Abstract
In the United States, research is limited on the mechanisms that link socioeconomic and structural factors to HIV diagnosis outcomes. We tested whether neighborhood income inequality, socioeconomic deprivation, and black racial concentration were associated with gender-specific rates of HIV in the advanced stages of AIDS (i.e., late HIV diagnosis). We then examined whether HIV testing prevalence and accessibility mediated any of the associations above. Neighborhoods with highest (relative to lowest) black racial concentration had higher relative risk of late HIV diagnosis among men (RR=1.86; 95%CI=1.15, 3.00) and women (RR=5.37; 95% CI=3.16, 10.43) independent of income inequality and socioeconomic deprivation. HIV testing prevalence and accessibility did not significantly mediate the associations above. Research should focus on mechanisms that link black racial concentration to HIV diagnosis outcomes.
Keywords: Income inequality, Socioeconomic deprivation, Black racial concentration, Late HIV diagnosis, HIV testing
1. Introduction
In the United Sates (U.S.), HIV diagnosis remains concentrated in geographic areas characterized by high economic inequality and neighborhood black racial concentration (Rebeiro et al., 2016; Nunn et al., 2014; Adimora and Schoenbach, 2005, AIDSvu, 2014, Centers for Disease Control and Prevention, 2013a). Those factors are contributing drivers to HIV infection in the population (Barnett and Whiteside, 2006; Gupta et al., 2008; Parker et al., 2000). Moreover, those factors more sufficiently explain geographic HIV-related disparities over and above individual-centered behavioral and biomedical determinants (e.g., injection drug use) (Decosas, 2002; Sutherland and Hsu, 2012; Gillespie et al., 2007; Millett et al., 2012; Maas et al., 2007). We know less, however, about the mechanisms that link socioeconomic and structural factors to HIV diagnosis outcomes (Kippax and Holt, 2009), particularly in the U.S. (El-Sadr et al., 2010).
The political economy of health is a powerful theoretical framework to guide research on socioeconomic and structural determinants of HIV diagnosis outcomes (Johnston et al., 2015; Hunter, 2007; Altman, 1999). A particular strength of this framework is a description about how political and economic power and socio-cultural factors interact to determine the unequal distribution of resources that constrain individuals’ agency (Minkler et al., 1994; Phelps, 1985). The political economy of health framework is particularly relevant for analyzing the role of socioeconomic determinants of HIV diagnosis within the U.S. For instance; the U.S. has the fourth highest income inequality among 34 other developed nations (OECD, 2014). In the U.S.; socioeconomic and structural factors are deeply rooted in historical (Schmitt, 2009; Norton and Ariely, 2011) and current political relations (Sanders, 2016; Lauter, 2015). Racial residential segregation is a fundamental cause of disparities in health (Osypuk and Acevedo-Garcia, 2010; Williams and Collins, 2001).
1.1. Socioeconomic and structural drivers of HIV diagnosis
Income inequality and socioeconomic deprivation are two key socioeconomic drivers of HIV diagnosis and transmission outcomes. Income inequality is a relative measure of economic opportunity, which reflects the gap across a continuum of high to low income (Kawachi et al., 1999). High income inequality has been associated with high HIV prevalence and incidence (Durevall and Lindskog, 2012; Lamontagne and Stockemer, 2010; Brodish, 2014; Lim et al., 2014). Socioeconomic deprivation is an absolute measure of economic inequality often operationalized through an index of factors that include education, unemployment, median household income, and percentage of families in poverty (Niyonsenga et al., 2013; Krieger et al., 2003). Higher socioeconomic deprivation within neighborhoods has been associated with higher rates of new HIV diagnosis (An et al., 2013) and late HIV diagnosis among individuals (Gueler et al., 2015). Neighborhood racial concentration is a structural factor that is strongly and positively correlated with socioeconomic deprivation (Quillian, 2012), and documented as an antecedent of economic inequality (Shapiro, 2004; Massey and Eggers, 1990). While the two are strongly correlated, limited empirical evidence exists on whether neighborhood racial concentration is a key determinant of HIV diagnosis and transmission outcomes (Nunn et al., 2014; Brawner, 2014).
1.2. Mechanisms linking income inequality, socioeconomic deprivation, and black racial concentration to HIV diagnosis
Individual HIV testing and community-level HIV testing accessibility are critical components of HIV prevention in the population (Coates et al., 2014; Moyer, 2013). In fact, currently, the Centers for Disease Control and Prevention (CDC) recommend screening for everyone in the population as routine part of their health care at least once a year (Centers for Disease Control and Prevention, 2010). Empirical studies showed that expanded HIV testing activities are associated with increases in identifying number of HIV-infected persons unaware of their HIV status (Centers for Disease Control and Prevention, 2011), decreases in rates of late HIV diagnosis in the population (Ransome et al., 2015), and more timely entry into HIV care (Castel et al., 2013). HIV testing and HIV testing accessibility are along the pathway between upstream socioeconomic and structural determinants and HIV infection (Pellowski et al., 2013) and transmission in the population (Poundstone et al., 2004).
1.3. Motivation for the current study
Despite the empirical evidence linking socioeconomic and structural determinants to HIV diagnosis and transmission, and linking HIV testing to protective effects on HIV outcomes; there is limited empirical research quantifying the extent to which HIV testing mediates the impact of those determinants on HIV outcomes. We theorize below on potential pathways from socioeconomic and structural determinants through HIV testing and the potential mediating impact on late HIV diagnosis.
First, income inequality can create political power imbalances within and across neighborhoods. Relative power theory (in contrast to conflict theory) posits that economic inequality has negative impacts on political engagement because money buys influence (Solt, 2008). Therefore, in neighborhoods with high income inequality, power imbalance could favor those with high socioeconomic status who may have the a greater political clout to ensure they prevail on conflicts of any issues (Solt, 2008). It is plausible also that individuals with high socioeconomic status could use their political power to lobby for the placement and proximity of HIV testing facilities further away from their residences to disassociate themselves from HIV risk and HIV-related stigma (the “NIMBY” (not in my backyard) phenomenon) (Takahashi, 1997, 1998). Income inequality also erodes social capital and weaken social ties (Kawachi et al., 2008), which can influence HIV testing among individuals (Grover et al., 2016) because those with weaker ties may have less access to relevant HIV knowledge and educational resources (Jesmin and Chaudhuri, 2013).
Neighborhoods with high socioeconomic deprivation are often isolated from mainstream social networks and economic opportunities, as a result of disinvestments in capital resources (Wilson, 2012; Massey, 2007). Isolation from mainstream resources such as HIV testing centers can thwart individual’s likelihood of timely HIV testing and subsequent diagnosis (Leibowitz and Taylor, 2007). Socioeconomic deprivation may also drive late HIV diagnosis through social observation (Latkin et al., 2010). Socioeconomically deprived neighborhoods tend also to have a higher distribution of persons with poor HIV prognosis (e.g., lower HIV survival rates and higher mortality) (Harrison et al., 2008; Wallace, 2003). It is plausible then that individuals in socioeconomically deprived communities are at greater exposure to observing persons plagued by worse HIV prognosis (e.g., lower virologic suppression)(Gueler et al., 2015). Therefore, HIV-infected individuals in socioeconomically deprived communities at higher exposure to observing persons with worse HIV prognosis may engender fatalism views about HIV and plausibly delay HIV testing (Simons et al., 2015), which subsequently leads to late HIV diagnosis. Neighborhoods with high black racial concentration disproportionately have poorer housing and social conditions and a greater number of barriers to accessing and attracting prevention services (Williams and Collins, 2001; Massey and Denton, 1993). Evidence also suggests that the success or failures of HIV prevention efforts in neighborhoods with high black racial concentration are influenced by stigma and attitudes among [fewer number of] non-blacks within those communities (Reid et al., 2014). Next, a direct association between high neighborhood black racial concentration and high rates of late HIV diagnosis is a function of two primary factors. These include greater exposure to a high HIV prevalence pool of individuals (i.e., especially among African Americans) in geographically isolated areas and sexual mixing patterns among persons of the same racial and ethnic group (Brawner, 2014; Adimora and Schoenbach, 2005; Chopel et al., 2015). The indirect association between neighborhood black racial concentration and HIV testing predicting late HIV diagnosis rates is complex. Neighborhoods with high black racial concentration are often characterized by “racialized risk environments” (Cooper et al., 2015), typified by indicators such as racialized housing, discrimination in medical and social services, and racialized policing and incarceration (Friedman et al., 2009). Features of the racialized risk environment such as incarceration have been correlated with higher HIV infection and transmission risk behaviors (Pouget et al., 2010). Another possible pathway is that high incarceration rates weaken social networks (Roberts, 2004), which in turn could limit one’s knowledge of HIV prevention resources in the community (Jesmin and Chaudhuri, 2013). A third potential pathway is that norms reflecting social distrust and HIV/AIDS conspiracy beliefs, which can inhibit accessing HIV prevention services (Bogart and Thorburn, 2005; Bogart et al., 2010), may be more pervasive in neighborhoods with high black racial concentration. On the other hand, research showed that some aspects racialized risk environments, including perceived discrimination, were associated with health promoting HIV prevention behaviors. For example, one study showed that higher perceived everyday racism, at the individual level, was associated with higher HIV testing rates among African Americans (Ford et al., 2009). Another study showed that higher perceived provider racial discrimination was associated with higher HIV testing among black men who have sex with men (Irvin et al., 2014).
