Abstract
Humans with histories of prolonged heavy alcohol use exhibit poorer performance on cognitive tasks associated with problem solving, short-term memory, and visuospatial reasoning, even following the cessation of drinking, when compared with healthy controls. It is unclear, however, whether the cognitive problems are a consequence of alcohol exposure or a contributing factor to alcohol-use disorders. Here, we examined the relationship between performance on a novel object recognition (NOR) task and total alcohol consumption (TAC) in adult male rhesus macaques (n = 12; ETH group; trained to self-administer alcohol). NOR performance in this group was assessed prior to induction of alcohol drinking (“pre”) and, again, after a 1-year abstinence period (“post”) and was compared to the performance of a second group (n = 6; Control group), which was alcohol-naïve. In the NOR task, difficulty was manipulated across three phases by varying specific object features and/or by varying duration of access to objects. For each monkey, we measured aspects of novelty-related behavior including novelty detection, novelty reactivity, and perseverative behavior. TAC during induction and a “free” access period in which the monkey could choose between water and a 4% w/v ethanol solution also was determined. We found that performance deficits in the NOR task were a consequence of high total alcohol intake instead of a predictor of subsequent high intake. Poor NOR performance in drinkers with the highest intakes was characterized by increased perseverative behavior rather than an inability to detect or react to novelty. Finally, the observed deficits are long-lasting – persisting even after a year of abstinence. Given the prevalent and persistent nature of alcohol-induced cognitive deficits in patients in treatment settings, understanding the nature of the deficit and its neural basis could ultimately offer novel treatment approaches based on the reversal of alcohol-induced impairment.
Keywords: alcohol-use disorder, cognitive deficit, novelty task, Rhesus macaque, self-administration
Introduction
Alcohol-use disorders (AUDs) are characterized by progressive cognitive decline in multiple domains (Beatty, Tivis, Stott, Nixon, & Parsons, 2000; Noël, Bechara, Dan, Hanak, & Verbanck, 2007). It is generally accepted that the greatest deficits are observed at the initiation of abstinence. However, the extent to and rate at which these deficits recover with prolonged abstinence remains controversial (Davies et al., 2005; Pitel et al., 2009). It is likely that while some cognitive functions improve following several weeks of abstinence, other functions show more persistent deficits that subside over months, or even years, of abstinence (for review/meta-analysis see Oscar-Berman & Marinković, 2007; Stavro, Pelletier, & Potvin, 2013). Because decision-making, response inhibition, learning and/or retention of new information, and cognitive flexibility are related to positive treatment outcome, it seems reasonable that alcohol-induced impairments in some or all of these domains may contribute to continued heavy alcohol use, high relapse rates, and poor treatment prognosis upon abstinence (Penick et al., 2010; Trick, Kempton, Williams, & Duka, 2014; Worley, Tate, Granholm, & Brown, 2014).
While wide-ranging cognitive deficits are a clear consequence of alcohol use, some evidence suggests that pre-existing cognitive deficits may be predictive of, or a risk factor for, subsequent heavy alcohol use. For example, several studies conducted in military personnel show that lower cognitive ability as measured by standardized intelligence tests in early adulthood is associated with an increased risk in middle age of AUDs and, in some cases, other psychiatric disorders (e.g., depression, generalized anxiety disorder; Gale et al., 2008; Latvala, Kuja-Halkola, D'Onofrio, Larsson, & Lichtenstein, 2016). Many studies also show that nonalcoholic, family history-positive individuals who are at high risk for developing AUDs have impaired visuospatial and perception skills, increased impulsiveness, and decreased executive functioning in the absence of significant alcohol exposure compared to family history-negative individuals (Gierski et al., 2013; Lovallo et al., 2013; Penick et al., 2010; for review see Cservenka, 2016). Cognitive problems are not a risk factor solely in adults, because adolescents with limited attentional abilities compared to control subjects also appear to have higher risk for developing problematic alcohol use (Tapert, Baratta, Abrantes, & Brown, 2002). Interestingly, pre-existing deficits in cognitive domains, including attention and impulsivity/behavioral inhibition, appear to be predictive of poor treatment outcome (Penick et al., 2010; Tapert, Baratta, Abrantes, & Brown, 2002).
The fact that cognitive ability and AUDs are so inter-related is not surprising, given that brain regions involved in key cognitive abilities are particularly vulnerable to alcohol-induced damage, and include the prefrontal cortex (Chanraud et al., 2007; Fowler et al., 2014) and areas of the temporal lobe, including the hippocampus and para-hippocampal cortical regions (Chanraud et al., 2007; Mechtcheriakov et al., 2007; Nagel, Schweinsburg, Phan, & Tapert, 2005; Taffe et al., 2010). The prefrontal cortex is integral to decision-making, attention, and other executive functions, whereas the hippocampus is a key region involved in learning and memory formation. The hippocampus also interacts with other brain structures (e.g., amygdala, nucleus accumbens) to mediate attention to memories that are highly relevant to the formation of drug-related memories, as well as to drug reinforcement itself (Robbins, Ersche, & Everitt, 2008; Sesack & Grace, 2010). Moreover, circuitry including the hippocampus and prefrontal cortex is critical for an individual's ability to extract relevant information from prior experiences to support goal-oriented behavior (e.g., alcohol seeking; Murty, Calabro, & Luna, 2016). Collectively, these findings suggest that damage to these areas could critically influence the persistence of drinking behavior as well as the failure to remain abstinent.
