ABSTRACT
The neural precursor cell expressed developmentally downregulated protein 4 (NEDD4) plays a pivotal oncogenic role in various types of human cancers. However, the function of NEDD4 in bladder cancer has not been fully investigated. In the present study, we aim to explore whether NEDD4 governs cell proliferation, apoptosis, migration, and invasion in bladder cancer cells. Our results showed that downregulation of NEDD4 suppressed cell proliferation in bladder cancer cells. Moreover, we found that inhibition of NEDD4 significantly induced cell apoptosis. Furthermore, downregulation of NEDD4 retarded cell migration and invasion. Notably, overexpression of NEDD4 enhanced cell growth and inhibited apoptosis. Consistently, upregulation of NEDD4 promoted cell migration and invasion in bladder cancer cells. Mechanically, our Western blotting results revealed that downregulation of NEDD4 activated PTEN and inhibited Notch-1 expression, whereas upregulation of NEDD4 reduced PTEN level and increased Notch-1 level in bladder cancer cells. Our findings indicated that NEDD4 exerts its oncogenic function partly due to regulation of PTEN and Notch-1 in bladder cancer cells. These results further revealed that targeting NEDD4 could be a useful approach for the treatment of bladder cancer.
KEYWORDS: apoptosis, bladder cancer, cell growth, invasion, NEDD4
Introduction
Bladder cancer is one of the common cancers in human, with approximately 400,000 new patients diagnosed every year in the world.1 More than 79,000 people will be expected to have bladder cancer and 16,870 patients with bladder cancer will die in 2017 in the United States.2 The main risk factor for bladder cancer is tobacco smoking.3 It has been known that most of bladder cancers are non-muscle-invasive bladder cancer (NMIBC).4 Half of NMIBC patients exhibit a recurrence after transurethral resection (TUR). Many patients progressed to muscle-invasive disease due to multiple recurrences.5-7 Thus, it is necessary to investigate the molecular mechanism of bladder tumorigenesis and discover new treatment strategies to improve outcomes and reduce recurrences in NMIBC.
Neural precursor cell expressed developmentally downregulated protein 4 (NEDD4), an E3 protein ligase enzyme, degrades some proteins through ubiquitination and endocytosis.8,9 Emerging evidence has demonstrated that NEDD4 plays oncogenic roles in tumorigenesis.10 Consistently, overexpression of NEDD4 is frequently observed in a variety of human malignancies such as gastric carcinomas,11 colorectal cancer,12 non-small cell lung carcinomas,13 breast cancer,14 and bladder cancer.15 NEDD4 has been found to play a critical role in tumorigenesis and metastasis.16,17 One study has revealed that PTEN (phosphatase and tensin homolog depleted on chromosome 10) has an inversely relationship with NEDD4 in breast and prostate cancer cell lines.18 Moreover, impeded NEDD4-mediated Ras degradation underlies Ras-driven tumorigenesis in a variety of cancer cells.19 Furthermore, NEDD4 binds LATS1 (large tumor suppressor kinase 1) and leads to its ubiquitination and degradation, subsequently results in the inhibition of Hippo pathway.20
Although these studies provide evidence for the oncogenic role of NEDD4 in various types of human cancers, the functions of NEDD4 in bladder cancer have not been extensively studied. Thus, in the present study, we aim to explore whether NEDD4 governs cell proliferation, apoptosis, migration, and invasion in bladder cancer cells. Our results showed that inhibition of NEDD4 led to cell growth suppression in bladder cancer cells. We also observed that NEDD4 siRNA treatment caused induction of cell apoptosis. Moreover, downregulation of NEDD4 inhibited cell migration and invasion. In line with these findings, overexpression of NEDD4 stimulated cell growth and inhibited cell apoptosis, and promoted cell migration and invasion in bladder cancer cells. Strikingly, inhibition of NEDD4 activated PTEN and inhibited Notch-1, whereas upregulation of NEDD4 decreased PTEN level and increased Notch-1 level in bladder cancer cells. Our results demonstrated that NEDD4 exhibited its oncogenic role partly due to upregulation of PTEN and downregulation of Notch-1 in bladder cancer cells. Our data indicated that inhibition of NEDD4 might be a useful strategy for the treatment of bladder cancer.
