Abstract
Scope
The present study was designed to examine the damage caused by high-fat diet and streptozotocin-induced diabetes on the testis of rats and the effects of curcumin against oxidative stress and apoptosis from high-fat diet and diabetes.
Methods
Diabetes was induced by intraperitoneal injection of streptozotocin (30 mg/kg in 0.1 M sodium citrate buffer, pH 4.5) in obese rats. The rats in the obese and diabetic groups were treated with a daily dose of curcumin by intragastric intubation (100 mg/kg body weight) for 8 weeks. Testis tissue sections were stained with hematoxylin–eosin, and apoptosis was identified in situ by using terminal deoxynucleotidyl transferase dUTP nick end labeling.
Results
Curcumin treatment improved the histological appearance of the testis and significantly reduced the apoptosis level in the testicular cells of the obese and the diabetic rats. The expression of proliferating cell nuclear antigen (PCNA) was restored in the testis tissues of diabetic rats at the end of curcumin treatment. Molecular analysis demonstrated that curcumin treatment significantly and simultaneously decreased Bax and increased Bcl-2 expressions, therefore elevating the ratio of Bcl-2/Bax. Furthermore, curcumin treatment significantly decreased malondialdehyde (MDA) and increased superoxide dismutase (SOD) levels in testis tissue samples of the diabetic rats.
Conclusion
Curcumin treatment preserved the morphology of testes; restored the expression of PCNA, MDA, and SOD; and inhibited testicular cell death in diabetic rats. The capability of curcumin in inhibiting oxidative stress and modulating the Bax/Bcl-2-mediated cell death pathway reveals its potential as a therapeutic agent against diabetes.
Keywords: apoptosis, curcumin, diabetes, oxidative stress, testis
Introduction
Diabetes mellitus (DM), a chronic endocrine metabolic disorder, has been a common health problem affecting millions of individuals worldwide and is frequently associated with many functional and structural complications in organs, including the male sexual and reproductive system in humans and animals.1–3 Low testosterone levels and varying degrees of testicular dysfunction have also been demonstrated in diabetic men and male animals,4 with oxidative stress playing an important role in affecting the testicular function.5
Curcumin, an active yellow-colored phenolic pigment of turmeric isolated from the rhizomes of the plant Curcuma longa, possesses a variety of antidiabetic, antitumor, anti-inflammatory, and antioxidant activities.6–8 Several publications have reported the protective effects of curcumin in laboratory animals against ovarian toxicity caused by ionizing radiation,9 testicular damage induced by cadmium,10 and testicular injury from metronidazole,11 all implying the beneficial role of curcumin.
The aim of this study was to examine the structural damage caused by obesity and streptozotocin (STZ)-induced diabetes on the testis of rats and the effects of curcumin against oxidative stress and apoptosis from high-fat diet and diabetes.
Materials and methods
Experimental animals
All animals received humane care according to the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. All experimental protocols were approved by the Ethical Committee on Animal Research at the Wenzhou Medical University. Forty healthy male Sprague Dawley (SD) rats were utilized in this study and kept in a room with controlled temperature at 23°C±2°C, with 12-hour light/dark cycle and humidity ranging from 50% to 55%. At 12 weeks of age, the rats were divided into five groups, and four of these groups were raised with a high-fat diet (containing 24% fat, 24% protein, and 41% carbohydrate), with soybean oil and lard as the primary source of the increased fat content in the diet. Two of the four high-fat diet groups were treated with STZ (30 mg/kg in 0.1 M sodium citrate buffer, pH 4.5) and the other two groups were injected with vehicle alone. Rats with blood glucose levels of ≥250 mg/dL were considered as being diabetic. The obese and diabetic rats were treated with either the vehicle or a daily dose of curcumin (100 mg/kg body weight) for 8 weeks by using intragastric intubation. All rats were sacrificed with an intraperitoneal overdose of pentobarbital at the end of the experiment, and relevant specimens were taken for further study.
