Abstract
Objective
Pre-curved electrode arrays (EAs) are commonly used in Cochlear Implants (CIs). Modiolar placement of such arrays has been shown to lead to better hearing outcomes. In this project, we retrospectively evaluated the modiolar positioning of EAs within a large CI imaging database. We aimed to discover the rate at which perimodiolar placement is successfully achieved, and to evaluate a new technique we propose to pre-operatively plan patient-customized EA insertion depths to improve perimodiolar placement at the time of surgery.
Study Design
Retrospective chart review and radiographic analysis.
Setting
Single tertiary academic referral center
Subjects and Methods
Ninety-seven CI ears were evaluated. Perimodiolar positioning of electrodes was quantified using pre- and post-implantation CT scans and automated image analysis techniques.
Results
Average perimodiolar distance was 0.59 ± 0.18 mm. Disagreement between the actual and our recommended insertion depth was found to be positively correlated with perimodiolar distance (r = 0.49, p < 0.0001).
Conclusions
These results show that the average CI recipient with a pre-curved EA has a number of electrodes distant to the modiolus where they are not most effective. Our results also indicate the approach we propose for selecting patient-customized EA insertion depth would lead to better perimodiolar placement of pre-curved EAs.
INTRODUCTION
With over 324,000 cochlear implant (CI) performed worldwide, CI surgery has become the preferred treatment for severe to profound hearing loss 1. In CI surgery, an electrode array (EA), which directly stimulates auditory nerves to induce the sensation of hearing, is implanted ideally into the scala tympani (ST) of the cochlea. The auditory nerves have a finely tuned tonotopic spatial arrangement where stimulation of nerves near the cochlear entrance, also known as the basal turn, induces sensation of high frequency sound and stimulation of nerves deeper within the cochlea, near the apex, induces sensation of lower frequency sounds 2. CIs take advantage of this tonotopicity with EAs designed to position electrodes along the length of the cochlea so that deeper electrodes can be activated to stimulate low frequency specific neurons and more shallow electrodes stimulating high frequency specific neurons. Current commercially available EAs can be broadly divided into two classes – straight and pre-curved. Historically, pre-curved EAs are more widely used and are designed such that the resting state shape of the array roughly matches the coiled shape of the “average” human cochlea noting that wide variation in cochlear shape has been reported 3. The shape of the cochlea can be seen in the surface of Figure 1a where the green curve traces along the inner wall of the cochlea shows where pre-curved EAs are intended to be positioned. Such peri-modiolar positioning places the electrodes closer to the spiral ganglion cells – the intended sites of stimulation leading to activation of the auditory nerve and hearing. Positioning of electrodes closer to the spiral ganglion has been hypothesized to improve hearing outcomes potentially by reducing current spread allowing a cleaner signal to be delivered to the neurons 4. While it remains inconclusive whether pre-curved or straight EAs lead to better outcomes, it has been shown that when pre-curved EAs are used, better outcomes are achieved with better perimodiolar positioning of the array in a study by Holden et al. 5. In that study, a correlation of R=0.402 was found between Consonant-Nucleus-Consonant (CNC) word recognition scores18 and their “wrapping factor” metric proposed to indirectly measure modiolar positioning of the array. The CNC word recognition score measures the percentage of words in a CNC word list correctly recognized when presented to the subject in a sound booth. An increase in CNC word scores represents an increase in a subject’s speech recognition, which can have a significant impact on hearing-quality-of-life. Thus, the study of Holden et al. suggests that any advance in the CI procedure that leads to better perimodiolar positioning of pre-curved EAs could lead to an increase in average speech recognition, which would have a significant impact in the CI community.
Figure 1.
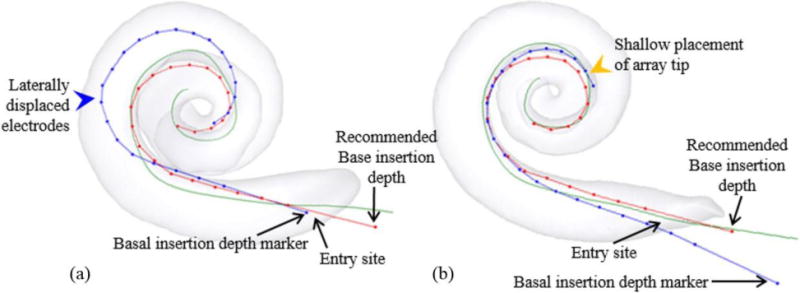
(a) An electrode insertion that is too deep (blue dotted curve). (b) An electrode insertion that is too shallow (blue dotted curve).
