Abstract
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, presents a variable clinical course, varying from asymptomatic to serious debilitating pathologies with cardiac, digestive or cardio-digestive impairment. Previous studies using two clonal T. cruzi populations, Col1.7G2 (T. cruzi I) and JG (T. cruzi II) demonstrated that there was a differential tissue distribution of these parasites during infection in BALB/c mice, with predominance of JG in the heart. To date little is known about the mechanisms that determine this tissue selection. Upon infection, host cells respond producing several factors, such as reactive oxygen species (ROS), cytokines, among others. Herein and in agreement with previous data from the literature we show that JG presents a higher intracellular multiplication rate when compared to Col1.7G2. We also showed that upon infection cardiomyocytes in culture may increase the production of oxidative species and its levels are higher in cultures infected with JG, which expresses lower levels of antioxidant enzymes. Interestingly, inhibition of oxidative stress severely interferes with the intracellular multiplication rate of JG. Additionally, upon H2O2-treatment increase in intracellular Ca2+ and oxidants were observed only in JG epimastigotes. Data presented herein suggests that JG and Col1.7G2 may sense extracellular oxidants in a distinct manner, which would then interfere differently with their intracellular development in cardiomyocytes.
Author summary
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, presents a variable clinical course, varying from asymptomatic to serious debilitating pathologies with cardiac, digestive or cardio-digestive impairment. It has been suggested that parasite differential tissue tropism is responsible for the development of the distinct clinical forms. Differences in parasite tissue tropism have been shown previously, using mixed infections in mice with two distinct T. cruzi populations, Col1.7G2 (T. cruzi I) and JG (T. cruzi II). In these infections hearts were preferentially colonized by JG. Increased JG adaptation to cardiac muscle was later confirmed in infection studies using isolated cardiomyocytes, where it was shown that selection was dependent on parasite intracellular development. However the mechanisms that determined this differential parasite intracellular growth was not described. Here we investigated whether host cell response upon T. cruzi infection was able to modulate parasite multiplication rate inside cells. We showed that, upon infection, cardiomyocytes increase the production of oxidative species, especially in cultures infected with JG and inhibition of oxidative stress severely interfered with the intracellular multiplication rate of JG. Data obtained suggests that JG and Col1.7G2 may sense extracellular oxidants in a distinct manner, which would enable JG to develop better inside cardiomyocytes.
Introduction
Chagas disease, caused by the protozoan Trypanosoma cruzi, is an important health problem affecting about 6 to 7 million people worldwide [1]. Infection in man is defined by two distinct clinical phases. The acute phase, corresponding to the initial period of infection, is characterized by high parasitemia and tissue parasitism, followed by the chronic phase of the infection, which persists throughout the life of the host and is characterized by low tissue parasitism as well as parasitemia [2]. Chronic infection has a variable clinical course, ranging from asymptomatic cases (indeterminate form), to severe clinical conditions with heart (chagasic cardiomyopathy) and / or digestive tract (megacolon or megaesophagus) maladies. In patients with cardiac and / or digestive disorders, symptoms may appear between 10 and 30 years after initial infection and are due to the persistence of parasites in specific tissues, such as cardiac and / or smooth muscle, with the development of an intense inflammatory process, deleterious to the organ (reviewed by [3]).
Chagas disease clinical variability is well known to depend not only on genetic factors of the parasite, whose population structure is quite variable, but also on genetic factors of the host [4–6]. Previous studies conducted by our group showed that distinct parasite populations are found in different organs of infected patients [7], reinforcing data on the existence of a differential tissue tropism, probably related to the development of the diverse clinical forms [8–10]. Later, we studied this tissue tropism by performing mixed infections in BALB/c mice with two clonal populations of T. cruzi, Col1.7G2 (T. cruzi I) and JG (T. cruzi II), and detection of parasites directly from infected tissues. A predominance of Col1.7G2 was found in the rectum, diaphragm, esophagus and blood while JG was predominant in the cardiac muscle [11]. Later, we showed that this tissue tropism could be influenced by the genetic background of the host, where mice with the same MHC haplotype presented the same selection profile of T. cruzi in different tissues [12, 13]. In vitro studies using infection in cultures of cardiac explants or primary cardiomyocytes, with Col1.7G2 and JG, indicated that tissue selection occurs due to the direct interaction between parasite and host cell, without direct influence of the host immune system [13, 14]. In these studies, a more accelerated and efficient intracellular development of JG with respect to clone Col1.7G2 was observed in explants and cultures of cardiomyocytes isolated from BALB/c, suggesting that not only invasion, but also and mainly intracellular multiplication is important to tissue selection. Additionally, it was shown that this behavior profile was dependent on the cell type studied [14]. These findings reinforce that not only the parasite, but also the host cell response to infection is involved in the differential tissue tropism of T. cruzi. However, the mechanisms that define this selection are still poorly understood.
During cell infection, infective trypomastigotes adhere to the surface of the host cell, being internalized in parasitophorous vacuoles, formed by lysosomal membrane [15]. Trypomastigote later escape from the vacuole to the cytoplasm of the cell and turn into the amastigote replicative form, colonizing the host cell [16–18]. Thus, during cell infection parasite passes through different environments, which can directly or indirectly influence its behavior within the cell. Data from the literature show that infected cells are able to respond to infection by activating several genes, through the production of cytokines and reactive oxygen species (ROS), which could interfere with parasite intracellular behavior [19–24]. Here we investigate how stress responses mediated by oxidants in cardiomyocyte may influence infection by T. cruzi clonal populations, JG and Col1.7G2, interfering with their intracellular multiplication rates.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Brazilian National Council of Animal Experimentation (http://www.cobea.org.br/) and Federal Law 11.794 (October 8, 2008). The institutional Committee for Animal Ethics of UFMG approved all the procedures used in this study. (CEUA/UFMG–Licenses 45/2009 and 261/2016)
Parasites and cells
Two clonal populations of Trypanosoma cruzi were used, Col1.7G2 and JG, belonging to T. cruzi lineages I and II, respectively. JG strain was originally isolated in 1995 by Professor Eliane Lages-Silva (UFTM) from a chronic patient with megaesophagus. Col.1.7G2 is a clone from Colombian strain, which was originally isolated by Federici in 1969 from a chronic patient with cardiac disorders. Both T. cruzi populations were previously analyzed and characterized as monoclonal, through the analysis of the eight microsatellite loci according to previously described methodology [25].
Epimastigote forms of Col1.7G2 and JG were maintained in LIT (Liver Infusion Tryptose) medium containing 20 mg/mL of hemin and supplemented with 10% Fetal Bovine Serum and 1% Penicillin/Streptomycin, in T-25 cm3 bottles, in a 28°C incubator, and subcultured every two days [26].
Tissue culture trypomastigotes (TCTs) from Col1.7G2 and JG were obtained from the supernatant of infected LLC-MK2 monolayers and purified as described previously [27].
Primary cultures of cardiomyocytes were prepared from hearts of BALB/c mice neonates (0–2 days), according to protocol previously described [28]. 2x105 purified cardiomyocytes were plated in each well of a 24 well pate, containing a 13 mm circular glass coverslips, and maintained in a 37°C CO2 incubator for 72 hours prior to infection. Alternatively, 5x104 cells were plated in each well of a XF-24 cell culture microplate (Seahorse Bioscience) and maintained in a 37°C CO2 incubator for 120 hours prior to infection.
Cultures of human cardiomyocytes (Pluricardio) were prepared from the differentiation of induced pluripotent stem cells (iPSC) obtained from Pluricell Biotechnologies. Cells were thawed and plated after counting by trypan blue exclusion method at a confluence of 2x105 cells per well in a 24-well plate. Cell differentiation was performed according to the company’s procedure, in 37°C CO2 incubator. All reagents required for maintenance and differentiation of cultures (extracellular matrix and culture media) were provided by the company. Five days after plating, after complete cell differentiation, cultures were used for the infection experiments.
Immortalized embryonic fibroblasts were originally isolated from C57BL/6 mouse embryos and spontaneously immortalized in culture [29]. These cells were maintained in culture by consecutive passages in 25 cm 3 flasks in DMEM (GIBCO) culture medium supplemented with 10% FBS and 1% antibiotic (penicillin/streptomycin). For the infection assays, 8x104 cells were plated in each well of a 24-well plate containing a 13 mm circular glass coverslips and maintained at 37°C in a CO2 incubator.
Treatments
For some experiments, parasites (epimastigote stage) were previously treated with H2O2. H2O2 solutions were prepared daily assuming an extinction coefficient of 81M-1 cm-1 at 230 nm. For this, epimastigotes were subjected to treatment with different concentrations of H2O2 (5 to 100 μM) for 6 days when the number of parasites was counted. Since 30 μM of H2O2 was the highest concentration in which JG and Col1.7G2 growth was still observed we used this concentration to perform the experiments. Treatment was performed by incubating 107 epimastigotes in 1mL PBS, pH 7.3 in the absence (control) or presence of 30 μM H2O2 at 28°C, for 30 minutes. Parasites were then washed and re-suspended in specific medium according to the experiment to be performed.
For some experiments, cardiomyocyte cultures were treated with catalase (Catalase-PEG C4963—SIGMA) at a final concentration of 40U/mL. Treatment was initiated 2 hours prior to infection and maintained during and after parasite exposure.
