Abstract
Neutrophils and macrophages are important constituents of the hepatic inflammatory infiltrate in non-alcoholic steatohepatitis. These innate immune cells express CD18, an adhesion molecule that facilitates leukocyte activation. In the context of fatty liver, activation of infiltrated leukocytes is believed to enhance hepatocellular injury. The objective of this study was to determine the degree to which activated innate immune cells promote steatohepatitis by comparing hepatic outcomes in wild-type and CD18-mutant mice fed a methionine-choline-deficient (MCD) diet. After 3 weeks of MCD feeding, hepatocyte injury, based on serum ALT elevation, was 40% lower in CD18-mutant than wild-type mice. Leukocyte infiltration into the liver was not impaired in CD18-mutant mice, but leukocyte activation was markedly reduced, as shown by the lack of evidence of oxidant production. Despite having reduced hepatocellular injury, CD18-mutant mice developed significantly more hepatic steatosis than wild-type mice after MCD feeding. This coincided with greater hepatic induction of pro-inflammatory and lipogenic genes as well as a modest reduction in hepatic expression of adipose triglyceride lipase. Overall, the data indicate that CD18 deficiency curbs MCD-mediated liver injury by limiting the activation of innate immune cells in the liver without compromising intrahepatic cytokine activation. Reduced liver injury occurs at the expense of increased hepatic steatosis, which suggests that in addition to damaging hepatocytes, infiltrating leukocytes may influence lipid homeostasis in the liver.
Introduction
Non-alcoholic steatohepatitis (NASH) is a disease characterized by accumulation of fat within hepatocytes accompanied by liver injury and inflammation. NASH occurs in approximately 6% of the population [1] and accounts for as much as two-thirds of the unexplained liver disease in the United States [2, 3] Inflammatory pathways play a central role in the development of NASH; Toll-like receptors (TLRs) on liver cells sense a variety of danger signals from within and outside the liver and cooperate with inflammasomes to induce liver cell death, inflammation and fibrosis [4–6] In many experimental models of NASH, the pivotal cells responsible for initiating liver disease are the Kupffer cells. These liver-resident macrophages regulate many of the features of NASH by secreting cytokines and chemokines that enhance hepatic steatosis, recruit circulating leukocytes to the liver, and stimulate collagen production by hepatic stellate cells [4, 5]. Once this complex series of events is set in motion, however, the exact contribution of recruited inflammatory cells to NASH is difficult to dissect. The objective of this study was to identify the specific impact of recruited leukocytes on the development of NASH in an animal model.
CD18, also known as β2-integrin, is a leukocyte adhesion molecule that plays a role in the transendothelial migration of leukocytes into sites of tissue injury. CD18 is expressed primarily on cells of the granulocyte lineage (neutrophils, monocytes and macrophages), where it forms heterodimers with β-integrin subunits (CD11a, b, c, and d) and facilitates binding of leukocytes to intercellular adhesion molecules on the surface of target cells [7, 8]. A mutation in the human CD18 gene (ITGB2) leads to a condition called leukocyte adhesion deficiency, which is characterized by neutrophilia and impaired neutrophil recruitment to sites of infection [7]. Mice with a mutation in the CD18 gene also have neutrophilia and impaired leukocyte emigration in experimental peritonitis and other inflammatory diseases [9–12].
Based on the above understanding of CD18 biology and NASH-related inflammation, we hypothesized that CD18 deficiency would protect mice from hepatic inflammation and liver injury in an experimental model of steatohepatitis. NASH can be induced in mice by feeding them an energy-rich, methionine-choline-deficient (MCD) diet for 3 weeks. MCD-mediated steatohepatitis develops rapidly in mice and bears close histologic resemblance to human NASH; [13–15] importantly, hepatic inflammation is pronounced in MCD-fed mice [16–18], and thus the model offers a platform for assessing the effect of infiltrating leukocytes on overall liver outcome.
Our results demonstrated that CD18 deficiency did not inhibit the hepatic cytokine activation characteristic of early NASH in MCD-fed mice. Nor did it impair leukocyte migration to the liver in response to MCD feeding. CD18 deficiency did, however, significantly reduce liver injury in MCD-fed mice, because of defective activation of recruited leukocytes. Unexpectedly, CD18-mutant mice developed more hepatic steatosis than wild-type mice in response to MCD feeding despite being spared from steatohepatitis. The reason for this is unclear, but persistent Kupffer cell activation, impaired triglyceride lipolysis and reduced activity of recruited leukocytes may all play contributory roles.
Materials and methods
Animals and diets
Adult male mice (21–25 g) expressing a mutant CD18 (Itgb2tm1Bay) on a C57BL/6 background were bred from a colony provided by Dr. Arthur Beaudet [9]. Wild-type C57BL/6 controls (WT) of the same gender and weight were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in groups of 3–5 with free access to food and water and a 12-h light/dark cycle. Weight-matched cohorts were fed either a chow diet (#5053; LabDiet, Richmond, IN) or an MCD formula (#518828; Dyets, Bethlehem, PA) for 3–8 weeks. At the end of each experiment, mice were fasted for 4 h and killed at approximately 12N by exsanguination under deep isoflurane anesthesia. All procedures were approved by the UCSF Institutional Animal Care and Use Committee.
Serum chemistries
Alanine aminotransferase (ALT) was assayed on an ADVIA 1800 autoanalyzer (Siemens Healthcare Diagnostics, Deerfield, IL) in the clinical chemistry laboratory at San Francisco General Hospital.
Histology and immunohistochemistry
Formalin-fixed liver sections were stained with H&E. Slides were reviewed blindly by a certified pathologist and scored for steatosis, ballooning and inflammation as described by Kleiner et al [19]. Granulocytes were identified in frozen sections of liver tissue using an antibody against Gr-1 (Ly6C/G, BD Biosciences, San Jose, CA) as previously described [20] and counterstained with hematoxylin. Cleaved caspase-3 (#9664, Cell Signaling Technology, Danvers, MA) and chlorotyrosine-protein adducts (#HP5002, Hycult Biotech, Plymouth Meeting, PA, USA) were identified in formalin-fixed sections by immunohistochemistry following sodium citrate unmasking. Infiltrating leukocytes and caspase-3-positive cells were quantified by counting stained cells in 10 consecutive microscopic fields to generate a mean value per liver. Chlorotyrosine adduct abundance was quantified as the percentage of stained tissue in a minimum of 500 μm2 liver tissue. Hepatic collagen was highlighted by Sirius Red staining and quantitated similarly by morphometry (Image J; http://rsb.info.nih.gov/ij/).
