Abstract
Glucose-6-phosphate dehydrogenase (G6PD) represents a common human enzyme defect, particularly prevalent in the Mediterranean, African e Asian area, where malaria was or is still endemic. Recently, we identified G6PD deficiency as a risk factor for developing invasive fungal disease (IFD) and particularly Candida Sepsis in patients undergoing intensive chemotherapy for acute myeloid leukemia (AML), suggesting that there is an urgent need for strategies to properly manage this kind of patients at high risk of invasive mycoses. Here we propose our algorithm for correct identification, prophylaxis, and treatment of IFD in patients with G6PD deficiency undergoing intensive chemotherapy for AML.
Keywords: G6PD, Aspergillosis, Candida, Acute Myeloid Leukemia, Fungal Infection
G6PD is a key enzyme in the pentose-phosphate pathway and the production of nicotinamide adenine dinucleotide phosphate (NADPH), protecting cells from oxidative stress and promoting neutrophil oxidative burst responses against microorganisms. About 140 mutations in the G6PD gene have been described, many of them influencing its activity. The most common G6PD variants are the African G6PD A-, frequently observed in tropical regions of Africa and North and South America, and the so called “Mediterranean” variant, widely found in Italy, Spain, Portugal and the Middle East.1 Females who have two copies of the G6PD gene on each X chromosome can present normal gene expression, a heterozygous pattern or, in rare cases a complete enzyme deficiency. Heterozygous females are genetic mosaics as a result of X-chromosome inactivation. Thus, clinical presentations are commonly seen in deficient male patients but are rare in heterozygous females. Traditionally the clinical picture of G6PD deficiency arises from his absence in red blood cells, with acute hemolytic anemia secondary to exogenous oxidative agents and neonatal jaundice.2 The role of G6PD deficiency in susceptibility to infections has rarely been investigated [3–6]. Biological background of this supposed increased risk is not clear. Previous studies of the G6PD Mediterranean variant showed that G6PD-deficient granulocytes display a reduced function in-vitro ranging from 25% to 33%.7,8 G6PDH enzyme catalyzes the first reaction in the pentose phosphate pathway, thereby providing reducing power to cells in the form of NADPH that is essential to NADPH oxidase enzyme.1,2 Therefore, patients with poor functioning of NADPH oxidase enzyme in phagocytes, are exposed to recurrent infections by catalase-positive organisms,9 like Candida and Aspergillus, it is likely that G6PD- patients with chemotherapy-induced neutropenia are particularly vulnerable to these germs.10 Recently, we have identified G6PD deficiency as a risk factor for invasive fungal disease (IFD) 11 in a large cohort of patients with acute myeloid leukemia (AML) undergoing intensive chemotherapy or hematopoietic stem cell transplantation (HSCT). In particular, we found that patients with G6PD deficiency (G6PD-) presented an incidence of IFD significantly higher than patients with wild type enzyme (35.7% vs. 5%). This finding was due to differences in the frequency of Mold Infections (17.8/% vs. 5%), but mostly of Candida Sepsis (17.8% vs. 0).
Thus, considering this there is a compelling need for prospective clinical trials to guide antimicrobial surveillance, prophylaxis, and treatment of G6PD- AML patients.
We recommend the determination of G6PD activity at AML diagnosis in all patients eligible for intensive chemotherapy. There are many available assays to evaluate G6PD activity, but we suggest to use quantitative tests to assess G6PD activity.12 We defined patients with activity <10% as deficient, but it is possible that, if evaluated with other assays, the threshold defining high-risk population may be higher, between 20 or 30%. Female with hyperleukocytosis and enzyme activity in the range of heterozygous people (e.g. between 11 and 84 % with G6PD/6PGD Automatic Analyzer (KUADRO), Nurex SRL) should be managed with close attention, because the G6PD activity may be over-estimated because of the high number of circulating white cells. In these cases, the molecular test may be indicated to confirm heterozygous status and exclude enzyme deficiency. The finding of G6PD activity in heterozygous range in a male patient should be considered as an interference due to circulating blast cells, hyperleukocytosis or recent transfusion, and this group of patients should be treated as those with complete deficiency.