Results
NEDD4 expression was inhibited by its siRNAs in bladder cancer cells
To explore whether NEDD4 could regulate cell growth in bladder cancer cells, we used NEDD4 siRNA to down-regulate NEDD4 in RT4 cells. We found that NEDD4 mRNA levels were significantly inhibited by NEDD4 siRNA transfection in RT4 cells (Fig. 1A). Our Western blotting results showed that NEDD4 siRNA transfection inhibited the protein levels of NEDD4 in RT4 cells (Fig. 1B). In the following study, NEDD4 siRNA1 was selected to deplete NEDD4 expression in RT4 cells.
Figure 1.
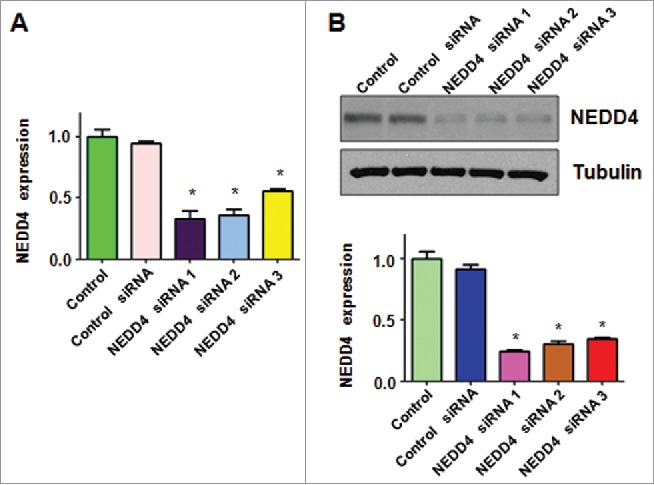
Depletion of NEDD4 by its siRNA in bladder cancer cells. A, Real-time RT-PCR was performed to measure the efficacy of NEDD4 siRNA in bladder cancer cells. **P < 0.01, ***P < 0.001 vs Control or Control siRNA. B, Top panel: Western blot analysis of NEDD4 in bladder cancer cells transfected with different NEDD4 siRNAs. Bottom panel: Quantitation of results from left panel. **P < 0.01, ***P < 0.001 vs Control or Control siRNA.
Down-regulation of NEDD4 inhibited cell proliferation in bladder cancer cells
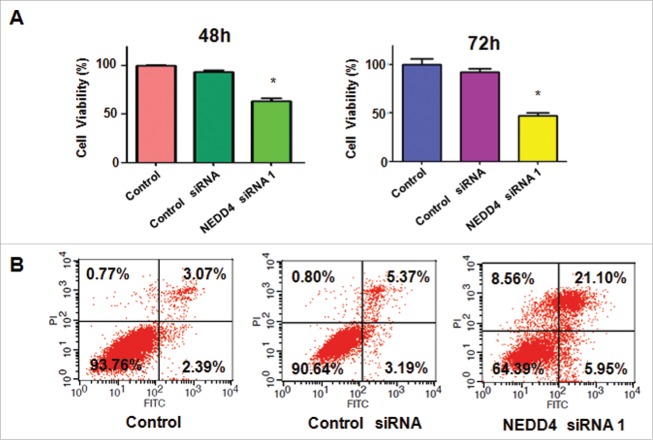
NEDD4 has been reported to enhance cell growth in human cancer cells.21 To investigate whether NEDD4 controls cell growth in bladder cancer cells, we performed MTT assay to measure the cell growth in RT4 cells after NEDD4 siRNA trnasfection. Our MTT results showed that NEDD4 siRNA transfection suppressed cell growth in RT4 cells compared with control group (Fig. 2A). This finding suggests that downregulation of NEDD4 could suppress cell growth in bladder cancer cells.
Figure 2.
Depletion of NEDD4 inhibited cell proliferation and induced apoptosis. A, MTT assay was performed in bladder cancer cells after treatment with NEDD4 siRNA for 48 h and 72 h. *P < 0.05 vs Control or Control siRNA. B, Cell apoptosis in bladder cancer cells treated with NEDD4 siRNA was measured by Flow cytometry.
Down-regulation of NEDD4 induced apoptosis in bladder cancer cells
To detect whether NEDD4 governs cell apoptosis in bladder cancer cells, cell apoptosis was measured in RT4 cells after NEDD4 siRNA transfection. Annexin V-FITC/PI (fluorescein isothiocyanate/propidium iodide) apoptosis assay was used to measure the percentage of apoptotic cells in RT4 cells transfected with NEDD4 siRNA. We found that cell apoptosis was increased from 8.56% in control siRNA treatment group to 27.05% in NEDD4 siRNA treatment group in RT4 cells (Fig. 2B). These results dissected that downregulation of NEDD4 enhanced cell apoptosis, which could contribute to inhibition of cell growth in bladder cancer cells.