Histopathologic evaluation
The testis specimens were fixed in 10% buffered formalin, embedded in paraffin, and sliced into 4 μm sections. Hematoxylin–eosin (H/E) staining was performed to evaluate the histological changes.
Biochemical assay
The protein content of the supernatant was determined using the Lowry method.12 Levels of malondialdehyde (MDA) and superoxide dismutase (SOD) were determined according to the methods of Draper and Hadley13 and Sun et al,14 respectively.
Detection of germ cell apoptosis
Apoptosis of the germ cells and Leydig cells was detected by the terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL) assay. Testicular tissue was fixed in 10% formalin, embedded in paraffin, and sliced into 5 μm sections. The slides were deparaffinized, rehydrated, and incubated with 20 mg/mL proteinase K for 15 minutes. Endogenous peroxidase activity was inhibited using 3% hydrogen peroxide. Sections were then incubated with TdT enzyme at 37°C for 1 hour. Staining was revealed using 3,3-diaminobenzidine (DAB) chromogen.
Immunohistochemical staining
Proliferating cell nuclear antigen (PCNA) immunostaining was performed using a standard protocol with monoclonal anti-PCNA antibody (catalog number 13110; 1:10,000 dilution; Cell Signaling Technology, Inc, Danvers, MA, USA). Appropriate negative and positive controls were also included for each run of immunostaining.
Western blot analysis
For Western blot analysis, an equal amount of protein (35 μg) from each tissue sample was separated on a 12% sodium dodecyl sulfate (SDS)–polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked in 5% skim milk (Sigma-Aldrich Co, St Louis, MO, USA) to prevent nonspecific binding at room temperature for 2 hours and then incubated at 4°C overnight with primary antibodies. The primary antibodies included anti-PCNA (catalog number 13110, 1:1,000 dilution), anti-Bax (catalog number 14796, 1:1,000 dilution), or anti-Bcl-2 (catalog number 2870, 1:10,000 dilution) (all purchased from Cell Signaling Technology, Inc). Subsequently, the membranes were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000 dilution) for 2 hours at room temperature and visualized using enhanced chemiluminescence (ECL) reagents. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control of protein loading to normalize each sample.
Statistical analysis
Data were presented as the mean values ± SD and analyzed using SPSS software. Image Quant 5.2 was used to analyze the Western blots. Comparisons were performed by one-way analysis of variance (ANOVA) and Student’s t-test for the different groups. A value of p<0.05 was considered statistically significant.
Results
Role of curcumin on testicular morphology of diabetic rats
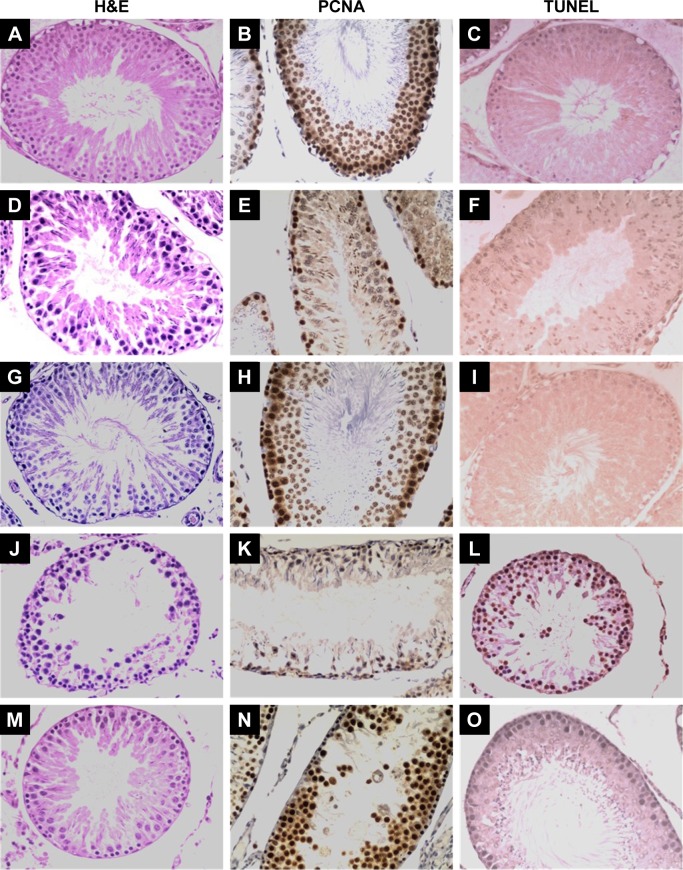
Assessment of the microstructure of the testes from the control group animals after H/E staining showed normal seminiferous tubules and spermatogenesis, whereas the testes from the high-fat-fed and diabetic groups had atrophy of seminiferous tubule, degeneration and vacuolization in spermatogonia, and disappearance of spermatids in the seminiferous tubular lumen (Figure 1). Notably, curcumin treatment markedly restored the morphology of seminiferous tubules of the testes in the high-fat-fed and diabetic rats (Figure 1), suggesting that curcumin could protect the testes of rats against damage caused by obesity and diabetes.
