Abstract
Background
Sentinel lymph node biopsy (SLNB) is one of the preferred treatments for breast cancer including clinically negative lymph node breast cancer. However, for 60–70% of patients this invasive axilla surgery is unnecessary. Our study aimed to identify the predictors for sentinel lymph node (SLN) metastasis in early breast cancer patients and provide evidence for rational decision-making in specified clinical situations.
Material/Methods
Medical records of 417 breast cancer patients who were treated with a breast surgical procedure and SLNB in Ningbo Medical Center Lihuili Eastern Hospital were retrospectively reviewed. Univariate analysis and multivariate logistic regression analysis were used to analyze the correlation between SLN metastasis and clinicopathological characteristics, including patient age, menstrual status, body mass index (BMI), family history, tumor size, laterality of tumor, histological grade, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), Ki67 index, and molecular subtypes of the tumor.
Results
In the cohort of 417 cases, the ratio of SLNM was 23.0%. Univariate analysis found that age, tumor size, histological grade, and Ki67 index were associated with SLN metastasis. However, age, tumor size, and histological grade were the only three independent predictors for SLN metastasis by multivariate logistic regression analysis. When these three factors were considered together, three different levels of SLN metastasis groups could be classified: low-risk group with the ratio of 14.3%, moderate-risk group with the ratio of 31.4%, and high-risk group with the ratio of 66.7%.
Conclusions
Our study demonstrated that age, tumor size, and histological grade were three independent predictive factors for SLN metastasis in early breast cancer patients. This finding may help surgeons in the decision-making process for early breast cancer patients before considering axilla surgical procedure.
MeSH Keywords: Breast Neoplasms, Multivariate Analysis, Sentinel Lymph Node Biopsy
Background
Axillary lymph node status is the most important prognostic factor for predicting survival in cases of invasive breast cancer [1–5], and axillary lymph node dissection (AxLND) has been widely accepted as the standard treatment in patients with invasive breast cancer because of its accurate assessment of lymph nodes status. However, sentinel lymph node biopsy (SLNB), as a minimally invasive surgical procedure, has emerged as the preferred technique for axilla staging especially in patients who are free of clinically detected lymph node metastasis [6–9]. Moreover, compared with the standard AxLND, there are two advantages to SLNB. First, it has better cosmetic results [10] and is associated with less arm and shoulder complications, including upper limb pain, sensory loss, lymphedema, and lymphangiosarcoma [11–13]. Second, it can provide a rapid intraoperative diagnosis and help surgeons to decide whether AxLND should be performed.
However, the reported incidence of SLN metastasis varies from 33.2–39% [14–16] and approximately 60–70% of patients suffered from unnecessary invasive axilla surgery. This raises the question of whether we can identify appropriate candidates for whom SLNB might be avoided. Prior work in this area has mostly focused on predictive factors for AxLN metastasis, whereas the role of predictors for clinically negative breast cancer patients is uncertain. To date, predictive factors for SLN metastasis have not yet been clarified. Therefore, we reviewed consecutive patients with early breast cancer who underwent breast and SLN surgical procedure in our hospital to determine the predictors for SLN metastasis.
Material and Methods
Patients
Inclusion criteria was as follows: 1) histologically proven invasive breast carcinoma; 2) no evidence of AxLN involvement or distant metastasis; 3) core needle biopsy (CNB) performed and pathological parameters, including tumor histological grade, ER, PR, HER2, Ki67 determined before surgery. SLNB with tracer methylene blue was implemented before breast surgical procedure, and AxLND (level I and II axillary lymph node) was then carried out if SLN was confirmed positive by frozen section examination intraoperatively. Every patient signed informed consent.
Exclusion criteria included: 1) male gender; 2) tumor size larger than 5 cm; 3) previous breast cancer history; 4) previous neoadjuvant therapy for breast carcinoma; 5) previous ipsilateral axilla surgery history; 6) bilateral breast cancer; 7) inflammatory breast cancer; and 8) pregnant with breast cancer.
