Abstract
Olfaction is an ancient sensory modality which is heavily involved in viscerally-important tasks like finding food and identifying mates. Olfactory processing involves interpreting stimuli from a non-continuous odor space, and translating them into an organized pattern of neuronal activity in the olfactory bulb. Additionally, olfactory processing is rapidly modulated by behavioral states and vice versa. This implies strong bidirectional neuromodulation between the olfactory bulb and other brain regions that include the cortex, hippocampus, and basal forebrain. Intriguingly, the olfactory bulb is one of the only brain regions where adult-born neurons are integrated into existing networks throughout life. The ongoing integration of adult-born neurons is known to be important for olfactory processing, odor discrimination, and odor learning. Furthermore, the survival and integration of the adult-born neurons is regulated by neuromodulatory signaling, sensory experience, and olfactory learning. Studies making use of new genetic markers to label and manipulate immature adult-born neurons reveal an increase in their population response to odors as they mature. Importantly, this reflects a period of developmental plasticity where adult-born neurons are especially sensitive to sensory experience and olfactory learning. In this review, we discuss the contribution of adult neurogenesis to olfactory bulb plasticity and information processing, with a focus on the developmental plasticity of adult born neurons, and how it is influenced by sensory experience and olfactory learning. Ultimately, recent studies raise important questions about behavioral-state-dependent effects on adult-born neurons, and the consequences of neuromodulation on the developmental plasticity of newborn neurons in the olfactory bulb.
Keywords: Adult neurogenesis, olfaction, development, experience, learning
Introduction
Olfaction is the primary sensory modality used by mice to understand their environment. Processing olfactory information has unique requirements, including the need to adapt throughout life due to experiences, rapidly modulate odor perception based on behavioral state, and sample a complex odor space [1]. Circuits in the mouse olfactory bulb (OB) satisfy these with spatially-organized activation of excitatory relay neurons [2] modulated by a highly-interconnected network of specialized inhibitory interneurons [3]. Of these, granule cells (GCs) represent the largest population, and function to provide feedforward, feedback, and lateral inhibition, control the extent and timing of excitatory output [4–11], and regulate synchronous oscillatory activity [12–16]. The functional organization of OB circuits is initially established during embryonic and early postnatal development, but is continuously modified throughout life by sensory experience, activity-dependent synaptic plasticity, and ongoing neurogenesis of inhibitory interneurons [17–20]. The continuous integration of adult-born interneurons (primarily GCs, but also periglomerular interneurons) is an important element of plasticity in olfactory processing, and it has been shown that olfactory processing depends on the integration of new GCs into the OB; reciprocally, olfactory stimulation controls the integration of granule cells as well [21–24]. Using genetic tools to target immature adult-born neurons in the OB, recent studies have begun to examine functional integration of specific populations of adult-born neurons [25–29]. Findings from these studies raise essential questions about mechanisms that regulate the integration of adult-born neurons, and the consequences of their integration for olfactory processing. In this review, we discuss the contribution of adult neurogenesis to OB plasticity and olfactory processing. We highlight a recent study by Quast et al. [25] which used genetic tools to examine the functional integration of adult-born granule cells (GCs), and describe how their integration is modified by olfactory learning and experience. Additionally, we emphasize important questions regarding the mechanisms of modulation, and the circuit level consequences of dynamically regulating the integration of adult-born interneurons into the OB.
Olfactory processing and sensory maps
Unlike visual and auditory systems, which sample information from a continuous frequency spectrum, the olfactory system responds to a wide variety of discrete, volatile chemicals which comprise a complex sensory space. During olfaction, odorant molecules activate olfactory sensory neurons (OSNs), each uniquely expressing one of about 1000 different olfactory receptors [30]. OSNs, each expressing a single type of olfactory receptor, transmit information to the OB by projecting to one or two spatially distinct glomeruli, depending on the olfactory receptor expressed [31–35]. At the glomeruli, OSNs synapse onto apical dendrites of excitatory mitral and tufted cells (M/TCs), which represent the principal output cells of the OB and function to relay information to the piriform cortex [36–39]. Thus, in this early stage of olfactory processing, a multidimensional odor space is translated into a two-dimensional sensory map of glomerular and M/TC activation in the OB [1, 2]. These olfactory sensory maps begin diffuse and overlapping, and are refined during embryonic and early postnatal development by molecular cues and sensory independent activity [40–42]. Later in development, the tuning curves of individual M/TCs (i.e. their preference for a particular odor) are sharpened and modified by odor experience [43]. The refinement of sensory maps and the sharpening of relay cell tuning curves, in turn, are important for odor discrimination and olfactory processing [44–46].