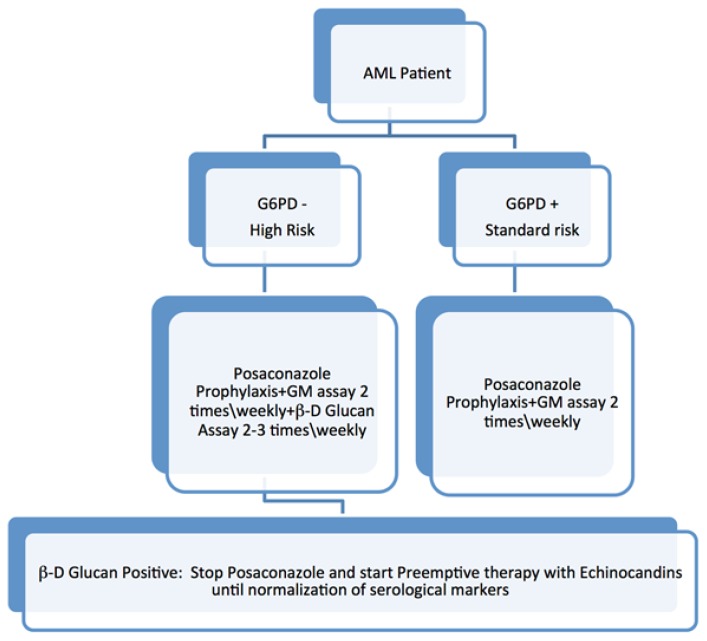
We showed that patients with enzyme activity below 10% are at higher risk of IFD, in particular, non-Albicans spp IC.11 This group of patients needs a more intensive surveillance strategy, with markers that allow prompt detection not only of impending infections from molds but also from yeasts. β-D Glucan assay was showed to have a high negative predictive value13 and to potentially detect invasive Candidiasis cases from days to weeks before positivity of blood cultures, thus considerably reducing the median time for starting antifungal therapy.14 So this marker could be of great impact in this kind of patients. In patients with G6PD deficiency undergoing intensive chemotherapy at our center, now we perform B.3Dglucan assay 2–3 times\weekly, associated with the Galactomannan assay twice weekly (Figure 1).
Figure 1.
Proposed serological surveillance algorithm in Acute Myeloid Leukemia patients with G6PD deficiency.
Some authors questioned the usefulness of β-D Glucan IFD screening in patients with hematologic malignancies, because of the high incidence of false-positive results due to exposure to some drugs (e.g. cefepime), other infections (e.g. Bacteria or Pneumocystis Jirovecii) or plasma and immunoglobulin administration.15 However, data from a large meta-analysis suggest that two consecutive positive β-D Glucan assays have a positive predictive value of 83.5% and a negative predictive value of 94.5%, and recently ECIL expert panel proposed a grade BII recommendation for the use in hemato-oncological patients.13 During chemotherapy-induced neutropenia, G6PD- patients with two consecutive positives β-D Glucan may benefit from stopping prophylaxis with Posaconazole and starting preemptive therapy with Echinocandins, even in the absence of signs of infection. Prospective studies, assessing the role of β-D glucan surveillance in combination with clinical, radiological and microbiological findings in this patient setting are lacking, and the positive predictive value of two consecutive B-D Glucan assay is only 83.5%,13 so it is possible that our approach is redundant and not cost-effective. However, we think that a 17.8% risk of Candida sepsis is too high to support a wait and watch strategy. We collect three sets of blood culture from the central venous catheter, to assess for fungal colonization. Preemptive therapy should be continued until normalization of serological markers, ideally, with two consecutive negative B-D Glucan assay, that means a negative predictive value of 94.6%.13 However, it is known that positive β-D Glucan results may persist long after blood cultures became sterile, and so also clinical variables have to be considered when deciding to stop pre-emptive therapy (e.g., neutropenia recovery, the absence of fever, stable condition, negative blood cultures). Posaconazole is still our choice for standard antifungal prophylaxis in G6PD- patients.
