The process of macroautophagy (herein referred to as autophagy) is evolutionarily highly conserved. It involves the sequestration of cytoplasmic components by double-membrane structures, referred to as autophagosomes, which fuse with lysosomes to degrade and recycle the engulfed material (reviewed in1). Under normal physiological conditions, autophagy occurs at basal levels to recycle organelles and long-lived proteins, thereby promoting cellular homeostasis. However, in response to stress, such as nutrient deprivation, DNA damage, or organelle damage, autophagy can be upregulated to ensure cellular survival, whereas in other cases, it can promote cell death and lethality.
Caenorhabditis elegans accumulate a pool of germline stem/progenitor cells during development.2 The decision for stem/progenitor cells to proliferate is controlled by several signals that include signals from the niche microenvironment, nutritional status, and the age of the animal. A single somatic cell, the Distal Tip Cell (DTC), which surrounds the distal region of the gonad, serves as a niche. The DTC produces a DSL (Delta-Serrate-LAG-2-like) ligand that activates the GLP-1/Notch receptor on distal germ cells to promote proliferation and prevent differentiation. The robust larval expansion of proliferative germline cells is highly sensitive to physiological conditions and is regulated by nutrient sensing pathways, such as insulin DAF-2/ IGF-1 like receptor (IIR), and DAF-7/TGFβ signaling.3,4 Interestingly, loss-of-function mutations in glp-1/Notch, or daf-2/IIR, result in a reduction in the number of proliferative stem cells. However these pathways act by diverse mechanisms. GLP-1/Notch signaling controls the decision to enter the differentiation program, but has no effect on the cell cycle.5 DAF-2/IIR signaling controls the cell cycle, but has no effect on the differentiation fate,3 and DAF-7/TGFβ controls the balance between proliferation and differentiation.4 Thus, the C. elegans germline serves as a great model to study the dynamics of proliferation, expansion, and differentiation of the stem/progenitor cell pool under the influence of several signaling pathways.
A recent publication by Ames et al. investigates the role of autophagy genes in the proliferation and differentiation of the C. elegans germline.6 This report established the importance of autophagy genes in the normal accumulation of germline stem/progenitor cells that occurs during development. Loss-of-function mutations in autophagy genes, such as bec-1/BECN1/Beclin 1, atg-16.2/ATG16L, atg-18/WIPI1/2, and atg-7/ATG7, resulted in a significant (up to 50%) reduction in the number of germline stem/progenitor cell pool. Interestingly, autophagy genes exhibited complex interactions with the DAF-2/IIR and DAF-7/TGFβ signaling pathways to control cell proliferation. For example, ATG-16.2/ATG16L and ATG-18/WIPI1/2 require the DAF-16/FOXO transcription factor, and the phosphatase and tensin homolog DAF-18/PTEN, whereas BEC-1 activity is dependent on DAF-18/PTEN and SKN-1/Nrf (nuclear-factor-erythroid-related factor), but independent of the DAF-16/FOXO transcription factor to promote germline proliferation. Moreover, whereas BEC-1 acts independently of the DAF-7/TGFβ pathway, ATG-7 promotes germline proliferation through DAF-7/TGFβ signaling. These data highlight the importance of a deeper understanding of the role of autophagy in stem cell proliferation and, suggests that autophagy proteins may function through autophagy-dependent and independent mechanisms to establish a germline stem cell pool.
Ames et al. reported that animals with compromised autophagy gene function do not have increased cell death or precocious differentiation that could explain the reduction in the germline stem cell pool. Rather, several autophagy proteins (BEC-1/BECN1/Beclin 1, ATG-16.2/ATG16L and ATG-18/WIPI1/2) are required for cell cycle progression.6 These conclusions are based on the observation that bec-1 mutants exhibit a significant, but transient, extension of the G2 phase, and a general slowdown of the whole cell cycle, suggesting that BEC-1 facilitates the G2 transition. Strikingly, BEC-1/BECN1/Beclin 1 can function non-cell-autonomously to promote germ cell proliferation and, tissue specific BEC-1 expression from a hypodermal, a muscle-specific and, to some extent, from a pan-neuronal promoter, rescued the germline proliferation defects of bec-1 mutants. Furthermore, hypodermal BEC-1 expression rescued the cell cycle defects observed in bec-1 mutants, such as a decrease in M-, S- and an increase in G2 indices.6 Curiously, overexpression of BEC-1 in muscle, neurons or intestine, in wild-type animals, decreased germline proliferation. Thus, autophagy genes can act non-cell-autonomously to promote germline proliferation, and the activity of autophagy genes has to be finely controlled in surrounding tissues to maintain stem cell homeostasis and proliferation (Fig. 1).