Down-regulation of NEDD4 retarded cell migration and invasion in bladder cancer cells
To determine whether downregulation of NEDD4 could retard cell motility in bladder cancer cells, we used Transwell chamber assays to measure the cell invasion in RT4 cells after NEDD4 siRNA transfection. We found that downregulation of NEDD4 inhibited cell invasive activity in bladder cancer cells (Fig. 3A). To validate the role of NEDD4 in cell migration, wound healing assay was used to detect the migratory activity in bladder cancer cells after downregulation of NEDD4. We observed that downregulation of NEDD4 decreased cell migration in bladder cancer cells (Fig. 3B). Taken together, downregulation of NEDD4 inhibited cell migration and invasion in bladder cancer cells.
Figure 3.
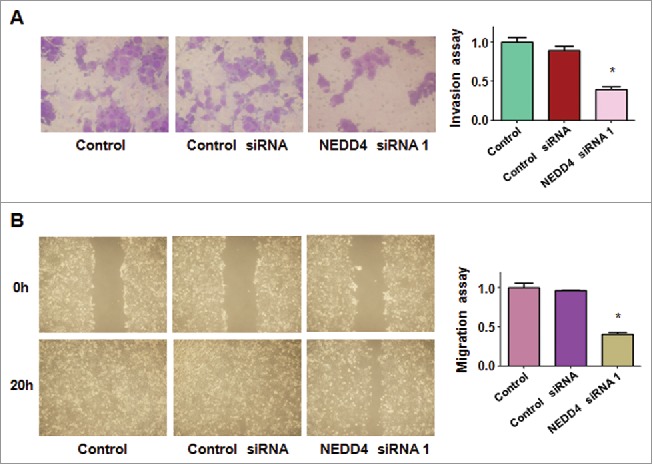
Depletion of NEDD4 suppressed motility activity in bladder cancer cells. A, Invasion assays were used to measure the migratory capacity in RT4 cells treated with NEDD4 siRNA. B, Wound healing assays was used to detect the migratory potential in RT4 after NEDD4 siRNA treatments.
Down-regulation of NEDD4 increased PTEN level, but decreased Notch-1 level in bladder cancer cells
NEDD4 has been reported to regulate the level of PTEN in several types of human cancers.22-24 To further determine whether downregulation of NEDD4 regulates the expression of PTEN in bladder cancer cells, western blotting analysis was used to measure the level of PTEN in bladder cancer cells after NEDD4 siRNA transfection. We found that downregulation of NEDD4 increased PTEN expression in bladder cancer cell lines (Fig. 4). Furthermore, downregulation of NEDD4 decreased the expression of Notch-1 in bladder cancer cells (Fig. 4). Our results indicated that NEDD4 could increase PTEN expression and subsequently suppress Notch-1 level in bladder cancer cells.
Figure 4.
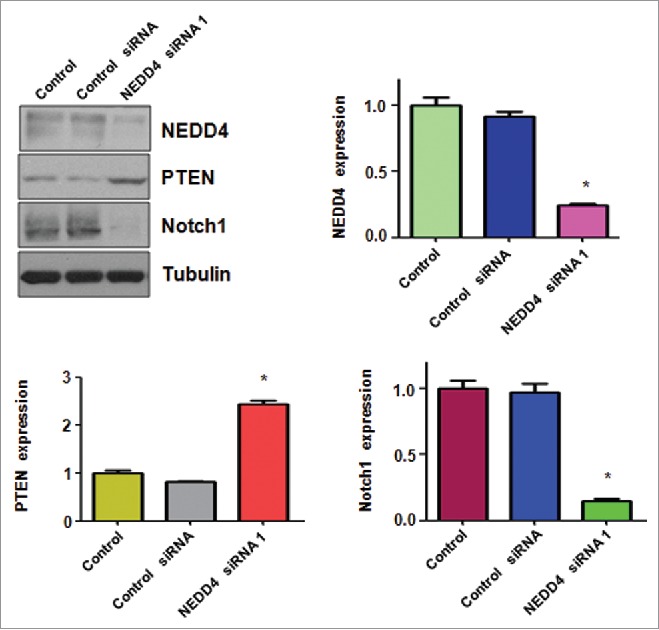
Depletion of NEDD4 inhibited Notch-1 and increased PTEN levels. Left top panel: Western blot analysis was performed to detect the expression of NEDD4, PTEN and Notch1 in bladder cancer cells transfected with NEDD4 siRNA. Right panel and bottom panel: Quantitation of results for Western blotting. *P < 0.01, vs Control or control siRNA.
