Abstract
The wide range in human skin color results from varying levels of the pigment melanin. Genetic mechanisms underlying coloration differences have been explored, but identified genes do not account for all variation seen in the skin color spectrum. Post-transcriptional and post-translational regulation of factors that determine skin color, including melanin synthesis in epidermal melanocytes, melanosome transfer to keratinocytes and melanosome degradation, is also critical for pigmentation. We therefore investigated proteins that are differentially expressed in melanocytes derived from either White or African American (AA) skin. Two dimensional gel electrophoresis (2-DGE) and mass spectrometry demonstrated that Heat Shock Protein 70-1A (Hsp70-1A) protein levels were significantly higher in AA melanocytes compared to White melanocytes. Hsp70-1A expression significantly correlated with levels of tyrosinase, the rate-limiting melanogenic enzyme, consistent with a proposed role for Hsp70-family members in tyrosinase post-translational modification. Additionally, pharmacologic inhibition and siRNA-mediated down-regulation of Hsp70-1A correlated with pigmentation changes in cultured melanocytes, modified human skin substitutes and ex vivo skin. Furthermore, Hsp70-1A inhibition led to increased autophagy-mediated melanosome degradation in keratinocytes. Our data thus reveal that epidermal Hsp70-1A contributes to the diversity of skin color by regulating the amount of melanin synthesized in melanocytes and modulating autophagic melanosome degradation in keratinocytes.
INTRODUCTION
The wide spectrum of human skin color phenotypes results from differences in epidermal melanin levels (Quevedo and Holstein, 2006). Melanocytes synthesize melanin in endolysosomal organelles called melanosomes (Marks and Seabra, 2001; Orlow, 1995), which contain a distinct set of melanin-synthesizing enzymes and structural proteins, including tyrosinase (TYR), oculocutaneous albinism type 2 protein (OCA2), tyrosinase- related protein 1 (TYRP1), DOPAchrome tautomerase (DCT/TYRP2), and Pmel17 (GP100). Maturation of melanosomes requires various membrane-trafficking factors including small Ras-like GTPases/Rab protein family members RAB- 27A, 32 and 38 (Bultema and Di Pietro, 2013). Melanin-filled melanosomes are translocated through dendrites (Scott, 2006) and transferred to neighboring keratinocytes (Byers, 2006) where they protect against ultraviolet (UV) damage and give skin its color. We have demonstrated correlation between RAB27A expression and human skin substitute pigmentation in vivo (Yoshida-Amano et al., 2012).
Melanosomes are more abundantly transferred from melanocytes to keratinocytes in African-American (AA) compared to White skin (Montagna et al., 1991). Apart from quantitative difference, melanosomes are larger and more densely pigmented in AA skin (Minwalla et al., 2001). The differences in phagocytic activity of keratinocytes may also contribute to skin color variations (Sharlow et al., 2000). Protease-activated receptor-2 (PAR-2) regulates phagocytic activity in keratinocytes and is expressed at higher levels in darker skin (Babiarz-Magee et al., 2004). The total number of melanosomes in keratinocytes has a considerable impact on skin coloration. We demonstrated that melanosome number can be further modulated by autophagy-mediated melanosome degradation in the keratinocyte, a novel mechanism for regulating skin color (Murase et al., 2013).
Notwithstanding the great inter- and intra-population diversity in skin color, the basic mechanisms underlying melanogenesis are comparable across all groups. While most studies have focused on intra-population variation among Europeans, genetic variants localized to a small subset of genes have been shown to be the primary determinants of inter-population color skin variation. These genes include regulatory proteins ASIP, KITLG, IRF4 and MC1R; and melanosomal proteins OCA2, SLC45A2 and SLC24A5 (Maroñas et al., 2015; Miller et al., 2007). Selected SNPs at these loci were shown to reasonably predict light or dark skin color in 72% of subjects (0.5% error rate) (Spichenok et al., 2011).
Differing degrees of skin pigmentation observed among ethnic groups can also be partially attributed to differences in tyrosinase activity, some of which is accounted for by genetic variants at the TYR (Jagirdar et al., 2014) and IRF4 loci (Praetorius et al., 2013). Tyrosinase, the rate-limiting enzyme required for melanin synthesis, is quantitatively more active in melanocytes from darker skin than lighter skin (Pomerantz and Ances, 1975; Iozumi et al., 1993; Iwata et al., 1990; Maeda et al., 1997), despite similar RNA expression levels (Naeyaert et al., 1991). Tyrosinase is synthesized, folded and post-translationally modified in the endoplasmic reticulum (ER), then further modified in the Golgi. Tyrosinase folding is facilitated by molecular chaperones including the Heat Shock 70 kDa Protein (Hsp70) family member BiP (Wang et al., 2005). In addition to promoting optimal protein folding, Hsp70 proteins also function in a variety of cellular biological processes (Kampinga and Craig, 2010).
Given that genetic association studies fail to capture all factors that determine phenotypic variability (Heng, 2010), we explored proteins that were differentially expressed between White- and AA skin-derived melanocytes using two dimensional gel electrophoresis (2-DGE) and mass spectrometry. We consequently identified an important role for Hsp70-1A in determining skin color.
RESULTS
Hsp70-1A is expressed at higher levels in AA-melanocyte lines compared to White-melanocyte lines
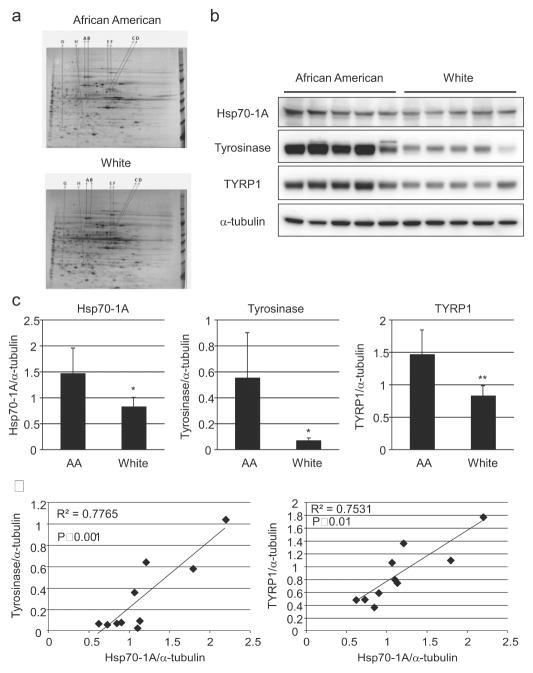
We performed differential protein analysis of six normal human melanocyte (NHEM) lines (three AA, three White) using 2-DGE followed by mass spectrometry. Gels were run in pairs (AA versus White) to account for experimental variability. Spot patterns were compared and the consequent differences were confirmed in three separate experiments. Eight spots, consistently differentially expressed in all three pairs, were selected for further analysis using mass spectrometry (Figure 1a). Hits were filtered for proteins with at least 2 unique peptides and a greater than 1% protein False Discovery Rate against a decoy database. Of the identified proteins, heat shock protein 70 (Hsp70-1A encoded by the HSPA1A gene) (Figure 1a, spot A) and tyrosinase-related protein 1 (TYRP1) (Figure 1a, spot C) were selected for further investigation.