Currently, both straight and pre-curved arrays are inserted using “soft” surgical techniques in which the array is threaded at a tangential angle into the ST through either the existing round window membrane or a separate cochleostomy site while attempting to inflict as little trauma as humanly possible on the soft tissue contained within the cochlea 6. For pre-curved EAs, the array is advanced straightened using either an internal or external stylet. This allows insertion tangential to the basal turn – an essentially straight entry – following which it is threaded off the stylet curving around the cochlea until a predetermined “average” depth is reached. This is a one-size-fits-all approach as the patient-specific cochlear size and shape are not considered. It may have merit if the variations in anatomy are clinically insignificant but it is well-known that considerable variation in cochlear anatomy exists across individuals 3, 7. Because the array inside the ST is invisible to the surgeon, how close it is placed to the modiolar wall of the ST is generally unknown during and after the surgery. Recently developed automated image analysis techniques permit using post-implantation CT images to detect the intracochlear position of CI electrodes relative to intra-cochlear anatomy 8–10. These make it feasible, for the first time, to evaluate CI electrode position on a large scale. The focus of our current work is to use these techniques to retrospectively evaluate the position of pre-curved CI arrays on a large cohort. Further, we retrospectively evaluate whether customized insertion depths would improve peri-modiolar placement.
METHODS
With IRB approval, we collected pre- and post-implantation CT scans (denoted as pre-CT and post-CT) of 97 CI ears. Eighty-two of the ears were implanted with a Cochlear (Sydney, Australia) Contour Advance array (A1), and 15 were implanted with an Advanced Bionics (Valencia, CA, USA) Mid-Scala array (A2).
Segmentation of intra-cochlear anatomy in the pre-CT was performed using an automatic active shape model-based method 8, 11. The electrodes were identified in post-CT using automatic or semi-automatic electrode extraction techniques 9, 10. The location of the electrodes relative to intra-cochlear anatomy was quantified by rigidly registering the pre- and post-implantation datasets using mutual information-based methods 12, 13. Intra-cochlear anatomy is identified in the pre- rather than the post-CT because cochlear anatomy is typically more difficult to identify in post-CT where the CI hardware causes significant beam hardening artifacts.
Estimating perimodiolar position
To evaluate how often a perimodiolar positioning of the electrodes was achieved in our cohort, we defined the modiolar position in the ST of our active shape model that we use to localize intra-cochlear anatomy 8 by manually defining a 3D modiolar hugging curve as a point sequence within the ST using in-house developed 3D object editing software. A cubic spline was then fitted to the manually selected points to generate a dense and smooth 3-D curve (green curve in Figure 1a) 14. This modiolar curve is automatically transformed to each patient ear using a Thin Plate Spline (TPS) registration 15 of the model ST to the patient ST. TPS is a landmark-based non-rigid registration method and requires a one-to-one point correspondence between the landmarks used to define the transformation – in this case the landmarks being the surface points of the patient and model ST surfaces. The modiolar curve computed using the TPS was visually verified to be correct in each case.
The average distance between the electrodes and the modiolar curve was used as a measure of array positioning and was calculated as the average distance between each electrode and its closest point on the modiolar curve. This distance, , is calculated as:
where is the number of electrodes, and is the distance between the electrode and its closest point on the modiolar curve . Figure 2a, 2b, and 2c show 3 cases where the values of are 0.173 mm, 0.497 mm, and 1.062 mm. Using this metric, large indicates the array is further from the modiolus, and close to zero indicates optimal perimodiolar positioning.
Figure 2.
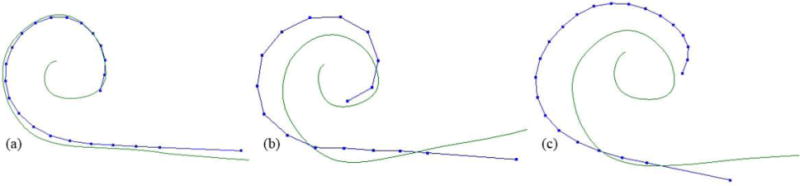
Shown are the modiolar curves (green), and the actual electrode positions for each of 3 subjects (blue dotted curves) with increasing average distance between electrodes and modiolus.
Figure 3.