Cell infection assay
Cardiomyocyte cultures pre-treated or not with catalase, as well as fibroblast cultures were exposed to purified JG or Col1.7G2 TCTs re-suspended in high-glucose DMEM at a multiplicity of infection (MOI) of 50. The infection was performed for 40 minutes at 37°C. After infection, monolayers were washed at least four times with PBS and either fixed with 4% (w/v) paraformaldehyde in PBS (0h–to determine the rate of parasite invasion) or re-incubated for 4, 8, 12, 24, 48 or 72 hours prior to fixation and processing for immunofluorescence assays (to determine parasitophorous vacuole escape– 4, 8 and 12 h or parasite intracellular multiplication rates– 24, 48 and 72 h). Alternatively, after parasite exposure (40 minutes at 37°C), cultures were washed and re-incubated for 48 and 72 hours before ROS measurements.
Immunofluorescence
After treatment, infection and fixation, coverslips containing attached cells were washed with PBS, incubated for 20 minutes with PBS containing 2% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich) and processed for an inside/outside immunofluorescence invasion assay as described previously [27]. Briefly, cells were fixed and extracellular parasites were immune-stained using a 1:500 dilution of rabbit anti-T. cruzi polyclonal antibodies in PBS containing 2% (w/v) BSA (PBS/BSA) followed by labeling with Alexa Fluor-546 conjugated anti-rabbit IgG antibody (Invitrogen).
For evaluation of parasitophorous vacuole escape, after extracellular parasite staining, cells were permeabilized using a solution containing 2% (w/v) BSA and 0.5% (v/v) saponin (Sigma-Aldrich) in PBS (PBS/BSA/saponin) for 20 minutes. Host cell lysosomes were then immunostained using a 1:50 dilution of rat anti-mouse LAMP-1 hybridoma supernatant (1D4B; Developmental Studies Hybridoma Bank, USA) in PBS/BSA/saponin for 45 minutes followed by labeling with Alexa Fluor-488 conjugated anti-rat IgG antibody (Invitrogen), as described previously [30]. Subsequently, DNA from both host cells and parasites were stained for 1 min with 10 μM of DAPI (Sigma-Aldrich), mounted on glass slide and examined on an Olympus BX51, Zeiss, Apotome or Nikon Eclipse Ti.
Evaluation of oxidant production by infected cells
A general measure of oxidant formation were performed using CM-H2DCFDA (5- (and-6) -chloromethyl-2', 7'-dichlorodihydrofluorescein diacetate, acetyl ester—Molecular Probes) probe, which fluoresces upon oxidation. For this, cell cultures, 48 and 72 hours post infection with JG or Col1.7G2, were washed once with PBS and exposed to CM-H2DCFDA at a final concentration of 10 μM in PBS. Immediately after the addition of CM-H2DCFDA, the plate was read on a Varioskan Flash (Thermo Scientific) at 37°C for monitoring the probe’s oxidization rate, with excitation and emission wavelengths of 485 and 520 nm, respectively. The data were analyzed using the program SkanIt Software 2.4.5. The probe oxidation curves were used to calculate the slope and are expressed as Relative Fluorescence Units (RFU)/min.
Cell respiration assay
Neonatal cardiomyocyte cultures plated in an XF-24 well culture microplate (Seahorse Bioscience) were infected with TCTs from JG or Col1.7G2, as described above for 48 hours. One hour before the experiment, media was replaced with unbuffered Dulbecco’s Modified Eagle Medium (DMEM, pH 7.4) supplemented with 4 mM L-glutamine, 5 mM glucose and 10 mM Pyruvate (Gibco). Olygomycin (5 μM, an inhibitor of ATP synthase (complex V)), Carbonyl Cyanide-p-trifluoromethoxyphenylhydrazone (FCCP 5 μM, uncoupling agent) and a mix of antimycin A (AA, complex III inhibitor) and rotenone (Complex I inhibitor) at a final concentration of 5 and 1 μM, respectively were injected sequentially through ports in the seahorse flux pack cartridges. Oxygen consumption rates (OCR) were analyzed for control and infected cardiomyocytes [31]. At least 5 replicates of each condition per plate and three independent replicates were analyzed. The non-mitochondrial oxygen consumption obtained after AA/Rotenone addition were subtracted to all OCR values. The mitochondrial respiratory control index (RCI) was calculated as the OCR value with FCCP divided by the OCR value with oligomycin (FCCP/Oligomycin).
Quantification of antioxidant enzymes from parasites
For the identification and quantification of anti-oxidant enzymes produced by the different T. cruzi populations, 1x107 epimastigote forms previously incubated or not with 30 μM H2O2 (30 min) were fixed with 3.7% (v/v) formaldehyde in PBS, centrifuged at 12,000 g at room temperature (RT), resuspended in a solution containing 0.1% (v/v) Triton in PBS and incubated for 30 minutes at RT for permeabilization. After permeabilization, samples were centrifuged and incubated overnight at 4°C with a 1/100 dilution of each of the rabbit polyclonal antibodies raised towards the different antioxidant enzymes analyzed (Ascorbate peroxidase, APX; Mitochondrial Peroxiredoxin, MPX; Trypanothione reductase, TR; Trypanothione synthetase, TS; mitochondrial iron superoxide dismutase A, FeSOD-A and cytosolic iron superoxide dismutase B, FeSOD-B), in PBS containing 0.1% (w/v) BSA and 0.5% (v/v) Tween (PBS/BSA/Tween). After this, samples were centrifuged, washed in PBS/BSA/Tween and incubated for 90 minutes with Alexa-Fluor 488-labeled anti-rabbit IgG secondary antibody (anti-APX, MPX, TR and TS) or anti-mouse IgG (anti-SODA and SODB) diluted 1:100 in PBS/BSA/Tween. After incubation with the secondary antibody, the samples were centrifuged, washed with PBS/BSA/Tween, re-suspended in the same solution and read on BD FACSCan or BD FACSCalibur flow cytometer. Acquired data were analyzed using the BD CellQuest Pro 6.0 or FlowJo program.
Determination of intracellular calcium concentrations, and superoxide production in epimastigotes
For intracellular calcium measurements, 5x107 parasites/mL were loaded with 5 μM fura-2AM at 28–30°C in fura buffer (116 mM NaC1, 5.4 mM KC1, 0.8 mM MgSO4, 5.5 mM glucose, 1 mM CaC12, and 50 mM Hepes, pH 7.0). After 1h, cells were washed and re-suspended in PBS and exposed or not to H2O2-treatment as described earlier. Afterwards cells were washed once in PBS, re-suspended in fura buffer and fluorescence determined in 107 cells/mL in fura buffer in a Hitachi F2500 fluorescence spectrophotometer with continuous stirring (excitation at 340 and 380 nm and emission at 510 nm) [32, 33].
For determination of epimastigote oxidant production in control and/or after H2O2 treatment, parasites (3 x108/mL) were loaded in Krebs-Henseleit buffer (KH buffer, 15 mM NaCO3, 5 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4, 1.5 mM NaH2PO4) at 28°C with 5 μM MitoSOX (3,8-phenanthridinediamine,5-(6-triphenylphosphoniumhexyl)-5,6-dihydro-6- phenyl, Molecular Probes). After 10 min of incubation with the probe, the cells were washed and re-suspended in KH buffer. Cells were then incubated with H2O2 for 30 min and washed, as described. The detection of oxidized MitoSOX (oxMitoSOX) in 5x107 cells/mL was performed in this buffer in the presence of 40 μM digitonin and 5 mM succinate to determine the production of these species by the mitochondrial respiratory chain. The fluorescence was detected using a Cytation 5 microplate reader with excitation and emission wavelengths of 510 and 580 nm, respectively [34].
Results
JG develops better in BALB/C neonatal primary cardiomyocyte cultures
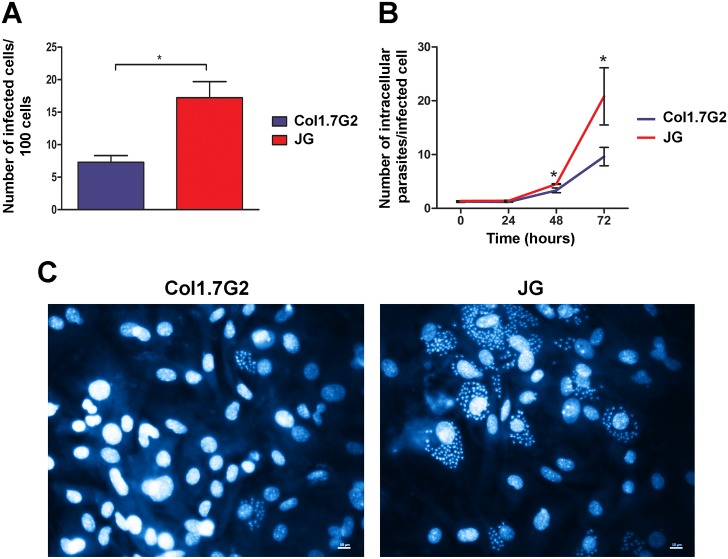
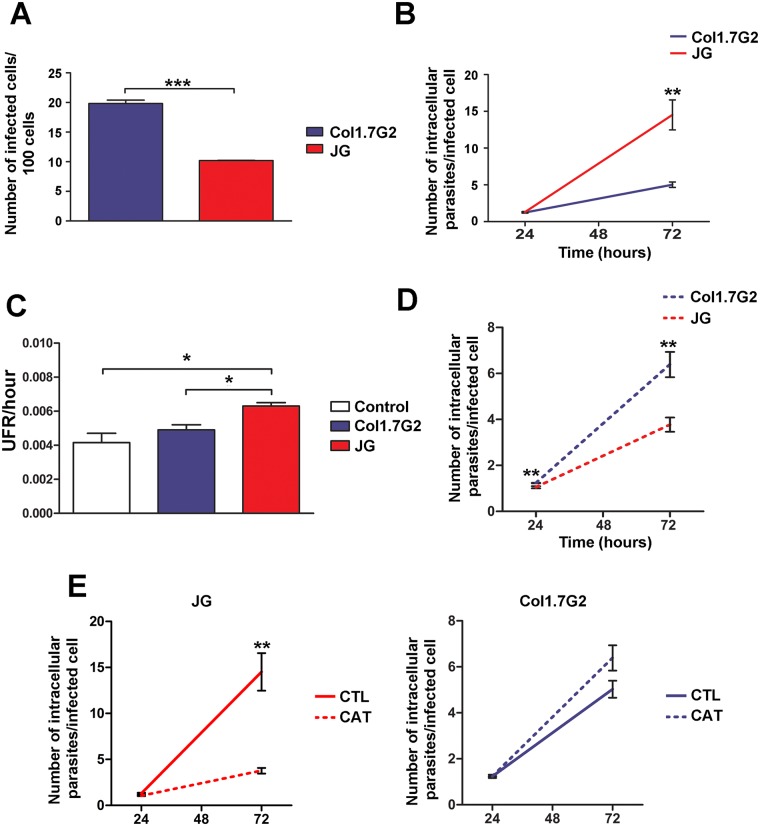
In order to confirm previous results obtained from primary embryonic cardiomyocyte cultures infected with JG or Col1.7G2 [14], cultures obtained from neonatal BALB/c mice were submitted to infection with the same T. cruzi populations and invasion and intracellular multiplication rates were analyzed. A different behavior regarding cell invasion was observed. JG infection rate was in this case higher than Col1.7G2. The number of infected cells was about 4.5 times higher for cultures exposed to JG, when compared to cultures infected with Col1.7G2 (Fig 1A). Intracellular multiplication rates, on the other hand, were in accordance with previous results from Andrade and colleagues (2010) [14]. Seventy-two hours post infection we observed that the number of intracellular parasites in JG infected cultures were higher than the number of intracellular parasites in cultures infected with Col1.7G2 (Fig 1B). 72 hours after exposure to the parasites, cultures infected with JG showed an approximately 2.5-fold increase in the number of intracellular parasites relative to those cultures infected with Col1.7G2 (Fig 1B). Fig 1C shows representative images of the number of intracellular parasites in cardiomyocyte 72 hours post invasion with each of the parasite populations. These results indicate that independently of the invasion rate, JG shows a better intracellular development in these cells when compared to Col1.7G2 (as noted by the slope of the curve).
