Abstract
Sleep variability has been associated with poor sleep quality in insomnia, but few studies have explored its effects on physical health. In this study of older adults with insomnia, we sought to determine the relationship of variability in objective (actigraphic) measures of sleep with subjective sleep quality and cardiometabolic disease risk measures and to evaluate the effect of non-pharmacologic intervention for insomnia on objective sleep variability. Participants in this study were randomly assigned to 16 weeks of a non-pharmacologic intervention (physical activity or structured social activity). Objective sleep measures were estimated using 7 days of wrist actigraphy completed at both baseline and post intervention (16 weeks). Sleep variability was defined as the standard deviation of objective sleep measures. Participants included seventeen community dwelling older adults with insomnia disorder and short sleep duration. The first analyses were cross-sectional correlations between objective sleep variability, subjective sleep quality and cardiometabolic disease risk measures at baseline. Significant effects in univariate analyses were tested in multivariable models controlling for age and average values of the respective objective sleep measure. Second, changes in sleep variability with the non-pharmacologic interventions were evaluated using repeated measures ANOVA. Cross sectional results indicated greater objective variability in wake after sleep onset (WASO) and fragmentation index at baseline were associated with poorer subjective sleep quality (p<0.05). Higher objective sleep duration variability at baseline was associated with higher HbA1c (p<.01) and sleep onset time variability at baseline was associated with higher BMI (p<0.05). In the pre-post analyses, objective sleep variability in sleep efficiency and WASO decreased from baseline to 16 weeks (p<0.05). Results support strong concurrent associations between objective sleep variability and self-reported sleep quality. Results also demonstrate improvement in some indices of sleep variability with non-pharmacologic intervention. Associations between sleep variability and concurrent cardiometabolic disease risk measures were less consistent. Objective sleep variability can be a useful measure to assess therapies aimed at improving sleep quality.
Keywords: Insomnia, aging, variability, sleep quality, metabolic
Introduction
Changes in the quality and predictability of sleep and other physiological rhythms are some of the hallmarks of aging. Age-related changes in sleep have been well documented even among healthy older adults, including earlier sleep and wake timing, increased sleep fragmentation, decreased slow wave sleep and total sleep time (Bliwise, Ansari, Straight, & Parker, 2005; Dijk, Duffy, Riel, Shanahan, & Czeisler, 1999). Insomnia disorder is defined as difficulty initiating, maintaining sleep or early morning awakenings that cause negative consequences to the individual, such as daytime fatigue, physical malaise or poor mood (International Classification of Sleep Disorders, 2014). Estimates suggest that nearly half of older adults report elevated insomnia symptoms (Foley et al., 1995; Reid et al., 2006).
Recently, sleep variability (i.e., the night-to-night changes in sleep measures within an individual), has garnered attention as an important feature of insomnia, with implications for diagnosis and treatment (Sanchez-Ortuno, Carney, Edinger, Wyatt, & Harris, 2011). Sleep variability has been calculated from sleep diary and actigraphic measures using different methods, such as the standard deviation as well as more complex computations to characterize change from day to day (Sanchez-Ortuno et al., 2011). Buysse and colleagues (2010) compared a sample of patients with chronic insomnia to non-insomnia control participants and demonstrated that despite similar mean values of sleep measures, participants with chronic insomnia had greater sleep variability in both sleep diary and wrist actigraphy measures. Interestingly, individuals with insomnia and comorbid psychiatric disorders have greater sleep variability in sleep diary and actigraphic measures when compared to those without psychiatric comorbidities (Sanchez-Ortuno et al., 2011; Straus, Drummond, Nappi, Jenkins, & Norman, 2015). Studies have also demonstrated individual differences in the degree of sleep variability among insomnia patients. Valliéres and colleagues demonstrated in two studies that some patients have poor sleep every night whereas other patients have no predictable pattern (Vallieres, Ivers, Bastien, Beaulieu-Bonneau, & Morin, 2005; Vallieres, Ivers, Beaulieu-Bonneau, & Morin, 2011). Greater variability in diary and actigraphic measures of sleep has also been linked to poorer global ratings of subjective sleep quality (Sanchez-Ortuno & Edinger, 2012) and lower well-being (Lemola, Ledermann, & Friedman, 2013).
