Abstract
The marine diatoms Thalassiosira proschkinae and T. spinulata are relatively small in size; their taxonomic identities have been debated owing to the diverse morphological variations. In the present study, we isolated both morphotypes from Korean coastal waters and examined their fine structures and conducted molecular sequence comparisons. The morphological and molecular analyses showed that T. proschkinae and T. spinulata were certainly distinct, and phenotypic plasticity of valve structure was not noted. Based on the morphological similarity and phylogenetic relationship, we transferred T. proschkinae and T. spinulata to another genus Minidiscus within Thalassiosirales that includes small-sized species and proposed new combination names, Minidiscus proschkinae (Makarova) Park & Lee comb. nov. and Minidiscus spinulatus (Takano) Park & Lee comb. nov., respectively. The genus description of Minidiscus was emended.
Introduction
Thalassiosira Cleve is one of the most speciose marine diatom genera, with over 180 species being listed by Algaebase [1]. Many species having the two known types of processes (fultoportula and rimoportula) have been newly described as belonging to the genus Thalassiosira, and the genus concept was gradually expanded with the highly diverse characters of new species. The broad concept of characters of the species belonging to the genus Thalassiosira has hindered the determination of the inter-specific relationships, and recent phylogenetic studies showed para- or polyphyletic relationships that was consisted of 10 different lineages within in the order Thalassiosirales [2–4]. To construct the phylogenetic classification of the order Thalassiosirales, some authors attempted to separate the ambiguous species of Thalassiosira into new genera such as Shionodiscus Alverson, Kang et Theriot [5] and Conticribra Stachura-Suchoples et Williams [6], or transfer some doubtful species such as Thalassiosira constricta Gaarder into the phylogenetically appropriate genus Bacterosira Gran [7]. Despite these efforts, this order still contains phylogenetically ambiguous taxa such as Thalassiosira, the phylogenetic position of the species of which needs to be determined.
Of the members of Thalassiosira, the small-sized species T. proschkinae Makarova was originally described from the Sea of Azov on August 24, 1962 by Makarova [8]; another small-sized species T spinulata Takano was first described from the Japanese waters by Takano [9]. Both the species shared morphological characters such as a small size of less than 10 μm, the presence of a rimoportula on the valve face, one central fultoportula, and one ring of marginal fultoportulae. Makarova [10] suggested that T. spinulata was conspecific with T. proschkinae and reduced the species as a variety of T. proschkinae, namely, T. proschkinae var. spinulata (Takano) Makarova. Hasle & Syvertsen [11] also agreed to Makarova’s consideration and mentioned that the two species could not be distinguished by light microscopy. However, the taxonomic relationship between the species has been clarified by the differences of areola structure and valve silicification [12–14]. The taxonomic relationship between T. proschkinae and T. spinulata regarding whether the areola structure is an intra-specific phenotypic variation or inter-specific distinguishing character needs to be determined [13].
In Thalassiosira, the rimoportula could be positioned either on the valve face or the valve margin. Hasle [15] divided the genus Thalassiosira into two groups based on the positions of rimoportula. Hasle & Syvertsen [11] improved the original subgrouping proposed by Hasle [15]: Subgroup A is characterized by the presence of a rimoportula near the valve mantle, usually with an external extension; Subgroup B has a rimoportula located on the valve face without external extensions. Alverson et al. [5] transferred 26 Thalassiosira species in subgroup B into the genus Shionodiscus, which is characterized by the rimoportula on the valve face and no extension of the processes externally. Since Thalassiosira species in subgroup B were transferred to the genus Shionodiscus, most Thalassiosira species have a rimoportula at the valve margin [14]. In addition to Shionodiscus, some genera have the rimoportula on the valve face in Thalassiosirales, namely, Minidiscus Hasle, Livingstonia Prasad, and Skeletonema Greville. The position of rimoportula on the valve face in T. proschkinae and T. spinulata hinder the identification of their phylogenetic position in the order Thalassiosirales.
In the present study, we clarified the taxonomic identities between T. proschkinae and T. spinulata based on the fine surface structures and DNA sequence similarities and investigated the phylogenetic position of the tested small Thalassiosira species within the Thalassiosirales lineage by conducting molecular comparisons. The fine structure and molecular sequence analyses revealed the phylogenetic relationship of the two Thalassiosira species leading to a reconsideration of the order Thalassiosirales.
Materials and methods
Ethics statement
The field studies did not involve endangered or protected species, therefore, no specific permission were required for the sampled locations and activities.
Environmental samplings and culture
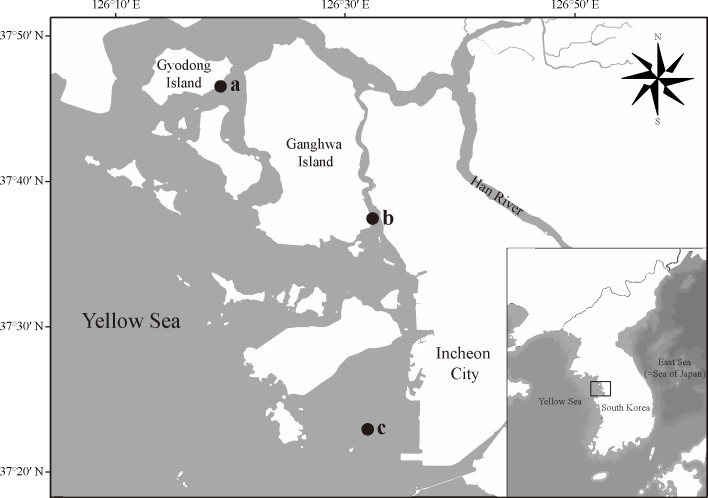
Phytoplankton samples were collected from the province of Incheon in Korea between 2007 and 2016 by using a 20-μm mesh net by horizontal and/or vertical towing. The seawater had a salinity of <30 psu and contained many inorganic sediments because the areas investigated are affected by the influx of freshwater from the Han River through the major channels as well as movement of seawater by tides. Diatom cells were isolated using the capillary method and transferred to 24-well culture plates containing the collected and sterilized seawater amended with f/2 nutrients. Cells were incubated at 15°C under 12:12h L:D, with an irradiance of approximately 30 μmol photons·m-2·s-1. When the cell density reached approximately 10,000 cells·ml-1, 2 ml of each isolate was transferred and maintained in a 250 ml Erlenmeyer flask containing 100 ml seawater with f/2 nutrients. The following Thalassiosira species were isolated (Fig 1): T. spinulata SMDC050 from the Incheon coast (37°23ʹ02.46ʺN, 126°31ʹ59.48ʺE) on Jul 27, 2007; T. spinulata SMDC303 from the coast at Gyodong Island (37°46′25.69″N, 126°19′02.27″E) on Jun 13, 2016; and T. proschkinae SMDC305 from the coast at Ganghwa Island (37°37′32.09″N 126°32′22.79″E) on Jun 15, 2016.
Fig 1. A map showing the sampling sites in Korea.
The small letters beside the black dots indicated the collecting sites where T. proschkinae and T. spinulata strains. T. spinulata SMDC303 (a), T. proschkinae SMDC305 (b), T. spinulata SMDC050 (c).
Morphological observations
Materials for scanning electron microscopy (SEM) were prepared for observing the intact and cleaned cells in two ways. The intact morphology from the isolates was observed by filtering live cells on a 3-μm polycarbonate membrane and washed with distilled water without any oxidation. The cleaned external and internal structures after the detachment of the organic matter were observed by adding some live cells to an equal volume of H2O2 and exposing them to ultraviolet light (312 nm) for 3 h on the UV-transilluminator (WUV-L50; witeg, Wertheim, Germany) and then washing them with distilled water. The cleaned cells were filtered and dried on a 3-μm polycarbonate membrane. The dried polycarbonate membranes were attached to aluminum stubs by using carbon conductive adhesive tapes and coated with 10 nm gold in an automatic sputter coater (Agar Scientific Ltd., Essex, UK). The prepared specimens were examined using FE-SEM (JSM7600F; Jeol, Tokyo, Japan) operating at 10 kV and 8 mm working distance. Valve dimensions from the SEM photographs were measured using ImageJ 1.51 software [16]. The terminology used for the structures discussed herein follows that of Ross et al. [17], Round et al. [18] and Theriot & Serieyssol [19].