Fig 1. (A-B) Quantification of invasion and intracellular multiplication rates of Col1.7G2 and JG in primary cultures of cardiomyocytes.
Primary cultures of cardiomyocytes from neonatal mice were exposed to Col1.7G2 or JG and the number of infected cells per 100 cells (A), as well as the number of intracellular parasites per infected cell (B), were evaluated in order to determine the invasion and intracellular multiplication rates, respectively. The data represent the mean of triplicates ± the standard error of the mean (SEM). Asterisks indicate statistically significant differences (* p≤0.05—Student's t-test). (C) Representative images of cell infection in primary cultures of cardiomyocytes from BALB/c neonatal mice, 72 hours after exposure to JG or Col1.7G2 (40X). The nuclear marker, DAPI, was used to identify the genetic material of both the parasite and the host cell. The scale bar corresponds to 10 μm.
JG and Col1.7G2 show the same parasitophorous vacuole escape kinetics
As it is known, when T. cruzi trypomastigotes invade the host cell it first resides in a parasitophorous vacuole, formed by lysosomal membrane, and later escapes from this vacuole falling into the host cell cytosol, where it transforms into the amastigote replicative form. Therefore the kinetics of parasitophorous vacuole escape could alter the transformation of internalized trypomastigotes into amastigote forms and consequently parasite intracellular development. In order to determine the kinetics of parasitophorous vacuole escape for JG and Col1.7G2 in primary neonatal cardiomyocyte cultures, infected cultures were washed and fixed at 0, 4, 8 or 12 hours after exposure to the parasites, as described. Parasites labeled with DAPI and lacking anti-T. cruzi antibody labeling were considered as intracellular parasites. To evaluate the proportion of intracellular parasites associated with the parasitophorous vacuole, the cells were also labeled with anti-LAMP-1 antibody, a protein present on the lysosomal membrane. Thus, intracellular parasites that co-localized with this marker were counted as inside the vacuole, the other intracellular parasites were considered free in the cytoplasm.
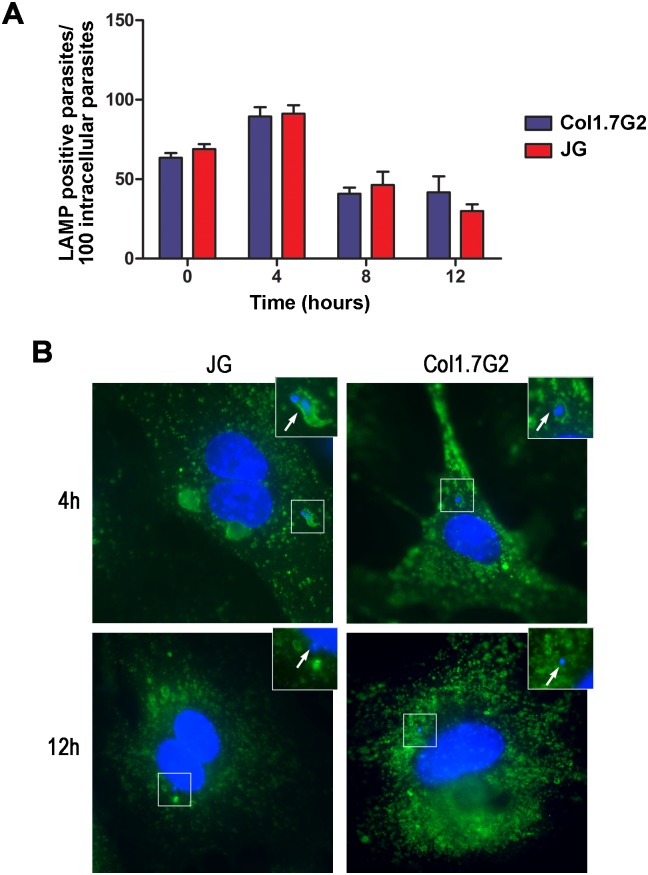
The number of parasites associated with the lysosomal marker, LAMP-1, shortly after (0h), 4, 8 and 12 hours after cell exposure to parasites is shown in Fig 2. No significant difference was observed in the kinetics of vacuole escape between JG and Col1.7G2. Soon after the invasion, for both T. cruzi populations, around 50% of the internalized parasites are associated with LAMP (Fig 2A). Four hours after parasite removal, the number of parasites associated with LAMP reaches 100%. This is due to the fact that the abundance of lysosomal markers associated to the vacuole increases in the first moments after the invasion, facilitating its visualization. Later, 8 to 12 hours post invasion, the number of parasites associated with LAMP starts to drop for both JG and Col1.7G2 infections, indicating that the parasites are escaping from the vacuole into the cytosol (Fig 2A). Representative images of parasites inside (4 hours) or outside the vacuole (12 hours), for both JG and Col1.7G2, are shown in Fig 2B.
Fig 2. Parasitophorous vacuole escape rate for T. cruzi clonal populations, Col1.7G2 and JG, upon infection in primary mouse cardiomyocytes.
(A) The graph shows the proportion of intracellular parasites associated with LAMP-1, a lysosomal marker, over the time of infection. The data represent the mean of triplicates ± the standard error of the mean (SEM). The data shown are representative of three individual experiments. (Student's t-test). (B) Representative images of intracellular parasites in primary cultures of cardiomyocytes from BALB/c neonatal mice, inside or outside the parasitophorous vacuole, 4 and 12 hours (respectively) after exposure to JG or Col1.7G2 (100X). The nuclear marker, DAPI, was used to identify the genetic material of both the parasite and the host cell and anti-LAMP-1, a lysosomal marker, was used to identify parasites inside vacuole. The scale bar corresponds to 10 μm.
JG-infected primary cardiomyocyte cultures produce more reactive oxygen species
In order to identify other possible factors that could account for the differential growth rate of JG and Col1.7G2 in neonatal cardiomyocytes, we evaluated the levels of production of oxidants in these cells upon infection with the two T. cruzi clonal populations. It is known that cardiomyocyte infection by the parasite can induce the production of ROS, which can modulate the intracellular development of the parasite [35, 36]. Analysis of the oxidant levels produced upon infection with JG and Col1.7G2 in cardiomyocyte cultures was done using the CM-H2DCFDA probe added to infected cultures, as described in material and methods. When oxidized, CM-H2DCFDA fluoresces and the amount of fluorescence produced is an indirect measure of the cellular production of ROS. CM-H2DCFDA fluorescence was measured 48 or 72 hours post infection, the period corresponding to the intracellular multiplication phase of the parasite.
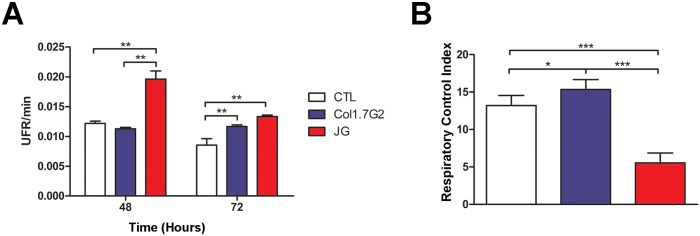
Forty-eight hours post infection no significant difference in the amount of oxidized probe was observed for those cultures infected with Col1.7G2, relative to the control (uninfected cultures) (Fig 3A). On the other hand, at the same time the levels of CM-H2DCFDA oxidation were about 1.6 fold higher for JG infected cultures when compared to the control or cultures infected with Col1.7G2, indicating increased oxidant production after infection with JG (Fig 3A). At 72 hours, both JG and Col1.7G2 infected cultures showed significantly higher levels of CM-H2DCFDA oxidization when compared to control non-infected cultures. (Fig 3A). However, the levels of oxidized CM-H2DCFDA in JG-infected cultures were still significantly higher than that observed for cultures infected with Col1.7G2 (Fig 3A).