Data collection
Medical records of eligible patients were reviewed and clinicopathological data were collected, including: patient age, gender, menstrual status, breast cancer family history, BMI; and tumor features such as tumor size, laterality of the tumor, histological grade, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), and Ki67 index. The criteria of HER2 positivity was defined as either IHC3+ or gene amplification by fluorescence in situ hybridization (FISH).
Statistical analysis
Univariate analysis with chi-squared test or Fisher’s exact test was performed to detect predictors for SLN metastasis. Then, multivariate analysis, including all variables from the univariate analysis that were related to SLN status, was performed to test factors’ independence. Statistical significance was defined as p<0.05; hazard ratio (HR) and 95% confidence intervals (CI) were also calculated. Statistical tests were two-sided, and analyses were performed using SPSS v.19.0 Software (SPSS, Chicago, IL, http://www.spss.com).
Results
Patient characteristics and SLNB
A total of 561 breast cancer patients who had breast surgery and SLNB at Ningbo Medical Center Lihuili Eastern Hospital between March 2009 and September 2016 were considered for the study. Of these, 144 cases were excluded from the study for the following reasons: in situ carcinoma (123 cases), bilateral breast cancer (six cases), male breast cancer (two cases), SLNB failure (eight cases), and only one SLN detected (five cases). Thereafter, 417 eligible cases were enrolled in the study. All patients were female with median age of 51 years old (range 23–80 years). Patient characteristics, tumor characteristics and surgical procedure are presented in Table 1 and included: patient age, menstrual status, BMI, breast cancer family history, tumor size, laterality of the tumor, histological grade, ER, PR, HER2, Ki67, and molecular subtype, and surgical procedure.
Table 1.
Clinicopathologic characteristics of early breast cancer patients.
| Numbers (n) | Percentage (%) | |
|---|---|---|
| Age (years) | ||
| <40 | 68 | 16.3 |
| 40–60 | 282 | 67.6 |
| >60 | 67 | 16.1 |
| Menstrual status | ||
| Premenopausal | 253 | 60.7 |
| Postmenopausal | 164 | 39.3 |
| BMI | ||
| ≤25 | 273 | 65.5 |
| >25 | 144 | 34.5 |
| Family history | ||
| Yes | 27 | 6.5 |
| No | 390 | 93.5 |
| Laterality of tumor | ||
| Left | 210 | 50.4 |
| Right | 207 | 49.6 |
| Tumor size (cm) | ||
| <1 cm | 78 | 18.7 |
| 1–2 cm | 230 | 55.2 |
| >2 cm | 109 | 26.1 |
| Histological grade | ||
| I | 62 | 14.9 |
| II | 270 | 64.7 |
| III | 85 | 20.4 |
| Histological type | ||
| IDC | 388 | 93.0 |
| ILC | 4 | 1.0 |
| Mucinous | 20 | 4.8 |
| Medullary | 5 | 1.2 |
| ER | ||
| Negative | 109 | 26.1 |
| Positive | 308 | 73.9 |
| PR | ||
| Negative | 127 | 30.5 |
| Positive | 290 | 69.5 |
| HER2 | ||
| Negative | 341 | 81.8 |
| Positive | 76 | 18.2 |
| Ki67 | ||
| Low expression index | 281 | 67.4 |
| High expression index | 136 | 32.6 |
| Molucular subtype | ||
| Luminal A-like | 118 | 28.3 |
| Luminal B-like | 200 | 48.0 |
| HER2 overexpression | 61 | 14.6 |
| Triple negative | 38 | 9.1 |
| Surgery | ||
| BCS + SLNB | 254 | 60.9 |
| BCS + ALND | 70 | 16.8 |
| Mastectomy + SLNB | 48 | 11.5 |
| MRM | 45 | 10.8 |
IDC – invasive ductal carcinoma; ILC – invasive lobular carcinoma; ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor 2; BCS – breast conserving surgery; SLNB – sentinel lymph node biopsy; ALND – axillary lymph node dissection; MRM – modified radical mastectomy.