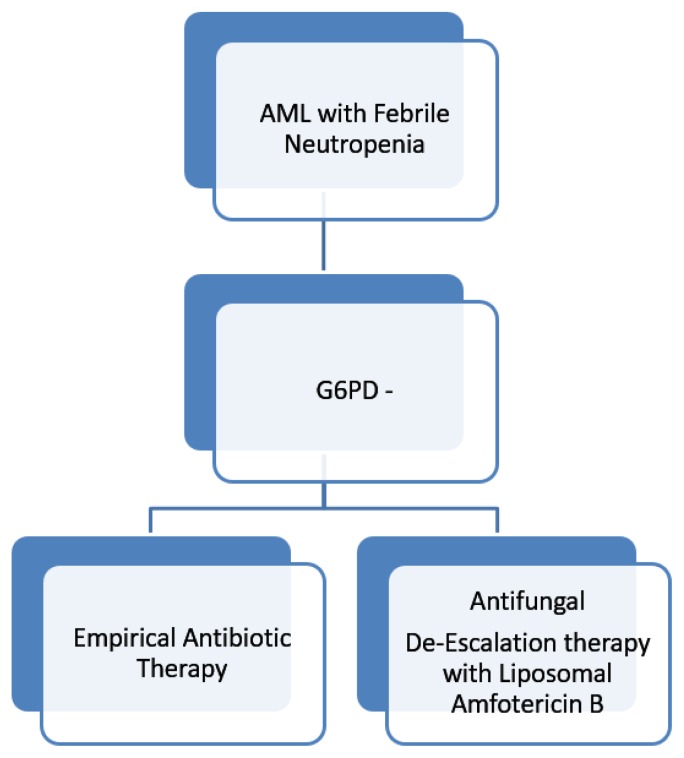
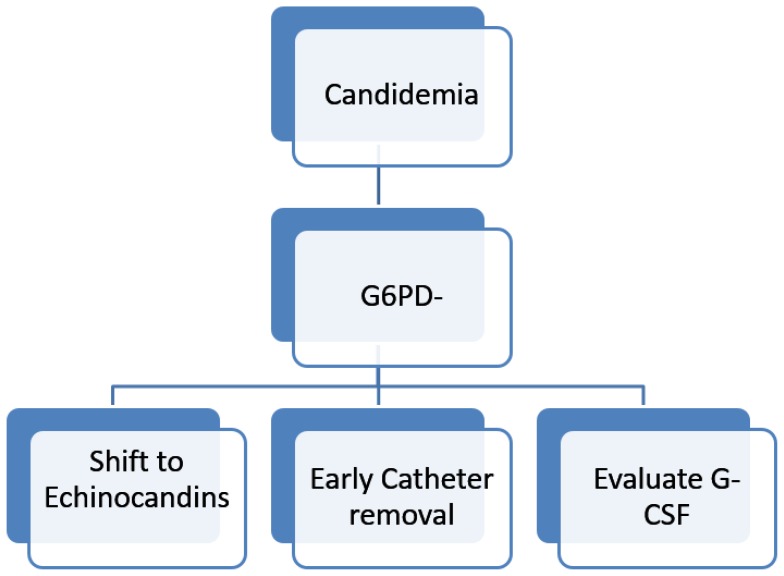
For G6PD- AML patients developing febrile neutropenia, after 72 hours of appropriate antibiotic therapy, we suggest starting an empirical de-escalation therapy, stopping prophylaxis with Posaconazole and administering a broad antifungal agent such as Liposomal Amphotericin B (Figure 2). Liposomal Amphotericin B has a good activity against Aspergillus, but is straightly recommended for treatment of Invasive Candidiasis,16 and so may give a good protection in this situation. Then, we pursue an aggressive diagnostic strategy, with the execution of TC scans of chest and sinuses and collection of three sets of blood culture, possibly during fever outbreak. For patients with signs or radiological evidence of lung disease, we execute bronchoalveolar lavage, with research of Galactomannan and culture and research for Aspergillus PCR and other pathogens. We pursue empirical therapy until identification of other causes of neutropenic fever, as recovery by the culture of bacteria or Aspergillus. In patients with persistent fever without clinical or radiological signs of infections and with two consecutive negative Beta D-Glucan assays, we recommend to stop empirical therapy and resume prophylaxis. Special efforts should be made in those center with a high prevalence of Zygomycetes, for which empirical therapy with Voriconazole should be avoided. A G6PD- patient diagnosed with IC should be aggressively managed (Figure 3). Recent published guidelines address management of invasive Candidiasis in hematologic patients.16 Echinocandins treatment with Caspofungin, Anidulafungin or Micafungin should be rapidly begun also in G6PD- patients. ECIL6 guidelines suggest with a grade BII recommendation to early remove the central venous catheter, and data from recent studies suggest that early catheter removal is associated with decreased mortality.17,18 In our experience, despite aggressive treatment with biofilm active agents as Echinocandins or Liposomal Amphotericin B, all patients who did not early remove catheter died, so we think that early catheter removal is mandatory in this subgroup of patients. The addition of G-CSF stimulation may further contribute to therapy, as we observed better outcome in patients who underwent G-CSF therapy during neutropenia. This finding deserves to be further evaluated with in-vitro studies.
Figure 2.
Proposed management algorithm of Acute Myeloid Leukemia patients with G6PD deficiency developing febrile neutropenia.
Figure 3.
Proposed management algorithm of Acute Myeloid Leukemia patients with G6PD developing Candida Sepsis.