Hepatocyte isolation and culture
Primary mouse hepatocytes were isolated by retrograde perfusion of the liver with Liver Perfusion Medium (Life Technologies, Grand Island, NY), followed by Liver Digest Medium (Life Technologies) at 37°C for 4 minutes. Livers were then excised and minced in DME + 5% FBS to create crude cell suspensions, which were filtered through sterile gauze. Cells were pelleted from the suspension by two rounds of low-speed centrifugation (150g, 2 minutes each). Hepatocytes were purified from the washed pellets by resuspension in culture medium and centrifugation through 50% Percoll (GE Healthcare Life Sciences, Piscataway, NJ). Viability was > 90%. Purified hepatocytes were plated in DME/F12 containing 5% fetal bovine serum. Two hours after plating, the cells were washed and replenished with Williams E medium either with methionine and choline (MCS) or without methionine and choline (MCD) (Caisson Labs, Logan, UT). Cells were harvested at 48 h for measurement of cellular triglyceride.
Isolation of liver immune cells
Mouse livers were perfused as described for hepatocyte isolation. When hepatocytes were separated from crude cell suspensions by low-speed centrifugation, the supernatants containing non-parenchymal cells were pelleted by centrifugation at 650g for 10 minutes. The 650g pellets were resuspended and separated on a 25%:50% Percoll gradient to isolate hepatic leukocytes.
Flow cytometry
Cells were pre-incubated with FC/block (10mg/ml, 2.4G2, BD Biosciences) and stained according to standard protocols with combinations of the following anti-mouse antibodies: CD18-FITC (clone C71/16, #553292, BD Biosciences), CD11b-PerCP-Cy™5.5 (clone M1/70, #550993, BD Biosciences), F4/80-PE-Cy7 (clone BM-8, #25–4801, eBioscience, San Diego, CA), Ly-6C-AF700 (clone AL-21, #561237, BD Biosciences) and Ly-6G-PerCP-Cy™5.5 (clone 1A8, #560602, BD Biosciences). Cells were analyzed using an LSRII flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR).
Quantitation of hepatic triglyceride
Lipids were extracted from fresh liver tissue using the Folch method [21]. Samples were processed and analyzed for total triglyceride as previously described.[22] For measurement of triglyceride in cultured hepatocytes, cells were scraped into a neutral salt solution pelleted by centrifugation, and homogenized in 2:1 chloroform:methanol using the same method as that for whole tissue. Triglyceride was measured using a commercial kit (TR0100, Sigma Chemical Company, St. Louis, MO), and results were normalized either to liver weight (for whole tissue) or cellular protein (for cultured hepatocytes).
Evaluation of gene expression by quantitative PCR
RNA was extracted from liver using TRIzol reagent (Life Technologies, Carlsbad, CA) and purified using the RNeasy kit (Qiagen, Valencia, CA). RNA integrity was verified by formaldehyde gel electrophoresis. cDNA was synthesized using iScript (BioRad, Hercules, CA); quantitative PCR (qPCR) was performed with TaqMan® assay kits (Life Technologies, Carlsbad, CA) using β-glucuronidase as the internal control gene.
Analysis of hepatic triglyceride secretion
Mice fed MCD diets for 14 days were injected intraperitoneally with Tyloxapol (Sigma) as previously described [23]. Blood samples were obtained via submandibular venipuncture prior to injection and at 4 h and 8 h post-injection; serum triglyceride concentrations were measured as previously described [22].
Myeloperoxidase assay
Myeloperoxidase activity was quantitated by using a tetramethylbenzidine (TMB)-based assay as previously described [24, 25]. Tissue samples were homogenized in phosphate buffer (20 mM, pH 7.4) and centrifuged (13,000g, 10 min, 4°C). The resulting pellets were resuspended in phosphate buffer (50 mM, pH 6.0) with 0.5% hexadecyltrimethylammonium bromide (Sigma). The suspension underwent four freeze-thaw cycles and then was briefly sonicated (10 sec). The samples were then centrifuged and the protein contents of the supernatants were determined (Pierce Micro BCA Protein Assay, Thermo Scientific, Waltham, MA). Equal amounts of protein from each sample were incubated with TMB (Enzo Life Science, Inc., Farmingdale, NY, USA) for 15 minutes at 37°C. The reaction was stopped with sulfuric acid and the absorbance was read at 405 nm.
Statistical analyses
Individual studies included 3–15 mice per group, as reported in the figure legends. Results were compared using one-way or two-way ANOVA with Bonferroni post-hoc testing, or Mann-Whitney tests as appropriate for the data. P values < 0.05 were considered statistically significant.
Results
CD18-mutant mice have normal livers at baseline
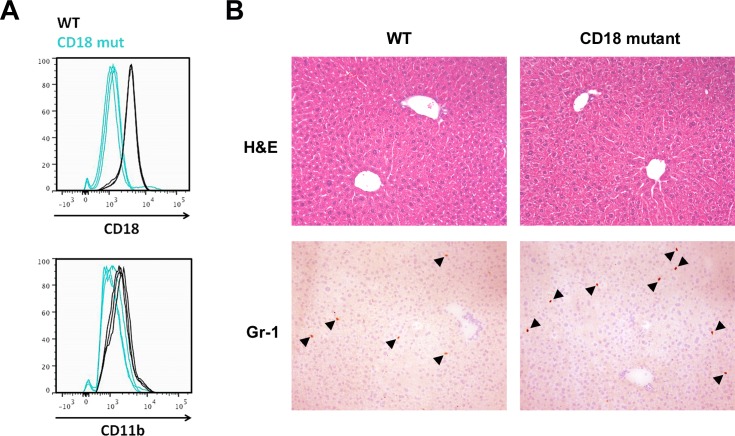
As expected, granulocytes isolated from the livers of CD18-mutant mice displayed weak CD18 staining (70% weaker than WT) as well as reduced immunoreactivity for the related β2-integrin subunit CD11b (Fig 1A). Under chow-fed conditions, CD18-mutant mice displayed normal liver histology, although mutant livers tended to contain more granulocytes than WT livers (5.4 ± 1.1 vs. 3.0 ± 0.5 cells per 10X field, P = 0.09, Fig 1B). Chow-fed CD18-mutant mice displayed no abnormality in serum ALT (65.3 ± 3.6 vs. 61.5 ± 6.5 IU/L, P > 0.05). Their hepatic triglyceride content was also indistinguishable from that of WT mice (5.6 ± 0.9 vs. 7.6 ± 0.8 mg/g liver, P > 0.05).