In all, 2,005 SLNs were detected, with the average of 4.81±2.15 (median: 5, range: 2–9). The majority of patients (95.2%) had more than two SLNs, and 4.8% of patients had only two SLNs. Figure 1 demonstrates the distribution of SLN numbers. Six patients had a negative primary diagnosis based on frozen section examination but were confirmed positive on final pathological examination, and another 90 patients were positive on both pathological examinations. Of these 96 (23%) were SLN-positive patients, 92 (22.0%) of patients had macro-metastasis, and four (1.0%) of patients had micro-metastasis. Figure 2 (pie picture) shows the percentages of SLN metastasis with different SLN metastasis numbers: 96 patients (23.0%) were SLN positive on final pathology results,
Figure 1.
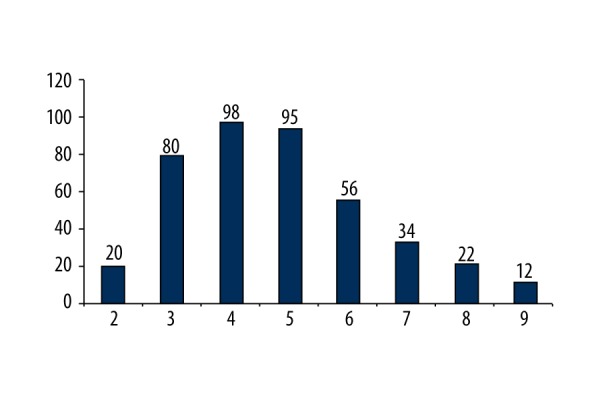
The distribution details of identified SLN number.
Figure 2.
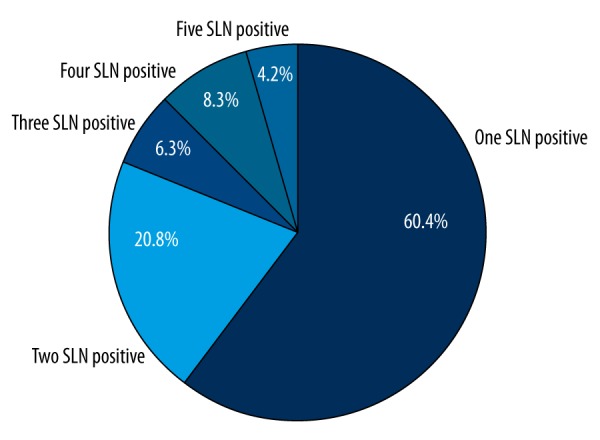
The percentage of different SLN positive numbers.
Association between clinicopathological factors and SLN status
Our study found that patient age, tumor size, histological grade, and Ki67 index were associated with the presence of SLN metastasis, while other factors had no relationship with SLN metastasis (Table 2). Four predictive factors which were confirmed to be associated with SLN metastasis by univariate analysis were brought into the multivariate logistic regression analysis model, which found that patient age, tumor size, and histological grade were independent predictive factors (Table 3).
Table 2.
Relationship of clinicopathologic factors for SLN metastasis.
| Variable | SLNM (n) | SLN-NM (n) | p Value | Hazard ratio | 95%CI |
|---|---|---|---|---|---|
| Age | |||||
| <40 years | 24 | 44 | |||
| ≥40 years | 72 | 277 | 0.009 | 2.098 | 1.198–3.677 |
| Menstrual status | |||||
| Premenopausal | 32 | 132 | |||
| Postmenopausal | 64 | 189 | 0.171 | 0.716 | 0.443–1.156 |
| BMI | |||||
| ≤25 | 58 | 215 | |||
| >25 | 38 | 106 | 0.235 | 0.753 | 0.470–1.205 |
| Family history | |||||
| Yes | 9 | 18 | |||
| No | 87 | 203 | 0.188 | 1.741 | 0.756–4.013 |
| Laterality of the tumor | |||||
| Left | 52 | 158 | |||
| Right | 44 | 163 | 0.392 | 1.219 | 0.772–1.926 |
| Tumor size | |||||
| ≤2 cm | 58 | 250 | |||
| >2 cm | 38 | 71 | 0.001 | 0.433 | 0.266–0.705 |
| Histological grade | |||||
| I–II | 62 | 270 | |||
| III | 34 | 51 | 0.000 | 0.344 | 0.206–0.576 |
| ER | |||||
| Positive | 76 | 232 | |||
| Negative | 20 | 89 | 0.178 | 1.458 | 0.841–2.526 |
| PR | |||||
| Positive | 68 | 222 | |||
| Negative | 28 | 99 | 0.754 | 1.083 | 0.657–1.785 |
| HER2 | |||||
| Positive | 16 | 60 | |||
| Negative | 80 | 261 | 0.652 | 0.870 | 0.475–1.594 |
| Ki67 | |||||
| Low index | 54 | 224 | |||
| High index | 42 | 97 | 0.014 | 0.557 | 0.349–0.889 |
| Molucular subtype | |||||
| Triple negative | 6 | 32 | |||
| Non-triple negative | 90 | 289 | 0.267 | 0.602 | 0.244–1.486 |
SLNM – sentinel lymphnode metastasis; SLN-NM – sentinel lymph node not metastasis; CI – confidence interval; ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor-2.