Next, there also may be a selection effect where high neighborhood income inequality, socioeconomic deprivation, and black racial concentration may drive higher HIV testing. This is because testing resources may be diverted to those communities to address the HIV high burden (Myers et al., 2012, New York City Department of Health and Mental Hygiene, 2011).
In this study, we investigate the role of neighborhood income inequality, socioeconomic deprivation, and black racial concentration on late HIV diagnosis in a large urban U.S. city. We then examine whether HIV testing prevalence and HIV testing accessibility mediate the associations between the determinants above and late HIV diagnosis. Given the empirical evidence on the topic, Fig. 1 shows a heuristic model displaying the proposed directions of associations from the exposures and mediators to late HIV diagnosis.
Fig. 1.
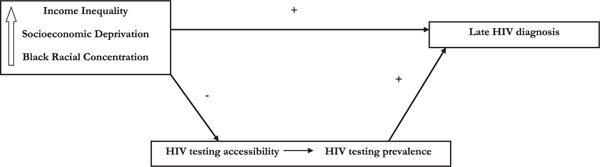
Heuristic model given the body of empirical evidence on the topic showing the proposed direction of associations among neighborhood income inequality, socioeconomic deprivation, black racial concentration, and the mediating associations of HIV testing on late HIV diagnosis.
2. Methods
We examined late HIV diagnosis because, in the United States, 24% (9,839/40,988) of newly diagnosed HIV were in the advanced stages of AIDS (i.e., late HIV diagnosis) (Centers for Disease Control and Prevention, 2015). At the population-level; late HIV diagnosis is associated with onward HIV transmission (Girardi et al., 2007; Wohlgemut et al., 2012) in part because of the increased risk of ongoing transmission associated with unsuppressed viral load among HIV-infected persons unaware of their HIV status (Quinn et al., 2000; Wilson et al., 2008).
New York City (NYC) is the study setting because the city has high income inequality (New York City Comptroller and Liu, 2012), high black racial concentration (Alba and Romalewski, 2012), and high prevalence (22%) (AIDSvu, 2016) and rate (10.65 per 100,000 persons) of late HIV diagnosis (Ransome et al., 2015). The units of analysis are ZIP codes because, in NYC, this unit approximates neighborhoods well (Silver and Messeri, 2014).
2.1. Sample
The New York City Department of Health and Mental Hygiene (NYC DOHMH) provided aggregate ZIP code-level gender-specific raw counts of HIV diagnosis from the HIV surveillance registry (The Registry). The Registry is a population-based source of all persons diagnosed with HIV infection since 2000 or AIDS since 1981 who meet CDC’s case definitions for surveillance and were reported to the NYC DOHMH (Fleming et al., 1999). Data provided included persons residing in NYC at the time of HIV diagnosis and excluded persons undomiciled or living in a shelter or non-residential, unknown or missing ZIP codes. A total of 1748 cases of late HIV diagnoses were available across 174 residential ZIP codes. Data are late HIV diagnosis from 2009 to 10 as reported to the NYC DOHMH by June 30, 2013.
2.2. Outcome variable
We defined late HIV diagnosis as a CD4 count test result of 200 cells/μl or less or an AIDS-defining illness within 12 months of the date of HIV diagnosis (Schneider et al., 2008). We calculated the rate of late HIV diagnosis per 100,000 population across ZIP codes using Census 2010 as the population denominator, in 2-year periods to improve the stability of rates within ZIP codes with small numbers of events.
2.3. Exposure variables
We measured neighborhood income inequality using the GINI coefficient, which ranges from 0 to 1 where 0 indicates perfect equality, and 1 indicates perfect inequality. We calculated a GINI coefficient for each ZIP code using published methods (Cohen, nd) by taking Census 2000 data on the 16 non-equally spaced income intervals from $10,000 and below to $200,000 and above, and the counts of the number of households within each income interval along with the total aggregate income and average household income. We were primarily interested in income inequality in 2000 because it has been shown to have 10-year or more lagged effects on health (Subramanian and Kawachi, 2004).
We created neighborhood socioeconomic deprivation (SED) using the following indicators: poverty level, unemployment status, educational attainment, and median household income, which prior research has shown to be reliable indicators (Gordon, 1995). Poverty level measures the percent of individuals with incomes below 100% of the Federal Poverty Level and adjusted specifically for NYC (New York City Department of Health and Mental Hygiene, 2013c). Unemployment measures the proportion of persons age 16 years and older without a job. Education measures the proportion of persons 18 years and older with less than a high school education. Median income measures household income adjusted for the value of the U.S. dollar in 2010. We obtained ZIP code-level unemployment, education, and median income from the American Community Survey (ACS) 5-year (2006–2010) estimates online (Infoshare Associates LLC, 2000). Consistent with prior HIV research (An et al., 2013), we operationalize socioeconomic deprivation as an index using principal components analyses (PCA) where higher values indicate greater deprivation.
We used neighborhood black racial concentration as a rough proxy for black racial residential segregation (White and Borrell, 2011) and operationalized the measure as the proportion of non-Hispanic black residents within each ZIP code. We retrieved those data directly from the ACS 5-year (2006–2010) estimates online (Infoshare Associates LLC, 2000).
2.4. Potential mediating mechanisms
HIV testing prevalence and HIV testing accessibility are compositional and contextual features, respectively (Macintyre et al., 2002) within the neighborhood environment that potentially could mediate an association between the socioeconomic factors and late HIV diagnosis rates (Cook et al., 2009). We retrieved HIV testing prevalence from the NYC Community Health Survey (CHS), which is an annual cross-sectional telephone survey administered by DOHMH (New York City Department of Health and Mental Hygiene, 2013b). We operationalize this measure as the weighted mean age-adjusted percent of individuals’ self-reported HIV test in the past 12 months, by gender, for 42 United Hospital Fund (UHF) for 2009–10, using custom weights provided by the DOHMH. UHFs are aggregate geographic areas that comprise between one and nine adjoining ZIP codes that correspond to catchment areas for certain healthcare facilities, and approximate community planning districts (United Hospital Fund Staff, 2002). CHS data were only available at the UHF, so we assigned the same UHF estimate to ZIP codes nested within them.
We geocoded HIV testing accessibility data within each ZIP code using data retrieved from CDC’s National HIV and STD testing resources website (Centers for Disease Control and Prevention, 2014). Data were for 182 organizations that provide free or low-cost HIV testing. We operationalized HIV testing accessibility as the density of HIV testing centers per square mile and proximity to subway lines, computed in two steps using Arc GIS software (Environmental Systems Research Institute (ESRI), 2014). First, to incorporate the high mobility among NYC residents afforded by a rapid transit subway system, we created a buffer zone of a half-mile from any subway line. Second, we weighted access to HIV testing centers within each ZIP code boundary based on the number of centers and the distance of each center to the subway line. Those procedures created a measure of weighted mean accessibility of HIV testing centers incorporating distance to the nearest subway line.
We classified the exposures and mediators into tertiles: high, medium, and low to identify potential thresholds effects. The HIV testing accessibility variable—a four level variable, was the exception. Since there were several neighborhoods with no testing centers, we created a separate category for none and then classified the remaining groups in tertiles.
2.5. Statistical analysis
2.5.1. Descriptive
We used Analysis of Variance (ANOVA) to assess the distributional properties of the exposures, and mediators across tertiles of Census 2000 income inequality. We produced a scatter plot of Census 2000 income inequality and late HIV diagnosis rates across NYC ZIP codes and highlight neighborhoods that fall along four dimensions of inequality and HIV (e.g., high-high, high-low, low-low, low-high).
We then randomly selected eight ZIP codes (two across high-high, high-low, low-low, low-high) to demonstrate variation in income inequality, late HIV diagnosis, socioeconomic deprivation, and black racial concentration across NYC geography. To aid in contextualizing the results and provide some geographic context, we created choropleth maps of late HIV diagnosis rates, HIV testing prevalence, and locations of test centers, income inequality, and socioeconomic deprivation, and black racial concentration, using ArcGIS software (Environmental Systems Research Institute (ESRI), 2014). Legends correspond to tertiles from the analysis, and darker shaded regions represent higher levels of that variable. We also highlight the eight selected ZIP codes in the choropleth maps. We report the spatial associations (Local Moran’s I) among the variables calculated in GeoDa software (Anselin et al., 2006), using Euclidean distance and a spatial weight matrix of k=10 nearest neighborhoods; statistical significance of clustering is assessed based on 499 permutations and p < 0.05 (Anselin, 2003).