Overexpression of NEDD4 promoted cell proliferation and inhibited cell apoptosis
To further validate the role of NEDD4 in bladder cancer cells, RT4 cells were transfected with NEDD4 cDNA to overexpress the NEDD4. As we expected, overexpression of NEDD4 enhanced cell proliferation in bladder cancer cells (Fig. 5A). In line with oncogenic role of NEDD4, overexpression of NEDD4 inhibited cell apoptosis in bladder cancer cells (Fig. 5C). We found that overexpression of NEDD4 enhanced cell invasion (Fig. 6A). Moreover, our wound healing assay results demonstrated that overexpression of NEDD4 promoted cell migration in bladder cancer cells (Fig. 6B). Mechanistically, overexpression of NEDD4 decreased PTEN level and increased the expression of Notch-1 in bladder cancer cells (Fig. 6C).
Figure 5.
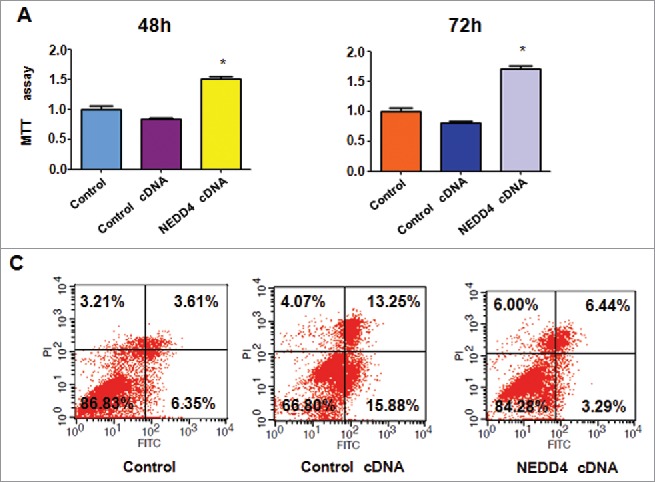
Overexpression of NEDD4 promoted cell proliferation and inhibited apoptosis. A, MTT assay was performed in bladder cancer cells after treatment with NEDD4 cDNA for 48 h and 72 h. *P < 0.05 vs Control or Control cDNA. B, Cell apoptosis in bladder cancer cells treated with NEDD4 cDNA was measured by Flow cytometry.
Figure 6.
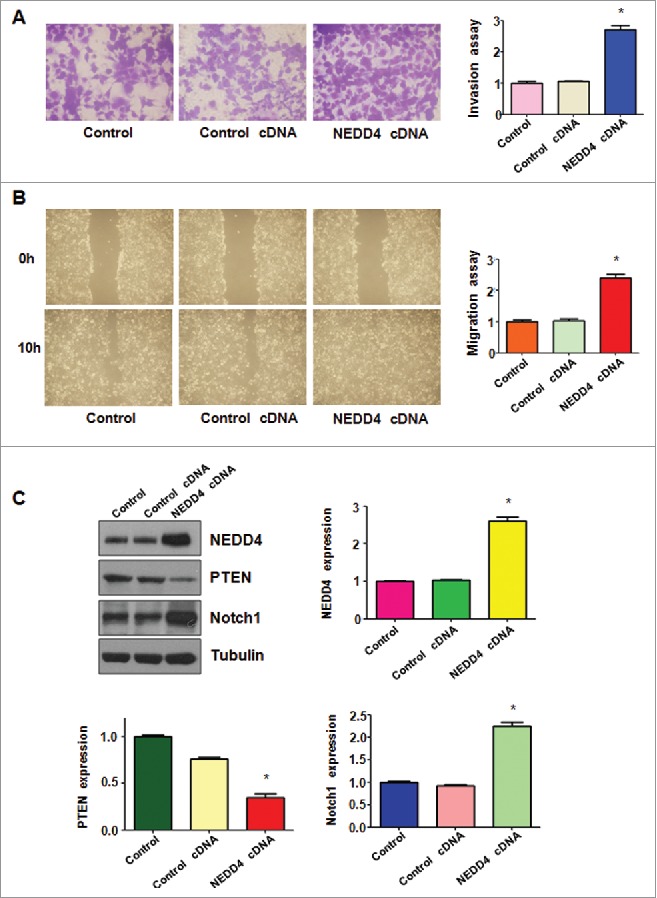
Overexpression of NEDD4 enhanced motility activity in bladder cancer cells. A, Invasion assays were used to measure the migratory capacity in RT4 cells treated with NEDD4 cDNA. B, Wound healing assays was used to detect the migratory potential in RT4 after NEDD4 cDNA treatments. C, Left top panel: Western blot analysis was performed to detect the expression of NEDD4, PTEN and Notch1 in bladder cancer cells transfected with NEDD4 cDNA. Right panel and bottom panel: Quantitation of results for Western blotting. *P < 0.01, vs Control or control cDNA.