Figure 1. Differences in protein expression in human melanocytes with varying pigmentation levels.
(a) NHEMs were cultured and proteins were harvested (3 AA- and 3 White-derived). Two-DGE was performed followed by silver staining. Indicated spots were extracted and subjected to mass spectrometry. (b) Expression of Hsp70-1A, tyrosinase, or TYRP1 in NHEMs was determined by Western-blot analysis and (c) densitometry (Relative intensity of each band normalized to α-tubulin is expressed to compare protein expression in AA and White melanocytes). Left: Hsp70-1A, Center: tyrosinase, Right: TYRP1. Values represent means (+/− SD), n = 5 (different melanocyte lines/group); **P<0.01; *P<0.05. (d) Correlations are shown between Hsp70-1A and tyrosinase (Left) or TYRP1 (Right) protein expression levels normalized to α-tubulin expression.
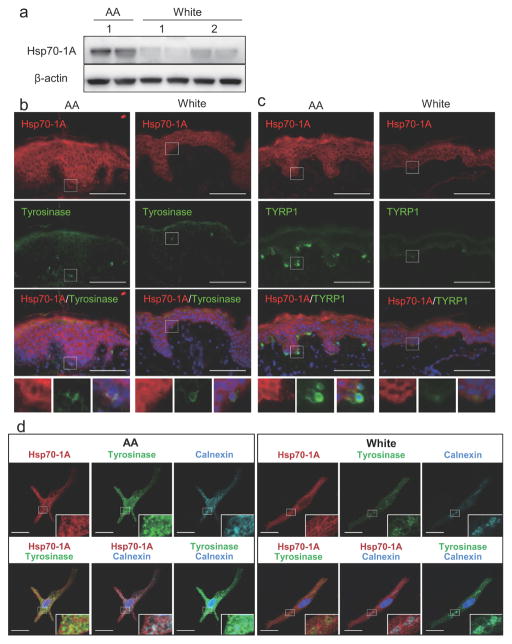
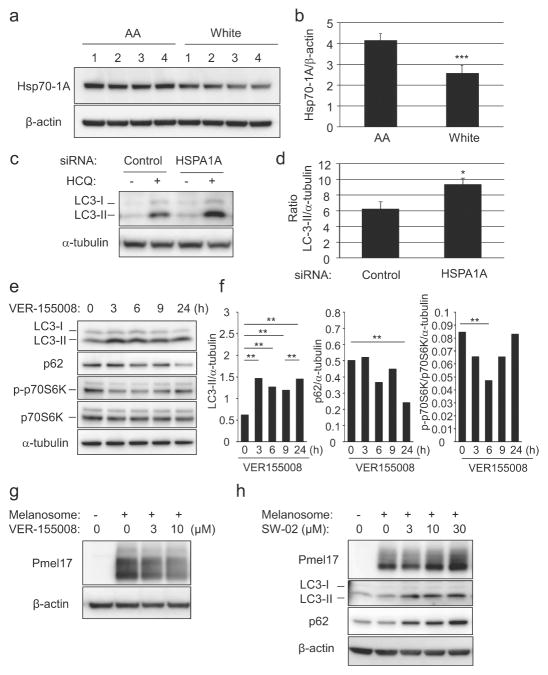
Data obtained from the mass spectrometry analysis were verified by Western-blotting in ten NHEM lines (not including lines used in proteomic analysis). We observed that the Hsp70-1A protein was significantly more abundant in AA-derived melanocytes as were tyrosinase and TYRP1, consistent with the proteomic analysis (Figure 1b and 1c). In addition, Hsp70-1A protein levels were significantly correlated with those of tyrosinase and TYRP1 (R2=0.78 and 0.75 respectively, Figure 1d). It is noteworthy that higher expression of Hsp70-1A protein was also observed in the epidermis of skin from AA donors as compared to that from White donors, consistent with differences in tyrosinase and TYRP1 protein levels (Figure 2a–2c).
Figure 2. Differential Hsp70-1A expression and co-localization with tyrosinase in AA and White NHEMs and skin.
(a) Proteins, harvested from skin obtained from AA- or White-donors, were subjected to Western-blotting for Hsp70-1A expression. β-actin = loading control. Immunohistologic analysis of (b) Hsp70-1A (red) and tyrosinase (green) or (c) Hsp70-1A (red) and TYRP1 (green) in AA- or White-derived skin was performed. Merged images of Hsp70-1A, tyrosinase or TYRP1 and DAPI nuclear staining are also shown. Lower insets show the magnified areas of the white rectangle-surrounding areas. Bar = 100 μm. (d) AA- or White-derived NHEMs were stained with Hsp70-1A- (red), tyrosinase- (green) and calnexin- (cyan) specific antibodies. Merged images with DAPI are also shown. Lower right insets show magnified areas. Bar = 20 μm.
Hsp70 family member BiP facilitates tyrosinase folding (Wang et al., 2005). We therefore investigated whether Hsp70-1A also associated with tyrosinase. Immunofluorescence staining and confocal microscopy consistently demonstrated partial Hsp70-1A co-localization with both tyrosinase and calnexin, an ER marker and known tyrosinase chaperone (Manga et al., 2011) (Figure 2d). It is also noteworthy that tyrosinase proteins were distributed more evenly from ER towards the cell periphery and more often co-localized with Hsp70-1A in AA-derived melanocytes than in White-derived melanocytes, where tyrosinase appeared to aggregate in calnexin-positive compartments.
