Abstract
This work aimed at investigating the effects of three domestic cooking methods (roasting, sprouting and boiling) on phytochemical contents (phenolic content, flavonoid, fibre), and anti-obesity (weight loss, hypoglycemic effect, serum lipids) potential of soybean seeds in obese male rats. Ten different forms were implemented, combining hulled/unhulled and raw/cooked soybean seeds using a basal and a hypercaloric diet as controls. Unhulled Roasted Soybean (URS) exhibited the highest phenolic content and a greater antioxidant activity by the FRAP assay than BHT at certain concentrations. Hulled boiled Soybean (HBS) showed the highest flavonoid content while Hulled Germinated Soybean (HGS) presented the lowest fibre content (P < 0.05). Unhulled Boiled Soybean (UBS) induced the best reduction in food intake while Unhulled Soybean Extract (USE) exhibited the greatest slimming effect. HBS exhibited the best cholesterol lowering ability; URS and Unhulled germinated Soybean (UGS) respectively induced the highest increase in HDL cholesterol levels and reduction in triglyceride levels. UBS demonstrated the highest ability to lower LDL cholesterol. UGS exhibited the highest ability to lower the postprandial blood glucose. Culinary treatments affect phytochemical content and anti-obesity potential of soybean seeds.
Keywords: Food science, Nutrition, Food analysis
1. Introduction
Obesity is a chronic disease resulting from a health threatening excess fat accumulation. Its prevalence has drastically increased in these last decades, and recent researches estimate that more than half of the world population should be obese in 2030 if nothing is done (Finkelstein et al., 2012). The disease is particularly expanding in Africa and other developing countries, as a direct consequence of socio-economic development and lifestyle modifications (Correia et al., 2014). Obesity also constitutes a key factor in the development of many other non-transmissible diseases such as diabetes mellitus, hypertension, cardiovascular disease and cancer (Correia et al., 2014). Management of obesity nowadays combines hypocaloric diets, chemical drugs and surgery, but hypocaloric diets are slow in producing desired results while drugs and surgery are expensive besides their dangerous side effects (Carette et al., 2012). This explains the renewed interest scientists show in functional food research in the management of obesity (Martirosyan and Singh, 2015) and other non-transmissible diseases (Miglio et al., 2008). Recent studies have indicated that soybean seeds can be used in the management of diseases like obesity and diabetes thanks to their content in bioactive phytochemicals such as alpha-amylase and alpha-glucosidase inhibitors, protease inhibitors, hemaglutinin and crude fibres, capable of disturbing the normal metabolism and helping in the management of obesity and other metabolic dysfunctions (Dixit et al., 2011; Uhegbu et al., 2013; Sharma and Baluja, 2015). Also, the presence in soybean seeds of phenolic compounds and mainly isoflavones, make them to be more efficient against metabolic diseases and their complications (Ganesan and Xu, 2017). But soybean seeds are never eaten raw (Sharma et al., 2013), they always, like many others legumes and vegetables undergo culinary treatments which can affect their bioactive phytochemicals content (Shah et al., 2011; Chutipanyaporn et al., 2014; Drinkwater et al., 2015), thus reducing their functional properties. Hulling, roasting, sprouting and boiling are the most common culinary treatments applied to soybean seeds. Thus this work aimed to evaluate the effects of hulling, sprouting, boiling and roasting on phytochemical contents of soybean seeds, in relation to their anti-obesity potential.
2. Material and methods
2.1. Experimental animals and diets
Three-week-old male Wistar rats were obtained from the Department Animal Centre and allowed to be accustomed to the new environment for 1 week. They were maintained in accordance with the guidelines of the OCDE (2008) and were randomly distributed into twelve groups of 10 animals each (including two controls). The animals were individually housed under controlled temperature (25 °C) and lighting (12:12-h light-dark cycle) and had free access to water and diet. The test groups and the positive control were fed a high fat high sucrose diet (17.6% fat and 7% sucrose-enriched) while the negative control received a basal diet. In order to induce the obesity, male Wistar rats were fed a high fat high sucrose (HFHS) diet for 12 weeks. HFHS groups (n = 110) were fed on a diet containing corn flour (52.9%), fish flour (20%), beef tallow (17.6%), bone (1%), vitamins (0.5%), salt (1%), sucrose (7%) while negative control (BD) were fed on a basal diet composed as follows: corn flour (77.8%), fish flour (20%), bone (0.1%), palm olein (1%), vitamins (0.1%), salt (1%). The HFHS animals had free access to a 2% sucrose solution and the rats with a Body Mass Index (BMI) greater than 0.68 g/cm2 were considered obese (Novelli et al., 2007) while the treatment lasted for 28 days.