The present study investigates the relationship between performance on a novel object recognition (NOR) task and alcohol drinking in rhesus monkeys. Specifically, we asked the question whether poor NOR performance predicts increased drinking levels and/or whether poor NOR performance results from heavier drinking. The NOR task was chosen because it relies critically on the hippocampus and surrounding areas. Moreover, the NOR task is sensitive to disruption by alcohol. In rodents and monkeys, acute (Matthews, Simson, & Best, 1995; Popke, Allen, & Paule, 2000), chronic (Crean, Vandewater, Katner, Huitron-Resendiz, & Taffe, 2011; Wright & Taffe, 2014), and binge-like (Cippitelli et al., 2010; Golub et al., 2015) alcohol exposure have been shown to impair performance on novel object/novel place recognition and other discrimination-type tasks. Importantly, human alcoholics typically show deficits in visuospatial learning and memory that, in some cases, appear to be negatively correlated to the amount of ethanol consumed (Nicolás et al., 1993; Sullivan, Rosenbloom, & Pfefferbaum, 2000; Zhang, Begleiter, Porjesz, & Litke, 1997). Interestingly, in both humans and laboratory animals, deficits in these types of tasks tend to manifest as abnormal perseveration and decreased cognitive flexibility (Chanraud et al., 2007; Ramos, 2013; Sullivan, Fama, Rosenbloom, & Pfefferbaum, 2002; Trick et al., 2014).
Materials and methods
Subjects
Alcohol-naïve adult male rhesus monkeys (Macaca mulatta, n = 18; Sources: Caribbean Primate Research Center, Puerto Rico and Mannheimer Foundation, Florida) served as subjects. Monkeys ranged in age from 4 to 6 years (mean age: 5.3 years) at the start of the study and had weights ranging from 9.7 to 16.6 kg. Initial NOR testing in all subjects and alcohol self-administration in a subset of subjects was conducted at Harvard Medical School/New England Primate Research Center (Southborough, MA); NOR re-testing in all subjects occurred at the University of Mississippi Medical Center (Jackson, MS).
Regardless of facility, monkeys were housed individually in a colony room with a 12-h light/dark cycle. Environmental conditions were controlled for temperature and humidity. Monkeys received a diet of monkey chow (Harlan Teklad Monkey Diet; Harlan Teklad, Madison, WI), supplemented with fruit, and water was available ad libitum. All animals were maintained in accordance with the guidelines outlined in the Guide for Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, Department of Health, Education and Welfare, Publication No. (NIH) 85-23, revised 2011. Research protocols were approved by the Harvard Medical School and the University of Mississippi Medical Center Institutional Animal Care and Use Committees.
Novel object recognition (NOR) task
A subgroup of monkeys (n = 12) was trained to self-administer alcohol (ETH group) as described below; the remaining monkeys (n = 6) remained alcohol-naïve and served as a control group. ETH monkeys were evaluated on the NOR task at two time points: 1) prior to alcohol induction and self-administration (i.e., while alcohol-naïve), and 2) following a 1-year period of abstinence. Control monkeys were tested at these same time points, though they lacked the intervening period of alcohol exposure. During all aspects of the study (including the abstinence period), all monkeys received daily enrichment according to the Environmental Enrichment and Psychological Well-Being program associated with the specific facility (i.e., HMS/NEPRC, UMMC). The NOR task was a variation of the task as described in Platt & Novak (1999). The task had three phases of difficulty (achieved by increasing the complexity of objects and/or reducing time to become familiar with objects). In the easy phase (week 1), monkeys were exposed 24 h/day to two identical objects hung on the cage front on days 1–4. In the moderate phase (week 2), monkeys were exposed 24 h/day to two different objects hung on the cage front on days 1–4. In the difficult phase (week 3), monkeys were exposed to two different objects hung on the cage front only during the 10 min of data collection on days 1–4. In every phase, on day 5 (test day), one familiar object was replaced with a novel object.
Observers, blinded to the monkeys' group assignment, recorded the occurrence of all contacts the monkeys made to each object for 10 min daily. “Contacting” was defined as any touching (with hands or feet) or mouthing (including licking and biting) of an object. Three separate but related criteria were assessed on test days. To evaluate novelty detection (criterion 1), observers indicated which object the monkey contacted first (i.e., novel versus familiar object). To evaluate novelty reactivity (criterion 2), observers compared the number of contacts made to the novel object versus the familiar object on the test day. To evaluate perseverative behavior (criterion 3), observers compared the number of contacts made to the novel object on the test day versus the familiar object in the same position on day 1. In each phase, monkeys received one point if their initial contact was to the novel object (i.e., they detected the novelty); one point if the number of contacts to the novel object were higher than to the familiar object on day 5 (i.e., they reacted to the novelty); and one point if the number of contacts to the novel object were higher than the number of contacts to the familiar object in the same position of day 1 (i.e., they habituated to the initial object across days). Totaling these points provided each animal with a NOR score. Thus, each monkey could receive a maximum NOR score of 9 (3 criteria/phase × 3 phases).