The activity of M/TCs is controlled, to a large extent, by GABAergic granule cells (GCs), which comprise the major inhibitory cell population in the OB [5]. GC dendrites form reciprocal synapses with lateral dendrites of the M/TCs in the external plexiform layer (EPL) [47]. At these dendrodendritic synapses, glutamate release from the M/TCs excites GCs and causes them to release GABA back onto the M/TCs [48, 49]. Similar to M/TCs, GCs are activated in a spatially-specific manner in response to odors. Mature GCs, however, tend to have broader sensory maps than M/TCs [25, 50]. In fact, lateral inhibition from broad GC sensory maps may directly constrain M/TC response areas and contribute to the definition of M/TC sensory maps [8, 45, 50]. GABA release from GCs not only provides direct feedback inhibition onto active M/TCs, but also mediates lateral inhibition through connections with more distant M/TCs [8]. This lateral inhibition, in turn, is critical for odor discrimination [45, 52]. In addition to controlling the spatial spread of M/TC activity, GCs regulate the timing of M/TC activation. GCs contribute to M/TC synchronization [16], and drive oscillatory activity in M/TC populations [13, 15, 51]. Specifically, GC activation is important for odor evoked M/TC oscillatory activity in the gamma and beta frequency ranges (40–100 Hz and 15–40 Hz respectively) [51]. Beta oscillations reflect communication between the OB and the olfactory cortex, and between the OB and the hippocampus [53]. Supporting a functional role for GC-mediated beta oscillations, the coherence of beta oscillations between OB and hippocampus is increased during odor learning [54]. Additionally, gamma oscillations – mediated by GCs and originating in the OB – have been shown to be important for odor discrimination [55–57]. The extent of GC sensory maps, therefore, provides an important level of control in olfactory processing by functionally linking and coordinating the oscillatory activity of broad populations of M/TCs.
Adult neurogenesis and ongoing OB plasticity
A unique feature of GCs is that they are continuously replaced by adult neurogenesis, enhancing plasticity in the adult OB [58]. Adult-born GCs originate in the subventricular zone (SVZ) and migrate tangentially via the rostral migratory stream (RMS) to the core of the OB, then radially to the superficial granule cell layer (GCL) [59]. The morphological and functional maturation of adult-born GCs mirrors postnatal development in several key ways. Firstly, during migration from the SVZ to the OB, newborn GCs are highly sensitive to tonic GABA signaling [60]. Secondly, developmental changes in intracellular chloride concentration are recapitulated in adult-born neurons [61]. High intracellular chloride concentration in newborn cells causes GABA signaling to be excitatory, which is necessary for neuronal migration and early stages of synaptogenesis [62]. Thirdly, adult-born interneurons express GABA receptors before glutamate receptors, and receive GABAergic synaptic inputs before glutamatergic inputs, a pattern which is also observed in embryonically-born interneurons [59]. Finally, as adult-born GCs integrate into OB networks, they exhibit a period of excessive synaptogenesis, followed by activity-dependent synapse refinement [24]. These similarities indicate that as adult-born interneurons mature and integrate into OB networks, they go through a bona fide activity-dependent critical period.
During this critical period, adult-born GCs are especially sensitive to GABAergic, glutamatergic, and neuromodulatory inputs. These signals control migration, drive synaptic integration, and promote adult-born GC survival [17, 24, 26, 61, 63]. The effect of neuromodulatory input is particularly relevant in this context, because it may represent a mechanism by which the integration of adult-born neurons is dynamically regulated in a behavioral state-dependent manner. This idea is supported by the findings that cholinergic neurons project to the OB, and that cholinergic signaling enhances GC survival [64]. However, the mechanisms by which neuromodulatory signals directly or indirectly affect GC integration have not been clearly demonstrated. Importantly, new genetic and imaging strategies allow for the selective targeting of adult-born neurons at different stages of development and integration [25–29]. Using these tools, it is possible to monitor newborn GCs as they integrate into OB networks, and examine the consequences of behavioral state-dependent neuromodulation.