Animals were grouped according to the treatment received as USE (Unhulled Soybean aqueous Extract), HSE (Hulled Soybean aqueous Extract), UUS (Unhulled Untreated Soybean), HUS (Hulled Untreated Soybean), URS (Unhulled Roasted Soybean), HRS (Hulled Roasted Soybean), UGS (Unhulled Germinated Soybean), HGS (Hulled Germinated Soybean), UBS (Unhulled Boiled Soybean), HBS (Hulled Boiled Soybean), HD (Hypercaloric Diet) and BD (Basal Diet). HD and BD received the vehicle (water) as controls. Treatments were made once a day by oral intubation between 7 am and 9 am local time with water as vehicle for both extracts and suspensions. All experiments were carried out according to the regulations and ethical approval of the Experimental Animal Welfare and Ethics Committee of the Institution.
2.2. Plant material
Yellow soybean seeds were purchased from a local market in the West region of Cameroon and transported to the Laboratory of Medicinal Plants Biochemistry, Food Science and Nutrition (LABPMAN) of the Biochemistry department of the University of Dschang where they were sorted out for the elimination of dirty and abnormal seeds before use.
2.3. Seeds’ aqueous extracts and culinary forms
Hulled and unhulled soybean seeds aqueous extracts (USE and HSE) were obtained by soaking powders of corresponding seeds in water for 24 h with gentle stirring, after which the mixtures were filtered using a Whatman No 4 filter paper. The resulting filtrates were dried at 45 °C using an air oven to obtain the respective aqueous extracts. To obtain the UUS and HUS, the corresponding seeds were grounded and sifted using a home sifter (400 μm) to produce a soft textured UUS and HUS powders respectively.
We obtained URS and HRS powders using the same procedure described for UUS and HUS with the only difference that the seeds were roasted for 10 min at 145 °C and cooled prior to grinding.
To obtain the UGS and HGS, four-hour soaked soybean seeds were placed to germinate at 27 °C in dark over moist filter paper on a moist sand bed and watered regularly for seven days. These unshelled and hulled germinated seeds were then washed and boiled for fifteen minutes. The boiled seeds were collected and dried for 24 h at 45 °C using an air oven, before grinding and sifting for powder’s collection.
UBS and HBS powders were obtained by grinding and sifting the dried product (24 h at 45 °C using an air oven) of cooked soybean seeds. Five hundred (500) g of hulled/unhulled soybean seeds were soaked for 4 h and then grounded, the resulting products were cooked at 95.5 °C for 45 min in 750 ml of water and cooled at room temperature before drying.
Roasting and cooking were done using stainless-steel pot and ladle. Dried extracts and powders were sealed using Aluminum foil, stored in a desiccator and used daily to prepare the suspensions administered to animals. Powders were weighed as to theoretically give an equivalent extract mass utilized for USE and HSE (500 mg/kg), based on the extraction ratio of 14.34%.