Few studies have investigated the association between sleep variability and measures of physical health. Growing evidence suggests that insomnia, particularly with short sleep duration, confers increased risk of hypertension (Fernandez-Mendoza et al., 2012; Vgontzas, Liao, Bixler, Chrousos, & Vela-Bueno, 2009), poorer metabolic function, diabetes risk (Knutson, Van Cauter, Zee, Liu, & Lauderdale, 2011; Vgontzas, Liao, Pejovic, et al., 2009) and all-cause mortality (Vgontzas et al., 2010). Okun and colleagues (2011) evaluated the relationship between sleep diary measures of bedtime, wake time and time in bed with inflammatory markers. They found that variability in sleep diary reports of bedtime and wake time were associated with higher TNF-α, a marker of low grade inflammation, in participants with and without insomnia. Given that sleep variability is common in insomnia and may be even more prominent in older adults, it is important to better understand the role of sleep variability in physical health among older adults (Luik, Zuurbier, Hofman, Van Someren, & Tiemeier, 2013).
To date, two studies have demonstrated improvements in sleep variability reported in sleep diaries with cognitive behavioral therapy for insomnia (CBT-I) (Sanchez-Ortuno & Edinger, 2012; Suh et al., 2012). However, the study by Sanchez-Ortuno (2012), which also included objective measures (actigraphy) did not observe change in objective sleep variability. There is one study demonstrating improvement in sleep variability with a physical activity intervention among older adults with poor sleep quality (Buman, Hekler, Bliwise, & King, 2011). In this study, 12 months of moderate intensity exercise was associated with improvement in sleep onset latency variability measured via sleep diary. These studies demonstrate that self-reported sleep variability can change with non-pharmacologic interventions, but the effects of interventions on objective measures of variability (polysomnography or wrist actigraphy) are less well known.
The purpose of this study was to evaluate correlates of objective (actigraphic) measures of sleep variability among older adults with insomnia participating in a non-pharmacologic treatment study. We previously published data from a randomized trial of healthy older adults with insomnia demonstrating greater improvements in subjective sleep quality, depressive symptoms and daytime sleepiness with exercise compared with non-physical activity (Reid et al., 2010). In the current study, we conducted a secondary analysis of this data to investigate two questions about sleep variability. First, we conducted cross-sectional analyses to evaluate associations between objective sleep variability with subjective sleep quality and cardiometabolic disease risk at baseline. We predicted that greater objective sleep variability would be associated with poorer concurrent ratings of subjective sleep quality and measures of cardiometabolic disease risk. Second, the design of this study (exercise compared with an active control group, non-physical activity) allowed us to evaluate the effects of these non-pharmacologic interventions on objective measures of sleep variability. Given that improvements in sleep have been demonstrated with both exercise and non-physical activity interventions, we predicted both groups may demonstrate improvements in objective sleep variability (Alessi et al., 2005; Kline et al., 2012; Naylor et al., 2000; Passos et al., 2011).
Methods
Participants
Data for this study were obtained from a project which tested the effects of sleep hygiene education with a 16-week aerobic exercise intervention versus sleep hygiene education and a 16-week non-physical (e.g. social, educational) activity intervention on sleep quality, daytime function, and metabolism in older adults with insomnia (Reid et al., 2010). This study was approved by the Northwestern University Institutional Review Board and all participants provided written informed consent.
Participants included community-dwelling physically inactive older adults (≥ 55 years) with symptoms of sleep disturbance for at least 3 months and a Pittsburgh Sleep Quality Index (PSQI) Score > 5 and Mini Mental Status Exam (MMSE) score > 26. Insomnia diagnosis was assessed by clinical interview with a sleep physician and objective criteria for study participation were confirmed with actigraphy. Objective criteria for insomnia were based on previously published criteria for insomnia with objective short sleep duration. Criteria were evaluated by 7 days of wrist actigraphy including the following cutoffs: sleep efficiency < 80% and/or waking earlier than desired if before 6:00 AM, and a total sleep time ≤ 6.5 hours (Vgontzas et al., 2001). Physical activity level was determined using an open ended question asked at phone screening, “How much physical activity do you participate in?” Responses were probed for activity type, intensity and duration. The criteria for being physically inactive was defined as self-reported participation in light to moderate intensity exercise <30 minutes per day, on < 2 days per week. Participants were also queried about their physical activity associated with work (if still working) to determine if they are involved in physical activity at work that would exclude them from being classified as physically inactive.
Exclusionary criteria included the following: a) Clinically significant comorbid sleep disorders by history or documented on screening polysomnography (apnea/hypopnea index > 10, periodic leg movement arousal index greater than 15, or REM behavior disorder); b) History of cognitive or other neurological disorders; c) History of DSM-IV criteria for any major psychiatric disorder, including mania, alcohol or substance abuse; d) Significant depressive symptoms as assessed by the Center for Epidemiological Studies Depression Scale (CES-D score > 22; Radloff, 1977); e) Unstable or serious medical conditions or cardiopulmonary disease that contraindicate exercise; f) Current or use within the past month, of psychoactive, hypnotic, stimulant or analgesic medications; g) Shift work or other types of self-imposed irregular sleep schedules; h) BMI > 38 kg/m2; i) History of habitual smoking (3 or more cigarettes per week) or caffeine consumption exceeding 300 mg per day.