Discussion
Emerging evidence has demonstrated that NEDD4 is critically involved in tumorigenesis. NEDD4 exerts its oncogenic-like properties through the ubiquitin-mediated degradation of multiple protein substrates.25 For example, NEDD4–1 interacts with and enhances Mdm2 (mouse double minute 2 homolog) poly-ubiquitination through the formation of K63-type polyubiquitin chains.26 Histone H3 ubiquitination by NEDD4 regulated H3 acetylation and tumorigenesis.27 NEDD4 directly binds to and polyubiquitinates PTEN, leading to its proteasomal degradation.15 Depletion of NEDD4 in DU145 cells with PTEN-positive caused a clear inhibition of tumor growth in nude mice, whereas depletion of NEDD4 in PC3 cells with PTEN-negative had no effect on tumor growth.15 In line with this, we also found that downregulation of NEDD4 in RT4 cells with PTEN-positive inhibited cell growth. Besides PTEN destabilization, NEDD4 was found to stabilize Mdm2, which suppresses tumor suppressor p53.26 NEDD4 was reported to regulate the stability of Mdm2 protein, leading to the suppression of p53 tumor suppressor.28 Zhou et al. found that NEDD4 promoted SAG/RBX2 (sensitive to apoptosis gene/RING box 2), a RING-type E3 component of SCF (Skp1-Cul1-F-box protein) E3 ligase, ubiquitination and degradation.29 Moreover, NEDD4 was found to inhibit the activity of the Hippo pathway via targeting LATS1 for degradation.20
NEDD4 is upregulated by 2 transcription factors FoxM1 (forkhead box M1) and p34SEI-1.30 FoxM1B regulated NEDD4 expression, resulting in cellular transformation and full malignant phenotype in immortalized human astrocytes.31 Jung et al reported that p34SEI-1 enhanced cancer cell survival and promoted tumorigenesis through downregulation of PTEN and subsequently activation of PI3K (phosphoinositide 3-kinase)/Akt pathway in breast cancer cells.30 Silencing of PMEPA1 (prostate transmembrance protein androgen induced 1) accelerated the cell growth through regulation of NEDD4 and AR (androgen receptor) in prostate cancer cells.32 Although multiple studies pointed out tumor-promoting functions of NEDD4, one group found that NEDD4 could be a tumor suppressor gene in specific types of human cancers, suggesting that the roles of NEDD4 in cancer appear to be more complex.10 Liu and colleagues reported that NEDD4 binds to oncoproteins N-Myc and c-Myc, and leads to their ubiquitination and degradation, thereby inhibiting the cell growth in neuroblastoma and pancreatic cancer.33 Therefore, in-depth investigation is required to determine whether NEDD4 exerts its function in cell-type dependent manner.
In our present study, downregulation of NEDD4 in bladder cancer cells inhibited cell growth, induced apoptosis, and blocked cell migration. This is direct evidence to show the oncogenic role of NEDD4 in bladder cancer cells. Inactivation of NEDD4 could be a useful strategy for the treatment of bladder cancer patients. One study showed that natural compound, indole-3-carbinol, disruption of NEDD4 ubiquitination activity triggers the stabilization of the wild-type PTEN to induce an antiproliferative response in melanoma.34 Recently, I3C analogs were characterized as potent small molecular inhibitors of NEDD4 ubiquitin ligase activity in human melanoma cells.35 Therefore, development of NEDD4 inhibitors is eager to treat human cancers with high expression of NEDD4.