Fig 3. ROS production and mitochondrial function in JG and Col1.7G2 infected cardiomyocytes.
(A) Relative fluorescence levels of oxidized CM-H2DCFDA present in primary BALB/c neonatal cardiomyocyte cultures, 48 and 72 hours after infection with Col1.7G2 or JG. Cultures of uninfected primary cardiomyocytes maintained for the same times were used as controls. The data represent the mean of duplicates ± the standard error of the mean (SEM). (B) Respiratory Control Index—RCI in primary cultures of BALB/c neonatal cardiomyocytes, infected with Col1.7G2 or JG, 48 hours after infection. Asterisks indicate statistically significant differences (* p<0.05, ** p≤0.01, *** p <0.001—Student's t-test).
It had been shown that oxidant production by T. cruzi infected cardiomyocytes could come from mitochondrial dysfunction [23]. Thus, we evaluated the mitochondrial function in cultures of primary mouse cardiomyocytes infected or not with trypomastigote forms of Col1.7G2 or JG. The respiratory control index (RCI) allows the evaluation of the mitochondrial capacity of substrate oxidation with low proton loss. Thus, the higher the RCI the lower is mitochondrial dysfunction and oxidant production. RCI measurements 48 hours post infection revealed greater mitochondrial impairment in cultures infected with JG. While cardiomyocytes infected with Col1.7G2 showed a small increase in the RCI when compared to control non-infected cultures, cardiomyocyte cultures infected with JG showed a significantly lower RCI when compared to control or Col1.7G2 infected cultures (Fig 3B). These results are in agreement with the higher rates of CM-H2DCFDA probe oxidation, indicating greater mitochondrial dysfunction and consequently higher production of oxidizing species cardiomyocyte cultures infected with JG.
JG epimastigote forms expresses lower amounts of antioxidant enzymes
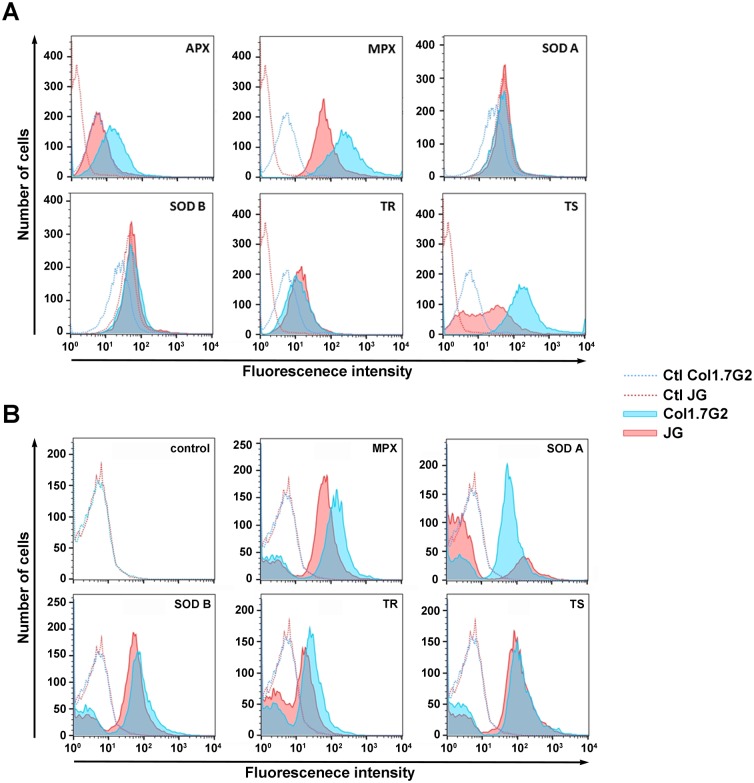
To assess the ability of JG and Col1.7G2 to cope with ROS produced upon infection in cardiomyocytes, the basal levels of different parasite anti-oxidant enzymes were assayed. Polyclonal antibodies directed to each of the anti-oxidant enzymes were used to label the parasites and the amount of labeling was read in a flow cytometer. Fig 4A shows the histograms of fluorescence intensities obtained in the epimastigote stage, for each T. cruzi population, for the different enzymes. The higher the expression of the enzymes the higher the number of cells presenting high levels of fluorescence. APX, MPX and TS anti-oxidant enzymes were found in higher amounts in the epimastigote forms of Col1.7G2 when compared to the same forms of JG in control conditions (Fig 4A).
Fig 4. Mean fluorescence intensity obtained from labeling of epimastigote forms of the two T. cruzi clonal populations.
Col1.7G2 (blue) and JG (red) with polyclonal antibodies against different T. cruzi anti—oxidant enzymes: Ascorbate peroxidase, APX; Mitochondrial Peroxiredoxin, MPX; Superoxide Dismutase A, SOD A; Superoxide Dismutase B, SOD B; Trypanothione Redutase, TR and Trypanothione Synthase, TS before (A) and after (B) treatment with 30 μM H2O2. Dotted lines represent control non-labeled parasites.
To determine if the profile of the antioxidant enzyme production by JG and Col1.7G2 would be the same after exposure of the parasites to oxidative stress, epimastigote forms from JG and Col1.7G2 were treated with H2O2 prior to the evaluation of enzyme expression. Even after exposure to the oxidant, a higher content of antioxidant enzymes was found for epimastigote forms of clone Col1.7G2. In this condition, higher expression of MPX, Fe-SODA and TR were found in Col1.7G2 epimastigotes when compared to JG (Fig 4B). Therefore, higher amounts of anti-oxidant enzyme was only observed for Col1.7G2, never for JG, either before or after exposure to an oxidative environment, indicating that JG could be more susceptible to oxidative stress.
Inhibition of host cell oxidative stress by treatment with catalase affects JG intracellular multiplication
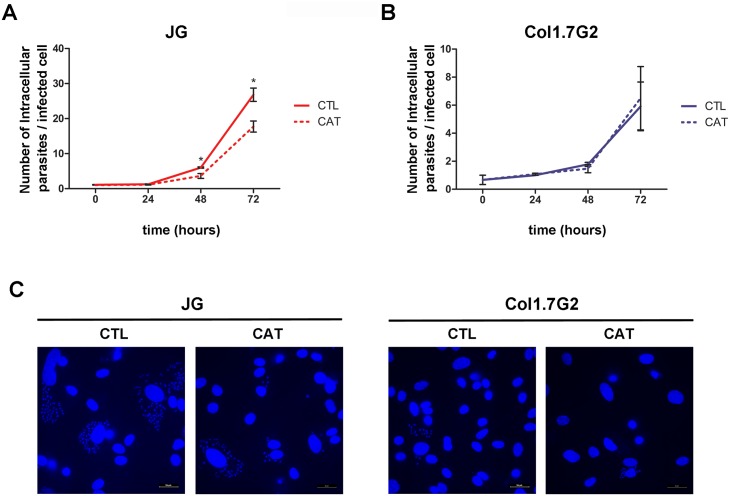
The results obtained above show that JG induces more ROS in infections of BALB/c neonatal cardiomyocyte cultures and has less anti-oxidant enzymes contents when compared to Col1.7G2. Nonetheless, intracellular multiplication of JG in these cells is faster when compared to Col1.7G2. These data suggest that, ROS production and oxidative stress generated during infection in cardiomyocytes may trigger JG intracellular development. To test this hypothesis we decided to investigate whether decreasing reactive oxygen species, such as hydrogen peroxide (H2O2) by treatment of cardiomyocyte cultures with catalase during T. cruzi infection, could interfere with the intracellular development of JG and or Col1.7G2 in these cells. For this, cultures of BALB/c neonatal cardiomyocytes were incubated or not with catalase, as described in the methodology. After treatment with catalase, a statistically significant decrease in the intracellular growth rate of JG was observed. JG infected cells had lower number of intracellular parasites along the course of infection when compared to non-treated cells (Fig 5A and 5C). Seventy-two hours post infection, the number of JG intracellular parasites for cultures treated with catalase was around 1.5 times lower than that obtained for control non-treated cultures, indicating a poorer intracellular development in a less oxidative environment (Fig 5A). On the other hand, treatment of cultures with catalase did not interfere with Col1.7G2 intracellular growth (Fig 5B and 5C), since the number of intracellular parasites along the course of infection was very similar in both treated and catalase treated conditions. These results suggest that the oxidative stress generated by the infection plays an important role in stimulating the intracellular development of the JG strain.
Fig 5. Quantification of intracellular multiplication rates of JG and Col1.7G2 in mouse cardiomyocyte cultures treated or not with catalase.
(A and B) Primary BALB/c neonatal cardiomyocyte cultures were treated (dashed line) or not (continuous line) with catalase, exposed to JG (A) or Col1.7G2 (B) and the number of intracellular parasites per infected cell along 72 hours of infection was evaluated. The data represent the mean of triplicates ± the standard error of the mean (SEM). Asterisks indicate statistically significant differences (* p≤0.01—Student t test). (C) Representative images of cell infection in primary BALB/c neonatal cardiomyocytes cultures, treated with catalase, 72 hours after exposure to JG or Col1.7G2. The scale bar corresponds to 10 μm.