Figure 1.
Light microscopy results of representative testicular tissue sections from different groups.
Notes: H&E panels; magnification ×400. In the controls (A), normal testicular architecture was seen; the testicular structure was severely damaged in the high-fat-fed (D) and diabetic rats (J); improvement in the seminiferous tubule structure was observed in curcumin-treated high-fat-fed (G) and diabetic rats (M). PCNA panels: In controls (B), spermatogonia and early-stage spermatocytes were strongly positive with PCNA staining; the number of PCNA-positive germinal cells was less in high-fat-fed (E) and diabetic (K) groups than in the control group; at the end of curcumin treatment, PCNA expression was restored in the testis tissues of the high-fat-fed (H) and diabetic (N) rats. TUNEL panels: there were almost no TUNEL-positive cells in the testis tissue sections from the control group (C); more numbers of TUNEL-positive cells were seen in the high-fat-fed (F) and diabetic (L) rats; treatment with curcumin markedly reduced the intensity and the number of TUNEL-positive germ cells in the high-fat-fed (I) and diabetic (O) groups.
Abbreviations: H&E, hematoxylin–eosin; PCNA, proliferating cell nuclear antigen; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Effect of curcumin on the antioxidant capacity
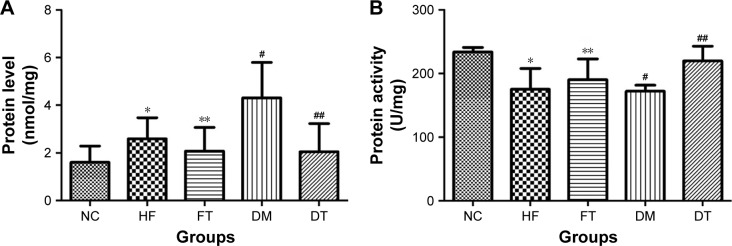
The MDA level was significantly increased in the testis tissues of the high-fat-fed and STZ-induced diabetic rats when compared with the testes of the control group rats (Figure 2; p<0.05 and p<0.01, respectively). Meanwhile, the antioxidant activity of SOD was significantly reduced in the testis tissues of the high-fat-fed and STZ-induced diabetic animals when compared with the control group rats (Figure 2). In contrast, curcumin treatment reversed the trend, significantly reduced the MDA level, and increased the SOD level in the testis tissues of the STZ-induced diabetic rats, while the changes were not significant in the high-fat-fed group, suggesting that curcumin attenuated the oxidative stress in the STZ-induced diabetic rats (Figure 2).
Figure 2.
Effect of curcumin on oxidative stress-related parameters.
Notes: (A) MDA level; (B) SOD level. The values presented are the mean ± SD. *p<0.05, #p<0.01 versus the control group; **p>0.05, ##p<0.05 versus the high-fat-fed and diabetic groups, respectively.