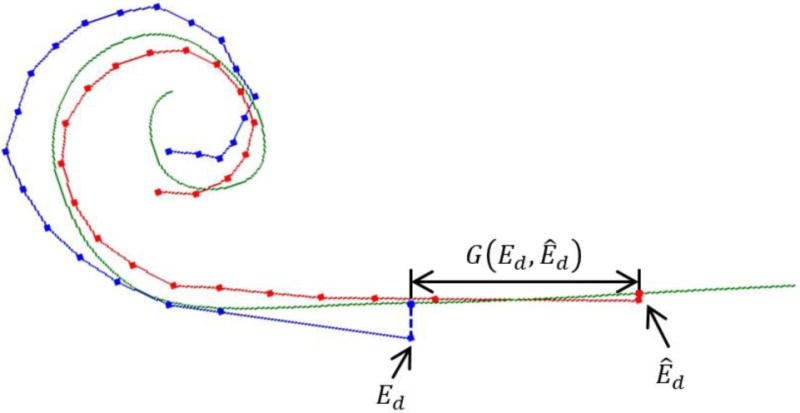
The geodesic distance between those two projected points along the modiolar curve is shown as .
Patient-customized electrode placement method
We hypothesize that one approach for increasing the probability of achieving optimal perimodiolar positioning is through patient-customized selection of the EA base insertion depth. Currently, the generically recommended insertion depth is specified by the manufacturer to be the depth at which the surgeon should stop inserting. The generic insertion depth is reached when a visual marker on the base of EA reaches the cochlea entry site. To implement a patient-customized base depth, the surgeon would alter this insertion depth to a pre-operatively defined, patient-customized depth conveyed in reference to the depth marker (e.g., “Insert the array until the base depth marker is 1 mm outside the entry site.”). Calculating this customized depth required two steps. First, we created EA shape models for each array, A1 and A2, by localizing the electrodes from the CT images of the EAs in air under no load. Second, the EA shape model was rigidly registered to the patient’s modiolar curve using an iterative closest point (ICP) registration technique 16. Figures 1a and 1b show the output of this process for two cases with the red dotted curves representing the EA shape models registered to the modiolar curves – we will refer to this registered EA shape model curve as the “optimal electrode position curve” for that individual. We then determine the patient-customized insertion base depth as the depth of the base of the optimal electrode position curve.
To reiterate, this approach outputs a recommended insertion depth at which the resting state shape of the array is in best agreement with the shape of the patient’s modiolar curve, i.e. is minimized, no matter where the depth marker ends up relative to the cochleostomy. Because it is likely that the array has returned to its resting shape once the base of the array has been inserted to this depth, deeper insertion of the EA would not result in further advancement of the tip of the array but push the middle portion of the array away from the modiolus. Figure 1a shows such a clinical case of over-insertion where the blue dotted curve represents the electrode position. The basal depth marker was inserted ~2mm past our recommended depth. This additional advancement likely resulted in the lateral displacement of the EA away from the modiolus. On the other hand, inserting the base of the array shallower than the recommended depth would lead to shallower depth of the tip of the array, which also could be detrimental to audiological outcomes as the range of nerves stimulated by the array may be reduced. A representative shallow insertion case is shown as the blue dotted curve in Figure 1b. The basal depth marker was placed ~2mm further from the entry site than recommended by our model. While the electrodes are perimodiolar, the tip of the array is much shallower than the expected depth demonstrated by the red curve.
Evaluation of patient-customized insertion depth technique
To evaluate whether our patient-customized approach would lead to more perimodiolar placement of the array, we perform a retrospective analysis to determine whether agreement between the actual base insertion depth achieved for patients and our recommended insertion depth is predictive of modiolar hugging placement of the EA. For each subject in our dataset, we measure the distance between the position of the depth marker on the surgically placed EA, , and the position where the marker is recommended to be by the techniques described above, (see Figure 3). To ensure the distance measured corresponds to a difference in insertion depth, we define as the distance in the direction tangential to the insertion by projecting and onto the modiolar curve and then measuring the geodesic distance between those two points along the modiolar curve. is a signed function with positive values indicating that is deeper than and negative values indicating is deeper than .
To evaluate whether agreement with the recommended insertion depth was associated with more modiolar hugging placement of the EA, Pearson correlation coefficients were calculated between and the absolute value of , . A significant positive relationship between and indicates that our patient-customized insertion depth would be better at achieving perimodiolar array positioning as compared to the generic insertion depth.