2.4. Phytochemicals and functional properties
2.4.1. Crude fibre
Aqueous extracts and powders of all soybean seeds culinary forms were analyzed for crude fibre content using the Ceramic Fibre Filter as described by A.O.A.C. (1990). These extracts and powders were previously treated to remove lipids using hexane (24 h soaking of 6 g of extracts and powders in 30 ml of hexane with gentle stirring). Briefly, 100 ml of 1.25% H2SO4 was added to 1 g of lipid free powder in a round bottom flask and the mixture boiled under reflux for 30 min. The hot solution was quickly filtered under suction. The insoluble matter was washed several times with hot distilled water until it was acid free. It was quantitatively transferred into the flask and 100 ml of hot 1.25% sodium hydroxide (NaOH) solution was added and the mixture boiled again under reflux for 30 min before it was quickly filtered under suction. The soluble residue was washed with boiling water until it was base free. Afterwards, it was dried to constant weight in the oven at 105 °C, cooled in a desiccator and weighed. The weighed sample (C1) was incinerated in a muffle furnace at 300 °C for about 2 h, cooled in the desiccator and weight measurement repeated (C2).The loss in weight of sample on incineration was given by C1–C2 while the crude fibre content was expressed as follows:
2.4.2. Phenolic content
Aqueous extracts and powders of all soybean seeds culinary forms were analyzed for total phenolic content using the Folin-Ciocalteu method as described by Gao et al. (2000). Respectively 0.44 ml and 0.02 ml of distilled water and Folin reagent were added to 0.02 ml of extract/suspension of the culinary form of soybean seeds (2 mg/ml). After 3 min resting, 0.4 ml of 20% Na2CO3 was added. The mixture was vortexed and incubated for 20 min at 40 °C using a water bath, thereafter the absorbance was read against a blank at 760 nm using a BioMate 6 UV–vis spectrophotometer (BIOMATE). The total phenolic content was determined using the standard curve (y = 0.022 x; r2 = 0.9945) obtained with Gallic acid. The contents were expressed as mg of Gallic Acid Equivalent/g of extract/powder.
2.4.3. Total flavonoid
Aluminum trichloride method, as described by Padmaja et al. (2011) was used to determine the total flavonoid content of extracts and powders of all soybeans seeds’ culinary forms. One hundred (100) μl of extract/suspension was mixed with 1.49 ml of distilled water before introduction of 0.03 ml of 5% NaNO2. After 5 min resting, 0.03 ml of 10% AlCl3 was added and the mixture allowed to rest. After 6 min, 0.2 ml of 1 M NaOH and 0.24 ml distilled water were respectively added and the mixture was vortexed and the absorbance was measured at 510 nm using a BioMate 6 UV–vis spectrophotometer (BIOMATE). The flavonoid content was determined using the standard curve (y = 0.1972 x; r2 = 0.9972) obtained with Catechin. The contents were expressed as mg CE/g of extract/suspension.
2.4.4. Ferric reducing ability of plasma (FRAP)
Ability of extracts and suspensions of soybean seeds’ culinary forms to reduce the ferric iron were tested as described by Benzie and Strain (1996). Briefly, 75 μl of extract/powders’ suspension was added to 2 ml of FRAP reagent (300 mM acetate buffer: pH 3.6; 10 mM TPTZ ((2, 4, 6-tris (2-pyridyl)-S-triazine)) in 400 mM of HCl; 10 mM ferric chloride). After 30 min of incubation at room temperature, the absorbance was read at 593 nm against a blank. BHT was used as standard.
2.4.5. Anthropometric measurements and food intake
The body weight of animals was measured daily using a scale (SCOUT PRO) and the body weight loss determined by calculation. The food intake was calculated by subtracting the refusal on the served portion of food.
2.4.6. Biological parameters
The serum lipid profile was determined using colorimetric methods (MONLAB kits). Standard protocols as described by Trinder (1969) for the total cholesterol were used, for the HDL cholesterol while the LDL cholesterol was estimated using the formula established by Friedewald et al. (1972).
With regard to Oral Glucose Tolerance Test (OGTT), after an overnight fasting (8 h), animals were given extracts or suspension of soybean seeds culinary forms at 400 mg/kg of body weight before receiving a D − glucose solution (2 g/kg of body weight). The blood glucose (expressed in mg/dl) was then measured (5–10 μl from tail tip) after 0 min (T0), 30 min (T1), 60 min (T2), 90 min (T3) and 120 min (T4) using a portable glucometer (Accu-Chek).
2.5. Statistical analysis
Statistical analysis was performed using SPSS program version 21. In vitro experiments were performed in triplicate. Results were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Bonferroni test was used for statistical analysis of the mean difference among groups. Because the repeated measurements on each rat in OGTT test and weight change were correlated, between-treatment changes were tested using one-way analysis of covariance (ANCOVA), adjusted for baseline values, with the initial value as a covariate. Differences were considered significant at p < 0.05 (at 95% confidence interval).