Table 3.
Multivariate analysis for SLN metastasis predictive parameters
| Variable | SLNM (n) | SLN-NM (n) | p Value | Hazard ratio | 95%CI |
|---|---|---|---|---|---|
| Age | |||||
| <40 years | 24 | 44 | |||
| ≥40 years | 72 | 277 | 0.011 | 2.188 | 1.198–4.001 |
| Tumor size | |||||
| ≤2 cm | 58 | 250 | |||
| >2 cm | 38 | 71 | 0.028 | 0.616 | 0.401–0.949 |
| Histological grade | |||||
| I–II | 62 | 270 | |||
| III | 34 | 51 | 0.003 | 0.408 | 0.224–0.743 |
| Ki67 | |||||
| Low index | 54 | 224 | |||
| High index | 42 | 97 | 0.574 | 0.851 | 0.485–1.494 |
SLNM – sentinel lymph node metastasis; SLN-NM – sentinel lymph node not metastasis; CI – confidence interval
The SLN metastasis ratio on different subgroups
The three risk factors for SLN metastasis were age younger than forty years old, tumor size larger than 2 cm, and histological grade III. The combination of three risk factors had eight different scenarios, and presented three different levels of SLN metastasis: 1) high-risk group, this group included patients with all three risk factors and SLN metastasis with a ratio of 66.7% (8/12); 2) moderate-risk group, this group included patients with one or two risk factors with a ratio of 31.4% (55/175); and 3) low-risk group, this group included patients with no aforementioned risk factors, with a ratio of 14.3% (33/230).
Discussion
Dual tracer technique with isotopic tracer and blue dye tracer has a higher detection rate compared to either of these tracers alone [17,18]. It is a great pity that isotopic tracer cannot be used routinely in China except in few large-scale medical centers, so blue dye tracer is the first and the sole choice for SLNB for early breast cancer patients. Three blue dye tracers used in clinical research and practice are isosulfan blue, patent blue dye, and methylene blue dye, but none of these are considered the gold standard for SLNB according to the current literatures [17,19–21]. In the present study, we used a single tracer technique with methylene blue dye and identified an average of 4.81 SLNs per patient, and three or more SLNs were detected in 95.2% of patients, which together demonstrated that SLNB with methylene blue tracer technique was a reasonable and feasible surgical procedure, supported further by the fact that when three and more SLNs were identified, the false negative rate decreased to the acceptable 5% level recommended by the American Society of Clinical Oncology (ASCO) guideline [22].
In our series of 417 early breast cancer patients, the presence of SLN metastasis was 23.0%, which was lower than in previous reports, with an incidence range reported in the literature of 33.2–39%) [14–16]. This difference may be due to the higher percentage of T1 tumor in our study (73.9%) compared to about 50% in other studies, which might account for the lower metastasis incidence in our study.
One significant finding was the three factors that were determined as independent predictor for SLN metastasis, both in univariate and multivariate analysis, namely patient age, tumor size, and histological grade. Another important finding was that the combination of patient age, tumor size, and histological grade can effectively discriminate patients with differential risk of SLN metastasis.