The behavior of JG and Col1.7G2 can be reproduced in cultures of human cardiomyocytes
The data obtained for primary cultures of cardiomyocytes from BALB/c mice suggest that the oxidative stress generated during infection benefits the growth of JG in these cultures. In order to verify if this data could be reproduced in cardiomyocytes from a different source, we performed cultures of human cardiomyocytes obtained from induced pluripotent human stem cells. First, the cultures of human cardiomyocytes were submitted to the same infection methodology with Col1.7G2 and JG and the rates of invasion and intracellular multiplication were evaluated. In these cultures, the rate of invasion observed for the two T. cruzi clonal populations, JG and Col1.7G2, was similar to the results previously obtained by Andrade et al. (2010), where Col1.7G2 had a higher number of infected cells when compared to cultures infected with JG (Fig 6A). With respect to parasite growth, 72 hours post-infection, JG-infected cultures showed higher intracellular proliferation rates (2.14 times) when compared to cultures infected with Col1.7G2 (Fig 6B), reproducing the results obtained by Andrade et al. (2010) [14] and data obtained here for BALB/c neonatal cardiomyocyte cultures (Fig 1B). JG growth in human cardiomyocyte cultures was about 2.14 times greater than Col1.7G2 (Fig 6B).
Fig 6. (A-B) Quantification of invasion and intracellular multiplication rates of Col1.7G2 and JG in human cardiomyocyte cultures.
Cultures of human cardiomyocytes derived from iPSCs were exposed to Col1.7G2 or JG and the number of infected cells per 100 cells (A), as well as the number of intracellular parasites per infected cell (B), were evaluated for the determination of invasion and intracellular multiplication rates, respectively. The data represent the mean of triplicates ± the standard error of the mean (SEM). (C) Relative fluorescence of oxidized CM-H2DCFDA present in human cardiomyocyte cultures, 48 hours after infection with Col1.7G2 or JG. Cultures of uninfected human cardiomyocytes maintained for the same time were used as controls. The data represent the mean of duplicates ± the standard error of the mean (SEM). (D) Quantification of intracellular multiplication rates of Col1.7G2 and JG in cultures of human cardiomyocytes treated with catalase. Human cardiomyocyte cultures from iPSCs were treated with catalase and then exposed to Col1.7G2 or JG and the number of intracellular parasites per infected cell was evaluated. The data represent the mean of triplicates ± the standard error of the mean (SEM). (E) Combination of data from graphs shown in B and D, showing JG or Col1.7G2 intracellular growth from independent experiments performed in catalase treated (dashed lines) and non-treated (continuous lines) human cardiomyocyte cultures. Asterisks indicate statistically significant differences (*** p≤0.001, ** p≤0.01 and * p≤0.05—Student's t-test).
Oxidative stress generated during infection in human cardiomyocyte cultures also contribute to JG intracellular development in these cells
Since the profile of JG and Col1.7G2 intracellular development in human cardiomyocytes reproduced the data obtained from infections in neonatal BALB/c cardiomyocyte cultures, we decided to investigate the induction of oxidants upon infection of these cells. Evaluation of oxidant production was also performed by incubation of cells with the probe CM-H2DCFDA, 48 hours post infection. Again, no significant difference was observed in the amount of oxidized probe for those cultures infected with Col1.7G2, relative to the control (uninfected cultures) (Fig 6C). On the other hand, at the same time a significantly higher amount of probe oxidation, about 1.52 fold higher, was observed for those cultures infected with JG, relative to the control or about 1.29 fold higher when compared to cultures infected with Col1.7G2, also indicating higher production of ROS upon infection of these cells with JG (Fig 6C). Additionally, we also investigated whether inhibition of oxidative stress would affect JG or Col1.7G2 infection in these cultures. While JG multiplied better than Col1.7G2 in non-treated cultures (Fig 6B), its growth was lower than Col1.7G2 in catalase treated cultures (Fig 6D). We also compared JG and Col1.7G2 intracellular growth obtained from experiments performed in human cardiomyocyte non-treated cultures (Fig 6B) with the new data obtained from the experiments performed with human cardiomyocytes treated with catalase (Fig 6D), which are shown in Fig 6E. As observed, a decrease in JG intracellular growth, but not in Col1.7G2 is found upon catalase treatment. These results imply that JG is also more responsive than Col1.7G2 to the repressive effects of catalase when infecting human cardiomyocyte cultures and reinforce the idea that the oxidative stress generated by the infection plays an important role in the intracellular development of at least some T. cruzi strain.
Infection in immortalized mouse fibroblasts does not generate oxidative stress and the intracellular growth profiles of JG and Col1.7G2 are similar
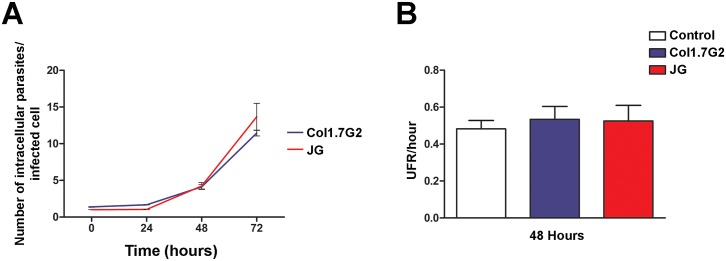
We next investigated whether infection in a different cell type, would alter JG and Col1.7G2 intracellular behavior. For this, we performed infections with JG and Col1.7G2 in immortalized mouse embryonic fibroblasts (MEFs) and evaluated parasite intracellular growth and oxidant production upon infection. Fig 7A shows the number of intracellular parasites per infected cell over a total period of 72 hours of infection in MEFs. As can be observed, there was no significant difference in the number of intracellular parasites between Col1.7G2 and JG in any of the analyzed points, being the growth curve of both T. cruzi populations similar to each other. In order to confirm that these cells did not respond to infection with oxidant generation, analysis of CM-H2DCFDA oxidization, 48 hours post infection with JG or Col1.7G2, was performed. For both cultures no statistically significant difference was observed in the levels of oxidized CM-H2DCFDA among control non-infected cultures and those infected with Col1.7G2 or JG (Fig 7B).
Fig 7.
(A) Quantification of intracellular multiplication rates of Col1.7G2 and JG in mouse embryonic fibroblasts (MEFs) cultures. Cultures of MEFs were exposed to Col1.7G2 or JG and the number of intracellular parasites per infected cell was evaluated. The data represent the mean of triplicates ± the standard error of the mean (SEM). (B) Relative fluorescence of oxidized CM-H2DCFDA present in MEFs cultures, 48 hours after infection with Col1.7G2 or JG. Cultures of uninfected MEFs maintained for the same time were used as controls. The data represent the mean of duplicates ± the standard error of the mean (SEM).
JG epimastigotes present higher levels of intracellular Ca2+ and oxidant production upon treatment with H2O2
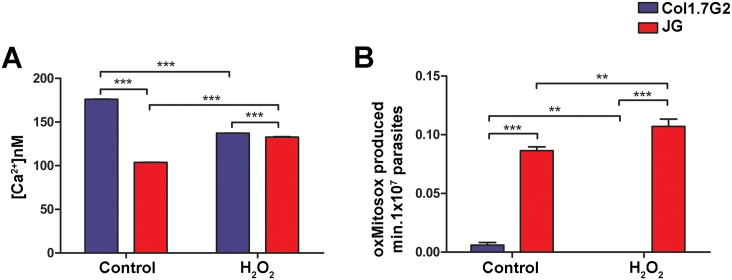
The above results suggest that oxidative stress may play in JG strain a role in the intracellular development of the parasite and may, in specific situations, be beneficial to its intracellular development. In the latter, oxidative stress could work as a signal triggering parasite intracellular growth [36, 37]. It has been shown in the literature that the increase in free intracellular Ca2+ levels in the cytoplasm of T. cruzi may represent an important signal, leading to an increase in the infective capacity of this parasite [33]. It has also recently been shown that the decrease of IP3 receptor expression in the parasite leads to a decrease not only in the infectivity, but also in parasite intracellular growth [38]. Thus, we decided to verify whether exposure of Col1.7G2 and JG to oxidative stress, by incubation with H2O2, could induce calcium signals in these parasites. Baseline levels of intracellular calcium, before treatment with H2O2 (control), were significantly higher for Col1.7G2, when compared to JG (Fig 8A). However, upon H2O2 treatment, only JG was capable of increasing the Ca2+ levels, as observed for Trypanosoma brucei [39] (Fig 8A).
Fig 8. Quantification of epimastigotes intracellular levels of calcium (A) and superoxide anion (B) upon JG and Col1.7G2 epimastigotes treatment with H2O2.
(A) JG and Col1.7G2 cells previously incubated with fura 2-AM, were treated or not (control) with 30 μM H2O2 for 30 minutes and the calcium levels in 107 parasites/mL was determined. (B) JG and Col1.7G2 parasites were incubated with MitoSOX and subsequently exposed to 30 μM H2O2 for 30 minutes, the oxMitoSOX level was measured in 107 parasites/mL. The data represent the mean of triplicates ± the standard error of the mean (SEM). Asterisks indicate statistically significant differences (**<0.01 *** p≤0.001—Student's t-test).
Another important signaling molecule is superoxide radical (O2•-). It has been shown that high levels O2•- is deleterious to cells, however in adequate concentrations O2•- may function as a stimulator of cell growth, as well as to inhibit apoptotic pathways [40, 41]. Thus, we also investigated the influence of H2O2 treatment on O2•-/H2O2 levels in epimastigote forms of JG and Col1.7G2 following MitoSOX probe oxidation. Baseline levels of MitoSOX oxidation for Col1.7G2 were very low, significantly lower than those observed for JG (Fig 8B). On the other hand, oxidant treatment of JG led to an increase in MitoSOX oxidation (Fig 8B). The above result may indicate that oxidant treatment may, by some unknown mechanism, enhance parasite O2•- production in the JG T. cruzi strain. Both intracellular O2•- and or H2O2 may be in part, responsible for the cellular signaling that boost parasite proliferation.