Procedure
Participants were recruited from the community using flyers, community presentations and newspaper advertisements. Potential participants were asked preliminary questions through a telephone screening, then, were further screened for eligibility criteria based on overnight polysomnography, questionnaires, and 7 days of wrist actigraphy and sleep diaries. Enrolled participants were randomized to one of two non-pharmacologic intervention groups: aerobic exercise plus sleep hygiene or non-physical activity and sleep hygiene. At baseline, participants completed 7 days of wrist actigraphy and then were admitted to the Clinical Research Unit (CRU) for a four-day admission which involved questionnaires, blood draws and performance testing. Exercise testing was conducted in an outpatient setting during the same week as the CRU visits, both at baseline and 16 weeks. At the end of the 16- week intervention period, participants completed another four-day CRU admission for a similar battery of tests and questionnaires. Participants wore the wrist actigraph throughout the 16 week study.
Interventions
Sleep Hygiene
All participants attended one appointment with a sleep specialist in which they were provided with verbal and written sleep hygiene instructions according to materials published by the American Academy of Sleep Medicine. Recommendations included reducing alcohol, caffeine and smoking, avoiding physical activity close to bedtime, winding down before bed and setting a consistent wake time. All patients were instructed by research staff to continue working to improve sleep hygiene during study visits every 2 weeks.
Exercise Intervention
After completion of the 6-week conditioning period, participants were instructed to exercise for either two 20-minute sessions or one 30 – 40 minute session at 75% of their maximum HR four times per week for the remaining 10 weeks. Exercise sessions were conducted in the afternoon/evening (1–7PM) and participants were not permitted to miss more than 1 exercise session per week. Participants engaged in at least two of three aerobic activities (walking, stationary bicycle, or treadmill) per week and engaged in each activity at a similar level of exertion, as measured by the Borg scale of Perceived Exertion (Borg, 1970) and a heart rate monitor. Participants attended the first 2–3 sessions with a certified fitness trainer, then the remaining sessions were conducted on their own. They were not prohibited from exercising in groups, but most participated in individual exercise at home or at a gym, most frequently, treadmill walking. Additional details of the exercise intervention can be found in a previous publication (Reid et al., 2010).
Non-physical activity
Participants in this group selected structured recreational or education activities (e.g., parks and recreation, YMCA, museum lectures, church activities) that met for at least 30 minutes, 3–5 times per week, for 16 weeks. Participants were provided with a list of suggested activities to assist with meeting the requirements for the intervention. For example, a participant could attend a cooking class at the YMCA, a lecture at the museum, and a bible study group. Study staff approved all activities and reviewed activity diaries at study visits.
Compliance was defined as a minimum of three exercise or non-physical activity sessions per week and was monitored using daily exercise and activity (non-physical activity) diaries. Diaries included the type of physical or non-physical activity, duration, time of day, and location. Compliance was verified by the research staff during twice monthly meetings with the participant.
Measures
Self-Report Questionnaires
Subjective sleep quality was measured by The PSQI (Buysse, Reynolds, Monk, Berman & Kupfer, 1989). This 19-item measure assessed self-reported sleep quality and disturbances over a one-month interval. A higher PSQI global score indicates greater sleep disturbance. Scores >5 are associated with clinically significant sleep disturbance (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Although not specific to insomnia, this measure has been designed for use in clinical populations and is validated in older adults (Smyth, 1999).
Sleep and Sleep Variability
Participants completed daily sleep diaries which were collected at two week intervals to assist in scoring actigraphy. Participants recorded bed time, wake time, number of awakenings during the night, and daily subjective rating of sleep quality from 1 (excellent) to 4 (poor).
Objective sleep measures were estimated using wrist actigraphy (AW-64 Actiwatch, Mini Mitter Co. Inc., Bend, OR). Actigraphs were worn on the non-dominant wrist and set with 30 second epoch length and medium sensitivity. Actigraphic sleep measures were calculated using Actiware-Sleep 3.4 software with default settings and include the following measures: sleep latency, sleep onset time, sleep offset time, minutes of wake after sleep onset (WASO), sleep duration, sleep efficiency and fragmentation index. The sleep fragmentation index was calculated by summing two percentages: 1) the percentage of 30-second epochs in the sleep period with movement (defined as an activity count ≥ 2) and 2) the percentage of all quiet blocks in the sleep period (consecutive epochs with activity count < 2) that are ≤ 1 min in duration. Bedtime and rise time were manually entered into the Actiware software by the scorer and used to define the rest period. Periods where the watch was clearly removed or reported as removed (e.g., bathing, swimming) were not included in analysis. A day was not considered valid if there was any off-wrist time reported during the period between the bedtime and rise time reported on the sleep diary. Actigraphic measures are designated in the text with the subscript “acti” (e.g., WASOacti)
Objective sleep variability was defined as standard deviation (SD) of the 7 days of wrist actigraphy recordings at baseline and 16 weeks (Okun et al., 2011). Variability values were considered valid if participants had at least 4 consecutive days recorded during the baseline and final week.
