Weygant N, May R, Aiello N, Rhim A, Zhao L, Zheng W, Lightfoot S, et al.. Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells. PLoS One 2015; 10(2):e0118933; PMID:25723399; https://doi.org/ 10.1371/journal.pone.0118933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived il-25 regulates an intestinal ilc2-epithelial response circuit. Nature 2016; 529(7585):221-5; PMID:26675736; https://doi.org/ 10.1038/nature16161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al.. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016; 351(6279):1329-33; PMID:26847546; https://doi.org/ 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Howitt MR, Lavole S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al.. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016; 351(6279):1329-33; PMID:26847546; https://doi.org/ 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]