2.5.2. Multivariable
We conducted the multivariable analyses in four steps. First, we used Pearson product moment correlation analyses to verify mediators (HIV testing prevalence and accessibility) were related to the exposure and HIV outcome. Second, we used Spearman correlations to examine potential multicollinearity among categorical distributions of neighborhood income inequality, socioeconomic deprivation, and black racial concentration. Third, we used regression diagnostics (Anselin, 2002; Anselin et al., 2006) to assess and remedy potential spatial dependence of income inequality predicting late HIV diagnosis rate. We found a small degree of autocorrelation in the residuals among neighborhoods for men only and created a spatial lag variable to include in the regression model, which eliminated that autocorrelation in re-analysis. Fourth, to model rates of late HIV diagnosis, we used population-averaged negative binomial regression (NBR) models where the raw count is modeled with the population denominator as an exposure offset (Long and Freese, 2006).
We selected NBR because of better accounting for overdispersion compared to alternate methods, which we assessed by inspecting the ratio of deviance to degrees of freedom across comparable models (Allison, 2012), and formally through statistics and line plots in STATA (Long and Freese, 2006). We accounted for the nested structure of ZIP codes within UHFs, using “xt” command in STATA (Allison, 2009; Long and Freese, 2006). In all models, we used the lowest category as the reference group. We do not report the spatial lag variable in the tables because the coefficients have no meaning. We stratified the analysis and report results for men and women separately because the relationship between income inequality and health at ecological-level varies by sex (Moss, 2002). In a (Crude model), we analyzed the crude relationship between the covariates and the outcome. We then examined the independent contributions of income inequality, socioeconomic deprivation, and black racial concentration on late HIV diagnosis rates (Model 1). Next, we examined the independent associations of HIV testing prevalence (Model 2) and HIV testing accessibility (Model 3) on the outcome. Then, we examined a fully adjusted model with the exposures and both HIV testing mechanisms (Model 4). In this preliminary analysis on the topic, we used regression methods compared to other mediation approaches such as path analysis because we were primarily interested in quantifying a difference in relative risk of the exposures, not tracing pathways among the variables. We operationalized mediation as the relative difference in risk between the highest level of statistically significant independent predictors between Model 1 and highest level of that predictor from Model 4, adjusted for both HIV testing mechanisms and the social determinants. We tested for a statistically significant mediating association using Monte Carlo methods for assessing mediation, via an interactive online tool that calculates a confidence interval around an indirect effect (Selig and Preacher, 2008). A statistically significant mediating association is present when the confidence interval of the coefficient for the indirect effect does not include zero. We assess model fit using the Bayesian Information Criteria (BIC) statistic holding a constant sample size across all models. A multivariable negative binomial regression takes the form: log(lateHIVdiagnosisij)=Intercept+Spatial Lag+b1(Income Inequality)+b2(Socioeconomic Deprivation) ++ b3(Black Racial Concentration), where i are ZIP codes nested within j (UHFs). We add HIV testing prevalence and HIV testing accessibility according to the model specifications listed above (i.e., models 2, 3, and 4).
3. Results
3.1. Descriptive
Principal components analysis of the socioeconomic deprivation (SED) index explained 76% of total variance. The correlations (in parentheses) of each item with the SED index are (0.92) poverty level, (0.74) proportion unemployed, (0.93) proportion with less than high school education, and (0.90) and median income. Spearman correlations among categorical GINI, % black, and SED index were not above 0.60 and overall variance inflation factor with those three in the model was less than 2; these thresholds pose no multicollinearity problems in adjusted models (Tabachnick and Fidell, 2007). The overall mean rate of late HIV diagnosis in 2009–10 was 10.3 per 100,000 with interquartile range [(IQR): 2.1–14.7 per 100,000], and the mean prevalence was 26% [IQR: 13.5–33.3%]. The average Census 2000 income inequality across NYC neighborhoods using the GINI index was 0.41 [IQR: 0.39–0.44]. (Results not displayed).
Table 1 shows that distribution of exposures, mediators, and late HIV diagnosis increased across low to high income inequality. The annualized crude rate of late HIV diagnosis among neighborhoods with high income inequality was twice as high (14.47 vs. 6.40 per 100,000, p < 0.001) compared to the rate among neighborhoods with low inequality. The proportion of neighborhoods below the federal poverty level with high income inequality was almost two and a half times as high compared to the proportion among neighborhoods with low inequality (25.9% vs. 8.96%, p < 0.001). Spearman correlations showed that income inequality was modestly correlated with socioeconomic deprivation (rho=0.55, p < 0.05) and black racial concentration (rho=0.38, p < 0.05) and so collinearity was not a problem for multivariable analyses. Fig. 2 shows the increasing trend of income inequality and late HIV diagnosis. A 0.1 increase in inequality is associated with an increase of 10.8 per 100,000 late HIV diagnosis (b=10.77, se=1.73, p=0.000). Fig. 3 represents a geovisual view of (a) late HIV diagnosis over Black racial concentration, (b) HIV testing accessibility (i.e., locations of free/low-cost testing centers) over HIV testing prevalence, (c) income inequality, and (d) socioeconomic deprivation for NYC. Late HIV diagnosis rates moderately and significantly clustered (Moran’s I=0.40, p < 0.002) as did HIV testing prevalence (Moran’s I=0.66, p < 0.002), income inequality (Moran’s I=0.33, p < 0.002), and socioeconomic deprivation (Moran’s I=0.57 P < 0.002).
Table 1.
Characteristics of study variables in the examination of association between income inequality and rates of late HIV/AIDS diagnosis, New York City ZIP codes (n=174), 2009–10.
| Income Inequality (mean GINI coefficient)a
|
|||||
|---|---|---|---|---|---|
| Low (0.38) | Middle (0.42) | High (0.45) | Total | p-valuef | |
| Outcome variableh | |||||
| Total number of late HIV/AIDS diagnosesb | 303 | 591 | 854 | 1748 | 0.000 |
| Annualized rate of late HIV/AIDS diagnosesc,d | 6.40 | 10.27 | 14.47 | 10.35 | 0.000 |
| Socioeconomic Deprivation Indicatorsh | |||||
| Below the federal poverty level, % | 8.96 | 17.70 | 25.94 | 17.48 | 0.000 |
| Unemployed (age >16 years), % | 4.74 | 5.61 | 6.14 | 5.49 | 0.000 |
| < High school education (age> 18 years), % | 13.32 | 19.23 | 24.58 | 19.01 | 0.000 |
| Median household incomed, $ | $75,872 | $57,336 | $43,792 | $59,078 | 0.000 |
| Socioeconomic Deprivation Indexh,e | −0.69 | 0.05 | 0.66 | 0.00 | 0.000 |
| Black Racial Concentration | |||||
| Percent of black/African American residentsh, % | 17.60 | 22.56 | 30.68 | 23.57 | 0.000 |
| Mediators | |||||
| HIV testing prevalence | |||||
| Tested for HIV in past 12, months in UHF, % | 26.09 | 32.49 | 35.48 | 31.33 | 0.000 |
| HIV testing accessabilityh,g | |||||
| Centers per square mile | 0.58 | 4.51 | 7.26 | 4.10 | 0.000 |
As measured by the GINI coefficient based on Census 2000 data.
As reported by the NYC DOHMH.
Rates are per 100,000 persons.
$=adjusted for US dollars in year 2010.
Reverse coded in creation of socioeconomic deprivation-index.
Based on principal components analysis (higher scores indicate higher deprivation).
Indicates significant differences among neighborhoods with low, medium, and high income inequality.
Measured by density of HIV testing centers incorporating distance to subway lines.
Across ZIP codes.
Fig. 2.
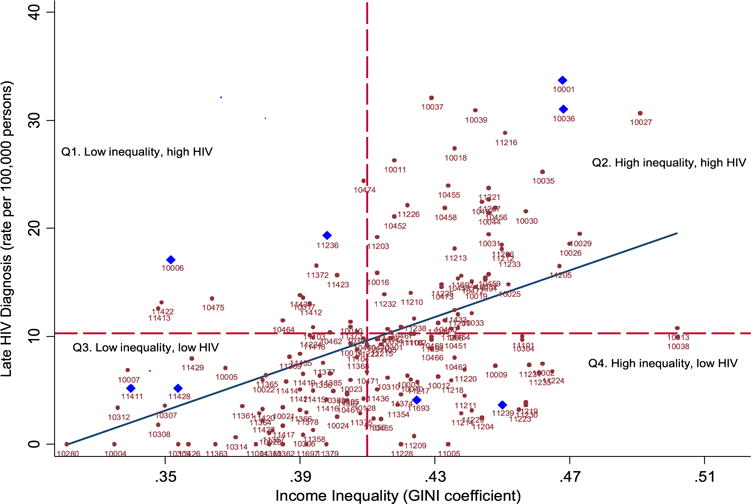
Scatter plot and linear fit between income inequality (x) and late HIV diagnosis rates (y) at the ZIP codes (n=174) Reference lines are dashed (X=0.41 for average Census 2000 GINI) and (Y=10.3 for average late HIV diagnosis rate 2009–10). Bold triangles highlight select ZIP codes across four quadrants.
Fig. 3.