Fig 1. Comparative features of WT and CD18-mutant mice fed chow diets.
(A) CD18 and CD11b expression measured by flow cytometry in leukocytes isolated from the livers of chow-fed WT and CD18-mutant (CD18 mut) mice. The fluorescence intensity of CD18 mut granulocytes was 70% lower than that measured in WT granulocytes, n = 3 per group. (B) Liver histology and Gr-1 immunohistochemistry in WT and CD18 mut mice. Arrowheads mark Gr-1-positive cells. For cell counts, see text. Original magnification 10X.
MCD feeding induces severe hepatic steatosis but only moderate steatohepatitis in CD18-mutant mice
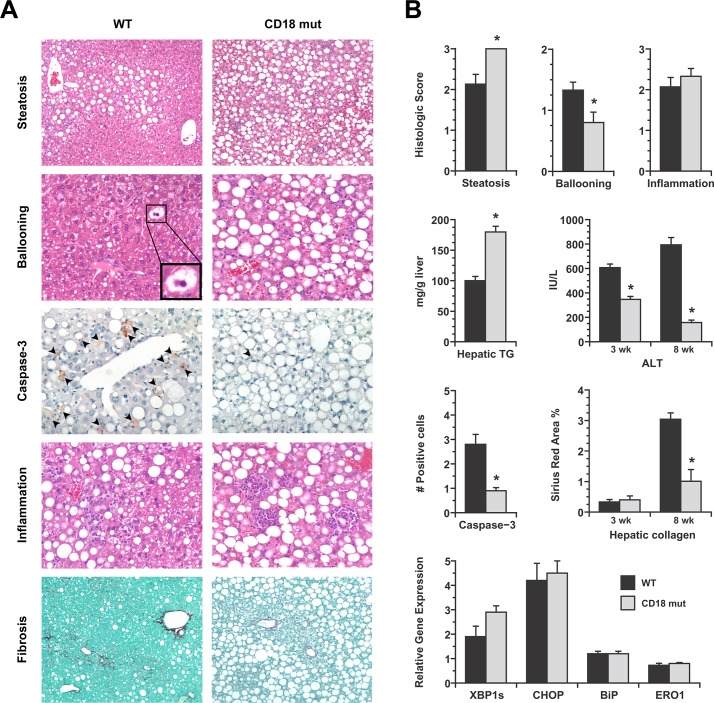
WT and CD18-mutant mice both developed weight loss in response to MCD feeding, which is typical for this experimental model [14]. There was no difference between the two groups in food intake (2.9 ± 0.2 vs. 3.1 ± 0.2 g/d in WT vs. CD18 mutant, P > 0.05) or body weight change over the experimental feeding period (-28.8% ± 0.4% vs. -26.7 ± 1.7% at 3 wk, -42.3 ± 1.8% vs. -38.9 ± 1.1% at 8 wk in WT vs. CD18 mutant, P > 0.05). Both groups of mice also developed liver injury in response to MCD feeding; however, important differences in disease severity were noted between the two genotypes (Fig 2). The most obvious difference was that hepatic steatosis was more severe in CD18-mutant livers than WT livers (Fig 2A). Hepatic triglyceride content was nearly two times higher in the CD18-mutant group (179.9 ± 9.2 vs. 100.0 ± 7.0 mg/g liver, P = 1.4 x 10−7) at 3wk (Fig 2B). Despite enhanced steatosis, hepatocellular injury was milder in CD18 mutants than WT mice, demonstrated by less histologic ballooning of hepatocytes, fewer liver cells exhibiting cleaved caspase-3 and a 40% or greater reduction in serum ALT (Fig 2A and 2B). There was no difference in ER stress marker expression between WT and CD18-mutant mice to account for the difference in liver injury (Fig 2B). By 8 weeks of MCD feeding, WT mice displayed clear evidence of liver fibrosis whereas CD18-mutant mice did not (Fig 2A and 2B).
Fig 2. Comparative features of WT and CD18-mutant mice after MCD feeding.
(A) Photomicrographs illustrate several features of liver disease in WT and CD18-mutant (CD18 mut) mice after MCD feeding for 3–8 wk. Steatosis (H&E, 3 wk, 10X) is more severe in CD18 mut livers whereas ballooning (H&E, 3 wk, 10X) is more evident in WT livers. WT livers also have more cells staining positively for cleaved caspase-3 (20X). Hepatic inflammation is visible in both WT and CD18 mut livers, although cells are diffusely distributed in WT livers and clustered in CD18 mut livers. Hepatic fibrosis (Sirius red, 8 wk, 10X) is more advanced in WT livers than CD18 mut livers. (B) Graphs illustrate comparative histologic scores for steatosis, ballooning and inflammation in WT and CD18 mut mice at 3 wk, hepatic triglyceride concentration at 3 wk, and serum ALT measurements at 3 wk and 8 wk. Additional histograms illustrate quantitation of caspase-3-positive cells (# cells per 20X field) and quantitation of hepatic fibrosis (Sirius red, percent area). Final histograms show hepatic mRNA levels for several ER stress markers in WT and CD18 mut liver, normalized to chow-fed WT liver. XBP1s, X-box protein-1 spliced form; CHOP, CEBP-homologous protein; BiP, binding immunoglobulin protein; ERO1, ER oxidoreductin. Values represent mean ± SE for n = 10–15. * P < 0.05 vs. WT.