Young breast cancer or breast cancer in young women refers to patients with breast cancer who are younger than 35–40 years old, and presented with more aggressive biological behavior and unfavorable prognosis when compared to their counterparts. In the present study, we used 40 years of age as the cutoff point, and found that patient age at the time of diagnosis was significantly associated with a high risk of SLN metastasis. This predictive effect of age on nodal involvement was consistent with earlier evidence that indicated that breast tumors can be more aggressive in younger women [23–25]. Inconsistent results have been reported in recent literature [26,27], and there are obvious differences in the cutoff point chosen for age, which was younger in our study (40 years old) than has been reported in some other studies (50 years old).
Tumor size was the second predictor for SLN metastasis in our study, which was found to be predictive of axillary lymph node metastasis. Other studies have found that even in patients with tumor size less than 5–10 mm, there was a significant nodal metastasis in 5–15% of cases [28–31]. A retrospective analysis of 893 cases showed that the rate of node metastasis increased from 11% to 36% as tumor size increased from 10 mm to 25 mm [32]. Similar results were also found in our study: 18.8% of patients with tumors no larger than 20 mm and 34.9% of patients with tumor larger than 20 mm had SLN involved.
Previous studies have shown that histological grade has important prognostic value, which is equivalent to that of lymph node status [22] and greater than that of tumor size [33,34]. A retrospective study demonstrated that patients with grade I, stage II disease had the same survival as those with grade III, stage I disease [35]. However, the value of histological grade on axillary lymph node metastasis has been controversial. Histological grade was the third predictor for SLN metastasis in the present study, which was in concordance with previous reports [36,37]. However, in a recent report, tumor histological grade was related with SLN metastasis by univariate analysis either in overall sample or in luminal subgroup, but lost predictive value by multivariate logistic regression analysis [26].
The most important result in our study was that when three predictors were considered together, all patients could be stratified into three SLN metastasis levels: 1) high-risk group, which included the patients with all of three risk factors; 2) moderate-risk group, which included the patients with one to two risk factors; and 3) low-risk group, which included patients with none of the aforementioned risk factors. Our results suggested that the combinations of patient age, tumor size, and histological grade could effectively discriminate between patients with differential SLN metastasis. In the present study, more than half of the patients were in the low-risk group, in which the incidence was 14.3%; and thus the remaining 85.7% of patients might avoid axilla surgery theoretically due to negative lymph node status. Our results also provided evidence for rational decision-making in specific clinical situations, especially for patients with low risk of lymph node metastasis. For instance, in elderly patients who cannot tolerate or who do not have any contraindications for general anesthesia, simple treatment under local anesthesia with wide excision of the primary tumor without axilla surgery may be a reasonable choice. In addition, an immediate autologous breast reconstruction should be primarily considered in low-risk patients, while a delayed reconstruction may be preferred in high-risk patients.
The limitations of the study should be acknowledged. First, this was a retrospective, single-institution study with small sample size, which may decrease the reliability of the present study findings. Second, in our study a single tracer technique with methylene blue dye was applied, which was not the preferred technique recommended by the National Comprehensive Cancer Network (NCCN). Third, lymphovascular invasion (LVI) has been described as an independent predictor of node involvement in prior reports [38–40], but it could not be evaluated in our study which used diagnostic core-needle-biopsy of the primary tumor, a technique that has limited ability to assess LVI, and thus we could not verified LVI in our study.
Conclusions
In conclusion, though it was a retrospective, single-institution study, the results presented may be useful for managing select groups of patients who, due to comorbidities or refusal, have avoided surgical interventions in the axilla. Currently, a randomized, double-blind, multicenter clinical trial is recruiting more than 1,000 patients to verify whether, in the presence of a negative preoperative axillary assessment, SLNB can be spared and the decision on adjuvant medical treatment be made according to the biology of the tumor.
Footnotes
Source of support: This study was funded by Ningbo Science and Technology Foundation (2015C50003)
Conflicts of interest
All authors have not received research grants from any company, and declared no conflicts of interest.