Alcohol self-administration
In the ETH subgroup of monkeys (n = 12), alcohol self-administration occurred in the home cage via a custom-designed operant drinking panel attached to one side of the cage. The panel was equipped with two response levers, two retractable sippers equipped with solenoids to minimize dripping (model #: ENV-652AM), a food pellet dispenser, and stimulus lights (Med Associates, Inc., Georgia, VT). Sippers were attached to stainless-steel liquid reservoirs fixed on the outside of the drinking panel via Tygon® tubing. Each press of a lever produced an audible click and was recorded as a response.
Monkeys were induced to drink alcohol in a step-wise fashion in a manner similar to Vivian et al. (2001), with each “step” lasting 30 days. Initially, only water was available from one spout. In this step, every lever press (fixed-ratio 1; FR1) during the 3-h session resulted in the extension of the sipper for 30 sec. Depression of the sipper by the monkey resulted in fluid delivery. Within the 30-sec sipper extension time, the monkey could stop drinking (i.e., release the spout) and resume drinking (i.e., displace the spout) as many times as he wanted. The actual duration and volume of intake, within the constraints of the schedule, were under the control of the subject. Between extensions of the drinking spout, all lights were off briefly, and responses had no scheduled consequences. The water phase served as a training period and allowed the monkeys to learn to use the operant panel.
In step 2, the volume of a 4% w/v ethanol solution (95% ethanol diluted with tap water; Pharmco Products, Brookfield, CT) required to deliver a dose of 0.5-g/kg alcohol was made available to drink on the opposite sipper; in step 3, the volume of 4% w/v ethanol solution was increased to deliver a dose of 1 g/kg; and, finally, in step 4, the volume of 4% w/v ethanol solution was increased again to deliver a dose of 1.5 g/kg. Other aspects of the self-administration procedure (e.g., sipper extension time, fixed-ratio) were the same as during the water-only availability step (step 1). Up through this point, all monkeys in the ETH group had an identical history of alcohol exposure. In the following month, ETH monkeys were given concurrent access to both water and a 4% w/v ethanol solution and could press either lever to obtain the fluid of their choice. Dose was calculated as (volume consumed [mL] × alcohol concentration [g/mL])/weight [kg]). Total alcohol consumed (TAC) during the month-long “free” access to water and 4% w/v ethanol solution was calculated for each monkey.
On the final day of concurrent access, monkeys were anesthetized with ketamine (10 mg/kg, intramuscular) immediately following the self-administration session, and 3–5 mL of blood from the femoral vein were collected in a sterile 10-mL tube (BD Vacutainer, sodium heparin 158 USP; BD, Franklin Lakes, NJ) for analysis of blood alcohol levels. Samples then were centrifuged at 1,150 × g for 8 to 12 min. The plasma was transferred to polypropylene tubes and frozen at −80 °C for later analysis. Triple determinations of blood alcohol levels were conducted using a rapid high-performance plasma alcohol analysis using alcohol oxidase with an AM1 series analyzer and Analox Kit GMRD-113 (Analox Instruments USA, Lunenburg, MA). This process detects blood alcohol levels ranging from 0–350 mg/dL using an internal standard of 100 mg/dL.
Data analysis
Differences in number of contacts to the novel versus familiar object on day 5 of each phase for the group were analyzed using one-tailed t tests. Group differences in NOR scores (expressed as a percentage of Pre “baseline” NOR performance) across time were analyzed using a mixed-design ANOVA (between-factor: ETH versus control; within-factor: pre versus post) followed by post hoc comparisons using Bonferroni's multiple comparisons test. A Pearson correlation was used to examine the relationship between total alcohol consumed (TAC) and NOR scores obtained pre-ethanol exposure. Differences in total alcohol consumed as a function of novelty-related criterion (mean ± SEM) were analyzed using a one-way ANOVA (between-factor: criterion score) followed by post hoc comparisons using Bonferroni's multiple comparisons test. The alpha level for all tests was 0.05.