3. Results and discussion
3.1. Phytochemical contents and antioxidant potentials
3.1.1. Crude fibre
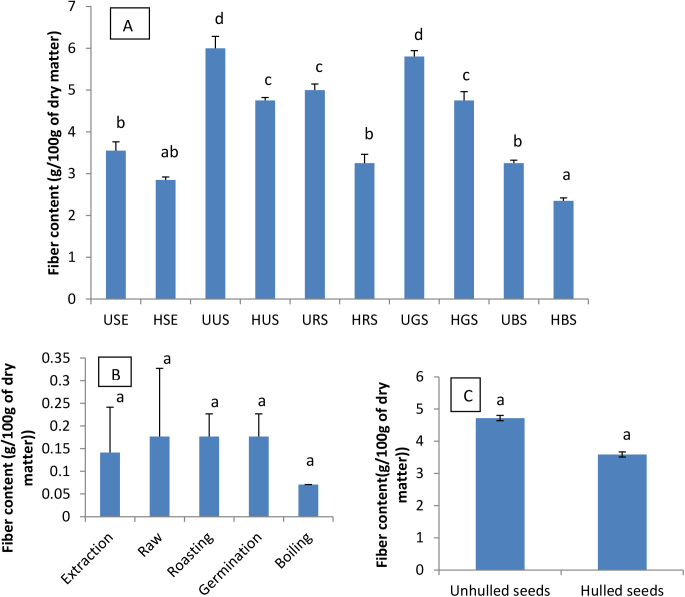
Fig. 1 shows the crude fibre content of lipid free soybean seeds extracts and culinary forms’ powders obtained by the Ceramic Fibre Filter (A.O.A.C., 1990). UUS exhibited the highest fibre content (6 g/100 g). This could be explained by the fact UUS did not undergo any culinary treatment or food processing; hence no loss in fibre has occurred. HBS exhibited the lowest fibre content (2.35 g/100 g). Overall, significant difference was observed between the fibre content of hulled and unhulled soybean seeds, and this result corroborates the findings of Písaříková and Zralý (2010). Sprouting did not significantly affect the crude fibre content of soybean seeds compared to other culinary treatments, and this result confirms the one previously obtained by Bau et al. (1997).
Fig. 1.
Crude fibre content (g/100 g dry matter) of lipid free soybean seeds extracts and culinary forms powders’ (A), effect of domestic cooking methods (B) and hulling (C) on crude fibre content. USE: Unhulled Soybean aqueous Extract, HSE: HulledSoybean aquoeus Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean.Values with different letters are significantly different at P < 0.05.
3.1.2. Total phenolic contents
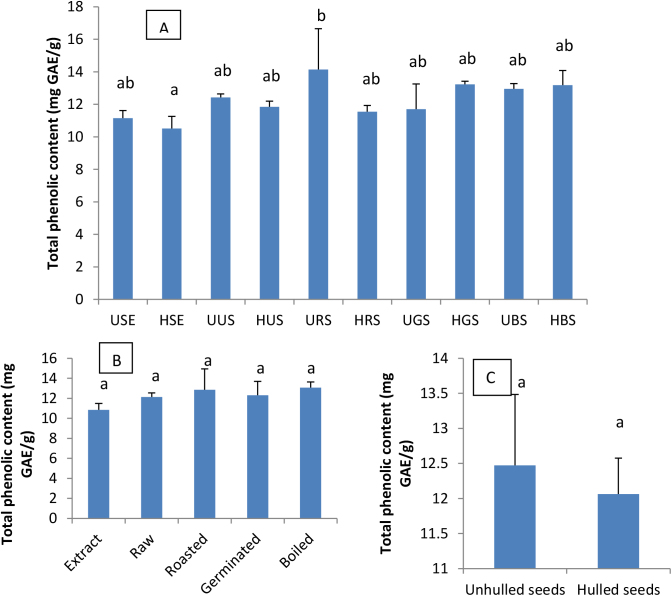
Antioxidant properties of phenolic compounds found in plants and consequently in functional foods, are nowadays well documented. It is therefore quite important to find out the cooking methods that better preserve them or even improve their contents in foods. Since almost all the complications associated with obesity are either direct or indirect consequences of the oxidative stress, the effects of domestic cooking methods of soybean seeds on their phenolic content were evaluated and the results are shown in Fig. 2. The phenolic contents expressed in mg of Gallic Acid Equivalent/g ranged from 10.51 (HSE) to 14.13 (URS). The values obtained in this study are lower than that reported in the literature and could be explained by the low extractability of phenolic compounds into water (Lemos et al., 2012) given that previous studies were done using the methanol extracts. Although no significant difference was observed in phenolic content of the different soybean seed culinary forms, roasting increased the phenolic content of soybean seeds and URS recorded the highest phenolic content (14.13 mg GAE/g) while HRS demonstrated the lowest content (11.54 mg GAE/g). Indeed, Xu and Chang (2008) already reported that heat could cause the evaporation of intracellular water, which might result in a greater availability of plant phenolic contents in the matrix. These results also corroborate the findings early reported by Lemos et al. (2012); Barakat and Rohn (2014), since no significant difference was observed between URS and UUS phenolic content.