Inhibition of Hsp70-1A reduced melanin content in cultured melanocytes and in cultured skin ex vivo
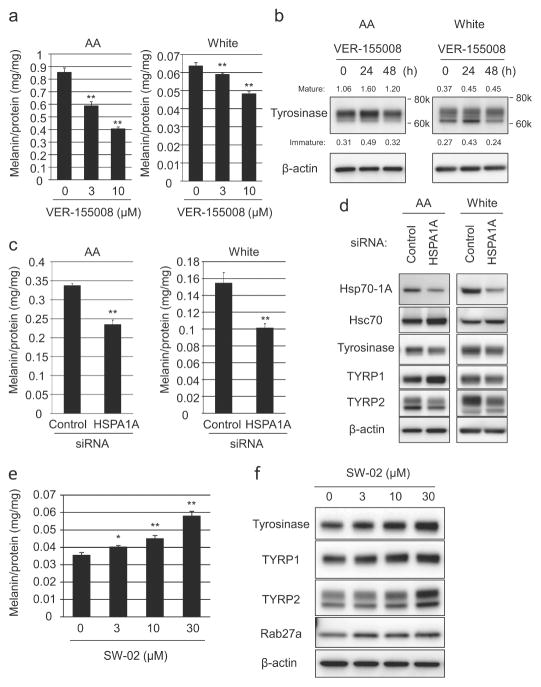
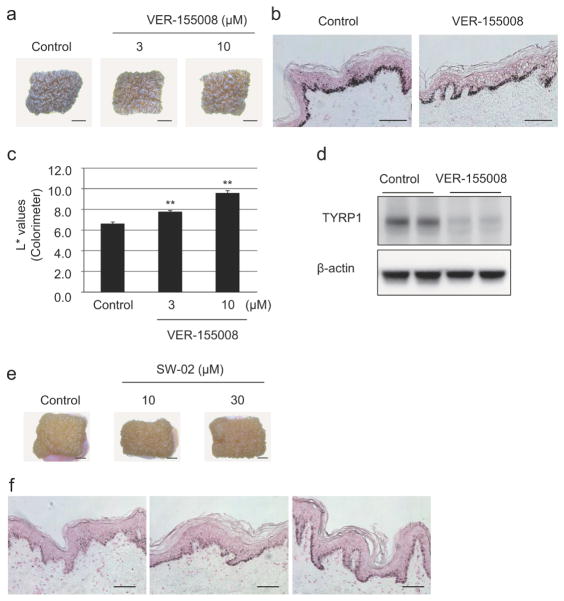
When AA- or White-derived melanocytes were treated with the Hsp70 inhibitor VER155008 (Williamson et al., 2009), melanin levels were strongly suppressed in a dose- and time-dependent manner, as were tyrosinase protein levels (Figure 3a and 3b), supporting a role for Hsp70-1A in tyrosinase regulation. Similar results were also observed with alternative Hsp70 inhibitors, YM-08 and Pifithrin-μ (Supplemental Figure 1a and 1b). In contrast to enriched expression of the 70-kDa mature form of tyrosinase in AA-derived melanocytes, White-derived prominently expressed a 66-kDa form previously shown to represent ER-retained tyrosinase (Chen et al., 2002). Inhibition of Hsp70-1A predominantly decreased expression of the mature form, suggesting inefficient tyrosinase processing that led to ER retention, which was more obvious in White-derived melanocytes (Supplemental Figure 1b and 1c). The specific effect of Hsp70-1A on pigmentation was confirmed by siRNA-targeted knockdown of HSPA1A (reproducible 60–70 % reduction in protein). Knockdown significantly inhibited melanin synthesis as well as expression of tyrosinase and TYRP2 in both AA- or White-derived melanocytes. Surprisingly, TYRP1 protein levels were either slightly increased or not changed at all (Figure 3c and 3d). Conversely, SW-02, an Hsp70 activator (Evans et al., 2006), induced elevated expression tyrosinase, TYRP1, TYRP2 and Rab27a and resulted in significantly increased melanin content in White-derived melanocytes (Figure 3e and 3f). The impact on melanogenesis in AA-derived melanocytes was less impressive; however these cells already express relatively high Hsp70-1A protein levels (Supplemental Figure 1f and 1g).
Figure 3. Hsp70-1A expression correlates with melanogenesis.
(a) AA- or White-derived NHEMs were treated with Hsp70-inhibitor VER-155008 (at increasing concentrations) for 7 days and melanin content determined (Mean +/− SD, n=3; ** P<0.01, * P<0.05 (ANOVA, Dunnett). (b) NHEMs were incubated with 3 μM VER-155008 (for increasing times) and tyrosinase expression determined by Western-blotting. Densitometry values normalized to β-actin are shown. (c) NHEMs were transfected with HSPA1A-specific or non-specific siRNAs and melanin quantitated after 7 days. (d) NHEMs were transfected with HSPA1A-specific or non-specific siRNAs for three days and Hsp70-1A, tyrosinase, TYRP1 or TYRP2 expression determined by Western-blotting. White skin-derived NHEMs were treated with Hsp70-activator SW-02 for (e) 7 days and melanin quantitated or (f) 2 days for Western-blot analysis of tyrosinase, TYRP1, TYRP2, or Rab27a expression. β-actin = loading control.
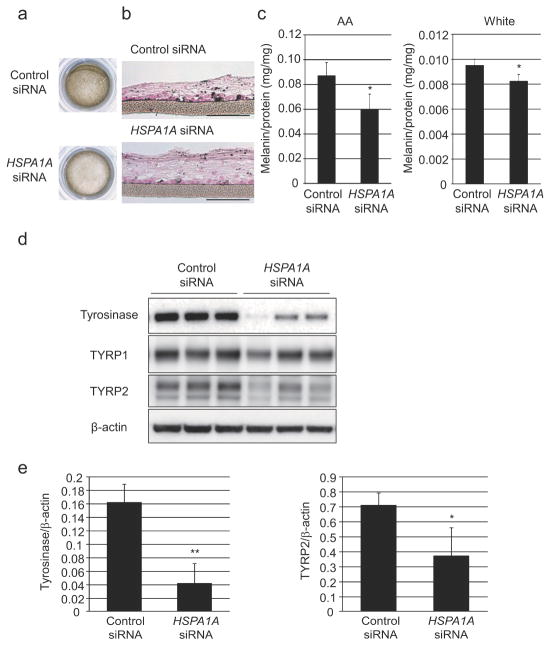
Knockdown of Hsp70-1A expression was also assessed in cultured three-dimensional human skin substitutes (3D-HSSs). AA- or White-derived NHEMs transfected with HSPA1A-targeting siRNA (significant reduction in Hsp70-1A expression, Figure 3d) were used to construct the 3D-HSSs. There was a visible decrease in pigmentation accompanied by significantly reduced melanin content as well as decreased expression of tyrosinase and TYRP2 (Figure 4a–4e) in Hsp70-1A knockdown models.
Figure 4. Hsp70-1A inhibition significantly lightens skin color in three-dimensional human skin substitutes.