Cardiometabolic Disease Risk Assessment
Plasma glucose and insulin were measured by a fasting blood draw taken at 8:45 AM of day 2 of the CRU visit. After collection, samples were centrifuged, aliquoted, and stored at −70ºC until assay. Plasma glucose levels were analyzed using the Beckman CX3D Glucose Analyzer (Beckman Coulter Inc., Brea, CA) using the glucose oxidase method. Insulin was measured using the Siemens Immulite 2000 chemiluminescent immunoassay. This assay is sensitive to 14.4 pmol/L. The oral glucose tolerance test (OGTT) was conducted on day 2 of the baseline and 16 week CRU visits. Fasting blood draws were performed at 8:45 AM and 8:58 AM. Then, 75 g Trutol was administered at 8:59 AM. Blood draws occurred at 30, 60, 90, 120 and 180 minutes after Trutol. Homeostatic Model of Assessment (HOMA) was calculated as a measure of insulin resistance (Matthews et al., 1985). We computed this value using the standard equation: (fasting glucose (mg/dL) × insulin (pmol/L)/405). Glycosylated hemoglobin (HbA1c) was calculated using the first fasting blood draw on day 1 of admission to the CRU.
High sensitivity C-reactive protein (CRP) was measured from fasting blood samples obtained from participants during the morning of day 2. After collection, samples were centrifuged, aliquoted, and stored at −70°C until assay. Plasma levels of CRP were analyzed using chemilluminescence methodology (Immulite 2000, Siemens USA, Malvern, PA), according to the manufacturer’s instructions.
Cortisol was measured via blood samples measured every 30 minutes during day 3. Samples were analyzed using chemilluminescence methodology (Immulite 2000, Siemens USA, Malvern, PA), according to the manufacturer’s instructions. After collection, samples were centrifuged, aliquoted, and stored at −70°C until assay. We averaged the 3 cortisol samples measured prior to lights out time to create a measure of pre-sleep cortisol.
BMI (kg/m2) was calculated using height and weight measurements taken at the baseline and post intervention (16 week) admission to the CRU.
Data Analysis
Data were analyzed using SPSS v. 20 (IBM, inc.). We first used t-tests for independent means to compare baseline values (demographics, subjective sleep quality and wrist actigraphy values) between the exercise and non-physical activity groups and to compare study drop outs to completers. Next, we conducted bivariate correlations between objective sleep variability with mean objective sleep measures, subjective sleep quality and cardiometabolic risk measures at baseline. To determine the independent effects of baseline objective sleep variability, we constructed multivariate models for significant correlations. Models tested the associations between objective sleep variability with subjective sleep quality and cardiometabolic risk measures at baseline, controlling for age and mean baseline objective sleep values. Finally, we conducted longitudinal analyses to evaluate changes in objective sleep variability from baseline to 16 weeks using repeated measures ANOVA. In these analyses, we combined the exercise and non-physical activity groups due to low power to detect differences between groups and tested the main effect of time (baseline and 16 weeks entered in the repeated measures model). In an exploratory analyses, we evaluated the effects of non-pharmacologic intervention group and the interaction of non-pharmacologic intervention group (exercise and non-physical activity) and time (baseline and 16 weeks) in the repeated measures ANOVA. Statistical significance was defined as p values <0.05 on two-tailed tests.
Results
Participant Characteristics
Demographic, sleep and cardiometabolic disease risk measures for participants who completed the study are listed in Table 1. Twenty-three participants met inclusion criteria and were enrolled in the study. The final sample included 10 participants in the exercise group and 7 participants in the non-physical activity group. Of the six participants who did not complete the study, four participants withdrew from the non-physical activity group, one was withdrawn from the exercise group due to non-compliance and one participant’s data was withdrawn due to failure to meet study criteria. There were no significant differences between completers and dropouts in age, baseline PSQI score or depressive symptoms. Average age of the participants was 61.5 (SD= 4.3) years. The majority of participants were women; there was one male enrolled in the study. We conducted analyses with and without this participant. Since there were no differences in the results by including the one male participant, we present the analysis with this participant included. Average baseline PSQI was 9.2 (SD= 2.3). The average BMI was in the overweight range. Participants had an average sleep durationacti of 6 hours and WASOacti of 57 minutes per night at baseline. None of the participants met criteria for diabetes based on fasting glucose values. There were no significant differences between the non-pharmacologic intervention groups in age, BMI, PSQI, actigraphy means, actigraphy standard deviations or cardiometabolic disease risk variables at baseline.