Fig. 2.
Total phenolic content of soybean seeds extracts and culinary forms powders (A), effect of domestic cooking methods(B) and hulling (C) on phenolic content. USE: Unhulled Soybean aqueous Extract, HSE: Hulled Soybean aquoeus Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean. Values with different letters are significantly different at P < 0.05.
3.1.3. Flavonoid content
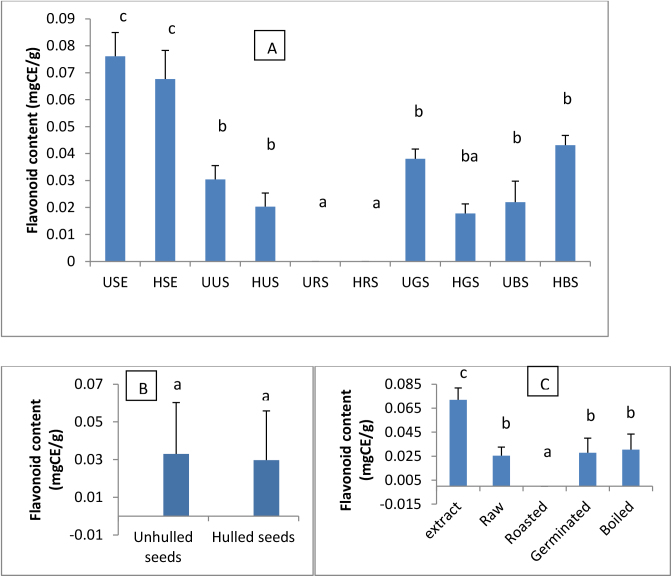
Flavonoid content of soybean seeds extracts and culinary forms are shown in Fig. 3. Overall, the soy extraction resulted in a better concentration of flavonoid. The lower flavonoid content for all the different seeds’ forms in the present study could mainly be the consequences of the forms and the solvent used, since powders were used and were extracted with water which is not the best solvent for flavonoid extraction. USE exhibited the highest flavonoid content. Boiling increased the flavonoid content of soybean seeds while roasting completely destroyed them. Lowering or complete destruction of flavonoid in roasted soybean seeds could be the consequence of thermal degradation since high temperatures are used in the process. Barakat and Rohn (2014) also reported a drastic reduction in flavonoid content of broccoli-based bars treated with heat (frying). No significant difference in flavonoid contents was induced by hulling of soybean seeds before treatments.
Fig. 3.
Flavonoid content of extracts and soybean seeds culinary forms(A), effect of hulling (B) and domestic cooking methods (C) on flavonoid content. USE: Unhulled Soybean aqueous Extract, HSE: Hulled Soybean aqueous Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean. Values with different letters are significantly different at P < 0.05.