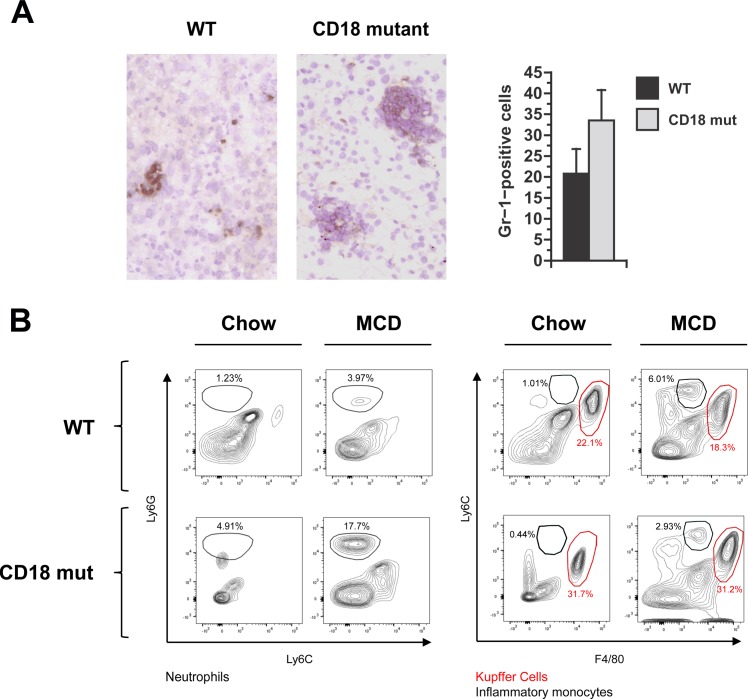
Hepatic leukocyte infiltration was evident in the livers of both WT and CD18-mutant mice. By histologic scoring, the degree of hepatic inflammation was comparable between the two groups (Fig 2B), but CD18 mutants displayed a unique pattern of inflammation characterized by prominent aggregates of inflammatory cells in the hepatic parenchyma (Figs 2A and 3A). Analysis of hepatic leukocytes by flow cytometry revealed that CD18-mutant mice accumulated more neutrophils in their livers than WT mice in response to MCD feeding (104,750 vs. 251,800 neutrophils in WT vs. CD18-mut, P = 0.04). Infiltration of inflammatory monocytes (Ly6Chi) was similar between the two groups (256,150 vs. 149,240 cells in WT vs. CD18-mut, P = 0.19) (Fig 3B). CD18 deficiency, therefore, did not impair the recruitment of either neutrophils or monocytes to the liver in response to MCD feeding.
Fig 3. Hepatic inflammation in WT and CD18-mutant mice in response to MCD feeding.
(A) Gr-1 staining and quantitation of Gr1-positive cells in WT and CD18 mut liver at 3 wk. Photomicrographs illustrate that Gr-1-positive cells are abundant in both WT and CD18-mutant (CD18 mut) mice after MCD feeding, although in different distributions. Original magnification 15X. Histograms illustrated Gr1-positive cell counts in WT and CD18 mut livers, performed as described in Methods. Values represent mean ± SE for n = 5. (B) Representative FACS plots of hepatic leukocytes from mice fed chow or MCD diets. MCD feeding enhances the proportion of neutrophils (Ly6Ghigh) and inflammatory monocytes (Ly6Chigh), while the lack of CD18 further enhances the accumulation of neutrophils but not inflammatory monocytes. Data are illustrative of n = 6 mice per group (24 per cohort), performed as 2 replicate experiments involving 12 mice each (3 per group).
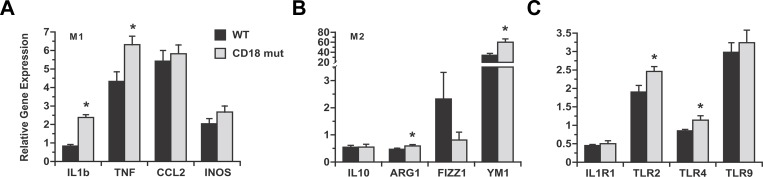
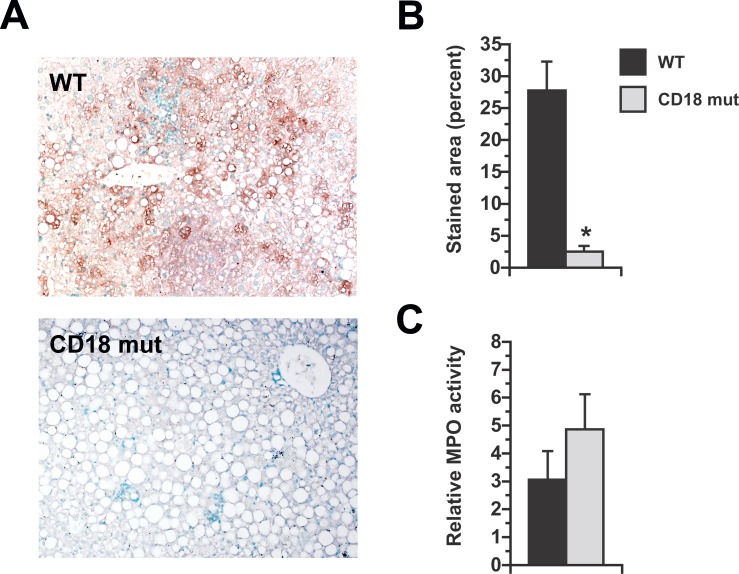
β2-integrins promote pro-inflammatory cytokine expression by leukocytes in addition to regulating cell migration. Notably, the influence of β2-integrins on cytokine induction can be independent of their effect on tissue invasion [26]. MCD feeding typically stimulates the expression of pro-inflammatory cytokine genes in the liver as it induces steatohepatitis [17, 27–30]; to determine whether CD18 influences inflammatory gene regulation in response to MCD feeding, we measured the levels of several pro- and anti-inflammatory genes in the livers of WT and CD18-mutant mice liver at 3 wk. We found that pro-inflammatory (M1) genes were similar or in some cases elevated in the CD18-mutant livers (Fig 4A). Anti-inflammatory (M2) genes were expressed at comparable levels in the livers of WT and CD18-mutant mice (Fig 4B). These results imply that CD18 is not required for MCD-mediated activation of pro-inflammatory signals in the liver and its absence does not alter the hepatic cytokine balance toward an anti-inflammatory phenotype. We also looked for alterations in hepatic expression of IL-1 receptor and TLRs in CD18-mutant mice after MCD feeding, as these have been implicated in the development of experimental steatohepatitis [5, 31–37]. Again, we found no reduction in these genes in CD18 mutants compared to WT mice (Fig 4C). Since CD18 deficiency failed to suppress any of these prerequisites to MCD-mediated steatohepatitis, but still reduced the severity of liver injury, we reasoned the improved liver outcome in CD18-mutant mice was due to impaired leukocyte function. Accordingly, we examined livers for chlorotyrosine-protein adducts, which are markers of leukocyte activation and oxidant release [38, 39]. Chlorotyrosine-protein adducts were abundant in WT livers but not in CD18-mutant livers, even in the vicinity of large leukocyte aggregates (Fig 5A and 5B). This was true despite comparable hepatic levels of myeloperoxidase, signifying that inflammatory cells of both genotypes are equipped with the machinery necessary to produce superoxide and hypochlorous acid [40, 41] (Fig 5C). Overall, the data indicate that in the MCD model of steatohepatitis, CD18 deficiency does not interfere with events that attract leukocytes to the liver, but does prevent recruited leukocytes from contributing to steatohepatitis. Indeed, they demonstrate that a substantial proportion of the liver injury that occurs in response to MCD feeding is a consequence of leukocyte-mediated damage to hepatocytes.