Materials and methods
Cell culture, reagents and antibodies
The human RT4 cells were cultured at 37°C in 5% CO2 in DMEM (Dulbecco's modified Eagle's medium) from Gibco company (Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS). MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was acquired from Sigma (St. Louis, MO). Anti-NEDD4 and anti-PTEN antibodies were obtained from Cell Signaling Technology. Antibodies against Tubulin and Notch-1 were bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Transfection
Cells were seeded in 6-well plates and transfected with control siRNA or NEDD4 siRNAs (Genepharma, Shanghai, China) using Lipofectamine 2000 according to manufacturer's protocol. Cells were also transfected with NEDD4 cDNA using Lipofectamine 2000.36
MTT assay
Cells were seeded at equal densities into 96-well culture plates. After 24 hours, cells were then treated with NEDD4 siRNA or NEDD4 cDNA for 48 hours. MTT assay was conducted by determining the absorbance at 560 nm using a Benchmark Microplate Reader (Bio-Rad, Hercules, CA, USA). All values were normalized to those of the control.37
Apoptosis assay
The transfected cells were cultured in 6-well plate for 48 h. Then, cells were harvested and washed with PBS, resuspended in 500μl binding buffer with 5 μl Propidium iodide (PI) and 5 μl FITC-conjugated anti-Annexin V antibody. Apoptosis was measured by a FACScalibur flow cytometer.37
Real-time RT-PCR analysis
Total RNA from transfected cells was isolated with Trizol and reversed-transcribed into cDNA by RevertAid First Strand cDNA Synthesis Kit according to manufacturer's protocol. The PCR reaction was described as previously.23,36 The expression of GAPDH was used as internal control.
Western blotting analysis
Cells were lysed in lysis buffer and protein concentrations were detected by Brandford Assay reagent. Equal amount of proteins were resolved on SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and then transferred to membranes. The membranes were immunoblotted as describe before.38 The anti-NEDD4 (1:2000), anti-PTEN (1:1000), anti-Notch-1 (1:2500), and anti-tubulin (1:3000) antibodies were used.
Wound healing assay
Cells were cultured in 6-well plates and grown to confluency. When cells converged almost 100%, monolayers of cells were scratched with small yellow pipette tips and washed with PBS. The scratched area was photographed with a microscope at 0 h and 20 h, respectively.37
Transwell invasion assay
An invasion assay was performed with BD BioCoat Matrigel invasion chambers. Briefly, tranfected cells were seeded in DMEM without serum in the upper chamber. The lower chamber was added with DMEM containing 10 % FBS. After overnight incubation, the non-invading cells were removed. The cells that had invaded through Matrigel matrix membrane were stained and photographed.39
Statistical analysis
The data were expressed as mean ± SD. Student's t-test was performed to evaluate statistical significance. The level of significance was taken as P < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87-108; https://doi.org/ 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67:7-30; https://doi.org/ 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- [3].Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol 2017; 71:96-108; PMID:27370177; https://doi.org/ 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- [4].Kamat AM, Bagcioglu M, Huri E. What is new in non-muscle-invasive bladder cancer in 2016? Turk J Urol 2017; 43:9-13; PMID:28270945; https://doi.org/ 10.5152/tud.2017.60376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997; 158:62-7; PMID:9186324; https://doi.org/ 10.1097/00005392-199707000-00017 [DOI] [PubMed] [Google Scholar]