3.1.4. Antioxidant activity: FRAP
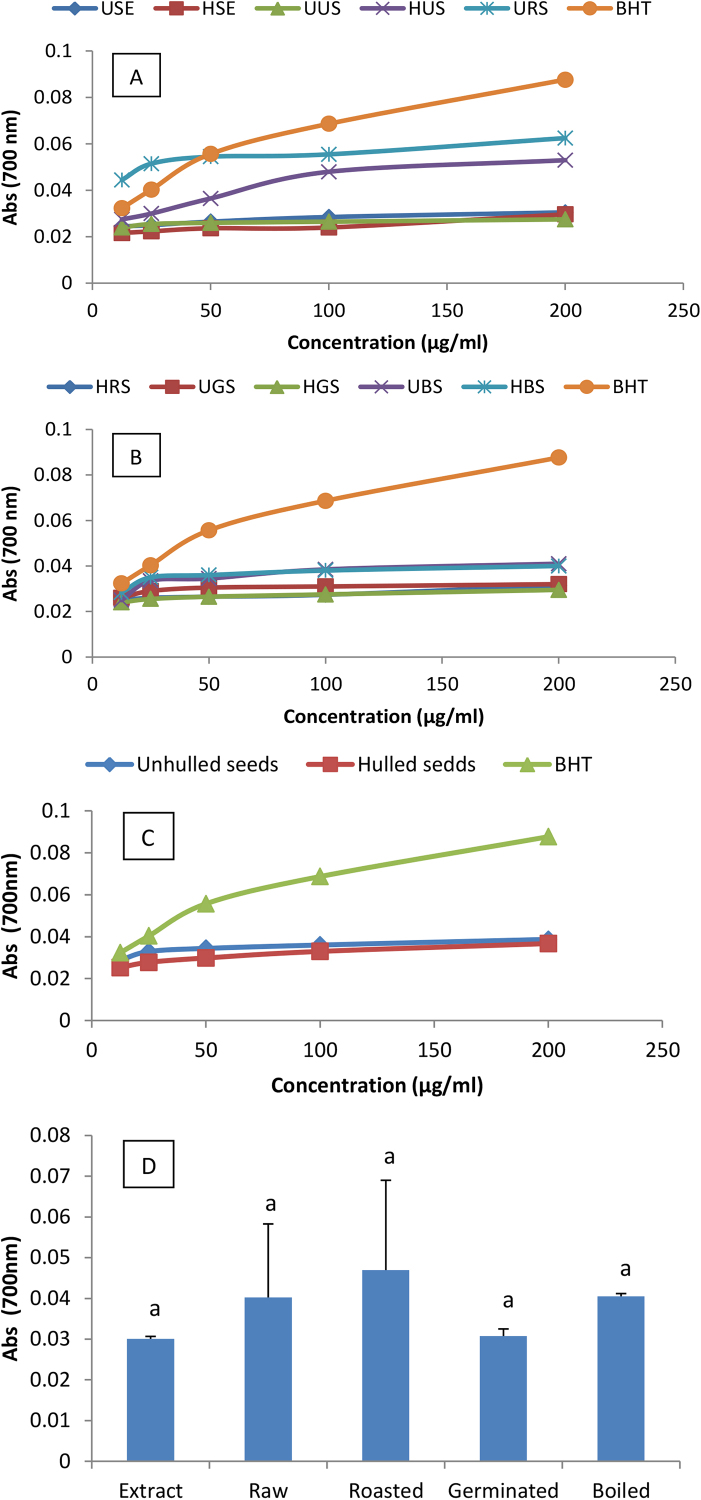
Antioxidant abilities of a substance can be estimated using different methods. The ferric iron reducing ability is one of the most commonly used methods to evaluate the antioxidant activity of a solution given that most known antioxidants act as electron donors to stabilize oxidative species. The ferric iron reducing capacity of extracts and suspensions of soybean seeds culinary forms are summarized in Fig. 4. Except for USE which exhibited a reducing activity greater than that of the control at concentrations 12.5 and 25 μg/ml, HSE and all the culinary forms exhibited a ferric iron reducing ability lower than that of BHT at all the concentrations. USE showed a significant difference in the reducing capacity compared to all the culinary forms of soybean seeds except for HUS. Hulling did not affect the ferric iron reducing ability of soybean seeds. Sprouting did not maintain the ferric iron reducing capacity while roasting was observed to better preserve it. The high ability of URS to reduce the ferric iron could be the consequence of its phenolic content since a high correlation have been demonstrated between phenolic contents and antioxidant abilities of soybean seeds and many other fruits, leaves, roots or seeds was demonstrated (Barakat and Rohn, 2014; Jinhu et al., 2016).
Fig. 4.
Ferric iron reducing capacity of extracts and soybean seeds culinary forms (A andB), effect of hulling (C) and domestic cooking methods (D) on ferric iron reducing capacity. USE: Unhulled Soybean aqueous Extract, HSE: Hulled Soybean aquoeus Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean. Values with different letters are significantly different at P < 0.05.