Table 1.
Baseline Participant Characteristics
| Exercise (n=10) | Non-physical activity (n=7) | All Participants | |
|---|---|---|---|
| Age, years | 61.4 (4.4) | 61.9 (4.6) | 61.6 (4.3) |
| Sex | 10 females/0 males | 6 females/1 male | 16 females/1 male |
| BMI, kg/m2 | 26.7 (4.9) | 27.5 (3.5) | 27.0 (4.3) |
| PSQI Global Score | 9.9 (2.3) | 8.1 (1.8) | 9.2 (2.3) |
| Sleep latencyacti (minutes) | 18.6 (18.6) | 25.2 (19.8) | 25.8 (18.6) |
| Sleep durationacti (hours) | 5.92 (0.60) | 6.22 (1.26) | 6.03 (0.88) |
| WASOacti (minutes) | 60.0 (30.0) | 55.8 (42.0) | 57.0 (34.2) |
| Sleep efficiencyacti, % | 85.1 (6.3) | 78.2 (11.3) | 82.5 (8.9) |
| Fragmentation indexacti | 35.2 (11.1) | 34.8 (13.7) | 35.1 (11.8) |
| Fasting glucose, mg/dL | 88.4 (7.1) | 91.5 (10.6) | 89.6 (8.4) |
| Fasting insulin, pmol/L | 46.5 (21.6) | 80.7 (57.4) | 60.6 (42.4) |
| HOMA-IR | 10.2 (4.5) | 18.4 (14.5) | 13.5 (10.4) |
| HbA1c, % | 5.7 (0.4) | 6.1 (0.6) | 5.8 (0.5) |
| Pre-sleep Cortisol (μg/dL) | 4.2 (2.0) | 5.4 (2.8) | 4.7 (2.4) |
| CRP mg/L | 0.18 (0.22) | 0.47 (.41) | 0.34 (.36) |
Acti= wrist actigraphy measure. BMI= Body Mass Index; PSQI= Pittsburgh Sleep Quality Index; WASO= Wake After Sleep Onset. HOMA-IR= Homeostatic model of assessment- insulin resistance. CRP= C-reactive protein. Note: Missing data n=2 exercise and n=3 non-physical activity for HbA1c, n= 1 exercise and n=1 non-physical activity for CRP. P values were >0.05 for all comparisons between groups on pre-treatment variables.
Correlations between objective mean and variability sleep measures at baseline
There were moderate to high correlations between mean and variability values (SD) of several objective sleep measures: sleep latencyacti (r=0.97, p<0.01), WASOacti (r=0.92, p<0.01), sleep efficiencyacti (r=−0.75, p<0.01) and fragmentation index (r=0.51, p<.05). Sleep variability measures were not associated with mean values for sleep durationacti, sleep onset timeacti, sleep offset timeacti or time in bedacti.
Correlations between objective sleep variability and self-reported sleep quality at baseline
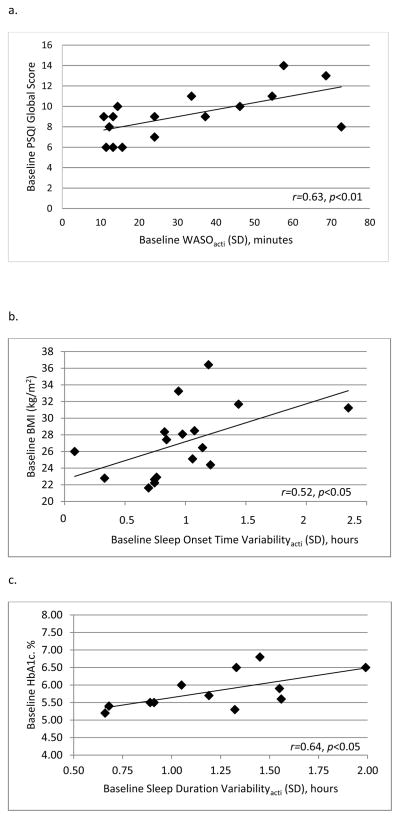
Due to our small sample size, we conducted outlier analyses for significant multivariable models evaluating correlations between objective sleep variability with self-reported sleep quality and cardiometabolic measures at baseline. None of the points were considered multivariate outliers (Mahalanobis Distance p values >0.05). In univariate analyses, higher objective sleep variability was associated with poorer subjective sleep quality on the global scale of the PSQI for WASOacti (r=0.63, p<0.01; Figure 1.a), sleep efficiencyacti (r=0.54, p<0.05), and fragmentation indexacti (r=0.62, p<0.01). The relationship between WASOacti variability and subjective sleep quality remained significant after controlling for age and mean baseline WASOacti (B= 9.67 SE=3.43, p<0.05, r2Δ=0.28), as did the relationship for fragmentation indexacti variability (B= 0.24 SE=0.95, p<0.05, r2Δ=0.29) and subjective sleep quality. The relationship between sleep efficiencyacti variability and subjective sleep quality was attenuated and became non-significant in the multivariable model (p=0.15). Sleep timingacti variability (onset, offset) and time in bed variability were not associated with subjective sleep quality.