Discussion
One of the great questions regarding T. cruzi infection is what defines the development or not of serious clinical forms resulting from the infection. As mentioned previously, T. cruzi infection in humans has a very variable clinical course, in which infected individuals may be asymptomatic or even develop severe clinical symptoms, presenting cardiac, digestive or cardio-digestive disorders (reviewed by [42]). Understanding the mechanisms involved with the pathogenesis of this disease is essential for better control of the infection. Evidence from the literature shows that this clinical variability is related to a differential tissue tropism of parasite populations, which depends directly on the parasite-host cell interaction, without direct interference of the immune system [11, 13, 43, 44]. Cellular infection can be divided into two stages: invasion and intracellular multiplication. According to previous data from our group, intracellular multiplication seems to be of fundamental importance for the definition of this selection [14]. Studying the behavior of two clonal populations Col1.7G2 (T. cruzi I) and JG (T. cruzi II) of T. cruzi during infection in primary cultures of BALB/c embryonic cardiomyocytes it was observed that JG, a T. cruzi strain with strong tropism to BALB/c hearts, presented higher intracellular multiplication rates in these cells when compared to Col1.7G2 [11, 14].
In order to investigate the factors influencing this differential intracellular behavior we used as a study model the in vitro infection of primary cultures of BALB/c cardiomyocytes with the same T. cruzi clonal populations used in the previous studies, JG and Col1.7G2. However, this time the cardiomyocytes were isolated from neonatal mice. Regarding invasion rates, the data obtained here diverged from the data previously published by Andrade et al. (2010) [14]. This divergence may be related to the fact that, although from the same type of animal, the stage of differentiation of the cells was distinct from the work published earlier [14]. Cardiomyocytes isolated from neonatal mice may express distinct proteins from the ones obtained from mouse embryos, which could account for the differences in invasion rates observed for these two cells [45]. Nonetheless, in BALB/c neonatal cardiomyocytes, JG still presented a higher multiplication rate when compared to Col1.7G2. These data reinforce the idea that the intracellular development of the parasite, as suggested before [14], may be more important for the determination of T. cruzi tissue tropism than cellular invasion itself. This hypothesis is also supported by the data obtained here from JG and Col1.7G2 infections in human cardiomyocyte cultures derived from iPSCs. Upon infection in these cells, even though Col1.7G2 invasion rates were higher than JG, parasite intracellular growth was greater for JG infected cultures.
Several factors could affect the intracellular development of T. cruzi, among them its intracellular traffic. During cell infection, T. cruzi uses the cell membrane repair mechanism to promote its internalization in non-professional phagocytic cells, forming a vacuole containing lysosomal markers and content [46, 47]. The acidic content of the vacuole allows the parasite to gradually escape into the cytoplasm of the cell, where it completes its transformation into the amastigote form and initiates its intracellular multiplication [15, 48–51]. It was possible that a faster escape could advance the transformation of the parasite into the amastigote form and thus allow it to start its multiplication sooner. In fact, trans-sialidase superexpressor parasites, which escape faster from their parasitophorous vacuoles, differentiate into the amastigote earlier than wild type parasites [52]. Our data showed that, there was no difference in the rate of parasitophorous vacuole escape between Col1.7G2 and JG. Therefore this could not account for the differences in parasite intracellular development observed in the cardiomyocytes.
Data from the literature show that infected cells are able to respond to infection by activating several genes, which could interfere with the intracellular behavior of the parasite [19, 20, 22, 24]. Thus, we decided to evaluate the response of the host cell to infection, trying to correlate this data with the intracellular development of T. cruzi. For this, we investigated whether the production of ROS upon infection could be responsible for the differential intracellular growth of JG and Col1.7G2 in the studied cardiomyocyte cultures. It had already been shown that infection of cardiomyocytes with T. cruzi leads to a disturbance in the membrane potential of the mitochondria generating ROS [23]. In fact, by using the CM-H2DCFDA probe, we observed an increase in ROS production in primary cultures of BALB/c and human cardiomyocytes infected with Col1.7G2 (72 hours) or JG (48 and 72 hours) post infection, when compared to control uninfected cultures. Additionally, for JG infected cultures, a higher amount of ROS was produced 48 hours post infection, indicating a faster and stronger response of cardiomyocytes infected to this clonal population.
The high increase in ROS detected in BALB/c cardiomyocyte cultures 48 hours post infection with JG was likely generated by mitochondrial dysfunction as revealed by the analysis of the respiratory control index (RCI) in BALB/c cardiomyocyte infected cultures, although other sources cannot be ruled out. At this time post infection, cultures infected with JG presented a significant decrease in the RCI. The RCI is the best general measure of mitochondrial function in cell populations that have sufficiently active glycolysis to support metabolism, while mitochondrial function is manipulated [31]. Thus, a decrease in RCI does indicate mitochondrial dysfunction. These results are in agreement with previous data from the literature showing that upon infection with T. cuzi mitochondrial potential is disturbed, inducing ROS production [23]. On the other hand, we could not detect mitochondrial dysfunction in Col.17G2 infected cardiomyocytes 48 hours post infection. In agreement with this, at this time post infection, we could also not detect an increase in ROS in Col1.7G2 infected cardiomyocytes, when compared to control non-infected cultures. We are not sure why Col1.7G2 did not cause changes in cardiomyocyte mitochondrial RCI 48 hours post infection, but it may have to do with differences in the strains used. It is well known that T. cruzi populations do vary in their behavior during infection in cells, which may account for changes in their ability to interfere with mitochondrial function. In fact, although lower than that observed upon JG infection, we did observe changes in the RCI 72 hours post infection. So it is possible that upon infection with this strain there is a delay in the induction of mitochondrial dysfunction and generation of oxidative stress. In fact, there is data in the literature showing that hearts of BALB/c mice infected with Colombian strain do present a significant increase in the oxidative stress [53].
T. cruzi has a sophisticated system of antioxidant defenses to protect parasite from oxidative stress [54]. Interestingly, for epimastigote forms, none of the anti-oxidant enzymes evaluated were more expressed in JG when compared to Col1.7G2. In fact, when there was a difference in enzyme expression, the higher expression was found in Col1.7G2. This was also the case for parasites that were previously exposed to oxidative stress by incubation with H2O2, which had been already shown to induce an increase in anti-oxidant enzyme levels [55]. In fact, for Coll.72G parasites previously exposed to H2O2, we observed an increase in Fe-SODA and MPX levels, showing that H2O2 was effective in inducing an increase in enzyme expression. So far, JG induced more ROS production in cardiomyocytes and was likely to be more susceptible to this generated oxidative stress.
ROS has been shown to have a dual effect on cells. Although data from the literature show that an increase in ROS can compromise the intracellular growth of several pathogens, including T. cruzi, the opposite has also shown to be true [56–61]. It has recently been demonstrated that the oxidative stress generated by T. cruzi infection can lead to an increase in the replication rate of this parasite [36, 37]. One of the possible explanations for the increase in the rate of parasite replication upon induction of oxidative stress could be the bioavailability of iron for use by the parasite [36, 62]. It is known that iron is important for several metabolic events, such as DNA replication, mitochondrial respiration and anti-oxidant defense [63]. Thus, although amastigote forms of the parasite have been shown to be capable of binding and importing transferrin [64] in the intracellular environment, the concentration of this protein is very low. It is possible that free iron is more easily acquired in this way and then contributes to a better adaptation of this parasite to the intracellular environment. In the case of trypanosomatids, superoxide dismutases, important anti-oxidant enzymes, are iron-dependent [65]. Alternatively, the presence of oxidative stress could generate specific signals that would contribute to a more adequate response of the parasite in the cell, stimulating its faster replication. In fact, Finzi et al. (2004) also showed that pre-treatment of T. cruzi epimastigote forms with low concentrations of H2O2 increased parasite proliferation [66].
Considering the above, in our case, ROS seems to be important to give JG advantage during infection in cardiomyocytes. This is reinforced by the fact that inhibition of ROS by incubation of mouse and human cardiomyocytes with catalase inhibits JG intracellular growth. In fact, the inhibition of JG growth seemed even more prominent in human cardiomyocyte cultures. However distinct batches of catalase were used in the two experiments, which could account for this difference. Nonetheless, these results imply that at least for some strains ROS may be involved in parasite intracellular growth. Additionally, this is also corroborated by the data obtained from infected fibroblasts, which in our experimental conditions do not produce ROS in response to infection. In these cells JG did not present any growth advantage when compared to Col1.7G2. Previous studies have shown that a recombinant strain of T. cruzi, an E. coli MutT superexpressor (an enzyme involved in DNA repair), is more efficient in cell colonization compared to wild type parasites. In that work it was suggested that 8-oxo-GMP, generated by degradation of 8-oxo-GTP by MutT, could serve as signal to produce parasites more adapted to the intracellular environment [67]. It has also been shown that low concentrations of ROS were sufficient to promote better infection in in vitro and in vivo experiments [37]. Overall, our results suggest that ROS may have, in some specific circumstances, a helpful role in T. cruzi cell proliferation in non-professional phagocytes, which had not been shown before.