3.2. Hypoglycemic potential: oral glucose tolerance test (OGTT)
Table 1 shows the blood glucose concentrations taken at a constant time interval during 2 h. Compared to the HD control, the postprandial blood glycaemia peak appears 30 min following the ingestion of the glucose solution (2 mg/kg of body weight). Furthermore, all the treatments lowered the postprandial glycaemia peak concentration, with USE having the best activity while UUS exhibited the lowest ability to down regulate the peak of the post-prandial blood glucose. Overall, the culinary treatments reduced the ability of the soybean to lower the postprandial glycaemia peak concentration compare to the extracts even though with the exception of UUS there was no significant differences.
Table 1.
Variations in blood glucose (mg/dl).
| Groups | Time (T*30 min) |
||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| USE | 105.8 ± 6.8 | 108.8 ± 5.3a | 114.1 ± 3.6ab | 125.7 ± 4.1 | 113.0 ± 2.8 |
| HSE | 106.0 ± 1.7 | 121.1 ± 5.4a | 109.0 ± 3.6ab | 121.8 ± 4.1 | 114.9 ± 2.8 |
| UUS | 100.3 ± 6.5 | 143.4 ± 5.2c | 128.1 ± 3.5cde | 111.6 ± 4.0 | 114.4 ± 2.7 |
| HUS | 100.5 ± 3.5 | 123.7 ± 5.2a | 126.4 ± 3.5cde | 114.4 ± 4.0 | 108.6 ± 2.7 |
| WRS | 100.6 ± 6.1 | 122.4 ± 5.2a | 115.6 ± 3.5ab | 106.6 ± 4.0 | 105.7 ± 2.7 |
| HRS | 100.1 ± 9.3 | 115.1 ± 5.2a | 118.0 ± 3.5abde | 98.4 ± 4.0 | 110.7 ± 2.7 |
| UGS | 104.1 ± 7.2 | 113.9 ± 5.3a | 113.5 ± 3.6ab | 116.3 ± 4.1 | 100.2 ± 2.8 |
| HGS | 105.3 ± 11.7 | 114.5 ± 5.3a | 115.2 ± 3.6ab | 111.1 ± 4.1 | 103.0 ± 2.8 |
| UBS | 100.3 ± 6.2 | 122.9 ± 5.2a | 126.6 ± 3.5ce | 114.3 ± 4.0 | 88.7 ± 2.7 |
| HBS | 91.5 ± 7.8 | 114.0 ± 5.5a | 126.2 ± 3.7cde | 105.4 ± 4.3 | 98.4 ± 2.9 |
| HD | 97.5 ± 6.1 | 152.9 ± 5.2c | 120.2 ± 3.5acde | 126.4 ± 4.0 | 89.0 ± 2.7 |
| BD | 87.3 ± 5.3 | 117.0 ± 6.0a | 126.9 ± 4.0cde | 98.5 ± 4.6 | 90.2 ± 3.1 |
USE: Unhulled Soybean aqueous Extract, HSE: Hulled Soybean aquoeus Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean, HD: Hypercaloric Diet, BD: Basal Diet. Statistical difference is between treatment and values with different letters are significantly different at P < 0.05.
3.3. Anti-obesity potential
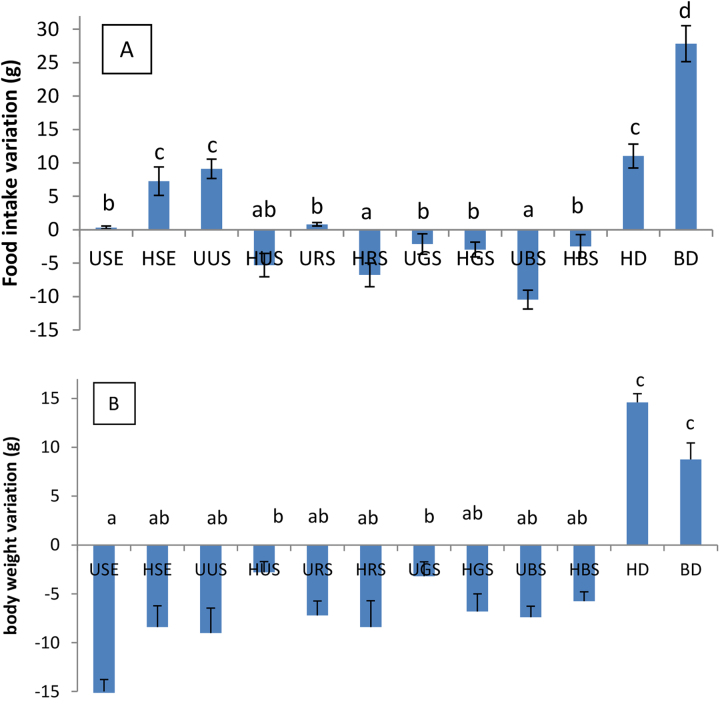
3.3.1. Effect of the treatments on the food intake
Variation of animals’ food intake within the 28 days of the treatment is shown in Fig. 5A. All the treated groups with exception of the groups receiving HSE and UUS, showed a significantly lower food intake compare to controls, suggesting that soybean seeds exhibit anorexigenic potential. Recent studies have shown that consumption of fibre-rich foods increase the synthesis of factors such as cholecystokinin, Glucagon-like-Peptide 1(GLP-1) and neuropeptide YY which limit food intake by reducing the production of ghrelin, the peptide responsible for transmission to the central nervous system of hunger sensation (Weickert and Pfeiffer, 2008). Otles and Ozgoz (2014) proposed that fibres and soluble ones specially, by their ability to adsorb water, can cause an increase in the volume and the viscosity of the stomach content, resulting in a slowdown of the gastric emptying and thus increasing and prolonging the satiety.
Fig. 5.