Figure 1.
Panel a. Association between subjective sleep quality and objective variability in WASO at baseline. WASO= Wake After Sleep Onset, Acti= actigraphy variable.
Panel b. Association between glycosylated hemoglobin (HbA1c) and objective variability in sleep duration at baseline (n=12). Acti= actigraphy variable.
Panel c. Association between BMI and objective variability in sleep onset time at baseline. Acti= actigraphy variable.
Correlations between objective sleep variability with cardiometabolic disease risk at baseline
Greater variability in sleep durationacti (r=0.64, p<0.05) was associated with higher HbA1c (Figure 1.b). This relationship remained significant after controlling for age and baseline mean sleep durationacti (B= 0.79 SE=0.31, p<0.01, r2Δ=0.35). Greater variability in sleep onset timeacti was associated with higher BMI (r=0.52, p<0.05; Figure 1.c). In the multivariable model, variability in sleep onset timeacti remained associated with BMI, independent of average baseline sleep onset timeacti and age (B= 0.001 SE=0.001, p<0.05, r2Δ=0.24). Fasting glucose, fasting insulin, HOMA, glucose from the OGTT at 120 minutes, pre-sleep cortisol and CRP were not associated with sleep variability measures.
Change in variability in objective sleep variability from baseline to 16 weeks
Table 2 lists baseline and post-intervention (week 16) objective sleep variability values among participants in both non-pharmacologic intervention groups pooled together. Sleep efficiencyacti variability and WASOacti variability significantly decreased from baseline to 16 weeks (effect for time p<0.05). We conducted exploratory analyses to test the effects of non-pharmacologic intervention group and group × time interactions. There were no significant group or group × time interactions. Effect sizes between groups were small (partial eta squares <0.001–1.0 and observed power ranged from 0.02–0.05).
Table 2.
Objective sleep variability (SD) at baseline to 16 weeks post intervention
| Sleep Variabilityacti | Baseline | 16 Weeks | P-value Effect for Time* |
|---|---|---|---|
| Time in bedacti (minutes) | 79 (19) | 60 (33) | 0.09 |
| Sleep onset timeacti (minutes) | 58 (29) | 66 (46) | 0.58 |
| Wake timeacti (minutes) | 62 (29) | 53 (29) | 0.43 |
| Sleep latencyacti (minutes) | 25 (23) | 23 (20) | 0.63 |
| Sleep durationacti (minutes) | 70 (21) | 62 (28) | 0.36 |
| WASOacti (minutes) | 33 (21.6) | 23 (13) | 0.03 |
| Sleep efficiencyacti (%) | 7.4 (4.04) | 5.1 (1.95) | 0.02 |
| Fragmentation index acti | 15.02 (6.44) | 13.19 (5.43) | 0.18 |
Effect for time in repeated measures ANOVA with baseline and 16 week intervention. Acti= actgraphy variable, WASOacti= wake after sleep onset calculated via actigraphy. P values listed are for the effects of time for participants in the exercise and non-physical activity groups combined. In exploratory models including group and time × group interactions, there were no significant effects for group or differences in change between groups for all objective sleep variability outcomes (p values >0.1).
Missing data due to missed sleep diary days: time in bed post (n= 2), sleep latency pre and post (n=3), sleep efficiency pre (n=1), sleep efficiency post (n=2). Time in bed is defined as bedtime to wake time, as reported in sleep diaries.
Discussion
Our results support and extend prior research on sleep variability in insomnia by demonstrating cross-sectional associations between objective sleep variability with subjective sleep quality among older adults with insomnia. Although many objective sleep variability measures had moderate to strong correlations with mean levels of the respective objective sleep measure at baseline, the associations between objective sleep variability and subjective sleep quality were not explained by mean levels alone. This indicates that objective sleep variability is independently associated with subjective sleep quality, rather than a feature of poor overall objective sleep quality. We also observed that some measures of objective sleep variability were correlated with cardiometabolic disease risk measures at baseline (HbA1c and BMI). However, the relationships between objective sleep variability and cardiometabolic disease risk measures were less consistent than associations with subjective sleep quality.