DNA analyses and phylogeny
Cultured samples of the three strains were harvested during exponential growth by using a series of centrifugation steps and stored at -70°C until the genomic DNA was extracted. The genomic DNA was extracted from cell pellets by using a DNeasy® Plant Mini Kit (Qiagen, Hilden, Germany). Nuclear rDNA fragments [i.e., nearly full-length small subunit ribosomal DNA (SSU rDNA) gene and the D1–D3 region of the large subunit ribosomal DNA (LSU rDNA)] and plastid-encoding genes (psbC and rbcL) were amplified and sequenced following protocols outlined in Alverson et al. [3] and Park & Lee [20]. Newly generated DNA sequences are available from GenBank using accession numbers KY912618, KY912621, KY912624, and KY912625 for T. proschkinae SMDC305; KY912616, KY912619, KY912622, and KY912626 for T. spinulata SMDC050; and KY912617, KY912620, KY912623, and KY912627 for T. spinulata SMDC303. These sequences were manually aligned to multiple sequence alignments containing 68 other broadly sampled species of Thalassiosirales [3, 7] (The alignment sequences file was provided as a phylip format in S1 File). A maximum likelihood phylogeny was inferred using RAxML ver. 8.0.20 by using the “-f a” option by performing 5,000 bootstrap replicates and inferring the best scoring tree [21]. Separate GTR+G models were applied to the SSU rDNA partition, LSU rDNA partition, the first + second codon positions for the combined psbC and rbcL genes, and the third codon positions for the combined psbC and rbcL genes.
Results
Morphology of Thalassiosira proschkinae SMDC305
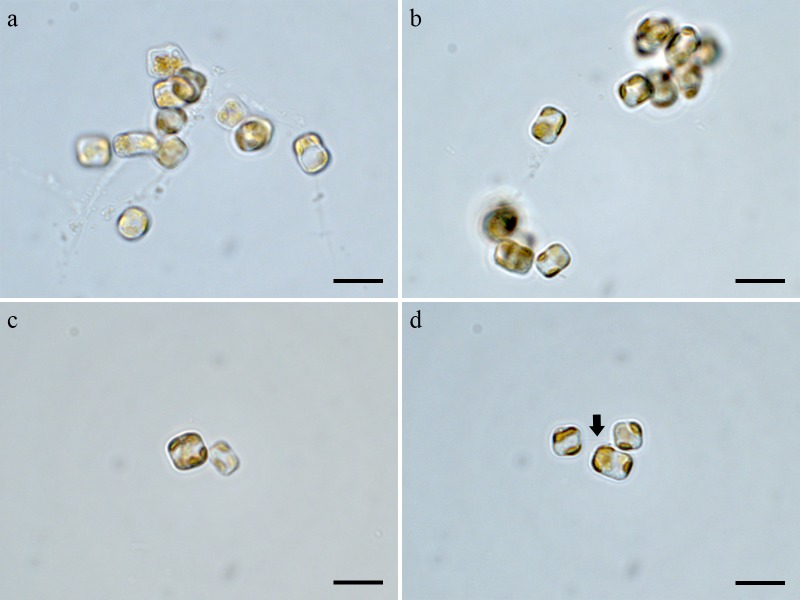
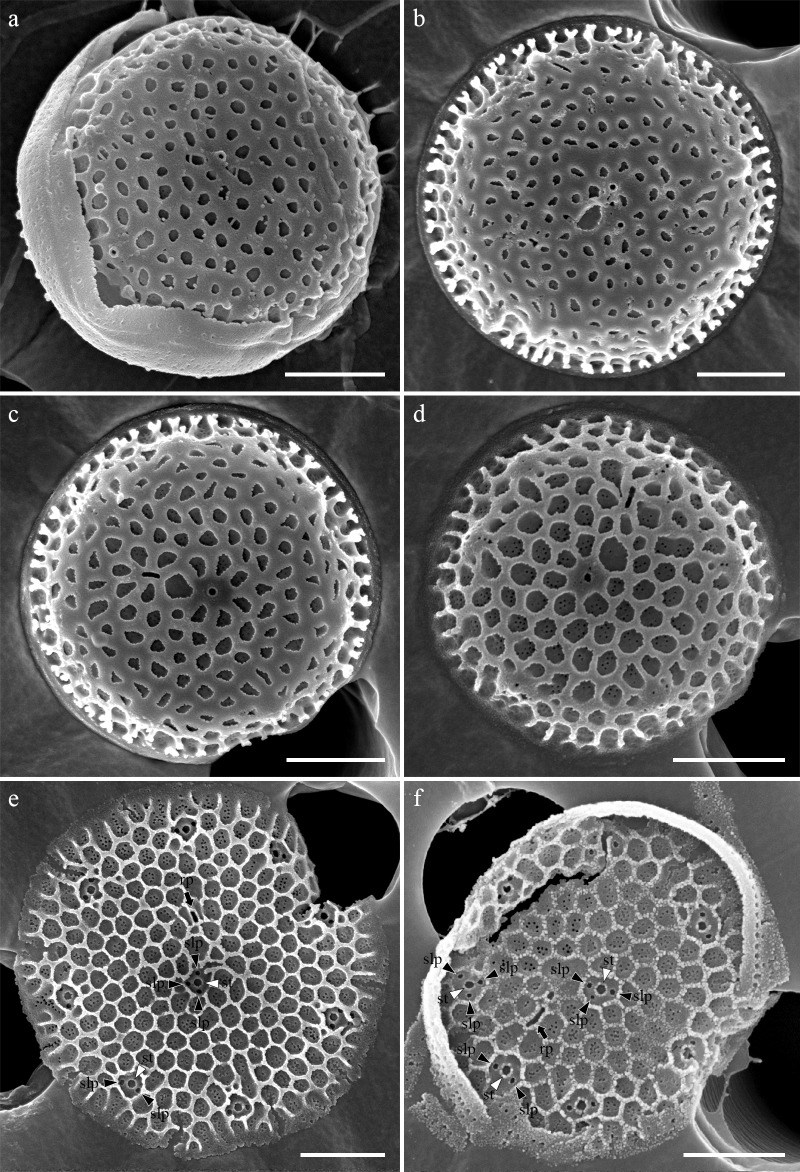
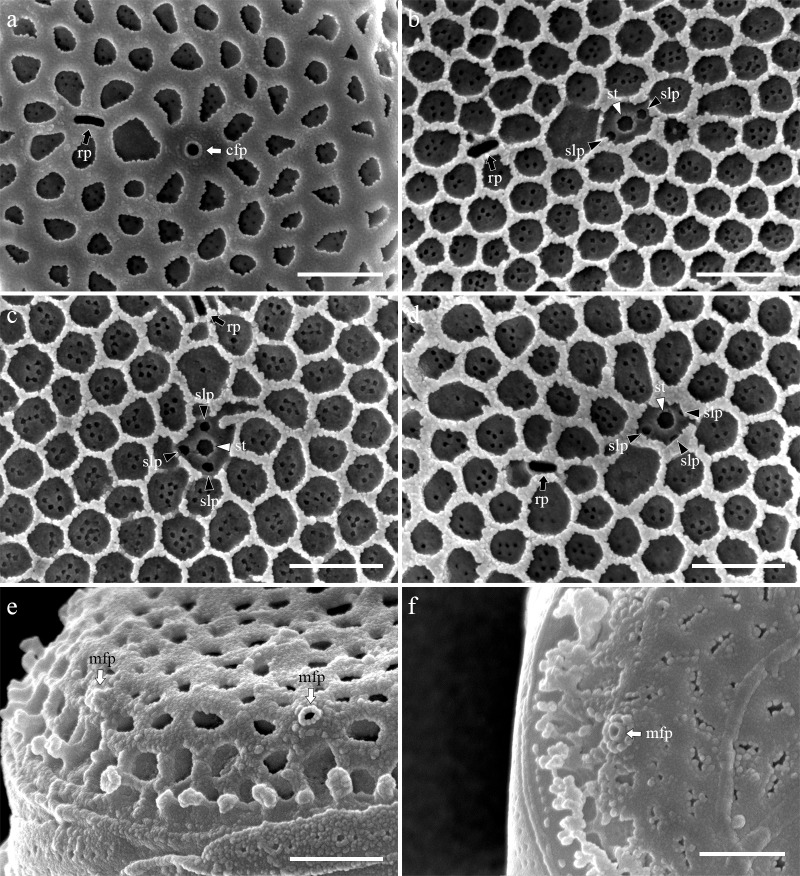
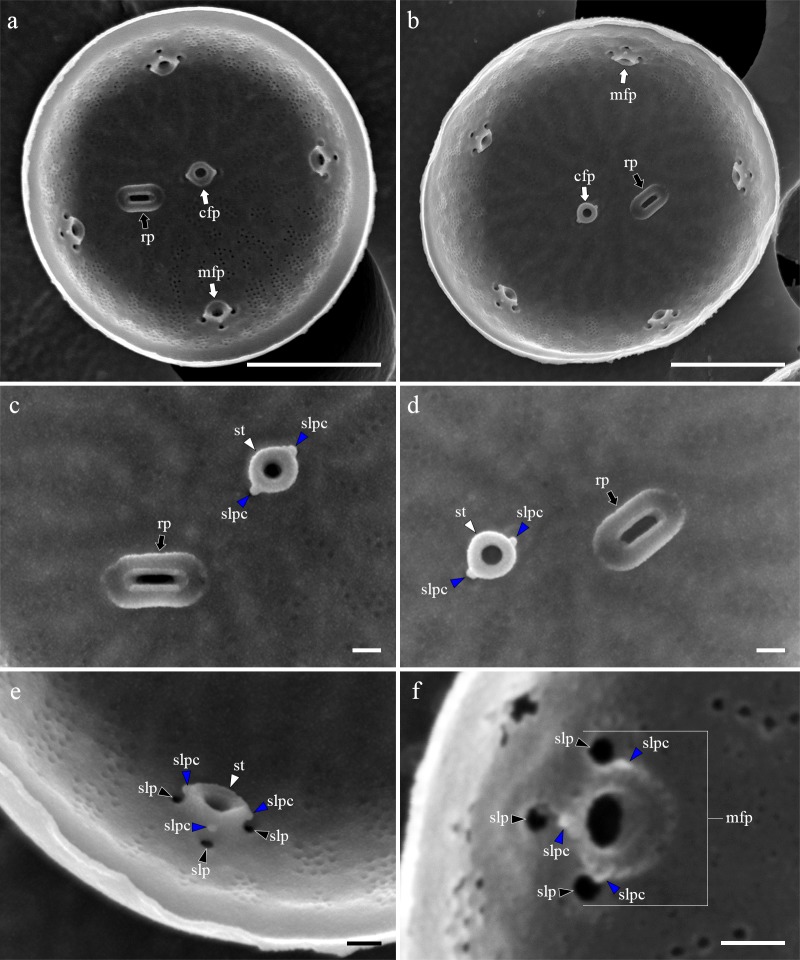
Cells were aggregated (Fig 2A and 2B), solitary (Fig 2B and 2C) or forming 2–3 cells chain colonies by mucilage thread extruded from central fultoportula (Fig 2D). Valves were circular with a flattened valve face and sharp-angled mantle, 2.46–8.46 μm in diameter. Areolation was linear to sub-linear (Fig 3A–3F). Areolae were loculate and structured by external foramen and internal cribrum. The foramina were an irregular circular to obtuse angled polygonal structures (Fig 4A–4F), and the