Results
Evaluation of NOR performance at initial time point
By-and-large, monkeys performed well on the NOR task at the initial evaluation (i.e., before any monkey had exposure to alcohol), although performance did decline somewhat as task difficulty increased (Tables 1 & 2). Across all phases, the majority of monkeys rapidly detected the novel object, as evidenced by directing their initial contact on the test day to the novel object (Tables 1 & 2, Criterion 1, Pre). Even during the Difficult phase, 14 of the 18 monkeys explored the novel object before the familiar object. As a group, monkeys also were reactive to the novel object (criterion 2) in the Easy and Moderate, but not Difficult, phases, directing significantly more contacts to the novel object compared to the familiar object on day 5 (Fig. 1 and Tables 1 & 2, Criterion 2, Pre; Easy: t(34) = −6.51, p < 0.001; Moderate: t(34) = −4.36, p < 0.001; Difficult: t(34) = −0.88, p = 0.19). Finally, across the Easy and Moderate phases, monkeys tended to habituate to the presence of objects on their cage, rather than perseverate in behavior (criterion 3). This phenomenon is evidenced by the steady decline in the number of touches to both objects across the first 4 days of both phases and, additionally, by a greater number of contacts to the novel object on day 5 compared to the object in the same position on day 1 (Fig. 1 and Tables 1 & 2, Criterion 3, Pre). In contrast, in the Difficult phase, monkeys did not habituate to the objects across days of exposure.
Table 1.
NOR performance by phase, criteriona, and time point in control monkeys without an alcohol history, expressed as the proportion of animals meeting each criterion.
| Criterion | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | Total: | |
| EASY: | ||||
| Pre | 6/6 | 6/6 | 6/6 | 18/18 (100%) |
| Post | 6/6 | 6/6 | 6/6 | 18/18 (100%) |
| MODERATE: | ||||
| Pre | 6/6 | 6/6 | 5/6 | 17/18 (94%) |
| Post | 6/6 | 6/6 | 5/6 | 17/18 (94%) |
| DIFFICULT: | ||||
| Pre | 5/6 | 5/6 | 4/6 | 14/18 (78%) |
| Post | 5/6 | 3/6 | 3/6 | 11/18 (61%) |
| Total Pre: | 17/18 (94%) | 17/18 (94%) | 15/18 (83%) | |
| Total Post: | 17/18 (94%) | 15/18 (83%) | 14/18 (78%) | |
Criterion 1 (novelty detection) = first contact on test day is to novel object; criterion 2 (novelty reactivity) = on the test day, contacts to novel object > contacts to familiar object; criterion 3 (perseverative behavior) = contacts to novel object > contacts to familiar object in same position on day 1
Table 2.
NOR performance by phase, criteriona, and time point in monkeys with alcohol history, expressed as the proportion of animals meeting each criterion.
| Criterion | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | Total: | |
| EASY: | ||||
| Pre | 12/12 | 12/12 | 12/12 | 36/36 (100%) |
| Post | 11/12 | 10/12 | 6/12 | 27/36 (75%) |
| MODERATE: | ||||
| Pre | 11/12 | 12/12 | 10/12 | 33/36 (92%) |
| Post | 7/12 | 6/12 | 1/12 | 14/36 (39%) |
| DIFFICULT: | ||||
| Pre | 9/12 | 8/12 | 7/12 | 24/36 (67%) |
| Post | 7/12 | 9/12 | 4/12 | 20/36 (56%) |
| Total Pre: | 32/36 (89%) | 32/36 (89%) | 29/36 (81%) | |
| Total Post: | 25/36 (69%) | 25/36 (69%) | 11/36 (31%) | |
Criterion 1 (novelty detection) = first contact on test day is to novel object; criterion 2 (novelty reactivity) = on the test day, contacts to novel object > contacts to familiar object; criterion 3 (perseverative behavior) = contacts to novel object > contacts to familiar object in same position on day 1
Fig. 1.

Reactivity to novel objects by rhesus monkeys prior to alcohol exposure (Criterion 2). As a group, monkeys distributed significantly more contacts to the novel object (right object) compared to the familiar object (left object) on the test day (day 5) in the easy (top) and moderate (middle), but not difficult (bottom) phases of the NOR task. Points represent mean + S.E.M. (n = 18). * = significant difference between contacts to right (novel) versus left (familiar) object on day 5.
Evaluation of NOR performance at re-test
After initial NOR testing, 12 monkeys underwent induction of alcohol self-administration. In general, all monkeys consumed the allotted dose of alcohol during each “step” of induction (Fig. 2, bars). Once trained to self-administer, this group had free access to alcohol versus water for 3 h per day (Fig. 2, line-and-scatter plot). Although intake across the first week of concurrent access exhibited a slight cyclic pattern and greater variability, alcohol intake subsequently stabilized for the group at approximately 2.3 g/kg/day. Stable self-administration is expected under limited-access conditions. After this alcohol exposure, monkeys entered a 1-year abstinence period. The other 6 monkeys remained alcohol-naïve across this same time period. NOR performance was re-assessed in all monkeys after the abstinence period.
Fig. 2.
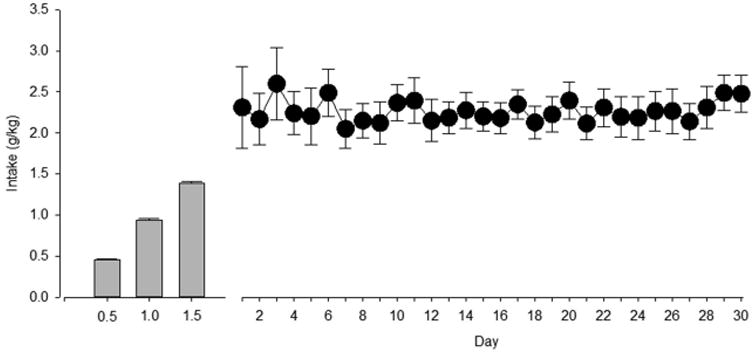
Average alcohol intake (g/kg) during induction (bars) and 30 days of concurrent access to water and a 4% w/v alcohol solution (points). Solutions were available for 3 h per day under a FR1 schedule. Points/bars represent mean ± S.E.M. (n = 12).