Figure 1.
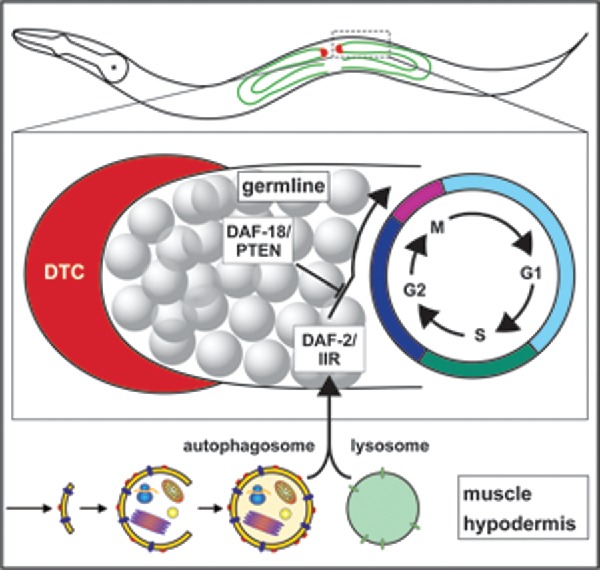
BEC-1-mediated autophagy functions in the muscle/hypodermis to promote cell cycle progression and stem cell proliferation. BEC-1 directly or indirectly interacts with components of the DAF-2/IIR signaling pathway to facilitate mitotic cell-cycle progression in the germline stem cells.
Recent studies have implicated autophagy in the control of homeostasis and the maintenance of a stem cell population in cancer stem cells. In a new report, a non-cell-autonomous role for autophagy was shown for stroma- pancreatic stellate cells in their support of the tumorous proliferation of pancreatic duct adenocarcinoma.7 Both Ames et al.,6 and Sousa et al.,7 highlight the fact that while in vitro studies have greatly expanded our molecular understanding of the mechanisms that regulate autophagy, they have failed to recognize the complexity of the processes, which may involve neighboring tissues and/or an organismal context. In multicellular organisms, cell environment, cell-to-cell contact, and cellular communication may affect the regulation and requirements for autophagy. Thus, it will be crucial in the future to expand in vivo studies to better delineate the crosstalk between different signaling pathways and autophagy in the context of the whole organism.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol 2009; 335:71-84; PMID:19802560; https://doi.org/ 10.1007/978-3-642-00302-8_3 [DOI] [PubMed] [Google Scholar]
- [2].Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol 2007; 23:405-33; PMID:17506698; https://doi.org/ 10.1146/annurev.cellbio.23.090506.123326 [DOI] [PubMed] [Google Scholar]
- [3].Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development 2010; 137:671-80; PMID:20110332; https://doi.org/ 10.1242/dev.042523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dalfo D, Michaelson D, Hubbard EJ. Sensory regulation of the C. elegans germline through TGF-beta-dependent signaling in the niche. Curr Biol 2012; 22:712-9; PMID:22483938; https://doi.org/ 10.1016/j.cub.2012.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hubbard EJ, Korta DZ, Dalfo D. Physiological control of germline development. Adv Exp Med Biol 2013; 757:101-31; PMID:22872476; https:doi.org/ 10.1007/978-1-4614-4015-4_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ames K, Da Cunha DS, Gonzalez B, Konta M, Lin F, Shechter G, Starikov L, Wong S, Bülow HE, Meléndez A. A non-cell-autonomous role of BEC-1/BECN1/Beclin1 in coordinating cell-cycle progression and stem cell proliferation during germline development. Curr Biol 2017; 27:905-13; PMID:28285998; https://doi.org/ 10.1016/j.cub.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, et al.. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016; 536:479-83; PMID:27509858; https://doi.org/ 10.1038/nature19084 [DOI] [PMC free article] [PubMed] [Google Scholar]