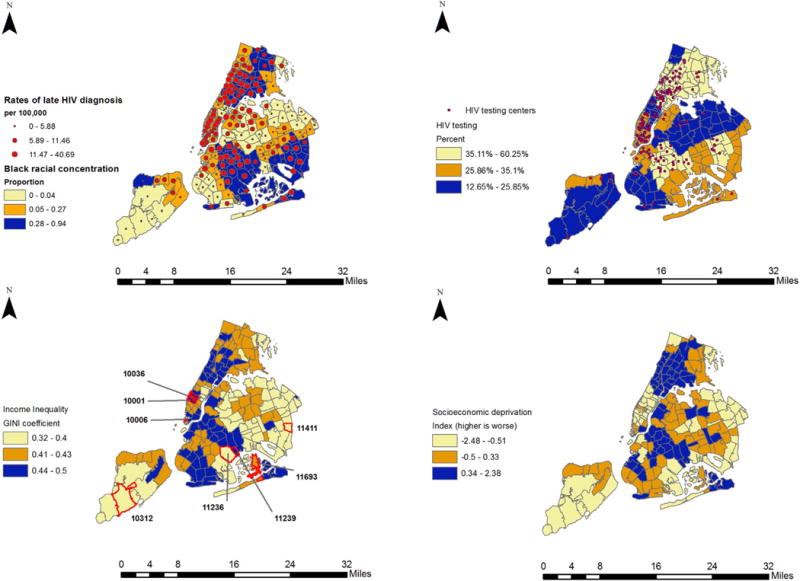
Choropleth maps (from top left to right) of Black racial concentration overlaid with late HIV diagnosis rates, HIV testing prevalence overlaid with location of HIV testing centers, income inequality, and socioeconomic deprivation index across, NYC ZIP codes (n=174). Selected ZIP codes on the income inequality map correspond to those in Fig. 2 and Supplement Table 1.
3.2. Multivariable
3.2.1. Men
Among men, outliers were less than four percent of the data. We ran the analyses with and without outliers and checked for differences in the income inequality unstandardized beta coefficient using the Z-statistic. There were no statistically significant differences in the coefficients so we retain the full sample. Table 2 shows that in crude models only, neighborhoods with high (compared to low) income inequality (Crude model, RR=1.94; 95% CI 1.28–2.29) and socioeconomic deprivation (Crude model, RR=1.75; 95% CI 1.26–2.26) had higher relative risk (RR) of late HIV diagnosis. However, higher black racial concentration was associated with late HIV diagnosis independent of those socioeconomic and structural determinants (Model 1, RR=1.86; 95% CI 1.15–3.00). HIV testing prevalence had an independent association with late HIV diagnosis, but only at medium compared to the lowest level (Model 2, RR=2.00; 95% CI 1.27–3.13). Compared to no HIV testing accessibility, a threshold effect was observed where low, medium, and high HIV testing accessibility in neighborhoods were linearly associated with higher rates of late HIV diagnosis (Model 3). Considering both HIV testing prevalence and HIV testing accessibility in a model with the exposures, highest black racial concentration in neighborhoods was independently associated with higher late HIV diagnosis rates (Model 4, RR=1.77; 95% CI 0.99–3.18). We then calculated the indirect effect of both HIV testing mechanisms between the highest level of black racial concentration and the association with late HIV diagnosis.
Table 2.
Multivariate association of income inequality, socioeconomic deprivation, black racial concentration, HIV testing prevalence and accessibility on rates of late HIV/AIDS diagnosis among men, New York City ZIP codes (n=174), 2009–10.
| Crude modela RR (95% CI) | Model 1a RR (95% CI) | Model 2a RR (95% CI) | Model 3a RR (95% CI) | Model 4a RR (95% CI) | |
|---|---|---|---|---|---|
| Income inequalityb | |||||
| Low 0.36–0.39) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium 0.41–0.42) | 1.45 (0.96, 2.20) | 1.27 (0.81, 1.99) | 1.33 (0.85, 2.08) | 0.90 (0.56, 1.46) | 0.90 (0.55, 1.45) |
| High 0.44–0.46) | 1.94 (1.28, 2.29)** | 1.51 (0.91, 2.49) | 1.68 (1.00, 2.82)* | 0.94 (0.54, 1.63) | 0.97 (0.55, 1.73) |
| Socioeconomic Deprivation indexc | |||||
| Low (−1.30 to −0.70) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium (−0.28 to 0.14) | 1.21 (0.84, 1.84) | 1.17 (0.75, 1.81) | 0.93 (0.59, 1.46) | 1.17 (0.73, 1.86) | 1.12 (0.70, 1.81) |
| High 0.60−1.65) | 1.75 (1.16, 2.64)** | 1.08 (0.62, 1.87) | 0.91 (0.51, 1.62) | 0.93 (0.52, 1.68) | 0.92 (0.49, 1.72) |
| Black racial concentration | |||||
| Low 0.00−0.04) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium 0.05−0.27) | 1.59 (1.05, 2.40)* | 1.39 (0.90, 2.14) | 1.17 (0.74, 1.85) | 1.38 (0.88, 2.15) | 1.24 (0.78, 2.00) |
| High 0.28−0.94) | 2.20 (1.47, 3.30)*** | 1.86 (1.15, 3.00)* | 1.57 (0.91, 2.73) | 1.98 (1.21, 3.25)** | 1.77 (0.99, 3.18)* |
| HIV testing prevalence (%)d | |||||
| Low 16−25) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Medium 27−34) | 2.05 (1.37, 3.06)** | 2.00 (1.27, 3.13)** | 1.32 (0.78, 2.00) | ||
| High 40−52) | 2.22 (1.46, 3.36)** | 1.59 (0.91, 2.73) | 1.77 (0.99, 3.18) | ||
| HIV testing accessibility (mean density of centers)e | |||||
| None | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Low (< 1) | 1.65 (1.00, 2.71)* | 1.73 (1.01, 2.95)* | 1.77 (1.03, 3.04)* | ||
| Medium 2–4) | 2.66 (1.62, 4.36)** | 2.56 (1.42, 4.60)** | 2.56 (1.42, 4.61)** | ||
| High 7–20) | 3.29 (1.94, 5.58)*** | 3.30 (1.78, 6.09)*** | 3.19 (1.70, 5.98)*** | ||
| Model Fit (BIC) | 971.33 | 952.62 | 942.58 | 933.75 |
For exposure variables, parentheses contain inter quartile range, which was rounded to nearest whole for HIV testing center variable. Crude model=Crude associatio.
Model 1=GINI+socioeconomic deprivation index+black racial concentration.
Model 2=Model 1+HIV testing prevalence.
Model 3=Model 1+HIV testing accessibility.
Model 4=Model 1+HIV testing prevalence+HIV testing accessibility.
Population average rate ratio from negative binomial regression.
Census 2000 GINI coefficient.
Index of poverty, median income, education, workforce participation.
Proportion tested for HIV in the past 12 months.
Mean density of free/low cost HIV/STI testing clinics.
Together, HIV testing prevalence and HIV testing accessibility attenuated only 5% (i.e., RR=1.77–1.86/1.77) of the independent association between highest black racial concentration and late HIV diagnosis. The HIV testing mechanisms did not have a statistically significant indirect effect on the outcome (i.e., confidence interval contained zero, results not displayed).
3.2.1.1. Women
Table 3 shows that in crude models only, neighborhoods with high (compared to low) income inequality (Crude model, RR=2.35; 95% CI 1.46–3.81) and high socioeconomic deprivation (Crude model, RR=3.49; 95% CI 2.12–5.79) had higher relative risk of late HIV diagnosis. Neither factors were associated with the outcome independent of black racial concentration. Medium (Model 1, RR=2.51; 95% CI 1.40–10.34) and highest levels of black racial concentration (Model 1, RR=5.37; 95% CI 3.16–10.34) were significantly associated with higher late HIV diagnosis. Neither HIV testing prevalence (Model 2) nor HIV testing accessibility (Model 3) were associated independently with late HIV diagnosis in models adjusted models for income inequality, socioeconomic deprivation, and black racial concentration. In a fully adjusted analysis, medium and highest levels of black racial concentration were independently associated with late HIV diagnosis rates (Model 4). We then calculated the indirect effect of both HIV testing mechanisms between the highest level of black racial concentration and the association with late HIV diagnosis. The independent association between highest level of black racial concentration predicting late HIV diagnosis was attenuated by 21% (i.e., RR=4.45–5.37/4.45) once HIV testing mechanisms were added. However, the HIV testing mechanisms were not significant predictors and did not have a statistically significant indirect effect on the outcome (i.e., confidence interval contained zero, results not displayed).
Table 3.