cribra consisted of several tiny pores (Fig 5A–5F). A single fultoportula was situated at the center of the valve (Fig 3A–3F), and it was structured as a small tube externally surrounded by silica granules (Fig 3A–3C, 4A); the strutted tube was internally surrounded by two to three satellite pores (Figs 4B–4D and 5A–5D). One ring of marginal fultoportulae was situated in the junction between the valve face and mantle (Fig 4E and 4F). In all, 6–8 marginal fultoportulae were present, with distances of 1.24–1.91 μm among them. The external openings of the marginal fultoportulae were similar to the central fultoportula (Fig 4E and 4F), and the strutted tube was internally surrounded by two satellite pores (Figs 3E, 3F, 5A and 5F), often three (Fig 5B and 5E). All fultoportulae had tiny triangular-shaped satellite pores covered with slightly raised cowling internally (Fig 5C–5F). A single rimoportula was located on the valve face adjacent to the central fultoportula, and one large areola was present between the central fultoportula and the rimoportula (Fig 4A–4D). The external opening of the rimoportula varied in forms and was elliptic (Fig 4A–4D) and was occasionally not distinguished from the adjacent areolae (Fig 3A and 3B). Internally, the rimoportula was positioned on the basal silica wall, without any stalk (Fig 5A–5D). The slit of rimoportula was thin and straight like the external opening (Fig 5A–5D). The slit was surrounded by the heavily silicified elliptic rim (Fig 5C and 5D). The internal shape of the rimoportula resembled an ellipsoidal doughnut (Fig 5C and 5D).
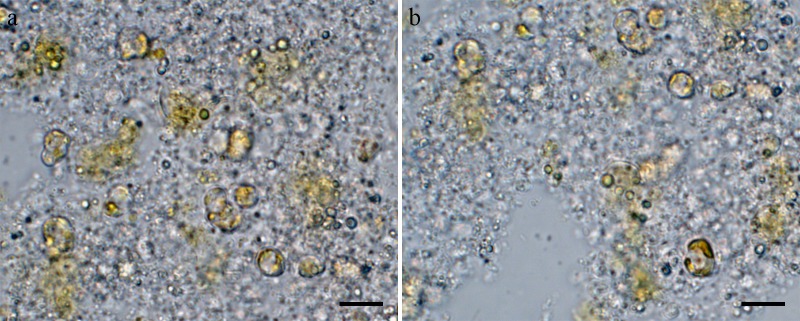
Fig 2. Light microscopy of Thalassiosira proschkinae SMDC305.
(a, b) Aggregated cells. (c) Solitary cell. (d) Chain colony of two cells by mucilage thread (arrow) extruded from central fultoportula. Scale bars are 10 μm.
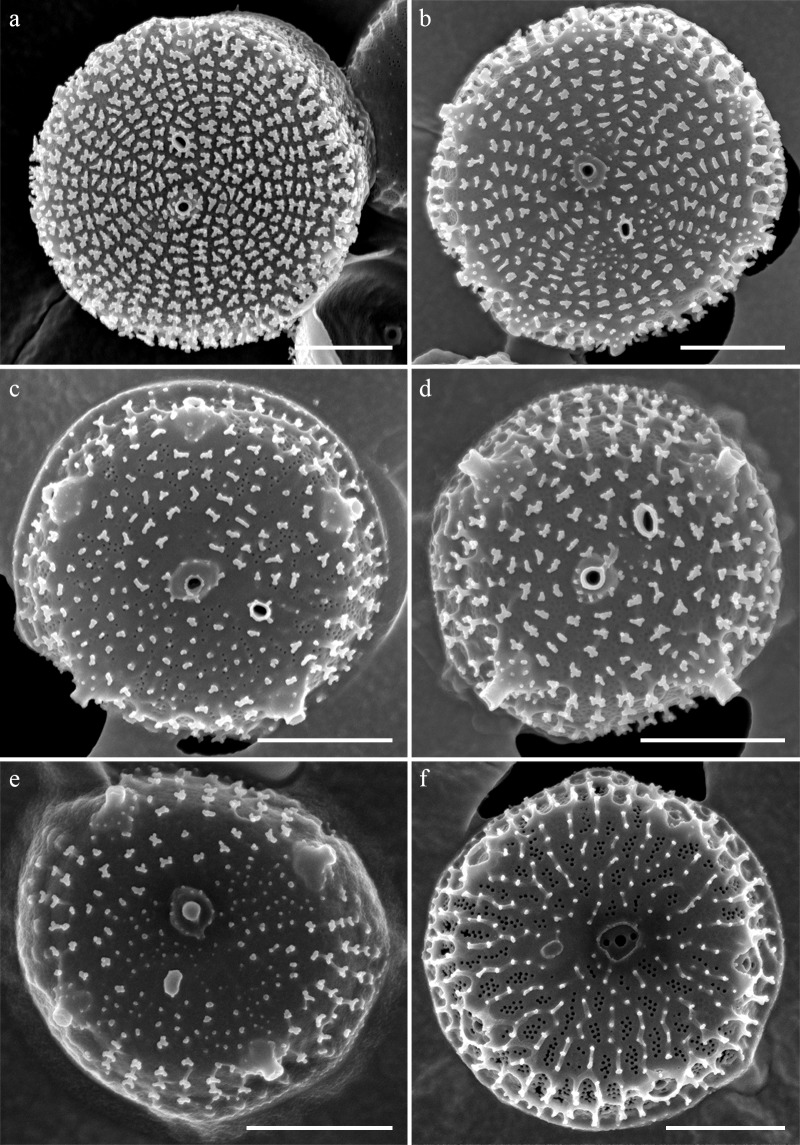
Fig 3. External whole valve of Thalassiosira proschkinae SMDC305.
(a–d) Heavily silicified valves. (e, f) Valve cleaned by acid treatment showing the external opening of rimoportula (rp, black arrow) and internal structure of strutted tube (st, white arrowheads) surrounded by satellite pores (slp, black arrowheads). Scale bars are 1 μm.