In general, NOR performance declined at re-test in all individuals (Tables 1 & 2, compare proportions/percentages Pre versus Post). NOR scores at the initial assessment ranged from 6–9 (mean ± S.E.M.: 8 ± 0.2) for the group; but at re-test, the range increased to 2–9 (mean ± S.E.M.: 5.8 ± 0.4) with 15 of the 18 monkeys exhibiting poorer performance at the latter time point (Fig. 3, top). While performance was worse in most monkeys at re-test, only the group of monkeys with histories of alcohol self-administration (ETH group) showed a significant decrease in average NOR score (Fig. 3, bottom; Group × Time interaction: F(1,16) = 5.871, p = 0.03; Bonferroni's multiple comparisons, p < 0.05; Normality Test [Shapiro-Wilk]: passed).
Fig. 3.

Changes in NOR task performance as a function of time and experimental history. Top: NOR scores tended to decrease in the majority of individuals upon re-testing. Each symbol/color pair represents an individual monkey. Bottom: Greater decreases in NOR scores (expressed as a percentage of baseline Pre scores) were observed in individuals with a history of alcohol self-administration compared to no history. Bars represent mean + S.E.M. * = significant difference between Pre and Post scores in ETH group; # = significant difference between Post scores in ETH and Control groups.
NOR performance and alcohol intake
As with NOR scores, we observed individual differences in cumulative alcohol intake (TAC). Although all monkeys self-administered alcohol, their intakes varied and ranged from approximately 30 g/kg (equal to ∼1 g/kg/day) to over 100 g/kg (equal to >3 g/kg/day) depending upon the monkey (Fig. 4, top). Based on the intake parameters outlined in Vivian et al. (2001), two of the monkeys can be classified as heavy drinkers (i.e., intakes of >3 g/kg/day), one was classified as a light drinker (i.e., intake of <1 g/kg/day), and the remaining 9 monkeys were classified as moderate drinkers (i.e., intakes between 1–3 g/kg/day). These intake parameters are based on human classification of alcohol use (Kalant & Poikolainen, 1999). On the final day of concurrent access, blood alcohol levels ranged from 51–183 mg/dL (mean ± S.E.M.: 110 ± 3.5 mg/dL); >80 mg/dL is considered the legal level of intoxication in the U.S. The differences in alcohol intake were not related systematically to individual differences in initial NOR performance because scores obtained prior to alcohol exposure did not predict subsequent heavy alcohol intake (r[10] = 0.34, p = 0.28; Fig. 4, bottom).
Fig. 4.
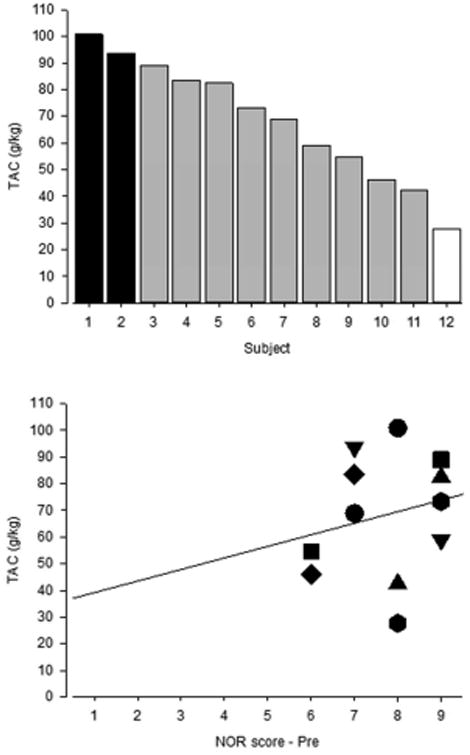
Relationship between initial NOR task performance and total alcohol intake during one month of concurrent choice of water and 4% w/v alcohol solution. Top: Total alcohol consumption (TAC; g/kg) in individual subjects. Each bar represents an individual. The color of the bar indicates average daily intake: black = heavy drinker, >3 g/kg/day; gray = moderate drinker, 1–3 g/kg/day; white = light drinker, <1 g/kg/day (cf. Vivian et al., 2001). Bottom: Lack of correlation between NOR scores at initial assessment and TAC. Symbols represent individual monkeys.