Reference
- 1.National Institutes of Health Consensus Development Panel. Consensus statement: treatment of early-stage breast cancer. J Natl Cancer Inst Monogr. 1992;(11):1–5. [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265(3):391–95. [PubMed] [Google Scholar]
- 3.Silverstein MJ, Gierson ED, Waisman JR, et al. Axillary lymph node dissection for T1a breast carcinoma. Is it indicated? Cancer. 1994;73(3):664–67. doi: 10.1002/1097-0142(19940201)73:3<664::aid-cncr2820730326>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52(9):1551–57. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Andree C, Schmidt VJ, Munder BI, et al. Detecting of breast cancer metastasis by means of regional lymph node sampling during autologous breast reconstruction – a screening of 519 consecutive patients. Med Sci Monit. 2012;18(10):CR605–10. doi: 10.12659/MSM.883486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: Update of a randomized controlled study. Lancet Oncol. 2006;7(12):983–90. doi: 10.1016/S1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- 7.Goyal A, Newcombe RG ALMANAC Trialists Group. Factors affecting failed localisation and false-negative rates ofsentinelnode biopsy in breast cancer – results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99(2):203–8. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 8.Krag DN, Anderson SJ National Surgical Adjuvant Breast and Bowel Project. Technical outcomes ofsentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–88. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 9.Gill G SNAC Trial Group of the Royal Australasian College of Surgeons (RACS) and NHMRC Clinical Trials Centre. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): A randomized controlled surgical trial. Ann Surg Oncol. 2009;16(2):266–75. doi: 10.1245/s10434-008-0229-z. [DOI] [PubMed] [Google Scholar]
- 10.Isik A, Karavas E, Peker K, et al. Male Mondor’s disease is a rare entity. Breast J. 2016;22(6):700–1. doi: 10.1111/tbj.12657. [DOI] [PubMed] [Google Scholar]
- 11.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer – a multicenter validation study. N Engl J Med. 1998;339(14):941–46. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 12.Kuwajerwala NK, Feczko C, Dekhne N, et al. Comparison of lymphedema in patients with axillary lymph node dissections to those with sentinel lymph node biopsy followed by immediate and delayed ALND. Am J Clin Oncol. 2013;36(1):20–23. doi: 10.1097/COC.0b013e31823a4956. [DOI] [PubMed] [Google Scholar]
- 13.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viale G, Zurrida S, Maiorano E, et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005;103(3):492–500. doi: 10.1002/cncr.20809. [DOI] [PubMed] [Google Scholar]
- 15.Chua B, Ung O, Taylor R, et al. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg. 2001;71(12):723–28. doi: 10.1046/j.1445-1433.2001.02266.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara E, Smeets A, Laenen A, et al. Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast. 2013;22(3):357–61. doi: 10.1016/j.breast.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Zakaria S, Hoskin TL, Degnim AC. Safety and technical success of methylene blue dye for lymphatic mapping in breast cancer. Am J Surg. 2008;196(2):228–33. doi: 10.1016/j.amjsurg.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 18.Blessing WD, Stolier AJ, Teng SC, et al. A comparison of methylene blue and lymphazurin in breast cancer sentinel node mapping. Am J Surg. 2002;184(4):341–45. doi: 10.1016/s0002-9610(02)00948-0. [DOI] [PubMed] [Google Scholar]
- 19.Lanitis S, Filippakis G, Sidhu V, et al. Atypical anaphylactic reaction to patent blue during sentinel lymph node biopsy for breast cancer. Ann R Coll Surg Engl. 2008;90(4):338–39. doi: 10.1308/003588408X285702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramin S, Azar FP, Malihe H. Methylene blue as the safest blue dye for sentinel node mapping: Emphasis on anaphylaxis reaction. Acta Oncol. 2011;50(5):729–31. doi: 10.3109/0284186X.2011.562918. [DOI] [PubMed] [Google Scholar]
- 21.Fattahi AS, Tavassoli A, Rohbakhshfar O, et al. Can methylene blue dye be used as an alternative to patent blue dye to find the sentinel lymph node in breast cancer surgery? J Res Med Sci. 2014;19(10):918–22. [PMC free article] [PubMed] [Google Scholar]