The results of our study demonstrate that objective sleep variability measures captures important information about global ratings of subjective sleep quality. Similar to previous studies, we found that objective sleep variability is highly correlated with subjective sleep quality whereas the mean levels of objective variables were not associated with subjective sleep quality (Sanchez-Ortuno & Edinger, 2012). Lack of association between mean values of objective sleep recordings and subjective ratings of sleep quality has been previously reported (Backhaus, Junghanns, Broocks, Riemann, & Hohagen, 2002; Buysse et al., 2008; Buysse et al., 1989; Grandner, Kripke, Yoon, & Youngstedt, 2006). This suggests that perhaps one of the most frustrating aspects of insomnia is not the consistent level of sleep disruption but the inconsistency of sleep.
Among older adults, variability may reflect a state of increasing instability due to age-related changes in sleep/wake regulation. According to the two process model of sleep, homeostatic sleep pressure and the circadian alerting signal interact to promote a consolidated sleep/wake pattern in the 24 hour day (Borbely, 1982; Daan, Beersma, & Borbely, 1984). Changes in either system may contribute to greater instability of sleep efficiency and WASO among older adults. For example, there is an age-related alteration in the homeostatic sleep regulation, as indicated by a decline in slow wave sleep and electroencephalographic (EEG) slow wave activity (Bliwise et al., 2005; Dijk & Beersma, 1989; Dijk, Beersma, & van den Hoofdakker, 1989). There may also be a lower daytime sleep propensity and reduced capacity for sleep among older adults (Klerman & Dijk, 2008). Furthermore, circadian changes include a phase advance (Carrier, Monk, Buysse, & Kupfer, 1997; Duffy, Dijk, Klerman, & Czeisler, 1998; Duffy et al., 2002) and reduced amplitude of the circadian rhythm (Dijk et al., 1999; Munch et al., 2005) as well as weaker wake promoting signals in evening hours (Cajochen, Munch, Knoblauch, Blatter, & Wirz-Justice, 2006; Munch et al., 2005). Together, changes in the homeostatic drive and the timing and amplitude of the circadian rhythm contribute to sleep fragmentation, early morning awakenings as well as variability of sleep among older adults. Studies evaluating rest/activity rhythms in middle aged and older adults have demonstrated diminished ability to sustain an active or inactive state over the 24-hour period, but also more stability of the 24-hour rhythm with aging (Luik et al., 2013).
Our study also demonstrates improvement in some measures of objective sleep variability (sleep efficiency and WASO) with non-pharmacologic intervention. Previous studies have reported improvements in subjective sleep variability with CBT-I (Sanchez-Ortuno & Edinger, 2012) and aerobic exercise (Buman et al., 2011). In CBT-I, use of sleep restriction, which increases sleep efficiency by limiting time in bed thus increasing sleep drive, reduces variability in sleep times by design. In our study, we cannot determine specifically which components of the interventions achieved the reductions in sleep variability. Participants in both interventions received sleep hygiene education, which includes recommendations for a consistent bedtime and wake time (Stepanski & Wyatt, 2003). Furthermore, common structured elements of both interventions may have enhanced circadian rhythmicity and/or increased homeostatic drive for sleep. Improvements in sleep with exercise have been documented in numerous studies, both in healthy and insomnia populations (King, Oman, Brassington, Bliwise, & Haskell, 1997; King et al., 2008; Passos et al., 2011; Reid et al., 2010; Youngstedt, O’Connor, & Dishman, 1997). Improvement in homeostatic sleep drive is also one of the hypothesized mechanisms by which exercise improves sleep (Youngstedt, 2005).
Results of this study should be interpreted in light of several limitations. The small sample size limits our power to evaluate differences between the groups as well as the cross-sectional associations. Also, the homogenous nature of our sample (predominately female, older adults with insomnia and short sleep duration) limits generalizability. The health of our sample (e.g. none of the participants had diabetes or high inflammation) may have also contributed to floor effects in some of the measures, including fasting glucose, insulin and CRP. Due to the small sample size, we could not evaluate if changes in objective sleep variability were associated with changes in cardiometabolic risk measures. Despite these limitations, the strength of effects we observed in this small sample in a healthy older population suggests that effect sizes are robust. Future studies are needed to confirm results in a larger sample, further understand the health consequences of sleep variability as well as to develop treatments aimed at reducing variability.
In conclusion, we observed that objective variability of WASO and fragmentation index were highly associated with subjective sleep quality. We also demonstrated improvements in WASO and sleep efficiency objective sleep variability with non-pharmacologic treatment. Further research is needed to understand the role of sleep variability in both healthy sleepers and those with insomnia. Individuals who have insomnia and high sleep variability may represent a distinct subtype with greater morbidity, and it may be useful to include sleep variability in future treatment studies. Further research is needed to understand the role of sleep variability in cardiometabolic disease risk and as an insomnia outcome measure.