In contrast, individual performance on the NOR task at re-test was related systematically to alcohol consumption. However, the three novelty-related criteria were not affected equally by a history of alcohol exposure. Although the percentage of monkeys meeting specific criteria declined for all measures, criterion 3 showed the largest reduction from 81% of subjects to 31% of subjects, whereas for criteria 1 and 2, percentages were reduced from 89% to 69% (Table 2). Criterion 3, in particular, appears to be especially dependent on level of alcohol intake (Fig. 5). While cumulative alcohol intake (TAC) did not differ for criterion 1 (novelty detection) or criterion 2 (novelty reactivity) based on performance (i.e., between groups of animals with scores of 1, 2, or 3), TAC did differ significantly as a function of criterion 3 scores (F(2,9) = 6.54, p = 0.02; Normality Test [Shapiro-Wilk]: passed; Equal Variance Test [Brown-Forsythe]: passed), which is an indicator of lack of habituation or perseverative behavior. Specifically, the group of monkeys scoring the worst on this criterion (i.e., a score of 0 out of 3) had a history of significantly higher mean alcohol intake and higher TAC than the group of monkeys exhibiting the best performance (i.e., a score of 2 out of 3).
Fig. 5.

Relationship between total alcohol intake and NOR task performance upon re-test. Total alcohol consumption (TAC, g/kg) specifically affected Criterion 3 (bottom) compared to Criterion 1 (top) or 2 (middle), with the highest alcohol intakes associated with the lowest criterion score (score of 0 out of 3). Points represent individual subjects; bars indicate mean alcohol intake. * = significant difference between TAC in best performers (score = 2 out of 3) versus worst performers (score = 0 out of 3).
Discussion
Numerous studies support the idea that deficits in multiple cognitive domains occur as a consequence of heavy alcohol exposure; however, other studies suggest that cognitive deficits may also be a predisposing risk factor for subsequent heavy alcohol drinking. Evaluation of these hypotheses in human populations can be problematic, given the necessary long-term and prospective nature of the studies, the difficulty of accurately estimating alcohol intake across time, and the heterogeneity of the study population. Laboratory studies, especially those using relatively long-lived subjects such as nonhuman primates, offer the advantages of precise control over environmental conditions and accurate measures of alcohol intake across extended periods of time. The present study evaluated the relationship between performance on a novel object recognition (NOR) task and alcohol drinking in rhesus monkeys.
Rhesus monkeys, as a group, are able to successfully perform the NOR task, especially when the task is relatively easy. This observation is supported by the finding that virtually all initial contacts on the test day are directed to the novel object (“detection”, criterion 1) and that significantly more contacts are directed to the novel object compared to the familiar object on the test day (“reactivity”, criterion 2), as well as compared to the object in the same location on day 1 (lack of “perseveration”, criterion 3). However, group performance tended to decline as the difficulty of the task increased across phases. Interestingly, it was in the Difficult phase, when the time of exposure to the objects was reduced, that performance suffered the most. This finding matches the results of a similar study with group-housed rhesus monkeys (Platt & Novak, 1999) and also is in agreement with other studies showing performance deficits that emerge as a function of increased task difficulty (e.g., over increasing delay/retention periods; Crean et al., 2011; Porrino, Daunais, Rogers, Hampson, & Deadwyler, 2005; Wright & Taffe, 2014).
Results from the present study provided no evidence of a systematic relationship between initial performance on the NOR task and subsequent levels of alcohol intake (e.g., low “pre” NOR scores did not predict high levels of alcohol intake), suggesting that poor performance on this cognitive task did not predispose an individual to high average daily intakes of high total alcohol intake. This finding perhaps is not surprising, given that the NOR task is related to visuospatial/object recognition learning and memory and, in only some cases (e.g., family-history positive individuals; Cservenka, 2016), do these cognitive processes appear to be associated with increased risk of developing AUDs. It cannot be discounted, however, that deficits in another specific domain may be more predictive of vulnerability or that a constellation of deficits across multiple domains may place an individual at increased risk.
In contrast, results did support a relationship between alcohol intake and subsequent NOR performance that was evidenced by significantly lower NOR scores in monkeys with a history of alcohol drinking compared to those with no drinking history. Importantly, the differences observed in “post” NOR performance cannot be attributed to baseline differences between the two groups of monkeys, because “pre” NOR scores were similar between the two groups. Rather, these results likely reflect the fact that visuospatial learning and memory are processes that are particularly sensitive to modulation by alcohol. A substantial body of literature in both animals and humans supports this idea. For example, compared to control subjects, rhesus monkeys that chronically consumed moderate amounts of alcohol (i.e., ∼1.8 g/kg/session) exhibited acquisition deficits (i.e., required more trials to reach criterion) on a visuospatial paired-associates learning task (Crean et al., 2011). Our results are also consistent with reports of human alcoholics performing worse than control patients on tests of visual recognition memory (e.g., delayed [non] match-to-sample; Zhang et al., 1997).
Interestingly, the observed performance deficits likely reflect the chronic nature of the alcohol exposure because, in monkeys at least, acute alcohol exposure often has been shown to either have no effect or to improve performance on visuospatial, object-related tasks (e.g., discrimination, reversal learning; Jedema et al., 2011; Wright, Glavis-Bloom, & Taffe, 2013).