In conclusion, G6PD- patients with AML represent a particular subgroup at high risk of IFD, especially IC. Our therapeutic algorithm may be helpful in the management of this kind of patients, while evidence from prospective clinical trials could give future evidence-based recommendations.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Luzzatto L, Nannelli C, Notaro R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am. 2016;30:373–93. doi: 10.1016/j.hoc.2015.11.006. https://doi.org/10.1016/j.hoc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Luzzatto L, Seneca E. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol. 2014;164:469–480. doi: 10.1111/bjh.12665. https://doi.org/10.1111/bjh.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spolarics Z, Siddiqi M, Siegel JH, et al. Increased incidence of sepsis and altered monocyte functions in severely injured type A- glucose-6-phosphate dehydrogenase-deficient African American trauma patients. Crit Care Med. 2001;29:728–36. doi: 10.1097/00003246-200104000-00005. https://doi.org/10.1097/00003246-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Osba YK, Mallouh AA, Hann RW. Incidence and causes of sepsis in glucose-6-phosphate dehydrogenase-deficient newborn infants. J Pediatr. 1989;114:748–52. doi: 10.1016/s0022-3476(89)80131-3. https://doi.org/10.1016/S0022-3476(89)80131-3. [DOI] [PubMed] [Google Scholar]
- 5.Meloni T, Forteleoni G, Ena F, et al. Glucose-6-phosphate dehydrogenase deficiency and bacterial infections in northern Sardinia. J Pediatr. 1991;118:909–11. doi: 10.1016/s0022-3476(05)82206-1. https://doi.org/10.1016/S0022-3476(05)82206-1. [DOI] [PubMed] [Google Scholar]
- 6.Rodey GH, Jacob HS, Holmes B, et al. Leucocyte G6PD levels and bacterial activity. Lancet. 1970;1:355–6. doi: 10.1016/s0140-6736(70)90729-4. https://doi.org/10.1016/S0140-6736(70)90729-4. [DOI] [PubMed] [Google Scholar]
- 7.Sanna F, Bonatesta RR, Frongia B, et al. Production of inflammatory molecules in peripheral blood mononuclear cells from severely glucose-6-phosphate dehydrogenase-deficient subjects. J Vasc Res. 2007;44:253–263. doi: 10.1159/000100903. https://doi.org/10.1159/000100903. [DOI] [PubMed] [Google Scholar]
- 8.Batetta B, Pulisci D, Bonatesta RR, et al. G6PD activity and gene expression in leukemic cells from G6PD-deficient subjects. Cancer Lett. 1999;140:53–58. doi: 10.1016/s0304-3835(99)00052-x. https://doi.org/10.1016/S0304-3835(99)00052-X. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S. Chronic granulomatous disease. J Clin Diagn Res. 2015;9:SD01–SD02. doi: 10.7860/JCDR/2015/12139.5945. https://doi.org/10.7860/JCDR/2015/12139.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JPA, Haynes K, Quinn J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr Opin Microbiol. 2009;12:384–391. doi: 10.1016/j.mib.2009.06.007. https://doi.org/10.1016/j.mib.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna M, Caocci G, Ledda A, et al. Glucose-6-Phosphate Deidhrogenase deficiency and risk of invasive fungal disease in patients with acute myeloid leukemia. Leuk Lymphoma. 2017 doi: 10.1080/10428194.2017.1312666. Epub ahead of print https://doi.org/10.1080/10428194.2017.1312666. [DOI] [PubMed] [Google Scholar]
- 12.Domingo GJ, Satyagraha AW, Anvikar A, et al. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Seidlein LMalar J. 2013;12:391. doi: 10.1186/1475-2875-12-391. https://doi.org/10.1186/1475-2875-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamoth F, Cruciani M, Mengoli C, et al. Third European Conference on Infections in Leukemia (ECIL-3). β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3) Clin Infect Dis. 2012;54:633–43. doi: 10.1093/cid/cir897. https://doi.org/10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 14.Pappas GP. Clinical Infectious Diseases Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. https://doi.org/10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racil Z, Kocmanova I, Lengerova M, et al. Difficulties in using 1,3-b-D-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies - high frequency of false-positive results and their analysis. Journal of Medical Microbiology. 2010;59:1016–1022. doi: 10.1099/jmm.0.019299-0. https://doi.org/10.1099/jmm.0.019299-0. [DOI] [PubMed] [Google Scholar]
- 16.Tissot F, Agrawal S, Pagano L. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433–444. doi: 10.3324/haematol.2016.152900. https://doi.org/10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122. doi: 10.1093/cid/cis021. https://doi.org/10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 18.Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, Ruiz Perez de Pipaon M, Hernandez-Caballero C, Lepe-Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp bloodstream infections. J Antimicrob Chemother. 2013;68(1):206–213. doi: 10.1093/jac/dks347. https://doi.org/10.1093/jac/dks347. [DOI] [PubMed] [Google Scholar]