Fig 4. Comparative expression of cytokine and inflammatory genes in WT and CD18-mutant mice after MCD feeding.
All data are normalized to the expression levels measured in WT mice on chow diets. (A) Hepatic expression of M1 inflammatory genes is up-regulated by MCD feeding in both WT and CD18 mut mice. M1 genes are induced as much or more in CD18-mutant mice than WT mice. M1 genes are induced as much or more in CD18-mutant mice than WT mice. CCL2, C-C chemokine ligand-2; INOS, inducible nitric oxidase synthase. (B) Hepatic expression of M2 inflammatory genes after MCD feeding for 3 wk. ARG1, arginase-1; FIZZ1, found in inflammatory zone protein; Ym1, chitinase 3-like-3. (C) Hepatic expression of IL-1 receptor-1 (IL1R1) is decreased, and Toll-like receptors (TLR) increased, by MCD feeding in WT and CD18-mutant mice. TLR induction is not impaired, and in some instances enhanced, in CD18-mutant mice. Values represent mean ± SE for n = 8–14. *P < 0.05 vs. WT.
Fig 5. Chlorotyrosine-protein adduct formation in the livers of MCD-fed mice.
(A) Immunohistochemical staining for chlorotyrosine-protein adducts in WT and CD18 mut mice fed MCD diets for 3 wk. Original magnification 15X. (B) Morphometric quantitation of adduct-stained area. (C) Liver myeloperoxidase (MPO) activity normalized to the level in chow-fed WT mice. Values represent mean ± SE for n = 5. *P < 0.05 vs. WT.
Hepatic lipid metabolism in WT and CD18-mutant mice
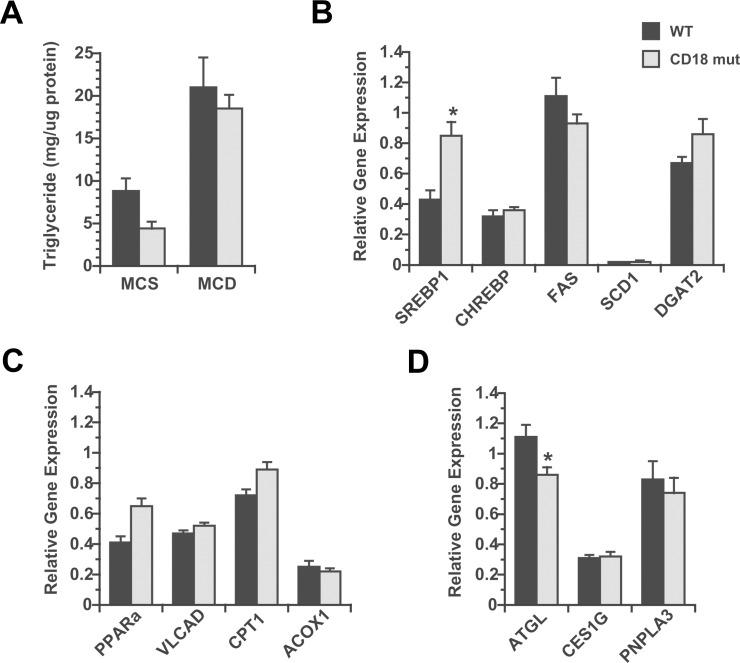
In an effort to explain why CD18-mutant mice developed exaggerated hepatic steatosis in response to MCD feeding, we isolated hepatocytes from chow-fed WT and CD18-mutant mice and cultured them for 48 h in MCD medium to induce lipid accumulation [42]. Mutant hepatocytes developed no more steatosis than WT hepatocytes, indicating that the tendency toward enhanced steatosis in vivo is not hepatocyte-intrinsic (Fig 6A). After MCD feeding in vivo, hepatic expression of IL-1 and TNF were significantly increased in CD18-mutant livers (Fig 4A), which could stimulate hepatic lipogenesis. Indeed, we found that mRNA encoding the lipogenic transcription factor SREBP1 was twice as abundant in CD18-mutant as WT mice (Fig 6B). By contrast, genes involved in fatty acid oxidation were largely similar in WT and CD18-mutant livers (Fig 6C). Investigating lipid hydrolysis, we found that mRNA encoding adipose triglyceride lipase (ATGL) was moderately but significantly reduced in the livers of CD18-mutant mice relative to WT mice after MCD feeding (Fig 6D). This suggests that slowed triglyceride lipolysis may also contribute to enhanced hepatic steatosis in the setting of CD18 deficiency.
Fig 6. Analysis of hepatic lipid homeostasis in response to MCD in culture and in vivo.
(A) Hepatic lipid accumulation in primary hepatocytes from chow-fed WT and CD18 mut mice, cultured in methionine-choline-sufficient (MCS) or methionine-choline-deficient (MCD) medium for 48 h. Values represent mean ± SE for n = 3. (B, C, D) Hepatic expression of genes pertinent to lipogenesis (B), fatty acid oxidation (C) and lipolysis (D) in WT and CD18 mut mice after 3 wk of MCD feeding in vivo, normalized to WT mice on chow diets. ACOX1, acyl-CoA oxidase-1; ATGL, adipose triglyceride lipase; CPT1, carnitine palmitoyl transferase-1; DGAT2, diacylglycerol transferase-2; FAS, fatty acid synthase; PNPLA3, patatin-like phospholipase domain containing 3; PPARa, peroxisome proliferator activated receptor-α; SCD1, stearoyl-CoA desaturase-1; VLCAD, very long chain acyl dehydrogenase. Values represent mean ± SE for n = 8–14. * P < 0.05 vs. WT.
Discussion
CD18 is a key mediator of neutrophil and macrophage activation in tissue injury. In this study, we used CD18-mutant mice to elucidate the contribution of activated neutrophils and macrophages to experimental steatohepatitis induced by an MCD diet. Our results confirmed our initial hypothesis that CD18-mutant mice would be protected from MCD-mediated liver injury; importantly, though, the protection was restricted to certain features of steatohepatitis. Hepatocyte injury was clearly reduced in CD18-mutant mice. By contrast, hepatic inflammation was not, and hepatic steatosis was actually worse than in WT mice. These findings indicate that CD18 is not necessary for the development of several MCD-related abnormalities including steatosis, pro-inflammatory cytokine induction and leukocyte recruitment. Rather, CD18 plays a singular role in leukocyte activation in MCD-fed mice, which by itself contributes significantly to their overall liver outcome.