AA- or White-derived NHEMs transfected with two different HSPA1A- or non-specific siRNAs were cultured with AA-derived NHEKs for 20 days to generate three-dimensional human skin substitutes (3D-HSSs). (a) Representative images of 3D-HSSs including AA-derived NHEMs are shown. 3D-HSSs were subjected to (b) Fontana-Masson staining (Scale bar = 100 μm) or (c) harvested and solubilized to quantitate melanin content. Three-dimensional-HSSs were cultured for 15 days and proteins were harvested. (d) Western-blot analysis and (e) densitometry were performed to determine tyrosinase and TYRP2 expression (β-actin = loading control). Graphs show relative expression after normalization against β-actin (Left: tyrosinase, Right: TYRP2). Values represent mean +/− SD from three different samples. ** P<0.01; * P<0.05 (t-test).
To confirm our findings, we treated cultured human breast skin derived from an AA female with Hsp70 inhibitor VER-155008 for 8 days. Fontana-Masson staining showed decreased melanin deposition in VER-155008 treated skin compared with untreated control. This decrease was correspondingly reflected in skin color changes (Figure 5a and 5b). The intensity of skin color lightness was also significantly increased by the inhibitor as shown by colorimeter measurement L* values (Figure 5c). TYRP1 protein was also remarkably decreased (Figure 5d). Conversely, Hsp70 activator SW-02 induced remarkable dose dependent skin darkening in White-derived skin tissues (Figure 5e and 5f).
Figure 5. Hsp70-1A activity correlates with pigmentation of cultured skin.
Skin from an AA donor was cultured with or without VER-155008 for 8 days (indicated concentrations). (a) Photographs show representative samples (Scale bar = 2 mm). (b) Treated skin was subjected to Fontana-Masson staining. Scale bars = 100 μm. (c) Colorimeter measurements of pigmentation were also taken (L* values, means +/− SD, n=3; **P<0.01 (ANOVA, Dunnett)). (d) Proteins were harvested and subjected to Western-blot analysis of TYRP1 expression. β-actin = loading control. Skin from a White donor was cultured with or without SW-02 for 8 days (indicated concentrations). (e) Photographs of representative samples are shown (Scale bars = 2 mm). (f) Fontana-Masson staining of SW-02 treated skin was performed. Scale bars = 100 μm.
Modulation of Hsp70-1A correlates with changes in autophagy-driven melanosome degradation in human keratinocytes
Given the significant decrease in pigmentation of skin treated with Hsp70 inhibitors and the broad distribution of Hsp70-1A in the skin, we hypothesized that the observed effects were due to changes in both melanocytes and keratinocytes. Hsp70 participates in a negative regulatory feedback loop that decreases cellular autophagy (Dokladny et al., 2013; 2015) and we recently reported that autophagy-mediated degradation of melanosomes in keratinocytes has a considerable impact on skin coloration (Murase et al., 2013). We therefore investigated a potential role for Hsp70-1A in keratinocytes. Consistent with difference in Hsp70-1A protein levels in intact epidermis, Hsp70-1A protein was significantly more abundant in AA skin-derived keratinocytes as compared with White skin-derived keratinocytes (Figure 6a and 6b).
Figure 6. Hsp70-1A expression inversely correlates with autophagy-mediated melanosome degradation.
(a). Hsp70-1A expression in four AA and four White skin-derived NHEKs was determined by Western-blotting and (b) densitometry (Mean +/−SD, ***P<0.001 (t-test)). (c) AA-NHEKs were transfected with HSPA1A-targeting or non-specific-siRNAs with or without HCQ. LC3 expression was determined by Western-blotting and (d) densitometry (Mean +/−SD, *P<0.05 (t-test)). (e) AA-NHEKs were cultured with or without VER-155008 (3μM). LC3-, p62-, phospho-p70S6K and p70S6K expression was determined by Western-blot analysis and (f) densitometry (*P<0.01; *P<0.05 (ANOVA, Holm-test)). NHEKs were cultured with isolated melanosomes for 24 hours, washed, then cultured with or without (g) VER-155008 (AA-NHEKs) or (h) SW-02 (White-NHEKs) for 48 hours. Pmel17, LC3 and p62 expression was determined by Western-blotting. β-actin/α-tubulin= loading control.
Autophagosome formation can be assayed by monitoring LC3-II levels, which correlate with cellular autophagosome numbers. Increased LC3-II expression thus reflects increased autophagy. LC3-II is however rapidly turned over by autolysosomal degradation. To assess longer-term effects, hydroxychloroquine (HCQ) is used to inhibit lysosomal activity to facilitate LC3-II measurement (Mizushima and Yoshimori, 2007). Transfection of AA-derived keratinocytes with HSPA1A-specific siRNA significantly increased accumulation of LC3-II following lysososome inhibition with HCQ compared to non-specific siRNA-treated controls (Figure 6c and 6d).
Keratinocyte treatment with Hsp70 inhibitor VER-155008 caused an increase in LC3-II levels, indicating increased autophagosome number. Due to the rapid effect of the inhibitor as compared to siRNA-mediated effects, it was not necessary to use HCQ.
To confirm that this increase also resulted in increased autophagic activity, levels of an autophagosome substrate, p62 protein, were monitored in VER-155008 treated cells. There was a significant decrease in p62 levels following the increase in LC3-II expression. To confirm our findings, we monitored p70S6K phosphorylation. mTORC1 both inhibits autophagy and phosphorylates p70S6K (Zhou and Huang, 2010). VER-155008 treatment of keratinocytes promoted a decrease in phospho-p70S6K levels, suggesting stimulation of autophagy upon Hsp70-1A inhibition. Thus VER-155008 treatment may increase autophagy through mTORC1 inhibition (Figure 6e and 6f).
Melanosome degradation in keratinocytes (measured by changes in levels of the melanosomal protein Pmel17 as previously described (Murase et al., 2013)) was significantly inhibited by treatment with Hsp70 activator SW-02 and accelerated by Hsp70 inhibitors (VER-155008, Pifithrin-μ, and YM-08) (Figure 6g, 6h, and Supplemental Figure 2). Therefore, Hsp70-1A may enhance skin pigmentation not only by stimulating expression of melanocyte-specific proteins and melanogenic activity in melanocytes, but also by repressing autophagy-mediated melanosome degradation in keratinocytes.
DISCUSSION
Skin pigment protects epidermal cells from UV damage and reduces skin cancer risk. Melanosomes form a melanin cap to shield the nuclei of keratinocytes (Park et al., 2009). Thus, despite the danger from cytotoxic melanin precursors (Graham et al., 1978), melanosomes play a crucial role in the skin. People whose ancestors resided more recently in lower-latitude areas tend to have darker skin color, while people whose ancestors recently resided at higher-latitudes tend to have relatively lighter skin color (Jablonski and Chaplin, 2000; Diamond, 2005). This change is hypothesized to be an evolutionary adaptation to counter reduced sun-induced vitamin D synthesis at higher latitudes where UV-induced skin damage is less problematic. A number of genes that determine levels of skin pigmentation have been identified through genetic association studies and investigation of hereditary pigment disorders. In this study, we have found that Hsp70-1A also plays a critical role in determining skin color differences between ethnic groups.