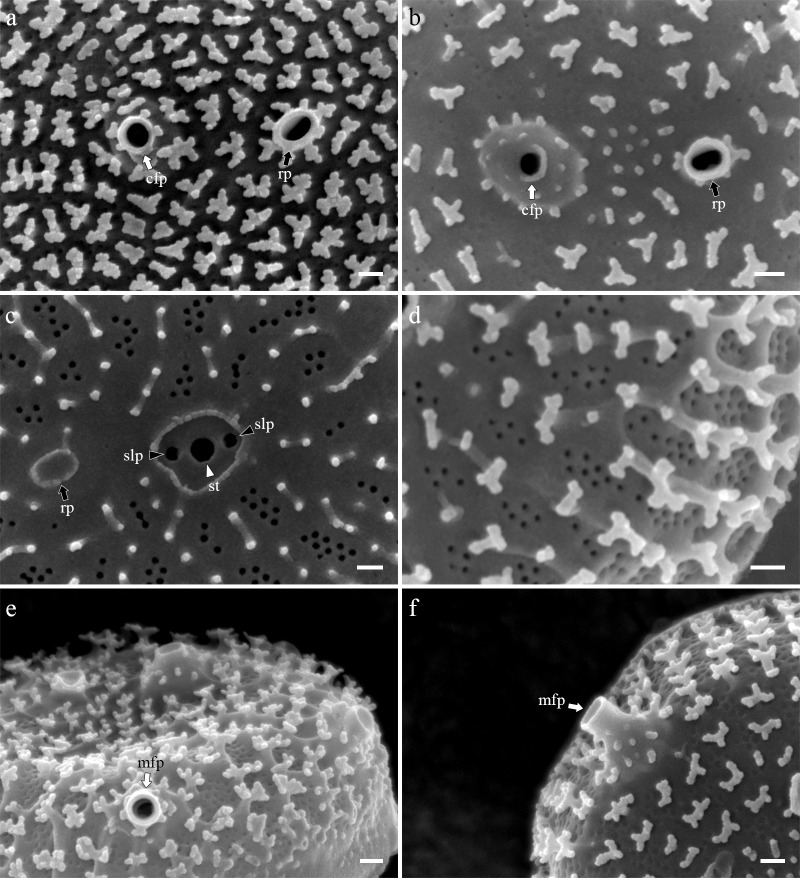
Fig 4. External valve view of Thalassiosira proschkinae SMDC305.
(a) External small tube of central fultoportula (cfp, white arrow) and elliptic opening of rimoportula (rp, black arrow). (b–d) Acid cleaned valves showing the elliptical opening of rimoportula (rp, black arrow) and the exposed internal structure of strutted tube (st, white arrowhead) surrounded by two to three satellite pores (slp, black arrowheads). (e, f) External small tube of marginal fultoportula (mfp, white arrows) at the junction of valve face and mantle. Scale bars are 1 μm.
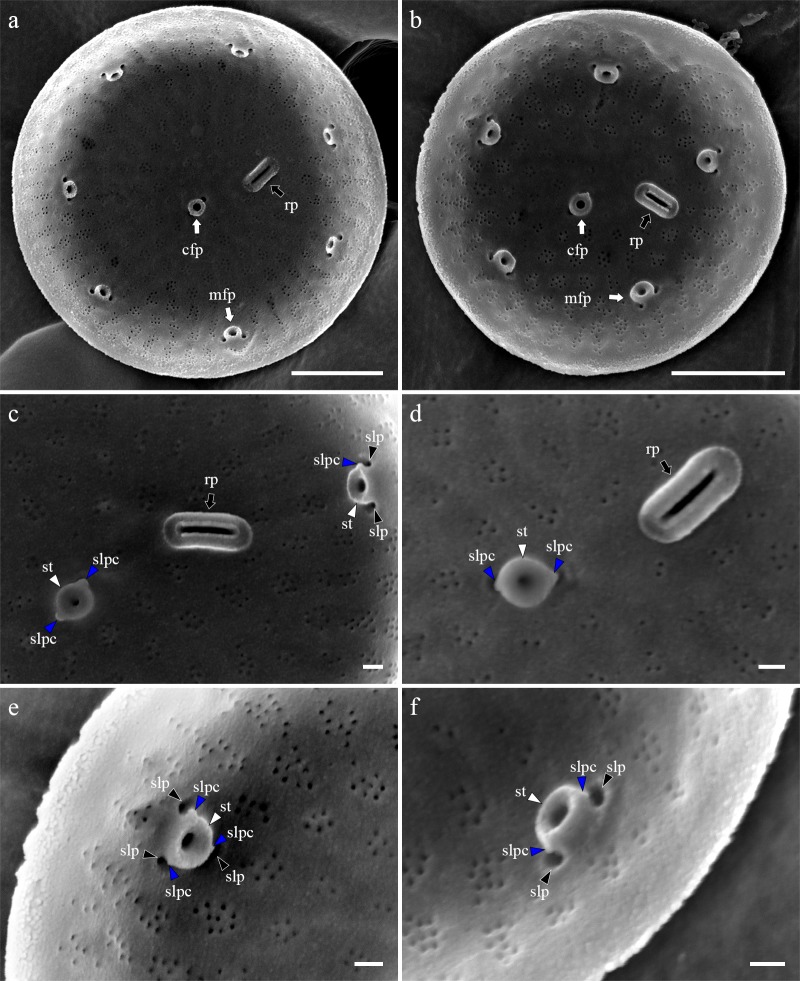
Fig 5. Internal valve view of Thalassiosira proschkinae SMDC305.
(a, b) Internal whole valve having the central fultoportula (cfp, white arrow), one ring of marginal fultoportula (mfp, white arrow), and radially sessile rimoportula (rp, black arrow). (c) Rimoportula (rp, black arrow) between the central (cfp) and marginal fultoportula (mfp). Each fultoportula consist of strutted tube (st, white arrowhead), satellite pore cover (slpc, blue arrowheads), and satellite pore (slp, black arrowheads). (d) Central strutted tube (st, white arrows) with two satellite pore covers (slpc, blue arrowheads) and elliptical donut-shaped rimoportula (rp, black arrow). (e, f) Marginal fultoportula consist of strutted tube (st, white arrowhead), satellite pore covers (slpc, blue arrowheads), and satellite pores (slp, black aorrwheads). Scale bars are 1 μm.
Morphology of Thalassiosira spinulata SMDC303
Cells were embedded in a mucilage (Fig 6A and 6B), Valves were circular with a flat valve face and obtuse-angled mantle and were 2.53–5.04 μm in diameter. Areolae almost incomplete in most cells and were neither loculate nor poroid (Fig 7A–7F). No true foramen was found externally (Fig 8A and 8B), and the cribra was radially continuous internally and occasionally naked externally (Fig 8C and 8D). Instead of the areolae, the valve face contained granules that were situated on the valve (Figs 7A–7F and 8A–8C), whereas Y-shaped ribs were observed on the valve margin (Fig 8D). A single fultoportula was situated at the center or subcenter of the valve (Fig 7A–7F) and it was structured externally as a stumpy tube surrounded by a hyaline area (Figs 7B–7E and 8B), and the strutted tube was surrounded internally by two satellite pores (Fig 9A–9S). One ring of marginal fultoportulae was situated in the junction between the valve face and valve mantle (Fig 8E and 8F). In all, 4–10 marginal fultoportulae were present, with distances of 1.11–1.93 μm between pairs of them. The external openings were similar to the central fultoportula (Fig 8E and 8F), but the strutted tube was internally surrounded by four satellite pores (Fig 9E and 9F). The satellite pore covers were semi-circular (Fig 9C and 9D) and attached near the tips of the strutted tube away from the satellite pores (Fig 9E and 9F). A single rimoportula was situated on the valve face adjacent to the central fultoportula (Fig 9A–9D). The external opening of the rimoportula was a sub-circular to elliptical tube, and it was smaller than the external tube of the central fultoportula (Fig 8A and 8B). Internally, the rimoportula was positioned on the basal silica wall, without any stalk (Fig 9A–9D). The internal opening was always elliptical, and the heavily silicified rim was located on the opening, like the external opening (Fig 9C and 9D). The internal shape of the rimoportula resembled an ellipsoidal doughnut (Fig 9C and 9D).
Fig 6. Light microscopy of Thalassiosira spinulata SMDC303.
(a, b) Cells embedded by mucilage. Scale bars are 10 μm.
Fig 7. External whole valve of Thalassiosira spinulata SMDC303.
(a, b) Heavily silicified valve. (c, d) Moderately silicified valve. (e, f) Poorly developed ribs. Scale bars are 1 μm.
Fig 8. External valve view of Thalassiosira spinulata SMDC303.
(a, b). External opening of central fultoportula (cfp, white arrow) and rimoportula (rp, black arrow). (c) Superimposed internal structure of central fultoportula consist of strutted tube (st, white arrowhead) and two satellite pores (slp, black arrows). (d) Y-shaped development of ribs between the areolae in the valve margin. (e) Circular opening of marginal fultoportula (mfp, white arrow). (f) Obtuse external tube of marginal fultoportula (mfp, white arrow). Scale bars are 0.1 μm.