Multivariate association of income inequality, socioeconomic deprivation, black racial concentration, HIV testing prevalence and accessibility on rates of late HIV/AIDS diagnosis among women, New York City ZIP codes (n=174), 2009–10.
| Crude modela RR (95% CI) | Model 1a RR (95% CI) | Model 2a RR (95% CI) | Model 3a RR (95% CI) | Model 4a RR (95% CI) | |
|---|---|---|---|---|---|
| Income inequalityb | |||||
| Low (0.36–0.39) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium (0.41–0.42) | 1.57 (0.96, 2.57) | 1.02 (0.58, 1.82) | 1.01 (0.56, 1.80) | 0.88 (0.48, 1.61) | 0.94 (0.51, 1.76) |
| High (0.44–0.46) | 2.35 (1.46, 3.81)*** | 1.09 (0.58, 2.49) | 1.14 (0.61, 2.14) | 0.92 (0.47, 1.81) | 1.09 (0.53, 2.22) |
| Socioeconomic Deprivation indexc | |||||
| Low (−1.30 to −0.70) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium (−0.28 to 0.14) | 1.37 (0.81, 2.34) | 1.15 (0.64, 2.05) | 1.07 (0.59, 1.95) | 1.11 (0.61, 2.01) | 1.03 (0.56, 1.90) |
| High (0.60–1.65) | 3.49 (2.12, 5.76)*** | 1.62 (0.82, 3.18) | 1.34 (0.64, 2.84) | 1.44 (0.71, 2.92) | 1.25 (0.58, 2.69) |
| Black racial concentration | |||||
| Low (0.00–0.04) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium (0.05–0.27) | 2.73 (1.57, 4.76)*** | 2.51 (1.40, 4.49)** | 2.24 (1.19, 4.20)* | 2.60 (1.45, 4.68)** | 2.30 (1.24, 4.26)** |
| High (0.28–0.94) | 7.38 (4.40, 12.47)*** | 5.37 (3.16, 10.34)*** | 4.44 (2.08, 9.47)*** | 6.15 (3.35, 11.30)*** | 4.45 (2.04, 9.73)*** |
| HIV testing prevalence (%)d | |||||
| Low (16–25) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Medium (27–34) | 1.92 (1.14, 3.26)* | 1.14 (0.62, 2.09) | 1.47 (0.78, 2.78) | ||
| High (40–52) | 5.02 (3.11, 8.10)*** | 1.52 (0.72, 3.22) | 1.86 (0.80, 4.32) | ||
| HIV testing accessibility (mean density of centers)e | |||||
| None | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Low (< 1) | 1.39 (0.76, 2.52) | 1.62 (0.81, 3.24) | 1.51 (0.75, 3.07) | ||
| Medium (2–4) | 2.44 (1.38, 4.32)** | 1.62 (0.78, 3.38) | 1.40 (0.66, 2.96) | ||
| High (7–20) | 2.49 (1.40, 4.42)** | 1.69 (0.81, 3.53) | 1.40 (0.64, 3.30) | ||
| Model Fit (BIC) | 583.80 | 589.47 | 592.73 | 600.38 |
For exposure variables, parentheses contain inter quartile range, which was rounded to nearest whole for HIV testing center variable. Crude model=Crude association
Model 1=GINI + deprivation index + black racial concentration
Model 2=Model 1+ HIV testing prevalence
Model 3=Model 1+ HIV testing accessibility
Model 4= Model 1+ HIV testing prevalence + HIV testing accessibility
Population average rate ratio from negative binomial regression.
Census 2000 GINI coefficient.
Index of poverty, median income, education, workforce participation.
Proportion tested for HIV in the past 12 months.
Mean density of free/low cost HIV/STI testing clinics.
4. Discussion
4.1. Overall findings
Our study examined the relationships among neighborhood income inequality, socioeconomic deprivation, and black racial concentration as predictors of late HIV diagnosis rates in a large urban United States city. In our data, at least 66% of NYC neighborhoods had GINI coefficients over 0.42, which is slightly lower than the 2012 US national average of 0.48. We observed in our study that income inequality and socioeconomic deprivation was associated with higher rates of late HIV diagnosis but only in crude models, not adjusted for covariates. The direction of association was consistent with crude results from one study that examined HIV incidence (Buot et al., 2014), one study that examined AIDS mortality rates among injection drug users (Friedman et al., 2013), and multivariable results from one study that examined late HIV diagnosis based on 2005–6 HIV data (Ransome et al., 2016).
4.2. Sex differences
Crude models showed stronger associations of high inequality, socioeconomic deprivation, and black racial concentration on late HIV diagnosis for men. In contrast, in multivariable models; for women compared to men, black racial concentration appeared to have a larger magnitude of association on late HIV diagnosis. One ecological study in NYC based on late HIV diagnosis data from 2005 to 2006 also found the same pattern of association between black racial concentration and late HIV diagnosis for women compared to men (Ransome et al., 2016).
The mediation models with HIV testing prevalence and accessibility also suggested sex differences that may be important for HIV prevention. Neighborhood HIV testing accessibility was a statistically significant predictor of late HIV diagnosis for men but not women. The non-significant finding among women could be related to HIV testing being more normative for women given universal HIV screening during pregnancy (Ransome et al., 2015). Alternately, within neighborhoods, women may be less likely than men to receive and benefit from advances in HIV prevention technologies (West et al., 2015).
4.3. Black racial concentration
While research from international settings showed that income inequality and socioeconomic deprivation are significant drivers of HIV diagnosis (Lamontagne and Stockemer, 2010; Fox, 2012), in the U.S., black racial concentration is also a key driver of HIV-related disparities (Robinson and Moodie-Mills, 2012). We found that black racial concentration robustly predicted late HIV diagnosis. These results add to evidence from earlier ecological studies on the topic in the U.S. First, Buot et al. (2014) examined the association among socioeconomic (e.g., income inequality and poverty) determinants and HIV incidence across 80 Metropolitan Statistical Areas (MSAs) in the U.S. The study found that in crude models, black racial residential segregation was positively correlated with HIV incidence (r=0.17). Gant et al. (2012) found that in crude models, white racial composition was negatively correlated with HIV diagnosis at the County level (r=−0.67). Those studies, however, used different proxies to capture a construct more broadly identified as racial residential segregation.
The robustness of black racial concentration in our study may be due to the high level of racial residential segregation in NYC compared to other large cities across the U.S. For example; according to one source using a black-white dissimilarity index, NYC ranks 3rd of among 22 most segregated cities in the U.S. with a score of 79.1 where a score above 60 is considered very high segregation (Baurd-Remba and Lubin, 2013).
The role, however, of black racial concentration in relation to income inequality and socioeconomic deprivation is complex. For instance, among the selected neighborhoods across quadrants of low-high inequality and HIV, we found that neighborhoods with above 50% black racial concentration were in low inequality-high HIV (Canarsie), low inequality-low HIV (Cambria Heights) as well as high inequality-low HIV (Starrett City). In contrast, some neighborhoods had less than 4% black racial concentration, and low inequality, yet high HIV diagnosis rate (Trinity). These findings underscore the complexity of understanding geographic terrain through spatial epidemiological approach (e.g., map visualization) in addition to evaluating effect sizes. diagnosis rate (Trinity). These findings underscore the complexity of understanding geographic terrain through spatial epidemiological approach (e.g., map visualization) in addition to evaluating effect sizes.
4.4. Mechanisms
Our study was also the first to examine whether HIV testing prevalence and HIV testing accessibility mediated the impact of socioeconomic and structural indicators on late HIV diagnosis. We found that these mechanisms did not have significant indirect effects on late HIV diagnosis. Furthermore, our findings do not support a model where neighborhoods with higher income inequality, socioeconomic deprivation, and black racial concentration would have fewer HIV testing resources, which in turn would be associated with higher late HIV diagnosis rates.
Historically highly black segregated neighborhoods faced difficulties attracting resources (Massey and Denton, 1993; Williams and Collins, 2001). Our findings more support a selection model where resources are directed to socioeconomically deprived neighborhoods to address and reduce high HIV burden. For instance; in NYC, the Department of Health and Mental Hygiene launched The Bronx Knows in 2008 and Brooklyn Knows in 2009, which are initiatives implemented to increase HIV testing resources in socioeconomically deprived neighborhoods in the city (New York City Department of Health and Mental Hygiene, 2013a; Myers et al., 2012). Findings that appear to be related to racial segregation, however, could be related to other social factors such as marriage rates, the proportion of welfare recipients and joblessness (Small and Newman, 2001; Charles, 2003). Thus one potential implication of our results is that reducing geographic disparities in late HIV diagnosis will require multifaceted interventions that address other social factors not limited to HIV stigma, co-infection of other sexually transmitted diseases (Vaughan et al., 2014) and features of the racialized risk environments such as incarceration. A greater focus on increasing HIV testing and linkage to HIV care in neighborhoods with high black racial concentration is necessary to address geographic related HIV disparities (Nunn et al., 2014).
4.5. Strengths and limitations
Although there was a temporal lag between the exposures (e.g., Census, 2000) and late HIV diagnosis (2009–10), statements of causal inference from our results are limited because data are cross-sectional. We did not have longitudinal data to assess the extent to which changes in the social and structural determinants are related to change in HIV testing and accessibility, and the association with changes in late HIV diagnosis. With ZIP codes as the unit of analysis, there is also potential area-level heterogeneity that may vary as a function of population size within ZIP codes (IQR: 25,147 to 66,362 persons). ZIP codes also correspond to postal service and not administrative boundaries that are overseen by a legislator, and thus it may be challenging to develop policies at that level. Related, aggregating the data to other geographic levels may not result in similar findings, which is known as the modifiable areal unit problem (MAUP) (Parenteau and Sawada, 2011). However, in exploratory analyses, we investigated these associations at the United Hospital Fund (UHF, n=42) and found the direction of association was the same, and the size of the relative risks was similar to those obtained using the ZIP code level (results not displayed, but available upon request).