Abbreviations: NC, normal control; HF, high-fat-fed; FT, curcumin-treated high-fat-fed; DM, diabetes mellitus; DT, curcumin-treated diabetes mellitus; MDA, malondialdehyde; SOD, superoxide dismutase.
Evaluation of germ cell apoptosis
As evaluated by the TUNEL assay, there were significantly more TUNEL-positive cells in the testis tissue sections of the high-fat-fed and the diabetic group rats, as compared with only a few TUNEL-positive cells in the control group rats (Figure 1). Moreover, curcumin treatment significantly decreased the number of apoptotic germ cells in the testis tissue of the rats of the high-fat-fed (Figure 1I) and the STZ-induced diabetic groups (Figure 1O).
Evaluation of curcumin on PCNA expression
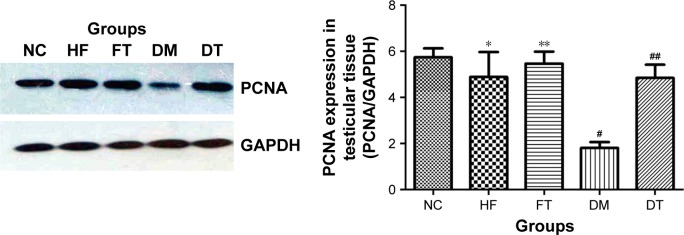
In the testis sections of the control group, the spermatogonia and early-stage spermatocytes were strongly positive with PCNA staining, whereas there were significantly less PCNA-positive germinal cells in the high-fat-fed and diabetic groups (Figure 1). Interestingly, curcumin treatment significantly increased the number of PCNA-positive germinal cells in the testes of the high-fat-fed and diabetic groups (Figure 1). Besides histological staining, PCNA expression in testis was also examined by Western blotting (Figure 3). Although the PCNA expression level was not significantly changed between the high-fat-fed group in relation to either the control group or the curcumin-treated high-fat-fed group, curcumin treatment of the diabetic rats did significantly increase their PCNA expression (Figure 3).
Figure 3.
PCNA was detected by Western blotting.
Notes: All data are presented as mean ± SD. *p>0.05, #p<0.01 versus the control group; **p>0.05, ##p<0.01 versus the high-fat-fed and diabetic groups, respectively.
Abbreviations: NC, normal control; HF, high-fat-fed; FT, curcumin-treated high-fat-fed; DM, diabetes mellitus; DT, curcumin-treated diabetes mellitus; PCNA, proliferating cellular nuclear antigen; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Effect of curcumin on mitochondria-dependent apoptotic pathway in STZ-induced diabetes
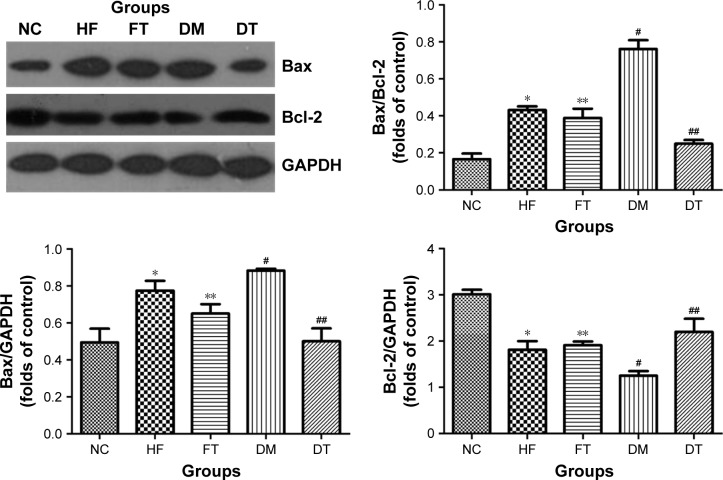
Bcl-2 expression level in the testis of high-fat-fed and diabetic rats was significantly lower (p<0.05), while Bax expression and the ratio of Bax/Bcl-2 were significantly higher compared with the control group (p<0.05) in Western blots (Figure 4). At the end of the curcumin treatment of diabetic rats, Bax expression and the ratio of Bax/Bcl-2 were significantly suppressed along with increased Bcl-2 expression, while no significant difference was found in the curcumin-treated high-fat-fed group (Figure 4).