The development of sensory maps in adult-born GCs
Historically, studies of adult neurogenesis have made use of birth dating techniques like BrdU, EdU, or retroviral labeling [24, 59, 65]. These techniques have provided a wealth of information about the survival and integration of adult born neurons, but both have limitations. With BrdU and EdU-based birth dating techniques, for example, it is impossible to visualize or manipulate newborn neurons in living tissue. Retroviral techniques allow for labeling and manipulating adult-born neurons in vivo, but sparse expression limits the usefulness of these techniques for implementing population-wide genetic manipulations. With the development of specific genetic markers for immature adult-born neurons, it has become possible to manipulate a large subpopulations of adult-born neurons in vivo. To examine population-wide activity of adult-born granule cells in the OB, Quast et al [25] used dlx5/6-Cre mice infected with a conditional adeno-associated virus (AAV) encoding either a Cre-dependent fluorescent marker (flex-GFP) or Cre-dependent calcium indicator (flex-GCaMP6). Dlx5/6 is a transcription factor active in immature interneurons [66], therefore, only immature interneurons expressed the conditional reporters two to three weeks after viral infection. Delivery of the AAV directly into the adult OB allowed specific targeting of only adult-born OB interneurons.
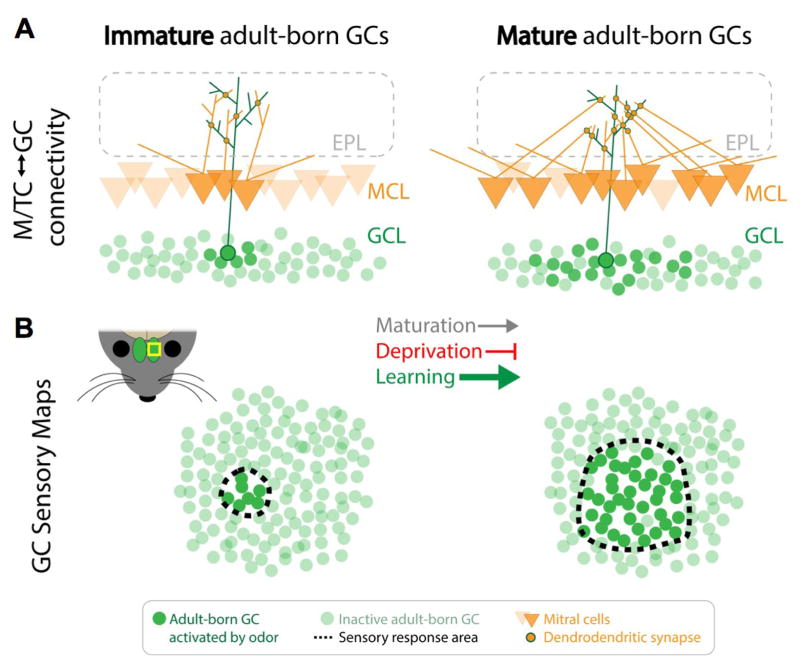
Examining the activation of labeled cells in response to odors, Quast et al. found that sensory maps of adult-born neurons are narrow in immature GCs, but become much broader in mature GCs (Figure 1B). Thus, in contrast to the development of M/TC sensory maps, the sensory maps of adult-born GCs begin narrow, and expand throughout development. Using electrophysiological assessment of mEPSCs onto individual GCs, it was determined that the expanded inhibitory maps reflect increased interconnectivity between GCs and a broad population of M/TCs (Figure 1A). It is unlikely that the expansion is caused by the physical growth of GC dendritic arbors, since mature GC dendrites extend only about 200 μm laterally. However, the lateral dendrites of M/TCs may extend over 1 mm [67]. Therefore, the expansion of GC sensory maps is likely due to synaptogenesis between GCs and lateral dendrites of distant M/TCs, which are made in the immediate vicinity of the immature GC. The increased interconnectivity, in turn, may give more broadly connected GCs the ability to simultaneously modulate the activity and synchronization of a larger population of M/TCs. Therefore, GCs that integrate with broader maps are positioned to exert more control over the total output of the OB.
Figure 1. The integration of adult born neurons into OB networks is influenced by sensory experience and learning.