Our study is strengthened by the use of population-based sources of late HIV diagnosis data, and standard area-level socioeconomic indicators readily available online. Next, we complemented the results from the multivariable analysis with geographic analysis and geovisual representations, which highlighted the complexity of space. We also examined HIV testing as a mechanism, which is at the forefront of HIV testing prevention policies in the U.S. (Centers for Disease Control and Prevention, 2013b).
5. Conclusions
Research should continue to focus on mechanisms that link black racial concentration to HIV diagnosis and transmission outcomes. Future studies should incorporate other methodologically sophisticated indices (e.g., surface-density) of racial residential segregation (Yang and Matthews, 2015; Kramer et al., 2010) and isolate potential causal effects on HIV diagnosis outcomes.
Supplementary Material
Acknowledgments
Y. Ransome was supported by the Alonzo Smythe Yerby Fellowship at the Harvard T.H. Chan School of Public Health. D. Nash was supported by grant #1R01MH101028-01 (National Institute of Mental Health (NIMH)).
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.healthplace.2016.09.004.
References
- Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191:S115–S122. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- AIDSVU. Illustrating HIV/AIDS in the United States [Online] AIDSVu; 2014. Available: ( http://aidsvu.org/wp-content/uploads/data-sets/AIDSVu-USA.pdf) (accessed 21.05.14) [Google Scholar]
- AIDSVU. City and state profiles [Online] AIDSVu; 2016. Available: ( http://aidsvu.org/local-statistics/) (accessed 18.01.16) [Google Scholar]
- Alba R, Romalewski S. The end of segregation? Hardly [Online] New York City: Center for Urban Research; 2012. Available: ( http://www.gc.cuny.edu/Page-Elements/Academics-Research-Centers-Initiatives/Centers-and-Institutes/Center-for-Urban-Research/CUR-research-initiatives/The-End-of-Segregation-Hardly) (accessed 30.01.16) [Google Scholar]
- Allison PD. Fixed Effects Regression Models. SAGE Publications Inc; Thousand Oaks, CA: 2009. [Google Scholar]
- Allison PD. Regression for count data: Logistic regression using SAS: Theory and application. SAS Institute Inc; Cary, NC: 2012. [Google Scholar]
- Altman D. Globalization, political economy, and HIV/AIDS. Theory Soc. 1999;28:559–584. [Google Scholar]
- AN Q, Prejean J, Mcdavid Harrison K, Fang X. Association between community socioeconomic position and HIV diagnosis rate among adults and adolescents in the United States, 2005 to 2009. Am J Pub Health. 2013;103:120–126. doi: 10.2105/AJPH.2012.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselin L. Under the hood issues in the specification and interpretation of spatial regression models. Agric Econ. 2002;27:247–267. [Google Scholar]
- Anselin L. An introduction to spatial autocorrelation analysis with GeoDa. Spatial Analysis Laboratory, University of Illinois; Champagne-Urbana, Illinois: 2003. [Google Scholar]
- Anselin L, Ibnu S, Youngihn K. GeoDa: an introduction to spatial data analysis. Geogr Anal. 2006;38:5–22. [Google Scholar]
- Barnett T, Whiteside A. AIDS in the twenty-first century: Disease and globalization. Palgrave Macmillan; New York, NY: 2006. [Google Scholar]
- Baurd-REMBA R, Lubin G. 21 maps of highly segregated cities in America [Online] Business Insider; 2013. Available: ( http://www.businessinsider.com/most-segregated-cities-census-maps-2013-4) (accessed 29.02.16) [Google Scholar]
- Bogart LM, Thorburn S. Are HIV/AIDS conspiracy beliefs a barrier to HIV prevention among African Americans? J Acquir Immune Defic Synd. 2005;38:213–218. doi: 10.1097/00126334-200502010-00014. [DOI] [PubMed] [Google Scholar]
- Bogart LM, Wagner G, Galvan FH, Banks D. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among African American men with HIV. J Acquir Immune Defic Synd. 2010;53:648–655. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner BM. A multilevel understanding of HIV/AIDS disease burden among African American women. J Obstet Gynecol Neonatal Nurs. 2014;43:633–643. doi: 10.1111/1552-6909.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodish PH. J Biosoc Sci. FirstView; 2014. An association between neighbourhood wealth inequality and HIV prevalence in Sub-Saharan Africa; pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buot MLG, Docena JP, Ratemo BK, Bittner MJ, Burlew JT, Nuritdinov AR, Robbins JR. Beyond race and place: distal sociological determinants of HIV disparities. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Greenberg AE, Befus M, Willis S, Samala R, Rocha N, Griffin A, West T, Hader S. Temporal association between expanded HIV testing and improvements in population-based HIV/AIDS clinical outcomes, District of Columbia. AIDS Care. 2013;26:785–789. doi: 10.1080/09540121.2013.855296. [DOI] [PubMed] [Google Scholar]
- Centers For Disease Control And Prevention. Vital signs: hiv testing and diagnosis among adults-United States, 2001–2009. Morb Mortal Wkly Rep. 2010;59:1541–1571. [PubMed] [Google Scholar]
- Centers For Disease Control And Prevention. Results of the expanded HIV testing initiative-25 jurisdictions. U. S., 2007–2010. Morb Mortal Wkly Rep. 2011;60:805–833. [PubMed] [Google Scholar]
- Centers For Disease Control And Prevention. HIV and AIDS in the United States by geograpic distribution [Online] Atlanta, GA: CDC; 2013a. Available: ( http://www.cdc.gov/hiv/statistics/basics/geographicdistribution.html) (accessed 21.05.14) [Google Scholar]
- Centers For Disease Control And Prevention. Expanded testing initiative [Online] Atlanta, GA: CDC; 2013b. Available: ( http://www.cdc.gov/hiv/policies/eti.html) (accessed 25.06.13) [Google Scholar]
- Centers For Disease Control And PreVENTION. National HIV and STD testing resources [Online] Rockville, MD: CDC National Prevention Information Network; 2014. Available: ( https://gettested.cdc.gov/) (accessed August 8.) [Google Scholar]
- CHARLES CZ. The dynamics of racial residential segregation. Ann Rev Socio. 2003;29:167–207. [Google Scholar]
- Chopel AM, Minkler M, Nuru-Jeter A, Dunbar M. Social determinants of late stage HIV diagnosis and its distributions among African Americans and Latinos: a critical literature review. J Health Dispar Res Pr. 2015;8:1. [Google Scholar]
- Coates TJ, Kulich M, Celentano DD, Zelaya CE, Chariyalertsak S, Chingono A, Gray G, Mbwambo JKK, Morin SF, Richter L, Sweat M, VAN Rooyen H, Mcgrath N, Fiamma A, Laeyendecker O, Piwowar-Manning E, Szekeres G, Donnell D, Eshleman SH. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2:e267–e277. doi: 10.1016/S2214-109X(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PA, Downing J, Wheater CP, Bellis MA, Tocque K, Syed Q, Phillips-Howard PA. Influence of socio-demographic factors on distances travelled to access HIV services: enhanced surveillance of HIV patients in north west England. BMC Public Health. 2009;9 doi: 10.1186/1471-2458-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HL, Linton S, Kelley ME, Ross Z, Wolfe ME, Chen YT, Zlotorzynska M, Hunter-Jone SJ, Friedman SR, Des Jarlais D. Racialized risk environments in a large sample of people who inject drugs in the United States. Int J Drug Policy. 2015;27:43–55. doi: 10.1016/j.drugpo.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosas . The Social Ecology of AIDS in Africa. Southern African AIDS Training Programme; Harare, Zimbabwe: 2002. [Google Scholar]
- Durevall D, Lindskog A. Economic inequality and HIV in Malawi. World Dev. 2012;40:1435–1451. [Google Scholar]
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America – forgotten but not gone. N Engl J Med. 2010;362:967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Systems Research Institute (ESRI) ArcGIS Desktop: Release 10.2. Redlands, CA: 2014. [Google Scholar]
- Fleming P, Ward JW, Janssen RS, DE Cock KM, Valdiserri RO, Gayle HD, Jones JL, Lehman JS, Lindegren ML, Nakashima AK. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Morb Mortal Wkly Rep. 1999;48:1–28. [PubMed] [Google Scholar]
- For Centers Disease Control And Prevention. Today’s HIV/AIDS Epidemic. HIV/AIDS Fact. Sheets. 2015. [Google Scholar]