- [6].Li F, Hong X, Hou L, Lin F, Chen P, Pang S, Du Y, Huang H, Tan W. A greater number of dissected lymph nodes is associated with more favorable outcomes in bladder cancer treated by radical cystectomy: a meta-analysis. Oncotarget 2016; 7:61284-94; PMID:27542252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kang W, Cui Z, Chen Q, Zhang D, Zhang H, Jin X. Narrow band imaging-assisted transurethral resection reduces the recurrence risk of non-muscle invasive bladder cancer: A systematic review and meta-analysis. Oncotarget 2017; 8:23880-90; PMID:27823975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goel P, Manning JA, Kumar S. NEDD4-2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins. Gene 2015; 557:1-10; PMID:25433090; https://doi.org/ 10.1016/j.gene.2014.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boase NA, Kumar S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene 2015; 557:113-22; PMID:25527121; https://doi.org/ 10.1016/j.gene.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zou X, Levy-Cohen G, Blank M. Molecular functions of NEDD4 E3 ubiquitin ligases in cancer. Biochim Biophys Acta 2015; 1856:91-106; PMID:26116757 [DOI] [PubMed] [Google Scholar]
- [11].Sun A, Yu G, Dou X, Yan X, Yang W, Lin Q. Nedd4-1 is an exceptional prognostic biomarker for gastric cardia adenocarcinoma and functionally associated with metastasis. Mol Cancer 2014; 13:248; PMID:25395181; https://doi.org/ 10.1186/1476-4598-13-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hong SW, Moon JH, Kim JS, Shin JS, Jung KA, Lee WK, Jeong SY, Hwang JJ, Lee SJ, Suh YA, et al.. p34 is a novel regulator of the oncogenic behavior of NEDD4-1 and PTEN. Cell Death Differ 2014; 21:146-60; PMID:24141722; https://doi.org/ 10.1038/cdd.2013.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amodio N, Scrima M, Palaia L, Salman AN, Quintiero A, Franco R, Botti G, Pirozzi P, Rocco G, De Rosa N, et al.. Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas. Am J Pathol 2010; 177:2622-34; PMID:20889565; https://doi.org/ 10.2353/ajpath.2010.091075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jung S, Li C, Jeong D, Lee S, Ohk J, Park M, Han S, Duan J, Kim C, Yang Y, et al.. Oncogenic function of p34SEI-1 via NEDD41mediated PTEN ubiquitination/degradation and activation of the PI3K/AKT pathway. Int J Oncol 2013; 43:1587-95; PMID:23970032 [DOI] [PubMed] [Google Scholar]
- [15].Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, et al.. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 2007; 128:129-39; PMID:17218260; https://doi.org/ 10.1016/j.cell.2006.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zou X, Levy-Cohen G, Blank M. Molecular functions of NEDD4 E3 ubiquitin ligases in cancer. Biochim Biophys Acta 2015; 1856:91-106; PMID:26116757 [DOI] [PubMed] [Google Scholar]
- [17].Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets 2014; 14:549-56; PMID:25088038; https://doi.org/ 10.2174/1568009614666140725092430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu J, Wan L, Liu P, Inuzuka H, Liu J, Wang Z, Wei W. SCF(beta-TRCP)-mediated degradation of NEDD4 inhibits tumorigenesis through modulating the PTEN/Akt signaling pathway. Oncotarget 2014; 5:1026-37; PMID:24657926; https://doi.org/ 10.18632/oncotarget.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zeng T, Wang Q, Fu J, Lin Q, Bi J, Ding W, Qiao Y, Zhang S, Zhao W, Lin H, et al.. Impeded Nedd4-1-mediated Ras degradation underlies Ras-driven tumorigenesis. Cell Rep 2014; 7:871-82; PMID:24746824; https://doi.org/ 10.1016/j.celrep.2014.03.045 [DOI] [PubMed] [Google Scholar]
- [20].Salah Z, Cohen S, Itzhaki E, Aqeilan RI. NEDD4 E3 ligase inhibits the activity of the Hippo pathway by targeting LATS1 for degradation. Cell Cycle 2013; 12:3817-23; PMID:24107629; https://doi.org/ 10.4161/cc.26672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets 2014; 14:549-56; PMID:25088038; https://doi.org/ 10.2174/1568009614666140725092430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hang X, Zhu S, Di H, Wu Z, Chu K, Wang J, Xin H, Yu G, Peng H, Miao X, et al.. NEDD4 Depletion Inhibits Hepatocellular Carcinoma Growth via Targeting PTEN. Cell Physiol Biochem 2016; 39:768-79; PMID:27467187; https://doi.org/ 10.1159/000445667 [DOI] [PubMed] [Google Scholar]