Fig 9. Internal valve view of Thalassiosira spinulata SMDC303.
(a, b) Internal whole valve having the central fultoportula (cfp, white arrow), one ring of marginal fultoportula (mfp, white arrow), and radially sessile rimoportula (rp, black arrow). (c, d) Central strutted tube (st, white arrowhead) with two satellite pore covers (slpc, blue arrows), and elliptical donut-shaped rimoportula (rp, black arrow). (e, f) Marginal fultoportula consist of strutted tube (st, white arrowhead), satellite pore covers (slpc, blue arrows) and satellite pores (slp, black arrowheads). Scale bars are 1 μm for Fig 6A and 6B and 0.1 μm for Fig 6C–6F.
Sequence comparison and phylogenetic position of T. proschkinae and T. spinulata
The intra- and interspecific differences of the molecular sequences between T. spinulata and T. proschkinae are shown in Table 1. The intraspecific differences of T. spinulata were calculated from the two strains, SMDC050 and SMDC305, but the intraspecific differences of T. proschkinae could not be calculated because only one strain of T. proschkinae was obtained. The intraspecific divergences of T. spinulata did not show any differences among the four markers. However, the interspecific divergences between T. spinulata and T. proschkinae showed various differences according to the markers. The SSU rRNA of both species were nearly identical; there was only 1 nucleotide difference in the 1,708 bp, and the D1–D3 region of the LSU rRNA differed by 6 bp of 825 bp. The nucleotide differences in the two chloroplast genes were higher than those in the nuclear rRNA markers: the psbC by 19 bp of 1,129 bp and the rbcL by 21 bp of 1,419 bp.
Table 1. Intraspecific genetic distances between the two strains of T. spinulata, and interspecific genetic distances between T. proschkinae and T. spinulata for the two nuclear rRNA and two chloroplast genes.
| Strains | 18S | 28S D1–D3 | psbC | rbcL |
|---|---|---|---|---|
|
T. spinulata SMDC303 vs. T. spinulata SMDC050 |
0.0000 | 0.0000 | 0.0000 | 0.0000 |
|
T. spinulata SMDC303 vs. T. proschkinae SMDC305 |
0.0006 (1707/1708) | 0.0074 (819/825) | 0.0120 (1398/1415) | 0.0122 (1110/1129) |
|
T. spinulata SMDC050 vs. T. proschkinae SMDC305 |
0.0006 (1646/1647) | 0.0074 (819/825) | 0.0120 (1398/1415) | 0.0122 (1110/1129) |
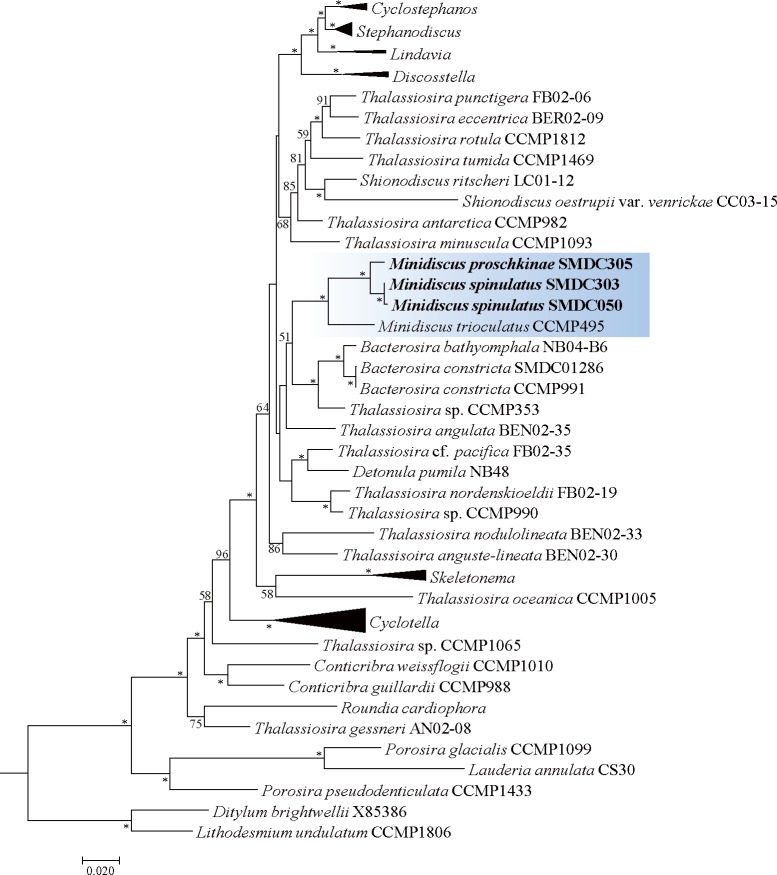
Multi-gene phylogenetic analysis strongly supported a relationship between T. proschkinae and T. spinulata. In addition, the phylogeny recovered a strongly supported sister relationship between the two small Thalassiosira species and Minidiscus trioculatus (Fig 10). This clade was related to the genus Bacterosira with moderate support (Fig 10).
Fig 10. A phylogenetic tree for Thalassiosirales inferred from the maximum likelihood (ML) by using RAxML.
Bootstrap values of ≥50% are shown, and nodes supported by bootstrap values of ≥95% are marked with an asterisk (*).
Discussion
Clarifying the taxonomic relationship between T. proschkinae and T. spinulata
Morphological comparison
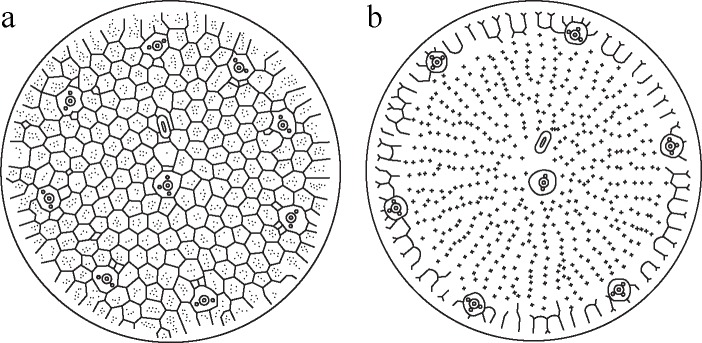
Certain morphological differences were observed in the two isolated morphotypes, T. proschkinae/T. spinulata in Korea. The two morphotypes showed differences in the external valve ornamentation, internal cribra, external opening of rimoportula and fultoportula, and the arrangement of satellite pores of marginal fultoportula (Fig 11). In particular, the external valve ornamentation and the arrangement of satellite pores of marginal fultoportulae were the critical characteristics to distinguish both the morphotypes, and we suggest that T. proschkinae and T. spinulata are distinct species.
Fig 11. Diagram of two small Thalassiosira species.
(a) T. proschkinae. (b) T. spinulata.
The areola structure of Thalassiosira species was traditionally characterized by the external foramen and internal cribra, and it was re-divided by the foramen shapes and the cribra [11, 22]. The foramen was divided into two types, loculate and poroid, by the level of covering of the roof of areola [11]. T. proschkinae consistently showed a distinct loculate areola structure that consists of the internally individual cribra and externally irregularly shaped foramen, whereas T. spinulata does not have a true areola, and the internally continuous cribra structure was exposed to the valve exterior. Although the areola structure showed variability according to the level of silicification, for instance, T. gravida/T. rotula complex [23], the areola structure of T. proschkinae and T. spinulata never showed the intermediate developing status in the present study. Martín-Cereceda & Cox [13] investigated the development of the valve structure of T. spinulata according to the changes in salinity and silicic acid concentration. They also did not observe mature areolae in T. spinulata. Therefore, the absence of the true areola is the species-specific character for T. spinulata, and thus T. proschkinae and T. spinulata are morphologically distinct species.
Two types of portula, rimoportula and fultoportula, are structured by the penetration of the base silica layer and can be easily distinguished by the complex internal structure. However, the external structure of the two portula is somewhat simple and expressed by pore- and tube-like structures [2]. The external structure of the two portula is the diagnostic characteristic for distinguishing the two small-sized Thalassiosira species. The external opening of rimoportula in T. proschkinae is a pore-like opening and situated between the loculate areolae, whereas that in T. spinulata is a conspicuous tube. The marginal fultoportula of the two small-sized Thalassiosira species have a tube-like opening, but the size of the external tube is different: the external tube of T. proschkinae is small and inconspicuous, whereas that of T. spinulata is large and remarkable.