Figure 4.
Mitochondrial-associated cell death was examined by Western blotting for the expression of Bax and Bcl-2, and the expression ratio of Bax to Bcl-2 is presented.
Notes: All data are presented as mean ± SD. *p<0.01, #p<0.01 versus the control group; **p>0.05, ##p<0.01 versus the high-fat-fed and diabetic groups, respectively.
Abbreviations: NC, normal control; HF, high-fat-fed; FT, curcumin-treated high-fat-fed; DM, diabetes mellitus; DT, curcumin-treated diabetes mellitus; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
The present study demonstrated that STZ-induced diabetes caused a series of testicular dysfunctions, and these functional deficits such as the deterioration of seminiferous tubules and the loss of spermatogenic cells can be ameliorated by treatment with curcumin.
As a useful cellular proliferation marker, PCNA expression has been used to characterize spermatogonia and early-phase primary spermatocytes in all stages of the seminiferous tubules of the testis tissues.21 In our study, PCNA was highly expressed in spermatogonia and early-stage spermatocytes of the control group rats, and its expression decreased in the diabetic rats. Curcumin treatment significantly increased PCNA expression in the testis tissues of diabetic rats, as evidenced by both immunohistochemical staining and Western blot results.
The proapoptotic Bax and antiapoptotic Bcl-2 are two critical molecules involved in cell death, and the ratio of Bax/Bcl-2 is the denominator that decides whether cells will undergo apoptosis. The STZ-induced diabetic rats had increased Bax (therefore, increased ratio of Bax/Bcl-2) and decreased Bcl-2 expression in their testis tissues, wherein the number of TUNEL-positive germinal epithelium cells also concomitantly increased. Treatment with curcumin significantly changed the equilibrium of the proapoptotic (represented by Bax) and antiapoptotic (represented by Bcl-2) molecules and effectively inhibited apoptosis of the testicular cells. Our findings (increased MDA level and decreased SOD activity in the testis tissues of diabetic rats) were consistent with a recent report describing that increased production of free radicals leads to apoptotic death of the testicular cells under diabetic condition.19 The apoptotic pathway in our case needs to be further studied to determine whether cytochrome c and caspase(s) are involved, similar to the mitochondria-dependent intrinsic apoptotic pathway as reported in other studies.18,20
Oxidative stress is regarded as an important mediator of apoptosis,20 and curcumin – acting as an antioxidant – has excellent reactive oxygen species (ROS)-scavenging ability.15–17 The level of MDA, a product of lipid peroxidation, might quantitatively reflect the damage that the free radicals inflict on cells. In addition, certain enzymes play a significant role in antioxidant defense to protect viable reproductive ability against oxidative stress. These enzymes, including SOD, glutathione peroxidase, glutathione reductase, and catalase, have the ability to convert free radicals to nonradical products. SOD is a major antioxidant enzyme that can eliminate harmful ROS in male reproductive organs. In the present study, MDA increased and SOD decreased in the testis of the diabetic rats, suggesting that testicular cells were under oxidative stresses. Curcumin significantly decreased lipid peroxidation locally at the testis, as evidenced by the lower MDA level in the testis tissue of the treated diabetic rats. Meanwhile, curcumin treatment also increased SOD expression in the testis of the diabetic rats.
Conclusion
Our experimental data have shown that curcumin treatment could preserve the morphology of testes; restore the expression of PCNA, MDA, and SOD; and inhibit death of testicular cells in diabetic rats. The capability of curcumin to release oxidative stress and modulate the ratio of Bax/Bcl-2 illustrates its potential as a promising therapeutic agent for the treatment of oxidative stress-mediated testicular dysfunction in diabetes.