Hsp70 family proteins generally function as molecular chaperones for the post-translational modification of target proteins and to prevent aggregate formation, promoting maintenance of cellular homeostasis following cellular stresses (Kampinga and Craig, 2010). Given that the Hsp70 family member BiP facilitates tyrosinase folding (Wang et al., 2005) in concert with chaperones such as calnexin (Manga et al., 2011), we propose that Hsp70-1A also plays a role in regulating tyrosinase levels by promoting tyrosinase maturation. We demonstrate that melanin levels correlate with levels of Hsp70-1A expression in melanocytes. In melanocytes derived from White donors, tyrosinase is more prominently co-localized with calnexin, an ER-resident protein, suggesting reduced maturation. When Hsp70-1A activity or expression is down-regulated, there is concomitant reduction in tyrosinase protein and increase in lower molecular weight tyrosinase that matches the size of the immature form. Conversely, when Hsp70 activity is increased, tyrosinase levels increase. Interestingly, Tyrp1, which has been shown to stabilize tyrosinase and promote tyrosinase maturation (Manga et al., 2000; Toyofuku et al., 2001) was also found to be expressed at higher levels in AA skin-derived melanocytes.
In addition to Hsp70-1A-induced enhancement of melanogenesis via tyrosinase regulation, we also found that Hsp70-1A expression inversely correlated with melanosome degradation in keratinocytes. Since Hsp70 is part of a negative regulatory feedback loop that reduces cellular autophagy (Dokladny et al., 2013; 2015) and autophagy-mediated degradation of melanosomes impacts skin coloration (Murase et al., 2013), we investigated whether Hsp70-1A expression correlated with keratinocyte autophagic activity. We found that lighter skin-derived keratinocytes have higher autophagic activity and lower Hsp70-1A protein expression compared to darker skin-derived keratinocytes. We hypothesize that the dampening effect of Hsp70 on autophagic activity, potentially mediated by the mTORC1 pathway, would lead to reduced melanosome degradation. Furthermore, lower levels of Hsp70-1A may also attenuate cellular ability to respond to stress and consequently lead to increased autophagy.
One study showed that the typical heat shock response instigates an Hsp70-mediated reduction in melanin synthesis by suppressing the melanocyte transcription factor MITF in B16 melanoma cells (Hoshino et al., 2010). This effect may be mediated by another Hsp70 family member; alternatively, the impact of Hsp70-1A may be cell type and/or stimulus dependent. The effect was only studied in a melanoma cell line that has responds differently compared to normal melanocytes when treated with agents such as phorbol esters (Bennett et al., 1987; Bertolotto et al., 1998). It has also been reported that Hsp70-family member BiP facilitates post-translational modification of tyrosinase (Wang et al., 2005), thus promoting melanin synthesis. Additionally, Gp96, an Hsp90 family member, regulates melanogenesis by distributing tyrosinase to late melanosomes (Zhang et al., 2014) suggesting that Hsp proteins could have antagonistic roles in fine-tuning melanogenesis. Further exploration would thus be warranted to reveal the precise functions of Hsp70-1A in controlling melanin levels.
Since autophagy is involved in melanogenesis, redox homeostasis, and premature senescence in melanocytes (Ganesan et al., 2010; Ho et al., 2011; Zhang et al., 2015), it would be worth understanding if Hsp70-1A plays a role in any of these cellular functions by modulating autophagy, conceivably by chaperoning the essential autophagosome-related proteins in a coordinated manner. It would also be of importance to identify potential mutations or polymorphisms that contribute to variations in Hsp70-1A levels and/or function. HSPA1A SNPs have been predicted to increase the risk of certain diseases, such as atherosclerosis and cerebral ischemia (Dulin et al., 2012; Wei et al., 2013), although none of these were implicated in melanogenesis-related function based on our relatively small number of melanocyte lines (data not shown). Further investigations are needed to clarify in detail how Hsp70-1A and Hsp70-family proteins control skin pigmentation and which molecules and/or mechanisms are the most feasible and approachable targets for controlling skin color for the treatment of pigmentation disorders.
Our studies have revealed an essential role for the Hsp70-1A protein in determining skin pigmentation. Hsp70-1A protein levels in melanocytes correlate with degree of pigmentation of donor skin. Hsp70-1A may play a role in regulating the rate of melanin synthesis by chaperoning melanogenic enzymes such as tyrosinase in melanocytes, as well as regulating autophagic activity that degrades pigment containing melanosomes in keratinocytes. These dual roles of Hsp70-1A in determining pigmentation both in melanocytes and in keratinocytes were considered to be clearly reflected in Hsp70 inhibitor-driven skin lightening and Hsp70-1A activator-driven skin darkening in ex vivo skin tissues. In addition, it has been reported that overexpression of Hsp70-1A inhibited UV-induced epidermal hyperplasia, degradation of extracellular matrix, and decreased skin elasticity that would characterize skin photoaging in SKH-1 mice (Matsuda et al., 2013), which led several cosmetic and/or skin care companies to launch products that counteract UV-induced premature skin aging via the activation and/or increased expression of Hsp70-1A (Rattan et al., 2013). Taken together, Hsp70-1A would be an essential player in skin protection against UV exposure, not only through its known efficacy in repairing cellular damage but also its previously unreported roles in regulating multiple stages of pigmentation.
Yin et al. (Yin et al., 2014), performed a microarray analysis comparing gene expression in skin from Asian, European and African donors. They identified several genes, but not HSPA1A, that were differentially expressed. To the best of our knowledge, HSPA1A has also not been identified genome-wide association studies (GWAS) studying skin color. There are a number of reason why this may be the case. For example genes with relatively small effects can be missed and analytic methods do not detect all associations. The “missing heritability” issue is a well-documented phenomenon of GWAS (Heng, 2010).
Given that skin color is one of the most apparent characteristics of an individual, the biological mechanisms regulating melanogenesis have received significant attention. Pigmentation disorders, such as post-inflammatory hyperpigmentation, vitiligo and albinism affect all ethnicities, and are the source of tremendous psychosocial trauma. Most of these disorders currently have no effective treatments. It is therefore imperative to understand the basic biology of pigmentation and the mechanisms that promote differences in skin color, particularly so that therapies can be developed. In an age of personalized medicine, these mechanisms will be particularly important when developing therapies that are effective for specific ethnic skin types.
MATERIALS AND METHODS
Cell Culture
MNT-1 melanoma cells were kindly provided by Dr. Pier Giorgio Natali (Regina Elena Institute, Rome, Italy).