It is interesting to note that not all aspects of novelty-related behavior are affected equally by a history of alcohol exposure. While novelty detection (criterion 1) and reactivity (criterion 2) declined somewhat after alcohol exposure (i.e., by 20 percentage points, each), it was criterion 3 that was affected to the greatest degree (i.e., by 50 percentage points). In comparison, although this measure declined at re-test in control monkeys, it was only by 5 percentage points. Because criterion 3 can be viewed as a measure of perseveration, the lower number of individuals meeting this criterion after a history of alcohol drinking likely reflects a change in how the monkeys responded to objects – from habituation (as reflected by a decline in number of contacts across days) to perseveration (as reflected by a stable number of contacts across days). Importantly, two observations suggest that the change in behavior can be attributed specifically to a history of alcohol exposure, rather than a test/re-test decline in performance. First, no significant reductions in overall NOR scores were observed in the control group that underwent initial NOR testing followed by a re-test after an interval of time that matched the alcohol group. Second, at re-test in the alcohol group, there was an intake-dependent effect on criterion-3 performance, with the monkeys with the greatest level of total alcohol consumption showing the worst performance. In fact, these particular monkeys never successfully met criterion 3 across any phase after alcohol exposure.
Our observation that deficits in the NOR task reflect increases in perseverative behavior is in agreement with other studies showing abnormal perseveration and reduced cognitive flexibility on novel object/novel place recognition and other discrimination-type tasks after alcohol (Chanraud et al., 2007; Ramos, 2013; Sullivan et al., 2002; Trick et al., 2014). There is a body of literature suggesting a functional role for the hippocampus and surrounding para-hippocampal cortical regions in reduced impulse control and/or impaired cognitive flexibility (e.g., Gray, 1982). As an example, monkeys with neonatal lesions of the perirhinal cortex exhibit increased perseverative errors in object-based working memory tasks with high proactive interference (Weiss, Nadji, & Bachevalier, 2016). In contrast, other studies suggest that the hippocampus and para-hippocampal regions do not play a key role in inhibition of behavior. For example, rats with lesions of the perirhinal cortex will habituate to sets of objects across time as measured by a time-dependent decrease in exploratory behavior (Olarte-Sanchez, Amin, Warburton, & Aggleton, 2015). Although the exact role of the hippocampus and surrounding regions in the observed deficits remains unclear, factors such as species and task differences could play a part in the divergent findings.
Finally, a significant result from the present study is that the deficits in the NOR task were observed approximately 1 year after alcohol drinking had been suspended. This timeframe extends previous findings showing impaired performance in a visual discrimination procedure in rhesus monkeys after 2 months of abstinence from alcohol (Wright & Taffe, 2014). To the extent that the NOR task is hippocampally-based, our result also is consistent with the observation that chronic alcohol disrupts neurogenesis in the monkey hippocampus months after alcohol discontinuation, a change that may ultimately set the stage for the alcohol-induced hippocampal neurodegeneration that has been observed across species (Chanraud et al., 2007; Mechtcheriakov et al., 2007; Nagel et al., 2005; Taffe et al., 2010). Given the prevalent and persistent nature of alcohol-induced cognitive deficits in patients in treatment settings, understanding the nature of the deficit and its neural basis could ultimately offer novel treatment approaches based on the reversal of alcohol-induced impairment.
Highlights.
Deficits on a novel object recognition task do not predict alcohol intake.
Deficits on a novel object recognition task are a consequence of alcohol intake.
Deficits on a novel object recognition task are long-lasting.
Deficits manifest as increased perseverative behavior to the incorrect stimuli.