Acknowledgments
The work for this project was funded by the following grants from the National Institute of Health: P01 AG11412, M01 RR00048, UL1RR025741, K23 HL091508, T32AG020506, 1K23HL109110. We also thank Erik Naylor, PhD, Lisa Wolfe, MD, Brandon Lu, MD, Rosemary Ortiz, Susan Benloucif, David Clough and Zach Warburg for their contributions to this project.
Contributor Information
Kelly Glazer Baron, Department of Neurology, Northwestern University, Chicago, Illinois.
Kathryn J. Reid, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Roneil G. Malkani, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Joseph Kang, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Phyllis C. Zee, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
References
- Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803–810. doi: 10.1111/j.1532-5415.2005.53251.x. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Ansari FP, Straight LB, Parker KP. Age changes in timing and 24-hour distribution of self-reported sleep. Am J Geriatr Psychiatry. 2005;13(12):1077–1082. doi: 10.1176/appi.ajgp.13.12.1077. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- Buman MP, Hekler EB, Bliwise DL, King AC. Exercise effects on night-to-night fluctuations in self-rated sleep among older adults with sleep complaints. J Sleep Res. 2011;20(1 Pt 1):28–37. doi: 10.1111/j.1365-2869.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, Monk TH. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, … Matthews KA. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Munch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23(1–2):461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the ‘middle’ years of life (20–59 y) J Sleep Res. 1997;6(4):230–237. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246(2 Pt 2):R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72(4):312–320. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol Aging. 1989;10(6):677–682. doi: 10.1016/0197-4580(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275(5 Pt 2):R1478–1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282(2):E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Classification of Sleep Disorders. 3. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277(1):32–37. [PubMed] [Google Scholar]
- King AC, Pruitt LA, Woo S, Castro CM, Ahn DK, Vitiello MV, … Bliwise DL. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63(9):997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep--implications for insomnia. Curr Biol. 2008;18(15):1118–1123. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Sui X, Hall MH, Youngstedt SD, Blair SN, Earnest CP, Church TS. Dose-response effects of exercise training on the subjective sleep quality of postmenopausal women: exploratory analyses of a randomised controlled trial. BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;8(8):e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223–1230. doi: 10.3109/07420528.2013.813528. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Munch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, … Cajochen C. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26(9):1307–1319. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Naylor E, Penev PD, Orbeta L, Janssen I, Ortiz R, Colecchia EF, … Zee PC. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23(1):87–95. [PubMed] [Google Scholar]
- Okun ML, Reynolds CF, 3rd, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73(2):142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos GS, Poyares D, Santana MG, D’Aurea CV, Youngstedt SD, Tufik S, de Mello MT. Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med. 2011;12(10):1018–1027. doi: 10.1016/j.sleep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11(9):934–940. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KJ, Martinovich Z, Finkel S, Statsinger J, Golden R, Harter K, Zee PC. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14(10):860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ortuno MM, Carney CE, Edinger JD, Wyatt JK, Harris A. Moving beyond average values: assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. Sleep. 2011;34(4):531–539. doi: 10.1093/sleep/34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ortuno MM, Edinger JD. Internight sleep variability: its clinical significance and responsiveness to treatment in primary and comorbid insomnia. J Sleep Res. 2012;21(5):527–534. doi: 10.1111/j.1365-2869.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- Smyth C. The Pittsburgh Sleep Quality Index (PSQI) J Gerontol Nurs. 1999;25(12):10–11. doi: 10.3928/0098-9134-19991201-10. [DOI] [PubMed] [Google Scholar]
- Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7(3):215–225. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- Straus LD, Drummond SP, Nappi CM, Jenkins MM, Norman SB. Sleep Variability in Military-Related PTSD: A Comparison to Primary Insomnia and Healthy Controls. J Trauma Stress. 2015;28(1):8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S, Nowakowski S, Bernert RA, Ong JC, Siebern AT, Dowdle CL, Manber R. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13(5):469–475. doi: 10.1016/j.sleep.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14(4):447–453. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- Vallieres A, Ivers H, Beaulieu-Bonneau S, Morin CM. Predictability of sleep in patients with insomnia. Sleep. 2011;34(5):609–617. doi: 10.1093/sleep/34.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, … Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, … Bixler EO. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–365. xi. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, O’Connor PJ, Dishman RK. The effects of acute exercise on sleep: a quantitative synthesis. Sleep. 1997;20(3):203–214. doi: 10.1093/sleep/20.3.203. [DOI] [PubMed] [Google Scholar]