A. Inhibitory granule cells (GCs), located in the granule cell layer (GCL) and excitatory mitral cells (orange triangles), located in the mitral cell layer (MCL), make dendrodendritic reciprocal synapses (orange and green circles) in the external plexiform layer (EPL, grey dashed line). Adult-born GCs (green circles) initially receive few excitatory inputs from mitral and tufted cells (M/TCs) (left). Over the course of their development and integration into OB networks, they receive progressively more inputs from M/TCs (right). B. The limited synaptic connectivity between M/TCs and immature adult-born GCs results in a smaller population of immature GCs activated in response to specific odors (left). This corresponds to a small sensory response area in immature GCs (dashed outline). As adult-born GCs mature, they respond to a broader array of odors, causing the GC sensory maps for individual odors to expand (right). The mouse head diagram (inset) shows the OB (green) and the location and orientation of sensory map recordings made through the thinned skull (yellow box). Importantly, the developmental expansion of GC sensory maps is modulated by sensory experience and olfactory learning. Sensory deprivation inhibits the expansion of GC sensory maps. Associative odor learning, on the other hand, potentiates the developmental map expansion.
Learning and sensory experience enhance the developmental expansion of GC sensory maps
The developmental expansion of GC sensory maps contrasts with the refinement of excitatory M/TC sensory maps. While M/TCs and GCs exhibit opposite changes in sensory map area over the course of development, both expansion and refinement appear to rely on sensory experience. This was determined in adult-born GCs by examining the development of GC sensory maps in the context of olfactory deprivation. Using dlx5/6-Cre mice injected with an AAV-flex-GCaMP6 virus, Quast et al. found that blocking olfactory input to one bulb by unilateral naris occlusion, inhibited GC sensory map expansion [25] (Figure 1). The effect of olfactory deprivation on GC integration into the adult OB mirrors the effect of deprivation on M/TCs during postnatal development. In the latter case, when animals were deprived of olfactory experience during postnatal development, M/TCs exhibited broader, unrefined sensory maps [68]. The observation that GC sensory map expansion is dynamically regulated by experience supports the idea that GC integration is an important mechanism of behavioral state-dependent neuromodulation in the adult OB.
Further supporting a role for behavioral state-dependent neuromodulation in the maturation of adult-born GCs, integration and survival of adult-born neurons in the OB is influenced by active olfactory learning, as well as by experience [69]. It follows that associative odor learning would influence the developmental expansion of GC sensory maps as well. Quast et al. demonstrated this using a Go/No-Go operant conditioning paradigm. During conditioning, water-deprived dlx5/6-cre mice injected with an AAV-flex-GCaMP6 virus learned to perform a nose poke in response to certain odors, eliciting a water reward when completed correctly. This allowed Quast et al. to examine the size of GC sensory maps in response to odors that were learned during the integration of those same GCs. They found that in the immature GCs, the sensory maps for learned odors were larger than the maps for unlearned or novel odors. This indicated that olfactory associative learning triggers early developmental expansion of the sensory map for that specific odor (Figure 1). Furthermore, associative learning had no effect on the sensory map area of GCs that were already mature at the time of learning. These results showed that associative olfactory learning controls the integration of adult-born GCs. At the same time, mature GCs are resistant to learning-induced map plasticity. Thus, the developmental plasticity of immature GCs provides a window of flexibility where learning may potentiate the integration of adult-born GCs into sensory processing circuits, which then become cemented once the newly-integrated GCs mature.
Conclusions
Contrary to popular belief, the human olfactory system is just as sophisticated as that of other mammals [70] and, in humans, a strong bidirectional link exists between the sense of smell, emotion, cognition, and memory. Along these lines, behavioral states powerfully influence olfactory processing and vice versa. This relationship, in turn, is critical for responding appropriately to visceral stimuli like spoiled food or potential mates. Olfaction is unique among sensory modalities, translating the activation of over 1000 unique chemoreceptors to a specific topography of neuronal activation in the OB. Circuits in the OB extensively process this information, refining the patterns and timing of neuronal activity, and ultimately tuning OB output. Maps of GC activation in response to odors are a key component of OB processing. Through extensive lateral connections, GCs control the activity of large populations of M/TCs. They also regulate the pattern and timing of M/TC activity, which ultimately controls OB output to downstream brain regions. The developmental plasticity and ongoing integration of adult born GCs greatly enhances the overall flexibility of OB circuitry throughout life. The recent findings that experience and learning alter the development of GC sensory maps indicate that the integration of adult-born GCs is influenced by state-dependent neuromodulation. This idea is supported by the abundance of neuromodulatory projections to the GCL and EPL, and that cholinergic signaling promotes the survival of newborn GCs. Considering this, it will be important to examine how neuromodulatory inputs like cholinergic projections from the basal forebrain influence dendrodendritic synapse development, and the synaptic integration underlying learning-induced GC map expansion. Ultimately, understanding how behavioral state-dependent neuromodulation controls the integration of adult-born GCs will shed light on mechanisms by which the brain attributes salience and contextual information to sensory stimuli.