NHEKs and NHEMs were isolated from human neonatal foreskins as described previously (Yoshida et al., 2007; Murase et al., 2009; Murase et al., 2013). NHEKs were incubated with melanosomes isolated from MNT-1 cells as described previously (Ando et al., 2010; Murase et al., 2013). Construction of three-dimensional human skin substitutes is reported in Supplemental Materials and Methods.
Human skin
Human neonatal foreskins or surgically removed adult female skins derived from 23- to 49-year-old White and AA females were provided by the National Disease Research Interchange (NDRI, Philadelphia, PA). This study was conducted according to the Declaration of Helsinki protocols and all tissues recovered for this study had appropriate donor consent in writing for donation to research. Each informed consent was obtained and informed in writing form for any donor of human tissue for the use of that tissue for research.
Two-dimensional gel electrophoresis (2-DGE) and Mass Spectrometry
Proteins extracted from NHEMs were subjected to 2-DGE as reported in Supplemental Materials and Methods.
siRNA transfection
HSPA1A-specic siRNA (ON-TARGETplus J-005168-07 or J-005168-08) and control siRNA (ON-TARGETplus Non-targeting pool) were purchased from GE Dharmacon (Lafayette, CO). NHEKs and NHEMs were transfected with either 10 nM siRNA against HSPA1A or 10 nM control siRNA, using HiPerfect Transfection Reagent (Qiagen) or Lipofectamine® RNAiMAX (Life Technologies).
Western-blot analysis
Proteins were harvested and protein concentrations determined as reported in Supplemental Materials and Methods. Whole-cell lysates were separated using 10 or 12% SDS-polyacrylamide gel electrophoresis then transferred to Millipore Immobilon® FL PVDF Membranes (EMD Millipore, Billerica, MA). Proteins were detected using antibodies as described in Supplemental Materials and Methods. Immunoreactive bands were visualized with ECL Prime Western-blotting detection reagents (GE Healthcare) and quantified using an ODYSSEY Fc Imaging system (LICOR Inc., Lincoln, NE). β-actin or α-tubulin were used as internal loading control standards.
Immunofluorescence microscopy analysis
Tissues and cells were fixed, permeabilized and stained as reported in Supplemental Materials and Methods. Images were obtained with a Leica DM5500B digital microscope (Leica Microsystems, Bannockburn, IL). NHEMs images were obtained with a Zeiss LSM710 LIVE Duo Confocal Microscope (Carl Zeiss Microscopy GmbH, Jena, Germany).
Measurement of melanin content in human skins, 3D HSSs, and in NHEMs
After solubilization in 200 μl Solvable™ (PerkinElmer, Waltham, MA) of washed cells or tissues, melanin contents were measured using an absorbance meter (Microplate Reader Model 550; Bio-Rad Laboratories) at 405 nm as described previously (Murase et al., 2013).
Fontana-Masson staining
The tissue-cultured human skin was fixed with 10% buffered formalin, and then embedded in paraffin. Melanin pigment was visualized using Fontana–Masson staining with an eosin counterstain as described previously (Hachiya et al., 2005).
Measurement of skin color
The intensities of pigmentation in human skin tissues were measured by a colorimeter (cyberDERM Inc., Broomall, PA) 8 days after the treatment and were expressed as the L* values.
Statistics
Statistical analysis of differences was calculated by Student’s t-test, paired t-test, or ANOVA. A p value < 0.05 was considered statistically significant. Significance on correlations was evaluated by regression analysis.
Supplementary Material
Supplemental Figure 1. Hsp70-1A activity correlates with melanogenesis in NHEMs.
(a) NHEMs were treated with Hsp70 inhibitors Pifithrin-μ or YM-08 for 7 days. Cellular melanin content was quantified (Mean +/− SD, n=4; **P<0.01; *P<0.05 (ANOVA, Dunnett)). (b) NHEMs were incubated with Hsp70 inhibitors at indicated doses for 2 days and tyrosinase expression determined by Western-blotting (β-actin = loading control). Densitometry values normalized to β-actin are shown. (c) White-NHEMs were incubated with 1μM Pifithrin-μ for 24 hours and immunofluorescence staining performed with Hsp70-1A-(red), tyrosinase-(green), and calnexin-(cyan) specific antibodies. Merged images with DAPI staining are also shown. Insets show magnified areas. Bar = 20 μm. (d) AA-NHEMs were treated with Hsp70 inhibitor VER-155008 for 3 days and viability determined using AlamarBlue® reagent (% relative to untreated control, Mean +/− SD, n=3). (e) White-NHEMs were treated with VER-155008 for 7 days and protein concentration of extracts determined (Mean +/− SD, n=4). (f) AA-NHEMs were treated with Hsp70 activator SW-02 for 7 days and cellular melanin quantified (Mean +/− SD, n=3; **P<0.01 (ANOVA, Dunnett)). (g) AA-NHEMs were treated with SW-02 for 2 days and Tyrosinase, TYRP1, TYRP2 and Rab27a expression determined by Western-blotting analysis. β-actin = loading control.
Supplemental Figure 2. Hsp70-1A activity inversely correlates with degradation of incorporated melanosomes in an autophagy-dependent manner in NHEKs.
White-derived NHEKs were treated with isolated melanosomes for 24 hours. After washing in PBS, cells were cultured with or without SW-02, VER-155008, Pifithrin-μ, or YM-08 at indicated concentrations for 48 hours. Harvested proteins were subjected to Western-blotting to monitor Pmel17 levels. α-tubulin = loading control.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (NIH), under Award Number AR41880 (Seth J. Orlow). This work was supported by the NYU School of Medicine Proteomics Resource Center- partially supported by the Laura and Isaac Perlmutter Cancer Center Support Grant, P30CA016087 and a Shared Instrumentation Grant from the NIH/ORIP S10OD010582. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Dr. Beatrix Ueberheide for guidance and assistance with proteomic experiments; Nazanin Roudiani, Martha Vega, Genevieve Torres, Shruti Nayak and Khushboo Abhichandani for technical assistance; and Dr. Seth J. Orlow for helpful discussions.
The abbreviations used are
- 2-DGE
2-dimensional gel electrophoresis
- AA
African-American
- ATG
autophagy-related protein
- BPE
bovine pituitary extract
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- Hsp
heat shock protein
- HCQ
hydroxychloroquine
- HSS
human skin substitute
- NHEK
normal human epidermal keratinocytes
- NHEM
normal human epidermal melanocytes
Footnotes
This work was undertaken in Cincinnati, OH, U.S.A., New York, NY, U.S.A. and in Tochigi, Japan.