RESULTS
The mean and standard deviation of were 0.59 ± 0.18 mm. A histogram of these data is shown in Figure 4a. These data suggest that more tightly modiolar hugging placement of the arrays ( < 0.5mm) is not the norm and that the average individual has a number of electrodes that are substantially distant to the modiolus. For a typical cochlea, the distance an electrode can vary between the modiolar and lateral walls is approximately 1.5mm. Overall, arrays with < 0.5mm can be considered mostly perimodiolar.
Figure 4.
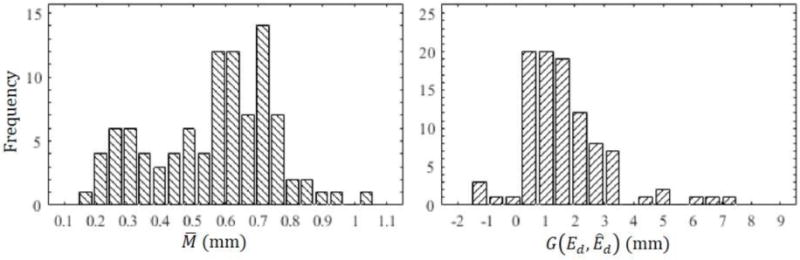
(a) Histogram of our modiolar distance results . (b) Histogram of the geodesic distance between recommended and actual insertion depths .
Figure 4b shows the histogram of , which has a mean and standard deviation of 1.44 ± 1.46 mm, suggesting that for the majority of individuals (91 out of 97 cases), our technique recommends inserting the array to a depth shallower than what was realized intraoperatively although there is substantial variation around the mean.
Figure 5 shows the scatter plot of versus . The black line shows the linear regression relation between them. was positively correlated with (r = 0.49, p < 0.0001), suggesting that cases with smaller values of , i.e., cases where the insertion depth matched our recommended depth, had better perimodiolar placement of the array. Figure 5 also suggests that achieving mostly modiolar hugging placement ( < 0.5mm) only occurs when is relatively small although is small in many cases where is large. We interpret this finding as follows: an insertion depth close to the patient-customized insertion depth is necessary but not sufficient for perimodiolar placement to occur.
Figure 5.
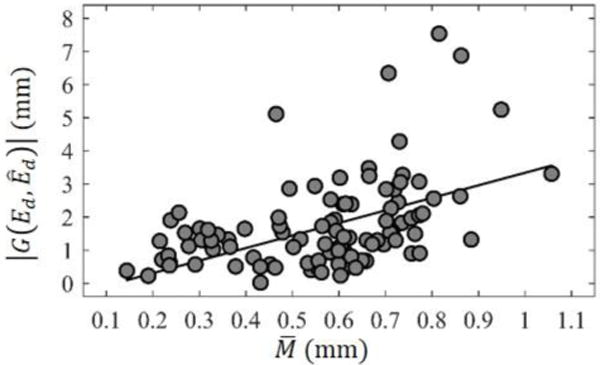
Scatter plot of the relationship between and with linear regression line shown in black.
CONCLUSIONS
Although CIs are remarkably successful, a significant number of individuals experience poor outcomes and restoration to normal fidelity is rare even among the best performers. This is likely due to many variables beyond the control of the surgeon or audiologist (e.g. neural survival), but at least one variable within surgical control is intracochlear placement. As their name suggests, perimodiolar electrodes are designed to be placed in close proximity to the modiolus to decrease channel interaction. Our results show that perimodiolar EAs, more often than not, do not sit adjacent to the modiolus where they are likely most effective.
To achieve more optimal perimodiolar positioning, we proposed a simple, image-guided approach to selecting a patient-customized electrode insertion depth based upon the final resting shape of the EA and the patient’s anatomy as assessed on pre-operative CT scanning. Our findings show correlation between perimodiolar placement of the array, , and the customized insertion depth as assessed by the absolute value of the different between actual and recommended insertion depth, , suggesting that if our patient-customized electrode insertion depth technique is implemented, it will increase the fraction of cases where perimodiolar placement of the electrode array is achieved. Our results indicate that over-insertion of EAs is the norm and under-insertion uncommon. Furthermore, it is rare that the generically recommended insertion depth is the best one for any individual suggesting that the one size fits all approach is rather a one size fits few approach. Even when arrays are inserted precisely to the manufacturer recommended depth, the over-insertion artifact of a laterally displaced mid-portion of the array is common as shown in the example case of Figure 1a. While we have shown that over-insertion of the EAs explains many cases where perimodiolar positioning is not achieved, our data indicate that an insertion depth close to the patient customized insertion depth is necessary but not sufficient for perimodiolar placement to occur, and thus clearly other factors contribute to achieving perimodiolar placement.