The satellite pore of the marginal fultoportulae was a fairly stable morphological character in the order Thalassiosirales [19]. In the present study, T. spinulata had three satellite pores of marginal fultoportulae, it agreed to the previous description [14]. However, T. proschkinae had two satellite pores in marginal fultoportula and sometimes rarely three reported by Park et al. [14]. Despite of the number of satellite pore in marginal fultoportula in the thalassiosiroid diatoms is a stable characteristic, this structural variation in T. proschkinae is unusual and provided the additional morphological evidence to separate T. proschkinae from T. spinulata that had three satellite pores in marginal fultoportula continuously. When T. proschkinae had three satellite in marginal fultoportula like T. spinulata, it can be distinguished by the pore position around the strutted tube. Of the three satellite pores, two were parallel along the valve margin, and one was located differently: in T. proschkinae, the pore was positioned towards the valve face behind the strutted tube; in T. spinulata, it was located towards the valve mantle. When the valves were observed in the valve plane without any tilts, T. proschkinae seemed to have two satellites because the other pore was hidden behind the strutted tube, whereas, in T. spinulata, all three satellite pores were visible. The number and position of satellite pores of marginal fultoportula supported two species are morphological distinct.
Global distribution of the two species
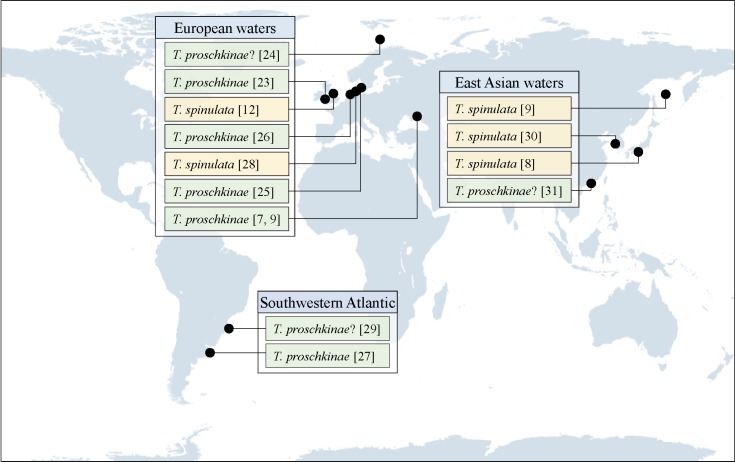
Since Makarova [10] suggested that T. spinulata was ranked on various T. proschkinae, most records worldwide for both the species have been mainly reported as T. proschkinae. Based on the above-mentioned morphological differences between T. proschkinae and T. spinulata, we re-identified the previous records on both the species (Table 2) and confirmed their distribution (Fig 12). Both the species have mainly been reported from the European waters, East Asian waters, and Southwestern Atlantic waters (Fig 12). Among the 14 records of T. proschkinae and T. spinulata, most records have reported T. proschkinae, but T. spinulata has also been reported from the areas where T. proschkinae occurred. Both the species have been mainly found from the estuaries characterized by low salinity (<30 psu) and high turbulence (Table 2). Although there were no descriptions regarding the co-occurrence of the two morphotypes simultaneously in the same area, we simultaneously found both the species in the estuaries of Korea. The co-occurrence of both the species indicated that they share habitat features and might have recently evolved as a sympatric speciation.
Table 2. Literatures on T. proschkinae/T. spinulata and re-identification based on the morphological criteria and their distribution.
| Literatures | Previous identification | Our interpretation | Distribution |
|---|---|---|---|
| Makarova et al. [8] (p. 922, pl. 1, fig 1–7) | T. proschkinae | T. proschkinae | Sea of Azov |
| Takano [9] (p. 33, figs 1B, 14–25) | T. spinulata | T. spinulata | Japanese coasts (Dokai Bay, Atsumi Bay, downstream of Smida River, Hamanako Lake) |
| Belcher & Swale [24] (p. 141, fig. 3) | T. proschkinae | T. proschkinae | British coasts |
| Makarova [10] (p. 80, pl. 51, figs 13–22) | T. proschkinae | T. proschkinae | Azov and Caspian Seas |
| T. proschkinae var. spinulata | T. spinulata | Okhotsk Sea | |
| Metzeltin & Witkowski, [25] (pl. 43) | T. proschkinae | uncertain (only LM micrographs) | Bear Island (North Atlantic) |
| Feibicke et al. [26] (p. 159, pls I and II) | T. proschkinae | T. proschkinae | The Schlei fjord of the Baltic Sea in the north of Germany |
| Muylaert & Sabbe, [27] (p. 110, figs 24–26) | T. proschkinae | T. proschkinae | The estuaries of the Schelde (Netherland) and the Elbe (Germany) |
| Hasle & Syvertsen, [11] (p. 84, pl. 13, figs a–c) | T. proschkinae | T. proschkinae | cosmopolitan |
| Sar et al. [28] (p. 215, figs 49, 50) | T. proschkinae | T. proschkinae | Pinamar and Villa Gesell, Argentina |
| Hoppenrath et al. [29] (p. 281, fig. 48) | T. proschkinae | T. spinulata | Helgoland, Germany |
| Tremarin et al. [30] (p. 1106, figs 24–27) | T. proschkinae | uncertain (only LM micrographs) | Paranaguá River, Brazil |
| Park et al. [31] (p. 75, pl. V, figs 27–32) | T. proschkinae | T. spinulata | Korean coasts |
| Martín-Cereceda & Cox [13] (p. 565, figs 1–23) |
Thalassiosira isolate | T. spinulata | British coast |
| Li et al. [32] (p.101, fig. 106) | T. proschkinae | T. proschkinae? | Zhelin Bay and Shantou off the Guangdong coast |
Fig 12. Biogeography of T. proschkinae and T. spinulata from 14 literature reports and the present study.
Comparison with morphologically similar taxa and identification of the phylogenetic position of the two small-sized Thalassiosira species
T. proschkinae and T. spinulata were characterized by a diameter of less than 10 μm, a rimoportula on valve face, and an internally ellipsoidal donut-shaped rimoportula. These morphological features were compared to the genera including small-sized Thalassiosira species having rimoportula on the valve face, such as Minidiscus, Shionodiscus, and Livingstonia.
The extremely small size of T. proschkinae and T. spinulata is a rare feature of some Thalassiosira species. Several species have less than 10 μm diameter: T. mala Takano, T. profunda (Hendey) Hasle, and T. exigua Fryxell & Hasle. When Takano [9] initially described T. spinulata, he already distinguished it from the small-sized Thalassiosira species by the rimoportula position. The Thalassiosira species that have the rimoportula on valve face were transferred to the genus Shinodiscus by Aleverson et al. [5], but several species (T. ferelineata Hasle & Fryxell, T. ignota Makarova, T. intrannula Herzig & Fryxell, T. maculata Fryxell & Johansen, and T. rosulata Takano) still remained in the genus Thalassiosira. These species differ from T. proschkinae and T. spinulata by larger size, no external extension of fultoportula, and the internally stalked rimoportula. Although further study is needed to confirm the taxonomic position of the species having the rimoportula on the valve face, this issue was not addressed in the present study and needs further investigation.
The genus Minidiscus is morphologically the most similar taxa to T. proschkinae and T. spinulata; it is characterized by species having diameter of less than 10 μm, a rimoportula on the valve face adjacent to a central fultoportula, and lack of a ring of marginal fultoportulae [18, 33]. T. proschkinae and T. spinulata share these morphological characters, as well as having an ellipsoidal doughnut-shaped rimoportula; however, the two small Thalassiosira species have a marginal ring of fultoportulae. Although the latter character does not coincide with the generic characteristics of Minidiscus, the positioning of the fultoportula in Minidiscus species is known to vary: M. chilensis Rivera has a fultoportula on the valve center [34]; M. comicus Takano has a fultoportula in midway between the valve center and margin [9]; and M. trioculatus has a varyingly positioned fultoportula depending on valve diameter [35]. Therefore, the absence of fultoportula in the valve margin of Minidiscus species represents a characteristic that differs among species and is not a generic characteristic. Except for the position of marginal fultoportula, T. proschkinae and T. spinulata are morphologically close to Minidiscus. In particular, the internally ellipsoidal-shaped rimoportula of T. proschkinae and T. spinulata is observed in all Minidiscus species.
Livingstonia palatkaensis Prasad is also characterized by a diameter of 10 μm, rimoportula on the valve face, and a ring of marginal fultoportulae [36] (Table 3). However, the absence of central fultoportula, the externally hood shape of marginal fultoportula, and the freshwater habitat of L. palatkaensis differentiates it from T. proschkinae and T. spinulata. Although Prasad & Nienow [36] did not compare L. palatkaensis with Minidiscus species, the shared characters between the two taxa is yet uncertain. The molecular sequence of L. palatkaensis might provide information to reveal the relationship between the two taxa.