- Ford CL, Daniel M, Earp JAL, Kaufman JS, Golin CE, Miller WC. Perceived everyday racism, residential segregation, and HIV testing among patients at a sexually transmitted disease clinic. Am J Public Health. 2009;99:S137–S143. doi: 10.2105/AJPH.2007.120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AM. The HIV-poverty thesis re-examined: poverty, wealth or inequality as a social determinant of HIV infection in Sub-Saharan Africa? J Biosoc Sci. 2012;44:459–480. doi: 10.1017/S0021932011000745. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Cooper HLF, Osborne AH. Structural and social contexts of HIV risk among African Americans. Am J Public Health. 2009;99:1002–1008. doi: 10.2105/AJPH.2008.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, West BS, Pouget ER, Hall HI, Cantrell J, Tempalski B, Chatterjee S, Hu X, Cooper HLF, Galea S, Des Jarlais DC. Metropolitan social environments and pre-HAART/HAART era changes in mortality rates (per 10,000 adult residents) among injection drug users living with AIDS. PLos One. 2013;8:e57201–e57201. doi: 10.1371/journal.pone.0057201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant Z, Lomotey M, Hall H, Hu X, Guo X, Song R. A county-level examination of the relationship between HIV and social determinants of health: 40 states. Open AIDS J. 2012;6:2006–2008. doi: 10.2174/1874613601206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie S, Greener R, Whiteside A, Whitworth J. Investigating the empirical evidence for understanding vulnerability and the associations between poverty, HIV infection and AIDS impact. AIDS. 2007;21:S1–S4. doi: 10.1097/01.aids.0000300530.67107.45. [DOI] [PubMed] [Google Scholar]
- Girardi E, Sabin CA, Antonella D’arminio Monforte M. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Synd. 2007;46:S3–S8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- Gordon D. Census based deprivation indices: their weighting and validation. J Epidemiol Commun H. 1995;49:S39–S44. doi: 10.1136/jech.49.suppl_2.s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover E, Grosso A, Ketende S, Kennedy C, Fonner V, Adams D, Sithole B, MNISI Z, Maziya SL, Baral S. Social cohesion, social participation and HIV testing among men who have sex with men in Swaziland. AIDS Care. 2016;28:795–804. doi: 10.1080/09540121.2015.1131971. [DOI] [PubMed] [Google Scholar]
- Gueler A, Schoeni-Affolter F, Moser A, Bertisch B, Bucher HC, Calmy A, Cavassini M, Ledergerber B, Wandeler G, Egger M. Neighbourhood socio-economic position, late presentation and outcomes in people living with HIV in Switzerland. AIDS. 2015;29:231–238. doi: 10.1097/QAD.0000000000000524. [DOI] [PubMed] [Google Scholar]
- Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet. 2008;372:764–775. doi: 10.1016/S0140-6736(08)60887-9. [DOI] [PubMed] [Google Scholar]
- Harrison KM, Ling Q, Song R, Hall HI. County-level socioeconomic status and survival after HIV diagnosis, United States. Ann Epidemiol. 2008;18:919–927. doi: 10.1016/j.annepidem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Hunter M. The changing political economy of sex in SouthAfrica: the significance of unemployment and inequalities to the scale of the AIDS pandemic. Soc Sci Med. 2007;64:689–700. doi: 10.1016/j.socscimed.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Infoshare Associates LlC. Infoshare Online [Online] New York, NY: 2000. accessed 25. 06.13. [Google Scholar]
- Irvin R, Wilton L, Scott H, Beauchamp G, Wang L, Betancourt J, Lubensky M, Wallace J, Buchbinder S. A study of perceived racial discrimination in black men who have sex with men (MSM) and its association with healthcare utilization and HIVtesting. AIDS Behav. 2014;18:1272–1278. doi: 10.1007/s10461-014-0734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesmin SS, Chaudhuri S. Why do some women know more? An exploration of the association of community socioeconomic characteristics, social capital, and HIV/AIDS knowledge. Women Health. 2013;53:669–692. doi: 10.1080/03630242.2013.822456. [DOI] [PubMed] [Google Scholar]
- Johnston D, Deane K, Rizzo M. The political economy of HIV. Rev Afr Polit Econ. 2015;42:335–341. [Google Scholar]
- Kawachi I, Kennedy BP, Wilkinson R. The Society and Population Health Reader: Income Inequality and Health. The New Press; New York, NY: 1999. [Google Scholar]
- Kawachi I, Subramanian SV, Kim D. Social Capital and Health. Springer Science + Business Media LLC; New York, NY: 2008. [Google Scholar]
- Kippax S, Holt M. The State of Social and Political Science Research Related to HIV: A Report for the International AIDS Society National Centre on HIV Social Research. The University of New South Wales; South Wales, Australia: 2009. [Google Scholar]
- Kramer MR, Cooper HL, Drews-Botsch CD, Waller LA, Hogue CR. Do measures matter? Comparing surface-density-derived and census-tract-derived measures of racial residential segregation. Int J Health Geogr. 2010;9:1. doi: 10.1186/1476-072X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian S. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures-the public health disparities geocoding project (US) Public Health Rep. 2003;118:240–258. doi: 10.1093/phr/118.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne B, Stockemer D. Determinants of HIV prevalence: a global perspective. Int Pol. 2010;47:698–724. [Google Scholar]
- Latkin C, Weeks MR, Glasman L, Galletly C, Albarracin D. A dynamic social systems model for considering structural factors in HIV prevention and detection. AIDS Behav. 2010;14:222–238. doi: 10.1007/s10461-010-9804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter D. Income inequality emerges as key issue in 2016 presidential campaign [Online] Los Angeles, CA: Los Angeles Times; 2015. Available: ( http://www.latimes.com/nation/la-na-campaign-income-20150205-story.html) (accessed 30.01.16) [Google Scholar]
- Leibowitz AA, Taylor SL. Distance to public test sites and HIV testing. Med Care Res Rev. 2007;64:568–584. doi: 10.1177/1077558707304634. [DOI] [PubMed] [Google Scholar]
- Lim TW, Frangakis C, Latkin C, Ha TV, Le Minh N, Zelaya C, Quan VM, Go VF. Community-level income inequality and HIV prevalence among persons who inject drugs in Thai Nguyen, Vietnam. PLoS One. 2014;9:e90723. doi: 10.1371/journal.pone.0090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S, Freese J. Models for Count Outcomes: Regression Models for Categorical Dependent Variables Using Stata. 2nd. StataCorp LP: College Station, Texas: 2006. [Google Scholar]
- Maas B, Fairbairn N, Kerr T, Li K, Montaner JSG, Wood E. Neighborhood and HIV infection among IDU: place of residence independently predicts HIV infection among a cohort of injection drug users. Health Place. 2007;13:432–439. doi: 10.1016/j.healthplace.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55:125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Massey DS. Categorically unequal: the American stratifications system. Russell Sage Foundation; New York, NY: 2007. [Google Scholar]
- Massey DS, Eggers ML. The ecology of inequality: minorities and the concentration of poverty, 1970–1980. Am J Socio. 1990;95:1153–1188. [Google Scholar]
- Massey DS, Denton NA. American Apartheid: Segregation and the Making of the Underclass. Harvard University Press; Cambridge, MA: 1993. [Google Scholar]
- Millett GA, Jeffries WL, Peterson JL, Malebranche DJ, Lane T, Flores SA, Fenton KA, Wilson PA, Steiner R, Heilig CM. Common roots: a contextual review of HIV epidemics in black men who have sex with men across the African diaspora. Lancet. 2012;380:411–423. doi: 10.1016/S0140-6736(12)60722-3. [DOI] [PubMed] [Google Scholar]
- Minkler M, Wallace SP, Mcdonald M. The political economy of health: a useful theoretical tool for health education practice. Int Q Community Health Educ. 1994;15:111–125. doi: 10.2190/T1Y0-8ARU-RL96-LPDU. [DOI] [PubMed] [Google Scholar]
- Moss NE. Gender equity and socioeconomic inequality: a framework for the patterning of women’s health. Soc Sci Med. 2002;54:649–661. doi: 10.1016/s0277-9536(01)00115-0. [DOI] [PubMed] [Google Scholar]
- Moyer VA. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51–60. doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- Myers JE, Braunstein SL, Shepard CW, Cutler BH, Mantsios AR, Sweeney MM, Tsoi BW. Assessing the impact of a community-wide HIV testing scale-up initiative in a major urban epidemic. J Acquir Immune Defic Synd. 2012;61:23–31. doi: 10.1097/QAI.0b013e3182632960. [DOI] [PubMed] [Google Scholar]
- New York City Comptroller. LiU JC. Income inequality in New York City [Online] New York, NY: New York City Comptroller’s Office; 2012. Available: ( http://comptroller.nyc.gov/wp-content/uploads/documents/NYC_IncomeInequality_v17.pdf) (accessed 30.01.16) [Google Scholar]
- New York City Department Of Health And Mental Hygiene. The Bronx Knows HIV Testing Initiative Final Report. Department of Health and Mental Hygiene; New York City, NY: 2011. [Google Scholar]
- New York City Department Of Health And Mental Hygiene. Selecting and applying a standard area-based socioeconomic status measure for public health data: analysis for New York CityEpi Research Report. New York City Department of Health and Mental Hygiene; 2013c. [Google Scholar]
- New York City Department of Health And Mental Hygiene. Brooklyn Knows HIV Testing Initiative [Online] New York City, NY: Department of Health and Mental Hygiene; 2013a. Available: ( http://www.nyc.gov/html/doh/html/living/brooklyn_test.shtml) (accessed June 25, 2013) [Google Scholar]