CD18 is the molecule that enables leukocytes to adhere firmly to vascular endothelial cells before migrating out of the circulation into sites of tissue injury [43]. Accordingly, one might expect CD18-mutant mice to exhibit less hepatic inflammation in response to MCD feeding. This was not the case in our experiments: indeed, CD18-mutant mice had hepatic inflammation scores and leukocyte counts comparable to those in WT mice after 3 wk of MCD exposure. The limited impact of the CD18 mutation on hepatic inflammation may be attributable to low-level expression of CD18 on leukocytes (Fig 1), which could be sufficient to support their transmigration into the liver. Alternatively, infiltration of leukocytes into MCD-fed livers could be CD18-independent, which is known to occur [10, 20, 44–47]. Whatever the reason, despite the fact that hepatic inflammation was not impaired in CD18-mutant mice, it was accompanied by far less liver injury than in WT mice. This is consistent with the notion that activation of innate immune cells, which is impaired in CD18 mutants, is a key event in MCD-mediated steatohepatitis. Leukocyte-mediated death of hepatocytes is an important event in other liver diseases such as hepatic ischemia-reperfusion injury [48, 49] and certain drug-induced and cholestatic disorders [20, 50, 51]. In these situations, engagement of the CD11/CD18 receptor on leukocytes helps trigger a respiratory burst that results in the formation of superoxide and hypochlorous acid, which damages adjacent cells [52–54]. Leukocyte-derived proteases can also mediate hepatotoxicity by killing hepatocytes directly or activating cytokines and growth factors to exacerbate inflammation [54–56]. In the current study, it was the inability of CD18-mutant leukocytes to generate toxic oxidants in the liver that coincided directly with protection from steatohepatitis. This underscores the importance of activated innate immune cells to the pathogenesis of fatty liver disease.
Our experiments place CD18 alongside other immunoreactive molecules that influence the development of steatohepatitis. Just as CD18-mutant mice are protected against MCD-mediated liver injury, mice with targeted disruption of genes encoding pro-inflammatory cytokines, cytokine receptors, and TLRs are also able to resist experimental fatty liver disease [28, 31, 57–59]. Many of these mice exhibit global reductions in all features of steatohepatitis, including steatosis, inflammation and hepatocellular injury, which is distinct from the outcome we observed in CD18-mutant mice. In these other strains, Kupffer cells have been implicated as key mediators of adverse liver outcomes [5, 29, 37, 60]. Interestingly, CD18-mutant mice showed no evidence of Kupffer cell dysfunction after MCD feeding. This important difference may account for the more specific yet still significant impact of CD18 deficiency on liver-related outcome. Mice deficient in CC-chemokine receptor-2 (CCR2), which prevents leukocyte recruitment to the liver, should theoretically exhibit a similar pattern of protection against steatohepatitis as CD18-mutant mice, because both have defects affecting events in the liver downstream of Kupffer cell activation. This is unfortunately difficult to confirm, because the protection afforded by CCR2 deficiency against steatohepatitis varies depending on the model used to induce liver injury [30, 59, 61]. Even so, our observations by themselves underscore the specific role played by newly infiltrated leukocytes in the pathogenesis of steatohepatitis. They indicate that as much as 40% of the liver injury in the MCD model is attributable to recruited leukocytes, whereas the remaining features of steatohepatitis arise from liver-autonomous events.
One intriguing finding in our study was that MCD feeding exaggerated hepatic steatosis in CD18-mutant mice. Since hepatocytes from WT and CD18-mutant mice responded identically to methionine and choline deprivation in culture (Fig 6A), leukocytes are likely driving the steatotic phenotype in CD18-mutant mice in vivo. There is a precedent to this concept, as mice lacking leukocyte adhesion molecules or certain NADPH oxidases have a reported propensity toward diet-induced obesity and alterations in hepatic lipid metabolism [62–64]. Even humans with a polymorphism in ITGB2 that results in reduced CD18 expression are at increased risk of obesity [65]. Taken together, these observations imply that activated leukocytes impose some tonic influence on metabolism that actually limits fat accumulation in hepatocytes. Applying this concept to human NASH, it is possible that prolonged hepatic inflammation is responsible for the decline in hepatic steatosis that occurs during disease progression [66–69].
Our work did not pinpoint a single dominant mechanism by which CD18 deficiency influenced hepatic lipid metabolism. CD18 mutants did exhibit increased IL-1 and TNF gene expression in the liver, which has been linked to hepatic steatosis [33, 60, 70]. We also found reduced ATGL gene expression in CD18-mutant mice. Global ablation of ATGL in mice exacerbates MCD-mediated hepatic steatosis [71], and liver-specific ablation of this enzyme leads to steatosis even on a normal diet [72]. It is possible that enhanced hepatic cytokine expression in CD18-mutant mice, combined with suppressed hepatic lipolysis, results in enhanced hepatic steatosis.
In conclusion, the current experiments demonstrate that CD18-mediated leukocyte activation is a central event in the pathogenesis of steatohepatitis in response to an MCD diet. Specifically, activated leukocytes recruited to the liver in response to MCD feeding greatly amplify the degree of hepatocellular injury in this NASH model. Events upstream of leukocyte activation remain largely unperturbed in CD18-mutant mice; this permits a distinction between liver outcomes that are either dependent on or independent of innate immune cell invasion. Interestingly, our results suggest that recruited leukocytes not only promote liver cell injury, but also modulate hepatic steatosis. This may be mediated by dual effects on hepatic lipogenesis and lipolysis.
Supporting information
(PDF)
Acknowledgments
The authors are indebted to Parshawn Lahiji, Jim Yan and Chris Her for expert technical assistance.