Clinically, our customized insertion depth approach recommends a base insertion depth that is typically shallower than the manufacturer specified depth. This shallower depth is more likely to position the most basal electrodes closer to the cochlear entrance where they are farther away from neural stimulation sites typically most in need of stimulation. While this may be a potential drawback of our approach, it can be overcome by deactivating these most basal electrodes. Recent studies support this idea as they have shown the benefit of deactivation of electrodes that are determined to be suboptimally positioned 17. With deactivation of electrodes placed near the entrance of the cochlea, we anticipate that the benefits of better perimodiolar positioning will outweigh this potential drawback, but this hypothesis will need to be verified in future studies. Another potential solution is redesigned EAs with electrodes concentrated in those regions where periomodiolar positioning can be achieved.
Summarizing, we have found that (1) pre-curved EAs tend to be over inserted leading to displacement away from the modiolus in the first turn of the cochlea, (2) this lateral displacement leads to worse hearing outcomes, and (3) we can achieve better perimodiolar positioning using patient-customized insertion depths. While exciting, we recognize that our report is retrospective in nature and that prospective studies will be necessary to substantiate these findings.
Acknowledgments
This work was supported in part by grants R01DC008408 and R01DC014462 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes.
INSTITUTIONAL REVIEW BOARD APPROVAL #: 090155
Footnotes
The paper has not been presented elsewhere.
References
- 1.Cochlear Implants. National Institute on Deafness and Other Communication Disorders. No. 11-4798. 2014 [Google Scholar]
- 2.Greenwood DD. A cochlear frequency-position function for several species−29 years later. J Acoust Soc Am. 1990;87(6):2592–605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 3.Pelosi S, Noble JH (co-first authors), Dawant BM, Labadie RF. Analysis of inter-subject variations in promontory and intracochlear anatomy for cochlear implantation. Otol Neurotol. 2013;34(9):1675–1680. doi: 10.1097/MAO.0b013e3182a1a7e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):444–448. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- 5.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342–60. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- 7.Hardy M. The length of the organ of corti in man. Am J Anat. 1938;62:291–311. [Google Scholar]
- 8.Noble JH, Labadie RF, Majdani O, et al. Automatic segmentation of intra-cochlear anatomy in conventional CT. IEEE Trans Biomed Eng. 2011;58(9):2625–2632. doi: 10.1109/TBME.2011.2160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble JH, Dawant BM. Automatic graph-based localization of cochlear implant electrodes in CT. Lecture Notes in Computer Science-Proceedings of MICCAI. 2015;9350:152–9. doi: 10.1007/978-3-319-24571-3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Dawant BM, Labadie RF, et al. Automatic Localization of Cochlear Implant Electrodes in CT. Lecture Notes in Computer Science-Proceedings of MICCAI. 2014;8673:331–8. doi: 10.1007/978-3-319-10404-1_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cootes TF, Taylor CJ, Cooper DH, et al. Active shape models−their training and application. Comp Vis Image Und. 1995;61(1):38–59. [Google Scholar]
- 12.Viola P, Wells WM., III Alignment by maximization of mutual information. Int J Comput Vis. 1997;24(2):137–154. [Google Scholar]
- 13.Maes F, Collignon A, Vandermeulen D, et al. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imag. 1997;16(2):187–98. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 14.Press WH, Flannery BP, Teukolsky SA, et al. Numerical Recipes in C. 2nd. Cambridge, UK: Cambridge Univ Press; 1992. pp. 412–419. [Google Scholar]
- 15.Goshtasgy A. Registration of images with geometric distortions. IEEE Tran Geosci Remote Sens. 1988;26(1):60–64. [Google Scholar]
- 16.Besl PJ, McKay ND. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intell. 1992;14(2):239–56. [Google Scholar]
- 17.Noble JH, Gifford RH, Hedley-Williams AJ, et al. Clinical evaluation of an image-guided cochlear implant programming strategy. Audiol Neurootol. 2014;19:400–11. doi: 10.1159/000365273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]