- New York City Department of Health And Mental Hygiene. The New York City Community Health Survey [Online] New York City, NY: Department of Health and Mental Hygiene; 2013b. Survey data on the health of New Yorkers. Available: ( http://www.nyc.gov/html/doh/html/data/survey.shtml) (accessed June 25, 2013) [Google Scholar]
- Niyonsenga T, Trepka MJ, Lieb S, Maddox LM. Measuring socioeconomic inequality in the incidence of AIDS: rural-urban considerations. AIDS Behav. 2013;17:700–709. doi: 10.1007/s10461-012-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton MI, Ariely D. Building a better America—One wealth quintile at a time. Perspect Psychol Sci. 2011;6:9–12. doi: 10.1177/1745691610393524. [DOI] [PubMed] [Google Scholar]
- Nunn A, Yolken A, Cutler B, Trooskin S, Wilson P, Little S, Mayer K. Geography should not be destiny: focusing HIV/AIDS implementation research and programs on microepidemics in US neighborhoods. Am J Public Health. 2014;104:775–780. doi: 10.2105/AJPH.2013.301864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. United States tackling high inequalities, creating opportunities for all [Online] 2014 Available: ( http://www.oecd.org/unitedstates/Tackling-high-inequalities.pdf) (accessed January 30, 2016)
- Osypuk TL, Acevedo-Garcia D. Beyond individual neighborhoods: a geography of opportunity perspective for understanding racial/ethnic health disparities. Health Place. 2010;16:1113–1123. doi: 10.1016/j.healthplace.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau MP, Sawada MC. The modifiable areal unit problem (MAUP) in the relationship between exposure to NO2 and respiratory health. Int J Health Geogr. 2011;10:1–15. doi: 10.1186/1476-072X-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RG, Easton D, Klein CH. Structural barriers and facilitators in HIV prevention: a review of international research. AIDS. 2000;14:S22–S32. doi: 10.1097/00002030-200006001-00004. [DOI] [PubMed] [Google Scholar]
- Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the US HIV epidemic. AM Psychol. 2013;68:197–209. doi: 10.1037/a0032694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ES. Political Economy: An Introductory Text. W. W. Norton & Company; New York, NY: 1985. [Google Scholar]
- Pouget ER, Kershaw TS, Ickovics JR, Blankenship KM. Associations of sex ratios and male incarceration rates with multiple opposite-sex partners: potential social determinants of HIV/STI transmission. Public Health Rep. 2010;125:70–80. doi: 10.1177/00333549101250S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poundstone K, Strathdee S, Celentano D. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol Rev. 2004;26:22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- Quillian L. Segregation and poverty concentration: the role of three segregations. Am Socio Rev. 2012;77:354–379. doi: 10.1177/0003122412447793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, LI C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Ransome Y, Galea SP, Roman, Kawachi I, Braunstein SL, Nash D. Is social capital associated with late HIV diagnosis?: an ecological analysis. J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000001043. Published Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransome Y, Terzian AS, Addison D, Braunstein SL, Meyers J, Abrahams B, Nash D. Expanded HIV testing coverage is asssociated with decreases in late HIV diagnoses. AIDS. 2015;29:1369–1378. doi: 10.1097/QAD.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiro PF, Gange SJ, Horberg MA, Abraham AG, Napravnik S, Samji H, Yehia BR, Althoff KN, Moore RD, Kitahata MM, Sterling TR, Curriero FC, FOR THE North American, A. C. C. O. R. & Design Geographic variations in retention in care among HIV-infected adults in the United States. PLoS One. 2016;11:e0146119. doi: 10.1371/journal.pone.0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AE, Dovidio JF, Ballester E, Johnson BT. HIV prevention interventions to reduce sexual risk for African Americans: the influence of community-level stigma and psychological processes. Soc Sci Med. 2014;103:118–125. doi: 10.1016/j.socscimed.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DE. The social and moral cost of mass incarceration in African American communities. Stanf Law Rev. 2004;56:1271–1305. [Google Scholar]
- Robinson R, Moodie-Mills AC. HIV/AIDS inequality: structural barriers to prevention, treatment, and care in communities of color. Center for American Progress; Washington, DC: 2012. [Google Scholar]
- Sanders B. Issues: Inc., ome and wealth inequality [Online] Burlington, VT: 2016. Available: ( https://berniesanders.com/issues/income-and-wealth-inequality/) (accessed January 30, 2016) [Google Scholar]
- Schmitt J. Inequality as policy: The United States since 1979 [Online] Center for Economic and Policy Research; 2009. Available: ( http://cepr.net/documents/publications/inequality-policy-2009-10.pdf) (accessed January 30, 2016) [Google Scholar]
- Schneider E, Whitmore S, Glynn KM, Domingue K, Mitsch A, Mckenna MT, Centers For Disease Control And Prevention Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged < 18 months and for HIV infection and AIDS among children aged 18 months to < 13 years, United States, 2008. Morb Mortal Wkly Rep. 2008;57:1–8. [PubMed] [Google Scholar]
- Selig JP, PREACHER KJ. Monte Carlo method for assessing mediation: An interactive tool for creating confidence intervals for indirect effects [Online] Quantpsy.org; 2008. Available: ( http://quantpsy.org/medmc/medmc.htm) (accessed 28.07.16) [Google Scholar]
- Shapiro TM. The Hidden Cost of Being African American: How Wealth Perpetuates Inequality. Oxford University Press; USA: 2004. [Google Scholar]
- Silver H, Messeri P. Concentrated poverty, racial/ethnic diversity and neighborhood social capital. In: Amina C, Davis JB, editors. Social Capital and Economics: Social Values, Power, and Social Identity. Routledge; New York, NY: 2014. [Google Scholar]
- Simons H, Lifson A, Bonilla Z. Barriers to early HIV testing for African-born immigrants and Latinos in Minnesota: A qualitative study. American Public Health Association; Conference Chicago, IL: 2015. [Google Scholar]
- Small ML, Newman K. Urban poverty after the truly disadvantaged: the rediscovery of the family, the neighborhood, and culture. Ann Rev Socio. 2001;27:23–45. [Google Scholar]
- Solt F. Economic inequality and democratic political engagement. Am J Polit Sci. 2008;52:48–60. [Google Scholar]
- Subramanian S, Kawachi I. Income inequality and health: what have we learned so far? Epidemiol Rev. 2004;26:78–91. doi: 10.1093/epirev/mxh003. [DOI] [PubMed] [Google Scholar]
- Sutherland D, Hsu YJJ. HIV/AIDS in China: the economic and social determinants. Routledge; New York, NY: 2012. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th. Pearson Education Inc; Boston, MA: 2007. [Google Scholar]
- Takahashi LM. Stigmatization, HIV/AIDS, and communities of color: exploring response to human service facilities. Health Place. 1997;3:187–199. doi: 10.1016/s1353-8292(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Takahashi LM. Homelessness, AIDS, and Stigmitization: The NIMBY Syndrome in the United States. Oxford University Press; New York, NY: 1998. [Google Scholar]
- United Hospital Fund Staff. New York City Community Health Atlas, 2002: section III. sources, methods, and definitions [Online] United Hospital Fund; New York City: 2002. (Available)( https://www.uhfnyc.org/publications/99007), (accessed August 25, 2016) [Google Scholar]
- Vaughan AS, Rosenberg E, Shouse RL, Sullivan PS. Connecting race and place: a county-level analysis of white, black, and hispanic HIV prevalence, poverty, and level of urbanization. Am J Public Health. 2014;104:e77–e84. doi: 10.2105/AJPH.2014.301997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RG. AIDS in the HAART era: NewYork’s heterogeneous geography. Soc Sci Med. 2003;56:1155–1171. doi: 10.1016/s0277-9536(02)00121-1. [DOI] [PubMed] [Google Scholar]
- West BS, Pouget ER, Tempalski B, Cooper HL, Hall HI, HU X, Friedman SR. Female and male differences in AIDS diagnosis rates among people who inject drugs in large US metro areas from 1993 to 2007. Ann Epidemiol. 2015;25:218–225. doi: 10.1016/j.annepidem.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Borrell LN. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place. 2011;17:438–448. doi: 10.1016/j.healthplace.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- Wilson WJ. The Truly Disadvantaged: The Inner City, The Underclass, and Public Policy. University of Chicago Press; Chicago, IL: 2012. [Google Scholar]
- Wohlgemut J, Lawes T, Laing RB. Trends in missed presentations and late HIV diagnosis in a UK teaching hospital: a retrospective comparative cohort study. BMC Infect Dis. 2012;12 doi: 10.1186/1471-2334-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC, Matthews SA. Death by segregation: does the dimension of racial segregation matter? PLoS One. 2015;10:e0138489. doi: 10.1371/journal.pone.0138489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