Abbreviations
- ACOX1
acyl-CoA oxidase-1
- ARG1
arginase-1
- ATGL
adipose triglyceride lipase
- ALT
alanine aminotransferase
- BiP
binding immunoglobulin protein
- CCL2
C-C chemokine ligand-2
- CCR2
CC-chemokine receptor-2
- CES1G
carboxylesterase 1G
- CD18
mut, CD18-mutant
- CHOP
CEBP-homologous protein
- CHREBP
carbohydrate responsive element-binding protein
- Col1a1
type I collagen α-1 chain
- CPT1
carnitine palmitoyl transferase-1
- DGAT2
diacylglycerol transferase-2
- ERO1
ER oxidoreductin
- FAS
fatty acid synthase
- FATP5
fatty acid transport protein 5
- FIZZ1
found in inflammatory zone protein
- IL1R1
IL-1 receptor-1
- INOS
inducible nitric oxide synthase
- MCD
methionine-choline-deficient
- MCS
methionine-choline-sufficient
- MPO
myeloperoxidase
- NASH
non-alcoholic steatohepatitis
- PNPLA3
patatin-like phospholipase domain containing 3
- PPARa
peroxisome proliferator activated receptor-α
- qPCR
quantitative PCR
- SCD1
stearoyl-CoA desaturase-1
- SREBP1
sterol regulatory element binding-protein 1
- TLR
Toll-like receptor
- VLCAD
very long chain acyl dehydrogenase
- WT
wild-type
- XBP1s
X-box protein-1 spliced form
- YM1
chitinase 3-like-3
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: R01 DK068450, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, (JJM); R56 DK088674, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, (JJM); R01 DK093646, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, (JLB); K08 DK098270, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, (ANM); T32 DK068414, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, (CCD); P30 CA082103, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Genome Analysis Core of the Hellen Diller Family Comprehensive Cancer Center; and P30 DK026743, https://www.niddk.nih.gov/, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, (JJM).
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466 [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–7. doi: 10.1111/j.1572-0241.2003.07486.x [DOI] [PubMed] [Google Scholar]
- 3.Ruhl CE, Everhart JE. Trunk fat is associated with increased serum levels of alanine aminotransferase in the United States. Gastroenterology. 2010;138(4):1346–56, 56 e1-3. doi: 10.1053/j.gastro.2009.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51(1):212–23. doi: 10.1016/j.jhep.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miura K, Seki E, Ohnishi H, Brenner DA. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic Fatty liver disease. Gastroenterology research and practice. 2010;2010:362847 doi: 10.1155/2010/362847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–303. doi: 10.1007/s10620-016-4049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275(31):23409–12. doi: 10.1074/jbc.R000004200 [DOI] [PubMed] [Google Scholar]
- 8.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O'Brien WE, et al. Gene targeting yields a CD18-mutant mouse for study of inflammation. The Journal of Immunology. 1993;151(3):1571–8. [PubMed] [Google Scholar]
- 10.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, et al. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med. 1997;186(8):1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188(1):119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titova E, Ostrowski RP, Kevil CG, Tong W, Rojas H, Sowers LC, et al. Reduced brain injury in CD18-deficient mice after experimental intracerebral hemorrhage. J Neurosci Res. 2008;86(14):3240–5. doi: 10.1002/jnr.21762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–75. doi: 10.1172/JCI8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47(10):2280–90. doi: 10.1194/jlr.M600198-JLR200 [DOI] [PubMed] [Google Scholar]
- 15.Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49(5):1068–76. doi: 10.1194/jlr.M800042-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Ip E, Dela Pena A, Hou JY, Sesha J, Pera N, et al. COX-2 induction in mice with experimental nutritional steatohepatitis: Role as pro-inflammatory mediator. Hepatology. 2006;43(4):826–36. doi: 10.1002/hep.21108 [DOI] [PubMed] [Google Scholar]
- 17.Lee GS, Yan JS, Ng RK, Kakar S, Maher JJ. Polyunsaturated fat in the methionine-choline-deficient diet influences hepatic inflammation but not hepatocellular injury. J Lipid Res. 2007;48(8):1885–96. doi: 10.1194/jlr.M700181-JLR200 [DOI] [PubMed] [Google Scholar]
- 18.Larter CZ, Yeh MM, Cheng J, Williams J, Brown S, dela Pena A, et al. Activation of peroxisome proliferator-activated receptor alpha by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation. J Gastroenterol Hepatol. 2008;23(2):267–75. doi: 10.1111/j.1440-1746.2007.05157.x [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi: 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 20.Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2006;291(2):G355–G63. doi: 10.1152/ajpgi.00458.2005 [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 22.Pickens MK, Yan JS, Ng RK, Ogata H, Grenert JP, Beyson C, et al. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res. 2009;50:2072–82. doi: 10.1194/jlr.M900022-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warne James P, Alemi F, Reed Alison S, Varonin Jillian M, Chan H, Piper Merisa L, et al. Impairment of Central Leptin-Mediated PI3K Signaling Manifested as Hepatic Steatosis Independent of Hyperphagia and Obesity. Cell Metabolism. 2011;14(6):791–803. doi: 10.1016/j.cmet.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Bhatia M, Saluja AK, Hofbauer B, Frossard J-L, Lee HS, Castagliuolo I, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proceedings of the National Academy of Sciences. 1998;95(8):4760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochemical and Biophysical Research Communications. 2005;327(2):509–15. doi: 10.1016/j.bbrc.2004.12.030 [DOI] [PubMed] [Google Scholar]
- 26.Wolf D, Bukosza N, Engel D, Poggi M, Jehle F, Anto Michel N, et al. Inflammation, but not recruitment, of adipose tissue macrophages requires signalling through Mac-1 (CD11b/CD18) in diet-induced obesity (DIO). Thromb Haemost. 2017;117(2):325–38. doi: 10.1160/TH16-07-0553 [DOI] [PubMed] [Google Scholar]
- 27.Leclercq IA, Farrell GC, Sempoux C, dela Pena A, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004;41(6):926–34. doi: 10.1016/j.jhep.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 28.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55(3):415–24. doi: 10.1136/gut.2005.071118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287(48):40161–72. doi: 10.1074/jbc.M112.417014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61(3):416–26. doi: 10.1136/gutjnl-2011-300304 [DOI] [PubMed] [Google Scholar]
- 31.Szabo G, Velayudham A, Romics L Jr., Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29(11 Suppl):140S–5S. [DOI] [PubMed] [Google Scholar]