Table 3. Comparison of the morphological characters of two small Thalassiosira species and the thalassiosiroid taxa having the rimoportula on valve face (Minidiscus, Livingstonia palatkaensis, and Shionodiscus) and Thalassiosira nordenskioeldii as the representative of the genus Thalassiosira.
| Morphological characters | Thalassiosira proschkinae | Thalassiosira spinulata | Minidiscus spp. | Livingstonia palatkaensis (monotype) | Shionodiscus spp. | Thalassiosira nordenskioeldii(type species) |
|---|---|---|---|---|---|---|
| Diameter | 2.5–8.5 | 2.5–5.0 | less than 10 μm | 2.5–6.0 | more than 10 μm | 10–50 |
| External valve structure | loculate areolae | streusel-like silicification | hyaline to areolae | loculate areolae | loculate areola | loculate areolae |
| Internal valve structure (cribra type) | individual | continuous radially with one to two row of pores | individual, continuous, unperforated | individual | individual | individual |
| Position of RP | valve face | valve face | valve face | valve face | valve face | valve margin |
| External opening of RP | areola | rimmed pore without any extension | rimmed pore without any extension | rimmed pore without any extension | pore without any extension | tube-like extension |
| Internal shape of RP | ellipsoidal ring without any stalk | ellipsoidal ring without any stalk | ellipsoidal ring without any stalk | ellipsoidal ring without any stalk | fan-shaped on stalk | asymmetric labium on stalk |
| Number of CFP | one | one | one | absent | one to several | one |
| Position of MFP | on junction between valve face and mantle | on junction between valve face and mantle | valve face to sub-margin, and absence | on junction between valve face and mantle | mantle | mantle |
| External opening of MFP | rimmed pore buried by siliceous ribs | tube-like extension | tube-like extension | hyaline hood | pore without any extension | tube-like extension |
| Number of satellite pores of MFP | two (three) | three | two to three | two | three to four | four |
| References | this study | this study | Quiroga & Chrétiennot-Dinet [12], Aké-Castillo et al.[37], Kaczmarska et al. [12, 35, 38] | Prasad & Nienow [36] | Alverson et al. [5] | Hasle [39] |
RP, rimoportula; CFP, central fultoportula; MFP, marginal fultoportula.
Shionodiscus also has a rimoportula on the valve face [5]. Although the position of rimoportula in Shionodiscus is similar to that in T. proschkinae and T. spinulata, the species belonging to this genus certainly differ from the two species by the internal structure of rimoportula, no fultoportula external extension without an outward extension, larger cell size, and external foramen shape [5] (Table 3). These features are the reason why T. spinulata and T. proschkinae remained in the genus Thalassiosira. Martín-Cereceda & Cox [13] presumed that T. proschkinae should probably be included in the genus Shionodiscus. Our morphological observation of T. proschkinae shows that the inclusion of T. proschkinae in Shionodiscus is not proper.
Multi-gene phylogeny for T. proschkinae and T. spinulata showed a close relationship to the other small-sized species, Minidiscus trioculatus that was phylogenetically related to T. nordenskioeldii and its allied species, T. pacifica and Detonula pumila in the previous phylogenetic analyses [4, 7]. With the addition of the sequence data of T. proschkinae and T. spinulata, the phylogenetic position of M. trioculatus is restructured as a sister clade to two small Thalassiosira species. Its relocation of M. trioculatus morphologically more proper than the relationship with T. nordenskioeldii and its allied species (Table 3). Taxon sampling in the phylogenetic analysis has emphasized as an important issue to improve the resolution towards the natural classification of diatom [40, 41]. The broad concept of the genus Thalassiosira still involves the ambiguous taxa as Thalassiosira [14]. Many Thalassiosira species are not assigned phylogenetic position, but their sequence data have not yet been analyzed. Recently, Park et al. [7] revised the genus Bacterosira by transferring it to T. constricta. Such studies from phylogenetically ambiguous species are needed to resolve the phylogenetic chaos of the order Thalassiosirales.
The morphological similarities and molecular sequences data between the two small-sized Thalassiosira and Minidiscus suggested that both the species should be transferred to Minidiscus. The genus Minidiscus was established to accommodate the species formerly known to Coscinodiscus trioculatus by Hasle [33]. She focused on the lack of the marginal fultoportulae and separated C. trioculatus from the genus Thalassiosira. Since the establishment of the genus, 10 Minidiscus species have been additionally described based on the genus concept by Hasle [33], namely, the valve size, fultoportula position on the valve, and hyaline margin. In addition, we noticed the similarity of rimoportula structure in Minidiscus species and it is proper to define the genus concept for Minidiscus. In conclusion, we transferred T. proschkinae and T. spinulata to the genus Minidiscus and renamed them Minidiscus proschkinae (Makarova) J.S. Park & J.H. Lee comb. nov. and Minidiscus spinulatus (Takano) J.S. Park & J.H. Lee comb. nov., respectively, and emended the description of the genus Minidiscus.
Minidiscus proschkinae (Makarova) comb. nov. J.S. Park & J.H. Lee. Basionym: Thalassiosira proschkinae Makarova 1979, p. 922, pl. 1, Figs 1–7.
Minidiscus spinulatus (Takano) comb. nov. J.S. Park & J.H. Lee. Basionym: Thalassiosira spinulata Takano 1981, p. 33, Fig 1B, 14–25. Synonym: Thalassiosira proschkinae var. spinulata (Takano) Makarova 1988
Minidiscus Hasle emended Park. Cell in solitary, chain or clogged. Cell diameter less than 10 μm. Valve face flat to convex. Valve ornamentation various, loculate areolae, hyaline without any perforation, unlinked ribs. One central fultoportula; one to ten fultoportulae on valve face to valve margin. All fultoportulae externally opened with short tube and surrounded by siliceous hyaline. A single rimoportula adjacent to a central fultoportula, having an externally circular or elliptical small pore and an internally ellipsoidal ring of sessile lips.
Supporting information
The multi-gene sequences of three strains, T. proschkinae SMDC305, T. spinulata SMDC050 and T. spinulata SMDC303, manually aligned based on the previous published 68 multi-gene alignment sequences [3, 7].
(PHY)
Acknowledgments
We would like to thank Prof. H.-U. Dahms for English editing and critical comments on the manuscript. The diatom samples including T. proschkinae and T. spinulata were provided from the Library of Maine Samples, Korea Institute of Ocean Sciences & Technology, South Korea. The microscope images were also obtained from the Library of Maine Samples, Korea Institute of Ocean Sciences & Technology, South Korea.
Data Availability
Sequences are available within GenBank under accession numbers KY912616-KY912627.