Recently, Vilar-Pereira and colleagues [53] have studied the role of antioxidants in cardiac function during T. cruzi infection in mice. For this they evaluated the production of ROS in the hearts of BALB/c mice infected with Colombian strain, by intravital microscopy at the chronic stage, before and after the treatment with different antioxidant agents. In this case, as mentioned before in this discussion, they found high amounts of oxidative stress in hearts of non-treated mice, possibly due to the fact that the experiments were performed in vivo at later time points, after several rounds of parasite infection. Additionally they showed electrical and mechanical dysfunction in these infected mice [53]. Treatment with different antioxidants was able to in fact improve hart function. However, they also demonstrated that only treatment with resveratrol was able to reduce parasite burden, but not treatment with the other antioxidant drugs. This is in contrast with previous findings from the same group showing that heart parasite burden is decreased in response to antioxidant treatment [36]. However in the latter they have used T. cruzi Y strain. Colombian and Y strain, such as the clone of Colombian strain (Col1.7G2) and JG used in our study, belong to two different T. cruzi lineages, T. cruzi I and II, respectively. It is possible that parasites from different lineages do respond differently to ROS. In this case, for T. cruzi II strains ROS may not have an effect in controlling or signaling to this parasite and that the effect of resveratrol may be by another signaling pathway, while T. cruzi I strains would be responsive to ROS. Our data supports this hypothesis since treatment does affect the T. cruzi I population, but not T. cruzi II. Whether T. cruzi II strains do not really respond to ROS or whether this response depends on the amount or the type of response triggered by the ROS production still remains to be elucidated.
There are several data in the literature showing that the presence of oxidative stress could generate signaling molecules. Here we show that parasite treatment with H2O2, leads to an increase in intracellular levels of calcium and also probably in O2•- and/or H2O2 production in JG, but not in Col1.7G2 T. cruzi clone, reinforcing that JG is more responsive to ROS, at least in this condition. Both molecules have been shown to interfere with cell death and replication [41, 68]. In relation to calcium levels, it has been shown that in epimastigotes the regulation of intracellular calcium is important for multiplication and metacyclogenesis [69]. In the literature it has also been shown that ROS are capable of increasing intracellular Ca2+ in parasites such as Trypanosoma brucei [39]. To the best of our knowledge this is the first time that it is shown that T. cruzi, in this case JG strain, can also respond to oxidative stress by altering intracellular calcium levels. In this case, the increase in calcium levels observed for JG was not sufficient for induction of cell death, but could be important for signaling some pathway related to cell proliferation. With respect to O2•-, there are reports showing that its increase may induce programmed cell death in T. cruzi and that parasites overexpressing mitochondrial Fe-SODA are more resistant [57]. However, there are reports in the literature showing that an increase in the concentration of this molecule may also signal for increased cell proliferation, as well as to work as an inhibitor of apoptotic pathways [40, 41]. In Dictyostelium discoideum, for example, the overexpression of SOD, with consequent consumption of O2•-, leads to the inhibition of multicellular aggregates [41]. What would determine whether ROS is responsible for death or proliferation would certainly be related to the amount to which parasites are exposed and the ability of the parasite to sense and trigger the intracellular signaling.
The data presented in this work suggest a mechanism responsible for the better development of JG, dependent on the parasite response to oxidant production, in cardiomyocyte cultures and may contribute to the understanding of the behavior of T. cruzi populations during infection in the host.
Acknowledgments
We are especially grateful to Centro de Aquisição e Processamento de Imagens (CAPI / ICB) and Centro de Microscopia da UFMG for the use of microscopes and imaging processing.
Data Availability
All relevant data are contained within the paper.
Funding Statement
This work has been funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-APQ-02269-14—LOA), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-479623/2009-0—LOA e 309764/2015-7—FRG), Fellowship from the Agencia Nacional de Investigación e Innovación—NIH (1R01AI095173- RR), Universidad de la República (CSIC and EI—LP and RR) and PEDECIBA (Uruguay—RR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Chagas disease (American trypanosomiasis). Fact Sheet. 2016.
- 2.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1(2):92–100. Epub 2002/03/02. doi: 10.1016/S1473-3099(01)00065-2 . [DOI] [PubMed] [Google Scholar]
- 3.Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Current opinion in infectious diseases. 2008;21(3):287–92. doi: 10.1097/QCO.0b013e3282f88b80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051–4. Epub 2009/12/23. . [DOI] [PubMed] [Google Scholar]
- 5.Macedo AM, Oliveira RP, Pena SD. Chagas disease: role of parasite genetic variation in pathogenesis. Expert Rev Mol Med. 2002;4(5):1–16. Epub 2004/02/28. doi: doi:10.1017/S1462399402004118 . [DOI] [PubMed] [Google Scholar]
- 6.Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz. 2004;99(1):1–12. Epub 2004/04/02. . [DOI] [PubMed] [Google Scholar]
- 7.Vago AR, Macedo AM, Oliveira RP, Andrade LO, Chiari E, Galvao LM, et al. Kinetoplast DNA signatures of Trypanosoma cruzi strains obtained directly from infected tissues. Am J Pathol. 1996;149(6):2153–9. Epub 1996/12/01. . [PMC free article] [PubMed] [Google Scholar]
- 8.Franco DJ, Vago AR, Chiari E, Meira FC, Galvao LM, Machado CR. Trypanosoma cruzi: mixture of two populations can modify virulence and tissue tropism in rat. Exp Parasitol. 2003;104(1–2):54–61. . [DOI] [PubMed] [Google Scholar]
- 9.Melo RC, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64(3):475–82. Epub 1978/06/01. . [PubMed] [Google Scholar]
- 10.Vera-Cruz JM, Magallon-Gastelum E, Grijalva G, Rincon AR, Ramos-Garcia C, Armendariz-Borunda J. Molecular diagnosis of Chagas' disease and use of an animal model to study parasite tropism. Parasitol Res. 2003;89(6):480–6. . [DOI] [PubMed] [Google Scholar]
- 11.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100(2):163–72. . [DOI] [PubMed] [Google Scholar]
- 12.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Trypanosoma cruzi: role of host genetic background in the differential tissue distribution of parasite clonal populations. Exp Parasitol. 2002;100(4):269–75. Epub 2002/07/20. . [DOI] [PubMed] [Google Scholar]
- 13.Freitas JM, Andrade LO, Pires SF, Lima R, Chiari E, Santos RR, et al. The MHC gene region of murine hosts influences the differential tissue tropism of infecting Trypanosoma cruzi strains. PLoS One. 2009;4(4):e5113 Epub 2009/04/02. doi: 10.1371/journal.pone.0005113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade LO, Galvao LM, Meirelles Mde N, Chiari E, Pena SD, Macedo AM. Differential tissue tropism of Trypanosoma cruzi strains: an in vitro study. Mem Inst Oswaldo Cruz. 2010;105(6):834–7. Epub 2010/10/15. . [DOI] [PubMed] [Google Scholar]
- 15.Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, et al. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71(7):1117–30. . [DOI] [PubMed] [Google Scholar]
- 16.Dvorak JA, Hyde TP. Trypanosoma cruzi: Interaction with vertebrate cells in vitro. I. Individual interactions at the cellular and subcellular levels. Exp Parasitol. 1973;34:268–83. [DOI] [PubMed] [Google Scholar]
- 17.Andrews NW, Whitlow MB. Secretion by Trypanosoma cruzi of a hemolysin active at low pH. Mol Biochem Parasitol. 1989;33:249–56. [DOI] [PubMed] [Google Scholar]
- 18.Andrews NW. From lysosomes into the cytosol: the intracellular pathway of Trypanosoma cruzi. Braz J of Med Biol Res. 1994;27(2):471–5. [PubMed] [Google Scholar]
- 19.Vaena de Avalos S, Blader IJ, Fisher M, Boothroyd JC, Burleigh BA. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J Biol Chem. 2002;277(1):639–44. doi: 10.1074/jbc.M109037200 . [DOI] [PubMed] [Google Scholar]
- 20.Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102(24):3003–8. Epub 2000/01/11. . [DOI] [PubMed] [Google Scholar]
- 21.Machado FS, Souto JT, Rossi MA, Esper L, Tanowitz HB, Aliberti J, et al. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2008;10(14–15):1558–66. Epub 2008/10/28. doi: 10.1016/j.micinf.2008.09.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costales JA, Daily JP, Burleigh BA. Cytokine-dependent and-independent gene expression changes and cell cycle block revealed in Trypanosoma cruzi-infected host cells by comparative mRNA profiling. BMC Genomics. 2009;10:252 Epub 2009/06/02. doi: 10.1186/1471-2164-10-252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Bhatia V, Wen JJ, Wu Y, Huang MH, Garg NJ. Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic Biol Med. 2009;47(10):1414–21. Epub 2009/08/19. doi: 10.1016/j.freeradbiomed.2009.08.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manque PA, Probst C, de SP MC, de PR CR, Ozaki LS, Pavoni DP, et al. Trypanosoma cruzi infection induces a global host cell response in cardiomyocytes. Infect Immun. 2011;79(5):1855–62. Epub 2011/02/24. doi: 10.1128/IAI.00643-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira RP, Broude NE, Macedo AM, Cantor CR, Smith CL, Pena SD. Probing the genetic population structure of Trypanosoma cruzi with polymorphic microsatellites. Proc Natl Acad Sci U S A. 1998;95(7):3776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellani O, Ribeiro LV, Fernandes JF. Differentiation of Trypanosoma cruzi in culture. J Protozool. 1967;14(3):447–51. . [DOI] [PubMed] [Google Scholar]