- 32.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–9. doi: 10.1016/j.jhep.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1beta in Mice. Gastroenterology. 2010;139(1):323–34.e7. doi: 10.1053/j.gastro.2010.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G433–41. doi: 10.1152/ajpgi.00163.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, et al. Lack of interleukin-1alpha or interleukin-1beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. JHepatol. 2011;55(5):1086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. The Journal of Clinical Investigation. 2012;122(10):3476–89. doi: 10.1172/JCI60777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57(2):577–89. doi: 10.1002/hep.26081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gujral JS, Hinson JA, Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp Hepatol. 2004;3 Suppl 1:S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2004;287(1):G243–G52. doi: 10.1152/ajpgi.00287.2003 [DOI] [PubMed] [Google Scholar]
- 40.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. Chlorination of Tyrosyl Residues in Peptides by Myeloperoxidase and Human Neutrophils. Journal of Biological Chemistry. 1995;270(28):16542–8. [DOI] [PubMed] [Google Scholar]
- 41.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the Reactions of Superoxide and Myeloperoxidase in the Neutrophil Phagosome: IMPLICATIONS FOR MICROBIAL KILLING. Journal of Biological Chemistry. 2006;281(52):39860–9. doi: 10.1074/jbc.M605898200 [DOI] [PubMed] [Google Scholar]
- 42.Sahai A, Pan X, Paul R, Malladi P, Kohli R, Whitington PF. Roles of phosphatidylinositol 3-kinase and osteopontin in steatosis and aminotransferase release by hepatocytes treated with methionine-choline-deficient medium. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G55–62. doi: 10.1152/ajpgi.00360.2005 [DOI] [PubMed] [Google Scholar]
- 43.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. The Journal of Clinical Investigation. 1989;83(6):2008–17. doi: 10.1172/JCI114111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackarel AJ, Russell KJ, Ryan CM, Hislip SJ, Rendall JC, FitzGerald MX, et al. CD18 dependency of transendothelial neutrophil migration differs during acute pulmonary inflammation. J Immunol. 2001;167(5):2839–46. [DOI] [PubMed] [Google Scholar]
- 45.Ridger VC, Wagner BE, Wallace WA, Hellewell PG. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol. 2001;166(5):3484–90. [DOI] [PubMed] [Google Scholar]
- 46.Bowden RA, Ding ZM, Donnachie EM, Petersen TK, Michael LH, Ballantyne CM, et al. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res. 2002;90(5):562–9. [DOI] [PubMed] [Google Scholar]
- 47.Blake KM, Carrigan SO, Issekutz AC, Stadnyk AW. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136(2):262–8. doi: 10.1111/j.1365-2249.2004.02429.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17(5):915–23. [PubMed] [Google Scholar]
- 49.Kobayashi A, Imamura H, Isobe M, Matsuyama Y, Soeda J, Matsunaga K, et al. Mac-1 (CD11b/CD18) and intercellular adhesion molecule-1 in ischemia-reperfusion injury of rat liver. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2001;281(2):G577–G85. [DOI] [PubMed] [Google Scholar]
- 50.Klintman D, Schramm R, Menger MD, Thorlacius H. Leukocyte recruitment in hepatic injury: selectin-mediated leukocyte rolling is a prerequisite for CD18-dependent firm adhesion. Journal of hepatology. 2002;36(1):53–9. [DOI] [PubMed] [Google Scholar]
- 51.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38(2):355–63. doi: 10.1053/jhep.2003.50341 [DOI] [PubMed] [Google Scholar]
- 52.Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, et al. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109(3):1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liles WC, Ledbetter JA, Waltersdorph AW, Klebanoff SJ. Cross-linking of CD18 primes human neutrophils for activation of the respiratory burst in response to specific stimuli: implications for adhesion-dependent physiological responses in neutrophils. J Leukoc Biol. 1995;58(6):690–7. [DOI] [PubMed] [Google Scholar]
- 54.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35(6):757–66. doi: 10.1080/01926230701584163 [DOI] [PubMed] [Google Scholar]
- 55.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61(6):647–53. [DOI] [PubMed] [Google Scholar]
- 56.Ho JS, Buchweitz JP, Roth RA, Ganey PE. Identification of factors from rat neutrophils responsible for cytotoxicity to isolated hepatocytes. J Leukoc Biol. 1996;59(5):716–24. [DOI] [PubMed] [Google Scholar]
- 57.Rivera CA, Gaskin L, Allman M, Pang J, Brady K, Adegboyega P, et al. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol. 2010;10:52 doi: 10.1186/1471-230X-10-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54(1):133–44. doi: 10.1002/hep.24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1310–21. doi: 10.1152/ajpgi.00365.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51(2):511–22. doi: 10.1002/hep.23337 [DOI] [PubMed] [Google Scholar]
- 61.Egan CE, Daugherity EK, Rogers AB, Abi Abdallah DS, Denkers EY, Maurer KJ. CCR2 and CD44 promote inflammatory cell recruitment during fatty liver formation in a lithogenic diet fed mouse model. PLoS One. 2013;8(6):e65247 doi: 10.1371/journal.pone.0065247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babic AM, Wang HW, Lai MJ, Daniels TG, Felbinger TW, Burger PC, et al. ICAM-1 and beta2 integrin deficiency impairs fat oxidation and insulin metabolism during fasting. Molecular medicine (Cambridge, Mass). 2004;10(7–12):72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong ZM, Gutierrez-Ramos J-C, Coxon A, Mayadas TN, Wagner DD. A new class of obesity genes encodes leukocyte adhesion receptors. Proceedings of the National Academy of Sciences. 1997;94(14):7526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, et al. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int J Obes (Lond). 2012;36(12):1503–13. [DOI] [PubMed] [Google Scholar]
- 65.Awaya T, Yokosaki Y, Yamane K, Usui H, Kohno N, Eboshida A. Gene-environment Association of an ITGB2 Sequence Variant With Obesity in Ethnic Japanese. Obesity. 2008;16(6):1463–6. doi: 10.1038/oby.2008.68 [DOI] [PubMed] [Google Scholar]
- 66.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29(3):664–9. doi: 10.1002/hep.510290347 [DOI] [PubMed] [Google Scholar]
- 67.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. [DOI] [PubMed] [Google Scholar]
- 68.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. Journal of hepatology. 2004;40(4):578–84. doi: 10.1016/j.jhep.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 69.Nagaya T, Tanaka N, Suzuki T, Sano K, Horiuchi A, Komatsu M, et al. Down-regulation of SREBP-1c is associated with the development of burned-out NASH. Journal of hepatology. 2010;53(4):724–31. doi: 10.1016/j.jhep.2010.04.033 [DOI] [PubMed] [Google Scholar]
- 70.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40(1):185–94. doi: 10.1002/hep.20283 [DOI] [PubMed] [Google Scholar]
- 71.Jha P, Claudel T, Baghdasaryan A, Mueller M, Halilbasic E, Das SK, et al. Role of adipose triglyceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology. 2014;59(3):858–69. doi: 10.1002/hep.26732 [DOI] [PubMed] [Google Scholar]
- 72.Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54(1):122–32. doi: 10.1002/hep.24338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.