Acknowledgments
This research was supported by NIH AA016179, AA016828, and OD11103. No funding source had involvement in study design, collection/analysis/interpretation of data, writing of the manuscript, or decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beatty WW, Tivis R, Stott HD, Nixon SJ, Parsons OA. Neuropsychological deficits in sober alcoholics: influences of chronicity and recent alcohol consumption. Alcoholism: Clinical and Experimental Research. 2000;24:149–154. [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, et al. Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiology of Learning and Memory. 2010;94:538–546. doi: 10.1016/j.nlm.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, Taffe MA. Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug and Alcohol Dependence. 2011;114:31–40. doi: 10.1016/j.drugalcdep.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug and Alcohol Dependence. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, et al. Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol and Alcoholism. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- Fowler AK, Thompson J, Chen L, Dagda M, Dertien J, Dossou KS, et al. Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced neurotoxicity. PLoS One. 2014;9:e106945. doi: 10.1371/journal.pone.0106945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Deary IJ, Boyle SH, Barefoot J, Mortensen LH, Batty GD. Cognitive ability in early adulthood and risk of 5 specific psychiatric disorders in middle age: the Vietnam experience study. Archives of General Psychiatry. 2008;65:1410–1418. doi: 10.1001/archpsyc.65.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, et al. Executive functions in adult offspring of alcohol-dependent probands: Toward a cognitive endophenotype? Alcoholism: Clinical and Experimental Research. 2013;37:E356–E363. doi: 10.1111/j.1530-0277.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- Golub HM, Zhou QG, Zucker H, McMullen MR, Kokiko-Cochran ON, Ro EJ, et al. Chronic Alcohol Exposure is Associated with Decreased Neurogenesis, Aberrant Integration of Newborn Neurons, and Cognitive Dysfunction in Female Mice. Alcoholism: Clinical and Experimental Research. 2015;39:1967–1977. doi: 10.1111/acer.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Jedema HP, Carter MD, Dugan BP, Gurnsey K, Olsen AS, Bradberry CW. The acute impact of ethanol on cognitive performance in rhesus macaques. Cerebral Cortex. 2011;21:1783–1791. doi: 10.1093/cercor/bhq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H, Poikolainen K. Moderate drinking: Concepts, definitions and public health significance. In: Macdonald I, editor. Health Issues Related to Alcohol Consumption. 2nd. Oxford: Blackwell Science Ltd.; 1999. pp. 1–25. [Google Scholar]
- Latvala A, Kuja-Halkola R, D'Onofrio BM, Larsson H, Lichtenstein P. Cognitive ability and risk for substance misuse in men: Genetic and environmental correlations in a longitudinal nation-wide family study. Addiction. 2016;111:1814–1822. doi: 10.1111/add13440. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcoholism: Clinical and Experimental Research. 2013;37:616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. Acute ethanol impairs spatial memory but not stimulus/response memory in the rat. Alcoholism: Clinical and Experimental Research. 1995;19:902–909. doi: 10.1111/j.1530-0277.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter M, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Calabro F, Luna B. The role of experience in adolescent cognitive development: Integration of executive, memory, and mesolimbic systems. Neuroscience and Biobehavioral Reviews. 2016;70:46–58. doi: 10.1016/j.neubiorev.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidities. Psychiatry Research. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolás JM, Catafau AM, Estruch R, Lomeña FJ, Salamero M, Herranz R, et al. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. Journal of Nuclear Medicine. 1993;34:1452–1459. [PubMed] [Google Scholar]
- Noël X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–786. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- Olarte-Sánchez CM, Amin E, Warburton EC, Aggleton JP. Perirhinal cortex lesions impair tests of object recognition memory but spare novelty detection. European Journal of Neuroscience. 2015;42:3117–3127. doi: 10.1111/ejn.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychological Reviews. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penick EC, Knop J, Nickel EJ, Jensen P, Manzardo AM, Lykke-Mortensen E, et al. Do premorbid predictors of alcohol dependence also predict the failure to recover from alcoholism? Journal of Studies on Alcohol and Drugs. 2010;71:685–694. doi: 10.15288/jsad.2010.71.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, Eustache F. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcoholism: Clinical and Experimental Research. 2009;33:490–498. doi: 10.1111/j.1530-0277.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- Platt DM, Novak MA. Perception of novel changes in a familiar environment by socially-housed rhesus monkeys. American Journal of Primatology. 1999;47:117–131. doi: 10.1002/(SICI)1098-2345(1999)47:2<117∷AID-AJP3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Allen SR, Paule MG. Effects of acute ethanol on indices of cognitive-behavioral performance in rats. Alcohol. 2000;20:187–192. doi: 10.1371/journal.pbio.0030299. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biology. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JM. Differential contribution of hippocampus, perirhinal cortex and postrhinal cortex to allocentric spatial memory in the radial maze. Behavioural Brain Research. 2013;247:59–64. doi: 10.1016/j.bbr.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addiction Biology. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24:611–621. [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Trick L, Kempton MJ, Williams SC, Duka T. Impaired fear recognition and attentional set-shifting is associated with brain structural changes in alcoholic patients. Addiction Biology. 2014;19:1041–1054. doi: 10.1111/adb.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, et al. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcoholism: Clinical and Experimental Research. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Weiss AR, Nadji R, Bachevalier J. Neonatal Perirhinal Lesions in Rhesus Macaques Alter Performance on Working Memory Tasks with High Proactive Interference. Frontiers in Systems Neuroscience. 2016;9179 doi: 10.3389/fnsys.2015.00179.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Tate SR, Granholm E, Brown SA. Mediated and moderated effects of neurocognitive impairment on outcomes of treatment for substance dependence and major depression. Journal of Consulting and Clinical Psychology. 2014;82:418–428. doi: 10.1037/a0036033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Glavis-Bloom C, Taffe MA. Acute ethanol reduces reversal cost in discrimination learning by reducing perseverance in adolescent rhesus macaques. Alcoholism: Clinical and Experimental Research. 2013;37:952–960. doi: 10.1111/acer.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Taffe MA. Chronic periadolescent alcohol consumption produces persistent cognitive deficits in rhesus macaques. Neuropharmacology. 2014;86:78–87. doi: 10.1016/j.neuropharm.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Begleiter H, Porjesz B, Litke A. Electrophysiological evidence of memory impairment in alcoholic patients. Biological Psychiatry. 1997;42:1157–1171. doi: 10.1016/s0006-3223(96)00552-5. [DOI] [PubMed] [Google Scholar]