- [23].Liu J, Wan L, Liu P, Inuzuka H, Wang Z, Wei W. SCF(beta-TRCP)-mediated degradation of NEDD4 inhibits tumorigenesis through modulating the PTEN/Akt signaling pathway. Oncotarget 2014; 5:1026-37; PMID:24657926; https://doi.org/ 10.18632/oncotarget.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eide PW, Cekaite L, Danielsen SA, Eilertsen IA, Kjenseth A, Fykerud TA, Agesen TH, Bruun J, Rivedal E, Lothe RA, et al.. NEDD4 is overexpressed in colorectal cancer and promotes colonic cell growth independently of the PI3K/PTEN/AKT pathway. Cell Signal 2013; 25:12-8; PMID:22974840; https://doi.org/ 10.1016/j.cellsig.2012.08.012 [DOI] [PubMed] [Google Scholar]
- [25].Liao CJ, Chi HC, Tsai CY, Chen CD, Wu SM, Tseng YH, Lin YH, Chung IH, Chen CY, Lin SL, et al.. A novel small-form NEDD4 regulates cell invasiveness and apoptosis to promote tumor metastasis. Oncotarget 2015; 6:9341-54; PMID:25823820; https://doi.org/ 10.18632/oncotarget.3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu C, Fan CD, Wang X. Regulation of Mdm2 protein stability and the p53 response by NEDD4-1 E3 ligase. Oncogene 2015; 34:281-9; PMID:24413081; https://doi.org/ 10.1038/onc.2013.557 [DOI] [PubMed] [Google Scholar]
- [27].Zhang X, Li B, Rezaeian AH, Xu X, Chou PC, Jin G, Han F, Pan BS, Wang CY, Long J, et al.. H3 ubiquitination by NEDD4 regulates H3 acetylation and tumorigenesis. Nat Commun 2017; 8:14799; PMID:28300060; https://doi.org/ 10.1038/ncomms14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shadfan M, Lopez-Pajares V, Yuan ZM. MDM2 and MDMX: Alone and together in regulation of p53. Transl Cancer Res 2012; 1:88-9; PMID:23002429 [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou W, Xu J, Zhao Y, Sun Y. SAG/RBX2 is a novel substrate of NEDD4-1 E3 ubiquitin ligase and mediates NEDD4-1 induced chemosensitization. Oncotarget 2014; 5:6746-55; PMID:25216516; https://doi.org/ 10.18632/oncotarget.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jung S, Li C, Jeong D, Lee S, Ohk J, Park M, Han S, Duan J, Kim C, Yang Y, et al.. Oncogenic function of p34SEI-1 via NEDD41mediated PTEN ubiquitination/degradation and activation of the PI3K/AKT pathway. Int J Oncol 2013; 43:1587-95; PMID:23970032 [DOI] [PubMed] [Google Scholar]
- [31].Dai B, Pieper RO, Li D, Wei P, Liu M, Woo SY, Aldape KD, Sawaya R, Xie K, Huang S. FoxM1B regulates NEDD4-1 expression, leading to cellular transformation and full malignant phenotype in immortalized human astrocytes. Cancer Res 2010; 70:2951-61; PMID:20332230; https://doi.org/ 10.1158/0008-5472.CAN-09-3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li H, Mohamed AA, Sharad S, Umeda E, Song Y, Young D, Petrovics G, McLeod DG, Sesterhenn IA, Sreenath T, et al.. Silencing of PMEPA1 accelerates the growth of prostate cancer cells through AR, NEDD4 and PTEN. Oncotarget 2015; 6:15137-49; PMID:25883222; https://doi.org/ 10.18632/oncotarget.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu PY, Xu N, Malyukova A, Scarlett CJ, Sun YT, Zhang XD, Ling D, Su SP, Nelson C, Chang DK, et al.. The histone deacetylase SIRT2 stabilizes Myc oncoproteins. Cell Death Differ 2013; 20:503-14; PMID:23175188; https://doi.org/ 10.1038/cdd.2012.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aronchik I, Kundu A, Quirit JG, Firestone GL. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol Cancer Res 2014; 12:1621-34; PMID:25009292; https://doi.org/ 10.1158/1541-7786.MCR-14-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Quirit JG, Lavrenov SN, Poindexter K, Xu J, Kyauk C, Durkin KA, Aronchik I, Tomasiak T, Solomatin YA, Preobrazhenskaya MN, et al.. Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells. Biochem Pharmacol 2017; 127:13-27; PMID:27979631; https://doi.org/ 10.1016/j.bcp.2016.12.007 [DOI] [PubMed] [Google Scholar]
- [36].Feng S, Yang G, Yang H, Liang Z, Zhang R, Fan Y, Zhang G. NEDD4 is involved in acquisition of epithelial-mesenchymal transition in cisplatin-resistant nasopharyngeal carcinoma cells. Cell Cycle 2017; 16:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 2015; 6:18027-37; PMID:26046466; https://doi.org/ 10.18632/oncotarget.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget 2016; 7:69770-82; PMID:27626499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou X, Su J, Feng S, Wang L, Yin X, Yan J, Wang Z. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget 2016; 7:79076-88; PMID:27738325 [DOI] [PMC free article] [PubMed] [Google Scholar]