- 27.Andrews NW, Hong KS, Robbins ES, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–84. [DOI] [PubMed] [Google Scholar]
- 28.Hissa B, Duarte JG, Kelles LF, Santos FP, del Puerto HL, Gazzinelli-Guimaraes PH, et al. Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells. PLoS Negl Trop Dis. 2012;6(3):e1583 Epub 2012/04/06. doi: 10.1371/journal.pntd.0001583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskelinen EL, Schmidt CK, Neu S, Willenborg M, Fuertes G, Salvador N, et al. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell. 2004;15(7):3132–45. Epub 2004/05/04. doi: 10.1091/mbc.E04-02-0103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade LO, Andrews NW. Lysosomal fusion is essential for the retention of Trypanosoma cruzi inside host cells. J Exp Med. 2004;200(9):1135–43. doi: 10.1084/jem.20041408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. doi: 10.1042/BJ20110162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–50. [PubMed] [Google Scholar]
- 33.Moreno SNJ, Silva J, Vercesi AE, Docampo R. Cytosolic free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med. 1994;180:1535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peloso EF, Goncalves CC, Silva TM, Ribeiro LH, Pineyro MD, Robello C, et al. Tryparedoxin peroxidases and superoxide dismutases expression as well as ROS release are related to Trypanosoma cruzi epimastigotes growth phases. Archives of biochemistry and biophysics. 2012;520(2):117–22. doi: 10.1016/j.abb.2012.02.020 . [DOI] [PubMed] [Google Scholar]
- 35.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem. 2011;286(8):6627–40. doi: 10.1074/jbc.M110.167247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paiva CN, Feijo DF, Dutra FF, Carneiro VC, Freitas GB, Alves LS, et al. Oxidative stress fuels Trypanosoma cruzi infection in mice. J Clin Invest. 2012;122(7):2531–42. doi: 10.1172/JCI58525 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goes GR, Rocha PS, Diniz AR, Aguiar PH, Machado CR, Vieira LQ. Trypanosoma cruzi Needs a Signal Provided by Reactive Oxygen Species to Infect Macrophages. PLoS Negl Trop Dis. 2016;10(4):e0004555 doi: 10.1371/journal.pntd.0004555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto M, Nara T, Mita T, Mikoshiba K. Morpholino antisense oligo inhibits trans-splicing of pre-inositol 1,4,5-trisphosphate receptor mRNA of Trypanosoma cruzi and suppresses parasite growth and infectivity. Parasitol Int. 2016;65(3):175–9. doi: 10.1016/j.parint.2015.12.001 . [DOI] [PubMed] [Google Scholar]
- 39.Ridgley EL, Xiong ZH, Ruben L. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem J. 1999;340 (Pt 1):33–40. . [PMC free article] [PubMed] [Google Scholar]
- 40.Buetler TM, Krauskopf A, Ruegg UT. Role of superoxide as a signaling molecule. News Physiol Sci. 2004;19:120–3. . [DOI] [PubMed] [Google Scholar]
- 41.Pervaiz S, Clement MV. A permissive apoptotic environment: function of a decrease in intracellular superoxide anion and cytosolic acidification. Biochem Biophys Res Commun. 2002;290(4):1145–50. doi: 10.1006/bbrc.2001.6274 . [DOI] [PubMed] [Google Scholar]
- 42.Coura JR, Borges-Pereira J. Chronic phase of Chagas disease: why should it be treated? A comprehensive review. Mem Inst Oswaldo Cruz. 2011;106(6):641–5. . [DOI] [PubMed] [Google Scholar]
- 43.Macedo AM, Pena SD. Genetic Variability of Trypanosoma cruzi:Implications for the Pathogenesis of Chagas Disease. Parasitol Today. 1998;14(3):119–24. Epub 2006/10/17. . [DOI] [PubMed] [Google Scholar]
- 44.Vago AR, Andrade LO, Leite AA, d'Avila Reis D, Macedo AM, Adad SJ, et al. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol. 2000;156(5):1805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koban MU, Moorman AF, Holtz J, Yacoub MH, Boheler KR. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res. 1998;37(2):405–23. . [DOI] [PubMed] [Google Scholar]
- 46.Fernandes MC, Andrade LR, Andrews NW, Mortara RA. Trypanosoma cruzi trypomastigotes induce cytoskeleton modifications during HeLa cell invasion. Mem Inst Oswaldo Cruz. 2011;106(8):1014–6. . [DOI] [PubMed] [Google Scholar]
- 47.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189(6):1027–38. doi: 10.1083/jcb.201003053 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Meirelles Mde N, de Araujo Jorge TC, de Souza W, Moreira AL, Barbosa HS. Trypanosoma cruzi: phagolysosomal fusion after invasion into non professional phagocytic cells. Cell Struct Funct. 1987;12(4):387–93. . [DOI] [PubMed] [Google Scholar]
- 49.Ley V, Robbins ES, Nussenzweig V, Andrews NW. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990;171:401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews NW. The acid-active hemolysin of Trypanosoma cruzi. [Review]. Exp Parasitol. 1990;71(2):241–4. [DOI] [PubMed] [Google Scholar]
- 51.Hall BF, Webster P, Ma AK, Joiner KA, Andrews NW. Desialylation of lysosomal membrane glycoproteins by Trypanosoma cruzi: a role for the surface neuraminidase in facilitating parasite entry into the host cell cytoplasm. J Exp Med. 1992;176:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin-de-Celis SS, Uemura H, Yoshida N, Schenkman S. Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell Microbiol. 2006;8(12):1888–98. Epub 2006/07/11. doi: 10.1111/j.1462-5822.2006.00755.x . [DOI] [PubMed] [Google Scholar]
- 53.Vilar-Pereira G, Carneiro VC, Mata-Santos H, Vicentino AR, Ramos IP, Giarola NL, et al. Resveratrol Reverses Functional Chagas Heart Disease in Mice. PLoS Pathog. 2016;12(10):e1005947 doi: 10.1371/journal.ppat.1005947 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piacenza L, Peluffo G, Alvarez MN, Martinez A, Radi R. Trypanosoma cruzi antioxidant enzymes as virulence factors in Chagas disease. Antioxid Redox Signal. 2013;19(7):723–34. doi: 10.1089/ars.2012.4618 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gadelha FR, Goncalves CC, Mattos EC, Alves MJ, Pineyro MD, Robello C, et al. Release of the cytosolic tryparedoxin peroxidase into the incubation medium and a different profile of cytosolic and mitochondrial peroxiredoxin expression in H2O2-treated Trypanosoma cruzi tissue culture-derived trypomastigotes. Exp Parasitol. 2013;133(3):287–93. doi: 10.1016/j.exppara.2012.12.007 . [DOI] [PubMed] [Google Scholar]
- 56.Murray HW, Rubin BY, Carriero SM, Harris AM, Jaffee EA. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985;134(3):1982–8. . [PubMed] [Google Scholar]
- 57.Piacenza L, Irigoin F, Alvarez MN, Peluffo G, Taylor MC, Kelly JM, et al. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J. 2007;403(2):323–34. doi: 10.1042/BJ20061281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fonseca-Silva F, Inacio JD, Canto-Cavalheiro MM, Almeida-Amaral EE. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One. 2011;6(2):e14666 doi: 10.1371/journal.pone.0014666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cifani N, Pompili B, Anile M, Patella M, Diso D, Venuta F, et al. Reactive-oxygen-species-mediated P. aeruginosa killing is functional in human cystic fibrosis macrophages. PLoS One. 2013;8(8):e71717 doi: 10.1371/journal.pone.0071717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novais FO, Nguyen BT, Beiting DP, Carvalho LP, Glennie ND, Passos S, et al. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J Infect Dis. 2014;209(8):1288–96. doi: 10.1093/infdis/jiu013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20(6):1000–37. doi: 10.1089/ars.2013.5447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews NW. Oxidative stress and intracellular infections: more iron to the fire. J Clin Invest. 2012;122(7):2352–4. doi: 10.1172/JCI64239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor MC, Kelly JM. Iron metabolism in trypanosomatids, and its crucial role in infection. Parasitology. 2010;137(6):899–917. doi: 10.1017/S0031182009991880 . [DOI] [PubMed] [Google Scholar]
- 64.Lima MF, Villalta F. Trypanosoma cruzi receptors for human transferrin and their role. Mol Biochem Parasitol. 1990;38(2):245–52. . [DOI] [PubMed] [Google Scholar]
- 65.Dufernez F, Yernaux C, Gerbod D, Noel C, Chauvenet M, Wintjens R, et al. The presence of four iron-containing superoxide dismutase isozymes in trypanosomatidae: characterization, subcellular localization, and phylogenetic origin in Trypanosoma brucei. Free Radic Biol Med. 2006;40(2):210–25. doi: 10.1016/j.freeradbiomed.2005.06.021 . [DOI] [PubMed] [Google Scholar]
- 66.Finzi JK, Chiavegatto CW, Corat KF, Lopez JA, Cabrera OG, Mielniczki-Pereira AA, et al. Trypanosoma cruzi response to the oxidative stress generated by hydrogen peroxide. Mol Biochem Parasitol. 2004;133(1):37–43. . [DOI] [PubMed] [Google Scholar]
- 67.Aguiar PH, Furtado C, Repoles BM, Ribeiro GA, Mendes IC, Peloso EF, et al. Oxidative stress and DNA lesions: the role of 8-oxoguanine lesions in Trypanosoma cruzi cell viability. PLoS Negl Trop Dis. 2013;7(6):e2279 doi: 10.1371/journal.pntd.0002279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium. 2011;50(3):211–21. doi: 10.1016/j.ceca.2011.03.003 . [DOI] [PubMed] [Google Scholar]
- 69.Lammel EM, Barbieri MA, Wilkowsky SE, Bertini F, Isola EL. Trypanosoma cruzi: involvement of intracellular calcium in multiplication and differentiation. Exp Parasitol. 1996;83(2):240–9. doi: 10.1006/expr.1996.0070 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are contained within the paper.