Acknowledgments
The manuscript has been proofread by Professor Xin-He Lai, Institute of Translational Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Soriguer F, Rubio-Martín E, Fernández D, et al. Testosterone, SHBG and risk of type 2 diabetes in the second evaluation of the Pizarra cohort study. Eur J Clin Invest. 2012;42(1):79–85. doi: 10.1111/j.1365-2362.2011.02559.x. [DOI] [PubMed] [Google Scholar]
- 3.Trindade AA, Simões AC, Silva RJ, Macedo CS, Spadella CT. Long term evaluation of morphometric and ultrastructural changes of testes of alloxan-induced diabetic rats. Acta Cir Bras. 2013;28(4):256–265. doi: 10.1590/s0102-86502013000400005. [DOI] [PubMed] [Google Scholar]
- 4.Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4(1):46–54. doi: 10.2174/157339908783502398. [DOI] [PubMed] [Google Scholar]
- 5.Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. 2016;26(4):285–292. doi: 10.1016/j.numecd.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Chen L, Shen Y, et al. Curcumin ameliorates ischemia-induced limb injury through immunomodulation. Med Sci Monit. 2016;22:2035. doi: 10.12659/MSM.896217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu MX, Zhao L, Deng C, et al. Curcumin suppresses proliferation and induces apoptosis of human hepatocellular carcinoma cells via the wnt signaling pathway. Int J Oncol. 2013;43(6):1951–1959. doi: 10.3892/ijo.2013.2107. [DOI] [PubMed] [Google Scholar]
- 8.Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit. 2007;13(12):BR286–BR292. [PubMed] [Google Scholar]
- 9.Aktas C, Kanter M, Kocak Z. Antiapoptotic and proliferative activity of curcumin on ovarian follicles in mice exposed to whole body ionizing radiation. Toxicol Ind Health. 2012;28(9):852–863. doi: 10.1177/0748233711425080. [DOI] [PubMed] [Google Scholar]
- 10.Aktas C, Kanter M, Erboga M, Ozturk S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol Ind Health. 2012;28(2):122–130. doi: 10.1177/0748233711407242. [DOI] [PubMed] [Google Scholar]
- 11.Noorafshan A, Karbalay-Doust S, Valizadeh A, Aliabadi E. Ameliorative effects of curcumin on the structural parameters of seminiferous tubules and Leydig cells in metronidazole treated mice: a stereological approach. Exp Toxicol Pathol. 2011;63(7–8):627–633. doi: 10.1016/j.etp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1995;193(1):265–275. [PubMed] [Google Scholar]
- 13.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 15.Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Parmar MS, Syed I, Gray JP, Ray SD. Curcumin, hesperidin, and rutin selectively interfere with apoptosis signaling and attenuate streptozotocin-induced oxidative stress-mediated hyperglycemia. Curr Neurovasc Res. 2015;12(4):363–374. doi: 10.2174/1567202612666150812150249. [DOI] [PubMed] [Google Scholar]
- 17.Bulboacă A, Bolboacă SD, Suci S. Protective effect of curcumin in fructose-induced metabolic syndrome and in streptozotocin-induced diabetes in rats. Iran J Basic Med Sci. 2016;19(6):585. [PMC free article] [PubMed] [Google Scholar]
- 18.Leon J, Acuna-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38(1):1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 19.Kanter M, Aktas C, Erboga M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2013;57(9):1578–1585. doi: 10.1002/mnfr.201200170. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Zhao H, Zhai X, et al. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol Mech Methods. 2013;23(1):42–47. doi: 10.3109/15376516.2012.731437. [DOI] [PubMed] [Google Scholar]
- 21.Kang MJ, Kim MK, Terhune A, Park JK, Kim YH, Koh GY. Cytoplasmic localization of cyclin D3 in seminiferous tubules during testicular development. Exp Cell Res. 1997;234(1):27–36. doi: 10.1006/excr.1997.3590. [DOI] [PubMed] [Google Scholar]