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando H, Niki Y, Yoshida M, Ito M, Akiyama K, Kim JH, et al. Keratinocytes in culture accumulate phagocytosed melanosomes in the perinuclear area. Pigment Cell Melanoma Res. 2010;23(1):129–133. doi: 10.1111/j.1755-148X.2009.00640.x. [DOI] [PubMed] [Google Scholar]
- Babiarz-Magee L, Chen N, Seiberg M, Lin CB. The expression and activation of protease-activated receptor-2 correlate with skin color. Pigment Cell Res. 2004;17(3):241–251. doi: 10.1111/j.1600-0749.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39(3):414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Bertolotto C, Bille K, Ortonne JP, Ballotti R. In B16 melanoma cells, the inhibition of melanogenesis by TPA results from PKC activation and diminution of microphthalmia binding to the M-box of the tyrosinase promoter. Oncogene. 1998;16(13):1665–1670. doi: 10.1038/sj.onc.1201685. [DOI] [PubMed] [Google Scholar]
- Bultema JJ, Di Pietro SM. Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles. Small GTPases. 2013;4(1):16–21. doi: 10.4161/sgtp.22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13(6):1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers HR. Melanosome processing in keratinocytes. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System. Oxford, UK: Blackwell Publishing; 2006. pp. 181–190. [Google Scholar]
- Diamond J. Evolutionary biology: geography and skin colour. Nature. 2005;435(7040):283–284. doi: 10.1038/435283a. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V, et al. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem. 2013;288(21):14959–14972. doi: 10.1074/jbc.M113.462408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Myers OB, Moseley PL. Heat shock response and autophagy--cooperation and control. Autophagy. 2015;11(2):200–213. doi: 10.1080/15548627.2015.1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin E, García-Barreno P, Guisasola MC. Genetic variations of HSPA1A, the heat shock protein levels, and risk of atherosclerosis. Cell Stress Chaperones. 2012;17(4):507–516. doi: 10.1007/s12192-012-0328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Wisén S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281(44):33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- Ganesan AK, Ho H, Bodemann B, Petersen S, Aruri J, Koshy S, et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DG, Tiffany SM, Vogel FS. The toxicity of melanin precursors. J Invest Dermatol. 1978;70(2):113–116. doi: 10.1111/1523-1747.ep12541249. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Sriwiriyanont P, Kaiho E, Kitahara T, Takema Y, Tsuboi R. An in vivo mouse model of human skin substitute containing spontaneously sorted melanocytes demonstrates physiological changes after UVB irradiation. J Invest Dermatol. 2005;125(2):364–372. doi: 10.1111/j.0022-202X.2005.23832.x. [DOI] [PubMed] [Google Scholar]
- Heng HH. Missing heritability and stochastic genome alterations. Nat Rev Genet. 2010;11(11):813. doi: 10.1038/nrg2809-c3. [DOI] [PubMed] [Google Scholar]
- Ho H, Kapadia R, Al-Tahan S, Ahmad S, Ganesan AK. WIPI1 coordinates melanogenic gene transcription and melanosome formation via TORC1 inhibition. J Biol Chem. 2011;286(14):12509–12523. doi: 10.1074/jbc.M110.200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Matsuda M, Yamashita Y, Takehara M, Fukuya M, Mineda K, et al. Suppression of melanin production by expression of HSP70. J Biol Chem. 2010;285(17):13254–13263. doi: 10.1074/jbc.M110.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozumi K, Hoganson GE, Pennella R, Everett MA, Fuller BB. Role of tyrosinase as the determinant of pigmentation in cultured human melanocyte. J Invest Dermatol. 1993;100(6):806–811. doi: 10.1111/1523-1747.ep12476630. [DOI] [PubMed] [Google Scholar]
- Iwata M, Corn T, Iwata S, Everett MA, Fuller BB. The relationship between tyrosinase activity and skin color in human foreskins. J Invest Dermatol. 1990;95(1):9–15. doi: 10.1111/1523-1747.ep12872677. [DOI] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39(1):57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- Jagirdar K, Smit DJ, Ainger SA, Lee KJ, Brown DL, Chapman B, et al. Molecular analysis of common polymorphisms within the human Tyrosinase locus and genetic association with pigmentation traits. Pigment Cell Melanoma Res. 2014;27(4):552–564. doi: 10.1111/pcmr.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez M, Tsukamoto K, Hearing VJ. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991;266(2):1147–1156. [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11(8):579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Yokokawa Y, Hatao M, Naganuma M, Tomita Y. Comparison of the melanogenesis in human black and light brown melanocyte. J Dermatol Sci. 1997;14(3):199–206. doi: 10.1016/s0923-1811(96)00575-0. [DOI] [PubMed] [Google Scholar]
- Manga P, Sato K, Ye L, Beermann F, Lamoreux ML, Orlow SJ. Mutational analysis of the modulation of tyrosinase by tyrosinase-related proteins 1 and 2 in vitro. Pigment Cell Res. 2000;13(5):364–374. doi: 10.1034/j.1600-0749.2000.130510.x. [DOI] [PubMed] [Google Scholar]
- Manga P, Orlow SJ. Informed reasoning: repositioning of nitisinone to treat oculocutaneous albinism. J Clin Invest. 2011;121(10):3828–3831. doi: 10.1172/JCI59763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2(10):738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Maroñas O, Söchtig J, Ruiz Y, Phillips C, Carracedo Á, Lareu MV. The genetics of skin, hair, and eye color variation and its relevance to forensic pigmentation predictive tests. Forensic Sci Rev. 2015;27(1):13–40. [PubMed] [Google Scholar]
- Matsuda M, Hoshino T, Yamakawa N, Tahara K, Adachi H, Sobue G, et al. Suppression of UV-induced wrinkle formation by induction of HSP70 expression in mice. J Invest Dermatol. 2013;133(4):919–928. doi: 10.1038/jid.2012.383. [DOI] [PubMed] [Google Scholar]
- Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, et al. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell. 2007;131(6):1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minwalla L, Zhao Y, Le Poole IC, Wickett RR, Boissy RE. Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J Invest Dermatol. 2001;117(2):341–347. doi: 10.1046/j.0022-202x.2001.01411.x. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Montagna W, Carlisle K, Beaverton MS. The architecture of black and white facial skin. J Am Acad Dermatol. 1991;24(6 Pt 1):929–937. doi: 10.1016/0190-9622(91)70148-u. [DOI] [PubMed] [Google Scholar]
- Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, et al. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. 2013;210(2):389–399. doi: 10.1084/jem.20121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase D, Hachiya A, Amano Y, Ohuchi A, Kitahara T, Takema Y. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J Biol Chem. 2009;284(7):4343–4353. doi: 10.1074/jbc.M805570200. [DOI] [PubMed] [Google Scholar]
- Murase D, Hachiya A, Takano K, Hicks R, Visscher MO, Kitahara T, et al. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J Invest Dermatol. 2013;133(10):2416–2424. doi: 10.1038/jid.2013.165. [DOI] [PubMed] [Google Scholar]
- Naeyaert JM, Eller M, Gordon PR, Park HY, Gilchrest BA, et al. Pigment content of cultured human melanocytes does not correlate with tyrosinase message level. Br J Dermatol. 1991;125(4):297–303. doi: 10.1111/j.1365-2133.1991.tb14161.x. [DOI] [PubMed] [Google Scholar]
- Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995;105(1):3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009;66(9):1493–1506. doi: 10.1007/s00018-009-8703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerants SH, Ances IG. Tyrosinase activity in human skin. Influence of race and age in newborns. J Clin Invest. 1975;55(5):1127–1131. doi: 10.1172/JCI108014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius C, Grill C, Stacey SN, Metcalf AM, Gorkin DU, Robinson KC, et al. A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell. 2013;155(5):1022–1033. doi: 10.1016/j.cell.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo WC, Holstein TJ. General biology of mammalian pigmentation. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System. Oxford, UK: Blackwell Publishing; 2006. pp. 63–90. [Google Scholar]
- Rattan SI, Kryzch V, Schnebert S, Perrier E, Nizard C. Hormesis-based anti-aging products: a case study of a novel cosmetic. Dose Response. 2013;11(1):99–108. doi: 10.2203/dose-response.11-054.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GA. Melanosome trafficking and transfer. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System. Oxford, UK: Blackwell Publishing; 2006. pp. 171–180. [Google Scholar]
- Sharlow ER, Paine CS, Babiarz L, Eisinger M, Shapiro S, Seiberg M. The protease activated receotor-2 upregulates keratinocyte phagocytosis. J Cell Sci. 2000;113(Pt 17):3093–3101. doi: 10.1242/jcs.113.17.3093. [DOI] [PubMed] [Google Scholar]
- Spichenok O, Budimlija ZM, Mitchell AA, Jenny A, Kovacevic L, Marjanovic D, et al. Prediction of eye and skin color in diverse populations using seven SNPs. Forensic Sci Int Genet. 2011;5(5):472–478. doi: 10.1016/j.fsigen.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Valencia JC, Kushimoto T, Ferrans VJ, Hearing VJ. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. FASEB J. 2001;15(12):2149–61. doi: 10.1096/fj.01-0216com. [DOI] [PubMed] [Google Scholar]
- Wang N, Daniels R, Hebert DN. The cotranslational maturation of the type I membrane glycoprotein tyrosinase: the heat shock protein 70 system hands off to the lectin-based chaperone system. Mol Biol Cell. 2005;16(8):3740–3752. doi: 10.1091/mbc.E05-05-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Wang W, He Y, Jiang L, Hou Z, Gao X, et al. Interaction between hypertension and HSP70 variants increase the risk of cerebral ischemia in Chinese Han population: an association study. Gene. 2013;513(2):239–243. doi: 10.1016/j.gene.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem. 2009;52(6):1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- Yin L, Coelho SG, Ebsen D, Smuda C, Mahns A, Miller SA, Beer JZ, Kolbe L, Hearing VJ. Epidermal gene expression and ethnic pigmentation variations among individuals of Asian, European and African ancestry. Exp Dermatol. 2014;23(10):731–5. doi: 10.1111/exd.12518. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Hachiya A, Sriwiriyanont P, Ohuchi A, Kitahara T, Takema Y, et al. Functional analysis of keratinocytes in skin color using a human skin substitute model composed of cells derived from different skin pigmentation types. FASEB J. 2007;21(11):2829–2839. doi: 10.1096/fj.06-6845com. [DOI] [PubMed] [Google Scholar]
- Yoshida-Amano Y, Hachiya A, Ohuchi A, Kobinger GP, Kitahara T, Takema Y, et al. Essential role of RAB27A in determining constitutive human skin color. PLoS One. 2012;7(7):e41160. doi: 10.1371/journal.pone.0041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Helke KL, Coelho SG, Valencia JC, Hearing VJ, Sun S, et al. Essential role of the molecular chaperone gp96 in regulating melanogenesis. Pigment Cell Melanoma Res. 2014;27:82–89. doi: 10.1111/pcmr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CF, Gruber F, Ni C, Mildner M, Koenig U, Karner S, et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J Invest Dermatol. 2015;135(5):1348–1357. doi: 10.1038/jid.2014.439. [DOI] [PubMed] [Google Scholar]
- Zhou H, Huang S. The complexes of mammalian target of rapamycin. Curr Protein Pept Sci. 2010;11(6):409–24. doi: 10.2174/138920310791824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Hsp70-1A activity correlates with melanogenesis in NHEMs.
(a) NHEMs were treated with Hsp70 inhibitors Pifithrin-μ or YM-08 for 7 days. Cellular melanin content was quantified (Mean +/− SD, n=4; **P<0.01; *P<0.05 (ANOVA, Dunnett)). (b) NHEMs were incubated with Hsp70 inhibitors at indicated doses for 2 days and tyrosinase expression determined by Western-blotting (β-actin = loading control). Densitometry values normalized to β-actin are shown. (c) White-NHEMs were incubated with 1μM Pifithrin-μ for 24 hours and immunofluorescence staining performed with Hsp70-1A-(red), tyrosinase-(green), and calnexin-(cyan) specific antibodies. Merged images with DAPI staining are also shown. Insets show magnified areas. Bar = 20 μm. (d) AA-NHEMs were treated with Hsp70 inhibitor VER-155008 for 3 days and viability determined using AlamarBlue® reagent (% relative to untreated control, Mean +/− SD, n=3). (e) White-NHEMs were treated with VER-155008 for 7 days and protein concentration of extracts determined (Mean +/− SD, n=4). (f) AA-NHEMs were treated with Hsp70 activator SW-02 for 7 days and cellular melanin quantified (Mean +/− SD, n=3; **P<0.01 (ANOVA, Dunnett)). (g) AA-NHEMs were treated with SW-02 for 2 days and Tyrosinase, TYRP1, TYRP2 and Rab27a expression determined by Western-blotting analysis. β-actin = loading control.
Supplemental Figure 2. Hsp70-1A activity inversely correlates with degradation of incorporated melanosomes in an autophagy-dependent manner in NHEKs.
White-derived NHEKs were treated with isolated melanosomes for 24 hours. After washing in PBS, cells were cultured with or without SW-02, VER-155008, Pifithrin-μ, or YM-08 at indicated concentrations for 48 hours. Harvested proteins were subjected to Western-blotting to monitor Pmel17 levels. α-tubulin = loading control.