Acknowledgments
This manuscript was supported by the McNair Medical Institute, and NIH-NINDS R01NS078294 to B.R.A.
Footnotes
Conflict of Interest: No conflicts declared.
References
- 1.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 2.Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23(3):499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 3.Mandairon N, et al. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. European Journal of Neuroscience. 2006;24(11):3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- 4.Urban NN. Lateral inhibition in the olfactory bulb and in olfaction. Physiology & behavior. 2002;77(4):607–612. doi: 10.1016/s0031-9384(02)00895-8. [DOI] [PubMed] [Google Scholar]
- 5.Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends in neurosciences. 2008;31(8):392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron. 2006;49(6):889–904. doi: 10.1016/j.neuron.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Aungst J, et al. Centre–surround inhibition among olfactory bulb glomeruli. Nature. 2003;426(6967):623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- 8.Margrie TW, Sakmann B, Urban NN. Action potential propagation in mitral cell lateral dendrites is decremental and controls recurrent and lateral inhibition in the mammalian olfactory bulb. Proceedings of the National Academy of Sciences. 2001;98(1):319–324. doi: 10.1073/pnas.011523098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra–and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. The Journal of physiology. 2002;542(2):355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arevian AC, Kapoor V, Urban NN. Activity-dependent gating of lateral inhibition in the mouse olfactory bulb. Nature neuroscience. 2008;11(1):80. doi: 10.1038/nn2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends in neurosciences. 2003;26(9):501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 12.Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31(4):639–651. doi: 10.1016/s0896-6273(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 13.Lagier S, Carleton A, Lledo PM. Interplay between local GABAergic interneurons and relay neurons generates γ oscillations in the rat olfactory bulb. Journal of Neuroscience. 2004;24(18):4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman D, Strowbridge BW. Both electrical and chemical synapses mediate fast network oscillations in the olfactory bulb. Journal of neurophysiology. 2003;89(5):2601–2610. doi: 10.1152/jn.00887.2002. [DOI] [PubMed] [Google Scholar]
- 15.Lagier S, et al. GABAergic inhibition at dendrodendritic synapses tunes γ oscillations in the olfactory bulb. Proceedings of the National Academy of Sciences. 2007;104(17):7259–7264. doi: 10.1073/pnas.0701846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49(2):271–283. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Nissant A, et al. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nature neuroscience. 2009;12(6):728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 18.Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life–death decisions, and the effects of sensory experience. Trends in neurosciences. 2005;28(5):248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends in neurosciences. 2003;26(5):243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 21.Breton-Provencher V, et al. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. Journal of Neuroscience. 2009;29(48):15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70(4):582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheusi G, et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proceedings of the National Academy of Sciences. 2000;97(4):1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. Journal of Neuroscience. 2002;22(14):6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quast KB, et al. Developmental broadening of inhibitory sensory maps. Nature Neuroscience. 2016 doi: 10.1038/nn.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia I, et al. Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons. Developmental cell. 2014;30(6):645–659. doi: 10.1016/j.devcel.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brill MS, et al. A dlx2-and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. Journal of Neuroscience. 2008;28(25):6439–6452. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brill MS, et al. Adult generation of glutamatergic olfactory bulb interneurons. Nature neuroscience. 2009;12(12):1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto M, et al. Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. Journal of Neuroscience. 2014;34(17):5788–5799. doi: 10.1523/JNEUROSCI.0674-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chess A, et al. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78(5):823–34. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P, et al. Visualizing an olfactory sensory map. Cell. 1996;87(4):675–86. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 32.Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annual review of neuroscience. 2011;34:467–499. doi: 10.1146/annurev-neuro-112210-112917. [DOI] [PubMed] [Google Scholar]