- 22.Lyman GH, Giuliano AE American Society of Clinical Oncology. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2015;23(30):7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Rivadeneira DE, Simmons RM, Christos PJ, et al. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: Analysis in more than 900 patients. J Am Coll Surg. 2000;191(1):1–6. doi: 10.1016/s1072-7515(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 24.Maibenco DC, Weiss LK, Pawlish KS, et al. Axillary lymph node metastases associated with small invasive breast carcinomas. Cancer. 1999;85(7):1530–36. [PubMed] [Google Scholar]
- 25.Fowble BL, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30(1):23–33. doi: 10.1016/0360-3016(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 26.La Verde N, Biagioli E, Gerardi C, et al. Role of patient and tumor characteristics n sentinel lymph node metastasis in patients with luminal early breast cancer: an observational study. Springerplus. 2016;5:114. doi: 10.1186/s40064-016-1720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie F, Yang H, Wang S, et al. A logistic regression model for predicting axillary lymph node metastases in early breast carcinoma patients. Sensors (Basel) 2012;12(7):9936–50. doi: 10.3390/s120709936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverstein MJ, Skinner KA, Lomis TJ. Predicting axillary nodal positivity in 2282 patients with breast carcinoma. World J Surg. 2001;25(6):767–72. doi: 10.1007/s00268-001-0003-x. [DOI] [PubMed] [Google Scholar]
- 29.Kambouris AA. Axillary node metastases in relation to size and location of breast cancers: Analysis of 147 patients. Am Surg. 1996;62(7):519–24. [PubMed] [Google Scholar]
- 30.Fein DA, Fowble BL, Hanlon AL, et al. Identification of women with T1-T2 breast cancer at low risk of positive axillary nodes. J Surg Oncol. 1997;65(1):34–39. doi: 10.1002/(sici)1096-9098(199705)65:1<34::aid-jso7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Kim SH, Suh YJ, et al. Predictors of axillary lymphnode metastases (ALNM) in a Korean population with T1-2 breast carcinoma: triple negative breast cancer has a high incidence of ALNM irrespective of the tumor size. Cancer Res Treat. 2010;42(1):30–36. doi: 10.4143/crt.2010.42.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cutuli B, Velten M, Martin C. Assessment of axillary lymph node involvement in small breast cancer: Analysis of 893 cases. Clin Breast Cancer. 2001;2(1):59–65. doi: 10.3816/CBC.2001.n.012. [DOI] [PubMed] [Google Scholar]
- 33.Sundquist M, Thorstenson S, Brudin L, et al. Applying the Nottingham Prognostic Index to a Swedish breast cancer population. South East Swedish Breast Cancer Study Group. Breast Cancer Res Treat. 1999;53(1):1–8. doi: 10.1023/a:1006052115874. [DOI] [PubMed] [Google Scholar]
- 34.Galea MH, Blamey RW, Elston CE, et al. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22(3):207–19. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- 35.Henson DE, Ries L, Freedman LS, et al. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index. Cancer. 1991;68(10):2142–49. doi: 10.1002/1097-0142(19911115)68:10<2142::aid-cncr2820681010>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Yenidunya S, Bayrak R, Haltas H. Predictive value of pathological and immunohistochemical parameters for axillary lymph node metastasis in breast carcinoma. Diagn Patho. 2011;6:18. doi: 10.1186/1746-1596-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng HS, Chen LS, Kuo SJ, et al. Tumor characteristics of breast cancer in predicting axillary node metastasis. Med Sci Monit. 2014;20:1155–61. doi: 10.12659/MSM.890491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barth A, Craig PH, Silverstein MJ. Predictors of axillary lymph node metastases in patients with T1 breast carcinoma. Cancer. 1997;79(10):1918–22. [PubMed] [Google Scholar]
- 39.Chadha M, Chabon AB, Friedmann P, et al. Predictors of axillary lymph node metastases in patients with T1 breast cancer. Cancer. 1994;73(2):350–53. doi: 10.1002/1097-0142(19940115)73:2<350::aid-cncr2820730219>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Harden SP, Neal AJ, Al-Nasiri N, et al. Predicting axillary lymph node metastases in patients with T1 infiltrating ductal carcinoma of the breast. Breast. 2001;10(2):155–59. doi: 10.1054/brst.2000.0220. [DOI] [PubMed] [Google Scholar]