Funding Statement
This work was supported by the KIOST projects ‘‘Management and application of marine biotoxins’’ (PE99515). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guiry MD, Guiry GM. AlgaeBase. World-wide electronic publication Galway: National University of Ireland; 2017. [cited 2017 06 April]. [Google Scholar]
- 2.Kaczmarska I, Beaton M, Benoit AC, Medlin LK. Molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula. J Phycol. 2006;42(1):121–138. doi: 10.1111/j.1529-8817.2006.00161.x [DOI] [PubMed] [Google Scholar]
- 3.Alverson AJ, Jansen RK, Theriot EC. Bridging the Rubicon: Phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms. Mol Phylogenet Evol. 2007;45(1):193–210. doi: 10.1016/j.ympev.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Alverson AJ, Beszteri B, Julius ML, Theriot EC. The model marine diatom Thalassiosira pseudonana likely descended from a freshwater ancestor in the genus Cyclotella. BMC Evol Biol. 2011;11(1):125–133. doi: 10.1186/1471-2148-11-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alverson AJ, Kang SH, Theriot EC. Cell wall morphology and systematic importance of Thalassiosira ritscheri (Hustedt) Hasle, with a description of Shionodiscus gen. nov. Diatom Res. 2006;21(2):251–262. http://dx.doi.org/10.1080/0269249X.2006.9705667 [Google Scholar]
- 6.Stachura-Suchoples K, Williams DM. Description of Conticribra tricircularis, a new genus and species of Thalassiosirales, with a discussion on its relationship to other continuous cribra species of Thalassiosira Cleve (Bacillariophyta) and its freshwater origin. Eur J Phycol. 2009;44(4):477–486. http://dx.doi.org/10.1080/09670260903225431 [Google Scholar]
- 7.Park JS, Alverson AJ, Lee JH. A phylogenetic re-definition of the diatom genus Bacterosira (Thalassiosirales, Bacillariophyta), with the transfer of Thalassiosira constricta based on morphological and molecular characters. Phytotaxa. 2016;245(1):1–16. http://dx.doi.org/10.11646/phytotaxa.245.1.1 [Google Scholar]
- 8.Makarova I, Genkal S, Kuzmin G. [Species of the genus Thalassiosira Cl. (Bacillariophyta), found in continental water bodies of the USSR]. Botanicheskii Zhurnal. 1979;64:921–927. [Google Scholar]
- 9.Takano H. New and rare diatoms from Japanese marine waters-VI. Three new species in Thalassiosiraceae. Bull Tokai Reg Fish Res Lab. 1981;105:31–43. [Google Scholar]
- 10.Makarova IV. Diatomovye vodorosli morei SSSR: Rod Thalassiosira Cl. Leningrad: Akademiya NAUK SSSR; 1988. [Google Scholar]
- 11.Hasle GR, Syvertsen EE. Marine diatoms In: Tomas CR, editor. In: Tomas CR (ed), Identifying marine diatoms and dinoflagellates Academic Press, Inc. Book. San Diego, California: Academic Press; 1997. p. 5–385. [Google Scholar]
- 12.Quiroga I, Chrétiennot-Dinet M-J. A new species of Minidiscus (Diatomophyceae, Thalassiosiraceae) from the eastern English Channel, France. Bot March 2004;47(4):341–348. doi: 10.1515/bot.2004.040 [Google Scholar]
- 13.Martín-Cereceda M, Cox EJ. Morphological variation in a small Thalassiosira species (Bacillariophyta) under different culture regimes. Bot March 2011;54(6):563–574. https://doi.org/10.1515/BOT.2011.063 [Google Scholar]
- 14.Park JS, Jung SW, Lee SD, Yun SM, Lee JH. Species diversity of the genus Thalassiosira (Thalassiosirales, Bacillariophyta) in South Korea and its biogeographical distribution in the world. Phycologia. 2016;55(4):403–423. https://doi.org/10.2216/15-66.1 [Google Scholar]
- 15.Hasle GR. The valve processes of the centric diatom genus Thalassiosira. Nytt Magasin for Botanikk. 1968;15:193–201. [Google Scholar]
- 16.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross R, Cox EJ, Karayeva N, Mann D, Paddock T, Simonsen R, et al. An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia, Beih. 1979;64:513–533. [Google Scholar]
- 18.Round F, Crawford R, Mann D. The Diatoms. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 19.Theriot E, Serieyssol K. Phylogenetic systematics as a guide to understanding features and potential morphological characters of the centric diatom family Thalassiosiraceae. Diatom Res. 1994;9(2):429–450. doi: 10.1080/0269249x.1994.9705318 [Google Scholar]
- 20.Park JS, Lee JH. Description of the pseudocryptic species Conticribra weissflogiopsis sp. nov. (Thalassiosirales, Bacillariophyta) isolated from brackish waters in Korea, based on its cingulum structure and molecular analysis. Phytotaxa. 2014;191(1):115–128. http://dx.doi.org/10.11646/phytotaxa.191.1.7 [Google Scholar]
- 21.Stamatakis A. RAxML version 8: a tool for phylogenetic anaylsis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasle GR, Lange CB. Freshwater and brackish water Thalassiosira (Bacillariophyceae): taxa with tangentially undulated valves. Phycologia. 1989;28(1):120–135. doi: 10.2216/i0031-8884-28-1-120.1 [Google Scholar]
- 23.Sar EA, Sunesen I, Lavigne AS, Lofeudo S. Thalassiosira rotula, a heterotypic synonym of Thalassiosira gravida: morphological evidence. Diatom Res. 2011;26(1):109–119. doi: 10.1080/0269249x.2011.573691 [Google Scholar]
- 24.Belcher JH, Swale EMF. Notes on some small Thalassiosira species (Bacillariophyceae) from the plankton of the lower thames and other British Estuaries (identified by transmission electron microscopy). Br Phycol J. 1986;21(2):139–145. doi: 10.1080/00071618600650161 [Google Scholar]
- 25.Metzeltin D, Witkowski A. Diatomeen der Bären-Insel. Süsswasser und marine Arten In: Lange-Bertalot H, editor. Annotated diatom micrographs. 4 Königstein: Koeltz Scientific Books; 1996. [Google Scholar]
- 26.Feibicke M, Wendker S, Geissler U. Thalassiosira proschkinae Makarova: a contribution to its morphology and autecology. Nova Hedwigia, Beih. 1990;100:155–169 [Google Scholar]
- 27.Muylaert K, Sabbe K. The Diatom Genus Thalassiosira (Bacillariophyta) in the Estuaries of the Schelde (Belgium/The Netherlands) and the Elbe (Germany). Bot March 1996;39:103–116. https://doi.org/10.1515/botm.1996.39.1-6.103 [Google Scholar]
- 28.Sar EA, Sunesen I, Castaños C. Marine diatoms from Buenos Aires coastal waters (Republica Argentina). I. Thalassiosiraceae. Nova Hedwigia. 2001;73(1):199–228. [Google Scholar]
- 29.Hoppenrath M, Beszteri B, Drebes G, Halliger H, Van Beusekom JEE, Janisch S, et al. Thalassiosira species (Bacillariophyceae, Thalassiosirales) in the North Sea at Helgoland (German Bight) and Sylt (North Frisian Wadden Sea)—a first approach to assessing diversity. Eur J Phycol. 2007;42(3):271–288. http://dx.doi.org/10.1080/09670260701352288 [Google Scholar]
- 30.Tremarin PI, Ludwig TAV, Moreira Filho H. Thalassiosirales (Diatomeae) do rio Guaraguaçu, Bacia Litorânea, PR, Brasil. Acta Bot Brasilica. 2008;22(4):1101–1113. http://dx.doi.org/10.1590/S0102-33062008000400021 [Google Scholar]
- 31.Park JS, Jung SW, Lee JH. A Study on the Fine Structure of the Marine Diatoms of Korean Coastal Waters—Genus Thalassiosira 4. Algae. 2009;24(2):67–77. https://doi.org/10.4490/algae.2009.24.2.067 [Google Scholar]
- 32.Li Y, Zhao Q, Lü S. The genus Thalassiosira off the Guangdong coast, South China Sea. Bot March 2013;56(1):83–110. https://doi.org/10.1515/bot-2011-0045 [Google Scholar]
- 33.Hasle GR. Thalassiosiraceae, a new diatom family. Norw J Bot. 1973;20:67–69. [Google Scholar]
- 34.Rivera P, Koch P. Contributions to the diatom flora of Chile II. In: Mann DG, editor. Proceedings of the Seventh International Diatom Symposium, Philadelphia, August 22–27, 1982 Koenigstein: Koeltz Science Publishers; 1984. p. 279–298.
- 35.Kaczmarska I, Lovejoy C, Potvin M, Macgillivary M. Morphological and molecular characteristics of selected species of Minidiscus (Bacillariophyta, Thalassiosiraceae). Eur J Phycol. 2009;44(4):461–475. doi: 10.1080/09670260902855873 [Google Scholar]
- 36.Prasad AKSK, Nienow JA. Livingstonia (Thalassiosirales, Bacillariophyta), a new genus of fultoportulate centric diatoms from an Atlantic coastal plain river in Florida, southeastern United States. Phycologia. 2011;50(3):264–280. doi: 10.2216/09-89.1 [Google Scholar]
- 37.Aké-Castillo JA, Hernández-Becerril DU, del Castillo MEM, Bravo-Sierra E. Species of Minidiscus (Bacillariophyceae) in the Mexican Pacific Ocean. Cryptogamie Algol. 2001;22(1):101–107. https://doi.org/10.1016/S0181-1568(00)01051-5 [Google Scholar]
- 38.Kaczmarska I, Mather L, Luddington IA, Muise F, Ehrman JM. Cryptic diversity in a cosmopolitan diatom known as Asterionellopsis glacialis (Fragilariaceae): Implications for ecology, biogeography, and taxonomy. American Journal of Botany. 2014;101(2):267–286. doi: 10.3732/ajb.1300306 [DOI] [PubMed] [Google Scholar]
- 39.Hasle GR. Some Thalassiosira species with one central process (Bacillariophyceae). Norw J Bot. 1978;25:77–110. [Google Scholar]
- 40.Alverson AJ, Theriot EC. Taxon sampling and inferences about diatom phylogeny. J Phycol. 2003;39:1 doi: 10.1111/j.0022-3646.2003.03906001_1.x [Google Scholar]
- 41.Theriot EC, Ashworth M, Ruck E, Nakov T, Jansen RK. A preliminary multigene phylogeny of the diatoms (Bacillariophyta): challenges for future research. Plant Ecol Evol. 2010;143(3):278–296. doi: 10.5091/plecevo.2010.418 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The multi-gene sequences of three strains, T. proschkinae SMDC305, T. spinulata SMDC050 and T. spinulata SMDC303, manually aligned based on the previous published 68 multi-gene alignment sequences [3, 7].
(PHY)
Data Availability Statement
Sequences are available within GenBank under accession numbers KY912616-KY912627.