- 33.Bozza T, et al. Odorant receptor expression defines functional units in the mouse olfactory system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(8):3033–43. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends in neurosciences. 2005;28(11):620–627. doi: 10.1016/j.tins.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Vassar R, et al. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79(6):981–91. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 36.Najac M, et al. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. Journal of Neuroscience. 2011;31(24):8722–8729. doi: 10.1523/JNEUROSCI.0527-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gire DH, et al. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. Journal of Neuroscience. 2012;32(9):2964–2975. doi: 10.1523/JNEUROSCI.5580-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourne JN, Schoppa NE. Three–dimensional synaptic analyses of mitral cell and external tufted cell dendrites in rat olfactory bulb glomeruli. Journal of Comparative Neurology. 2017;525(3):592–609. doi: 10.1002/cne.24089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaaga CE, Westbrook GL. Parallel processing of afferent olfactory sensory information. The Journal of physiology. 2016;594(22):6715–6732. doi: 10.1113/JP272755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu CR, et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42(4):553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary DD, Yates PA, McLaughlin T. Molecular development of sensory maps: representing sights and smells in the brain. Cell. 1999;96(2):255–269. doi: 10.1016/s0092-8674(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 42.Guthrie KM, Gall C. Anatomic mapping of neuronal odor responses in the developing rat olfactory bulb. Journal of Comparative Neurology. 2003;455(1):56–71. doi: 10.1002/cne.10452. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher ML, Wilson DA. Olfactory bulb mitral-tufted cell plasticity: odorant-specific tuning reflects previous odorant exposure. Journal of Neuroscience. 2003;23(17):6946–6955. doi: 10.1523/JNEUROSCI.23-17-06946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price J, Powell TS. The synaptology of the granule cells of the olfactory bulb. Journal of Cell Science. 1970;7(1):125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- 45.Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proceedings of the National Academy of Sciences. 1995;92(8):3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linster C, et al. Perceptual correlates of neural representations evoked by odorant enantiomers. Journal of Neuroscience. 2001;21(24):9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trombley PQ, Shepherd GM. Synaptic transmission and modulation in the olfactory bulb. Current opinion in neurobiology. 1993;3(4):540–547. doi: 10.1016/0959-4388(93)90053-2. [DOI] [PubMed] [Google Scholar]
- 48.Jahr C, Nicoll R. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980;207(4438):1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- 49.Schoppa NE, et al. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. Journal of Neuroscience. 1998;18(17):6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan J, et al. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron. 2010;65(6):912–926. doi: 10.1016/j.neuron.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. Journal of Neurophysiology. 2003;90(6):3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- 52.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286(5440):711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 53.Gervais R, et al. What do electrophysiological studies tell us about processing at the olfactory bulb level? Journal of Physiology-Paris. 2007;101(1):40–45. doi: 10.1016/j.jphysparis.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. Journal of neurophysiology. 2007;98(4):2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Orive J, Bazhenov M, Laurent G. Intrinsic and circuit properties favor coincidence detection for decoding oscillatory input. Journal of Neuroscience. 2004;24(26):6037–6047. doi: 10.1523/JNEUROSCI.1084-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nusser Z, et al. Disruption of GABA A receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. Journal of neurophysiology. 2001;86(6):2823–2833. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297(5580):359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 58.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. Journal of Comparative Neurology. 1969;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 59.Carleton A, et al. Becoming a new neuron in the adult olfactory bulb. Nature neuroscience. 2003;6(5) doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 60.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. Journal of Neuroscience. 2004;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belluzzi O, et al. Electrophysiological differentiation of new neurons in the olfactory bulb. Journal of Neuroscience. 2003;23(32):10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ge S, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends in neurosciences. 2007;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Whitman MC, Greer CA. Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. Journal of Neuroscience. 2007;27(37):9951–9961. doi: 10.1523/JNEUROSCI.1633-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes to Cells. 2006;11(10):1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 65.Wichterle H, et al. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 66.Kohwi M, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1-and Dlx5/6-expressing progenitors. Journal of Neuroscience. 2007;27(26):6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shepherd GM. The synaptic organization of the brain. Oxford University Press; 2003. [Google Scholar]
- 68.Guthrie K, Wilson D, Leon M. Early unilateral deprivation modifies olfactory bulb function. Journal of Neuroscience. 1990;10(10):3402–3412. doi: 10.1523/JNEUROSCI.10-10-03402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alonso M, et al. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. Journal of Neuroscience. 2006;26(41):10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGann JP. Poor human olfaction is a 19th-century myth. Science. 2017;356(6338):eaam7263. doi: 10.1126/science.aam7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mesholam RI, et al. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Archives of neurology. 1998;55(1):84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]