Variations of food intake (A) and body weight (B) within the treatment period (28 days). Values presented are cumulative. Weight variation: values are obtained by subtracting the initial body weight (day 1) to the final weight (day 28). USE: Unhulled Soybean aqueous Extract, HSE: Hulled Soybean aquoeus Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean, HD: Hypercaloric Diet, BD: Basal Diet. Values with different letters are significantly different at P < 0.05.
Comparisons between the controls show that BD had a food intake greater than that of the positive control (HD). These results corroborate what was previously reported by Novelli et al. (2007), and may be due to the oil content of the different diets given to animals. Indeed, BD was fed on a basal diet containing little lipids while HD were fed on a high fat, high carbohydrate diet. This could then explain the low food intake observed as foods with high lipid content have been reported to be less palatable besides the fact they can reduce the synthesis of ghrelin, the peptide signaling hunger to the central nervous system (Novelli et al., 2007; Mengmei and Taihua, 2016).
3.3.2. Effect of the treatments on the body weight
The variation in body weight of animals under treatment within the 28 days is presented in Fig. 5. All treated groups showed a negative body mass variation. Untreated animal group (HD) fed with HFHS diet resulted in a significant increase in body weight gain (p < 0.05) when compared to control group, fed with normal diet. This could be explained by the difference in the calorie density of foods served to animals. In fact, compared to BD, the HD group received a high energy density diet which was more prone to induce weight gain. Similar results have been observed in other studies (Prangthip et al., 2013; Kshirsagar et al., 2015). USE exhibited the highest ability to induce a weight loss (-15.5 g). URS was the culinary form that induced the highest loss in body weight (-8.4 g) while HUS did it to a lesser extent. Induction of weight loss could be explained by a limitation of the food intake due to their fibres content, forcing the body to utilize fat reserves, thus inducing a slimming effect. Also, this potential could be conferred by the presence of phenolic compounds, mainly including isoflavones in soybean seeds. Indeed, isoflavones can reduce adipogenesis via the Peroxysome-Proliferator Activated Receptors (PPARs) and specially PPARα and PPARγ (Milind et al., 2014). The slimming potential of soybean seeds culinary forms could also be related to their fibre content since soluble fibres specially are able to adsorb nutrients in the gastro intestinal tract, then limiting their absorption. Indeed, McCue et al. (2005) suggested that aqueous extracts of soybean seeds could greatly inhibit alpha-amylase; a key enzyme implicated in the catabolism of carbohydrates, via phenolic compounds since they observed in vitro, a great correlation between the phenolic content of extracts and their ability to inhibit alpha-amylase. They also reported the ability of soybean seeds to inhibit alpha-amylase could be increased by heat treatments, sprouting or microbial bioprocess. This may then explain why it was a non-treated form (UUS) that exhibited the lowest activity in reducing the postprandial blood glucose either at 30 or 60 min after ingestion of the glucose solution.
3.3.3. Effect of the treatment on the lipid profile
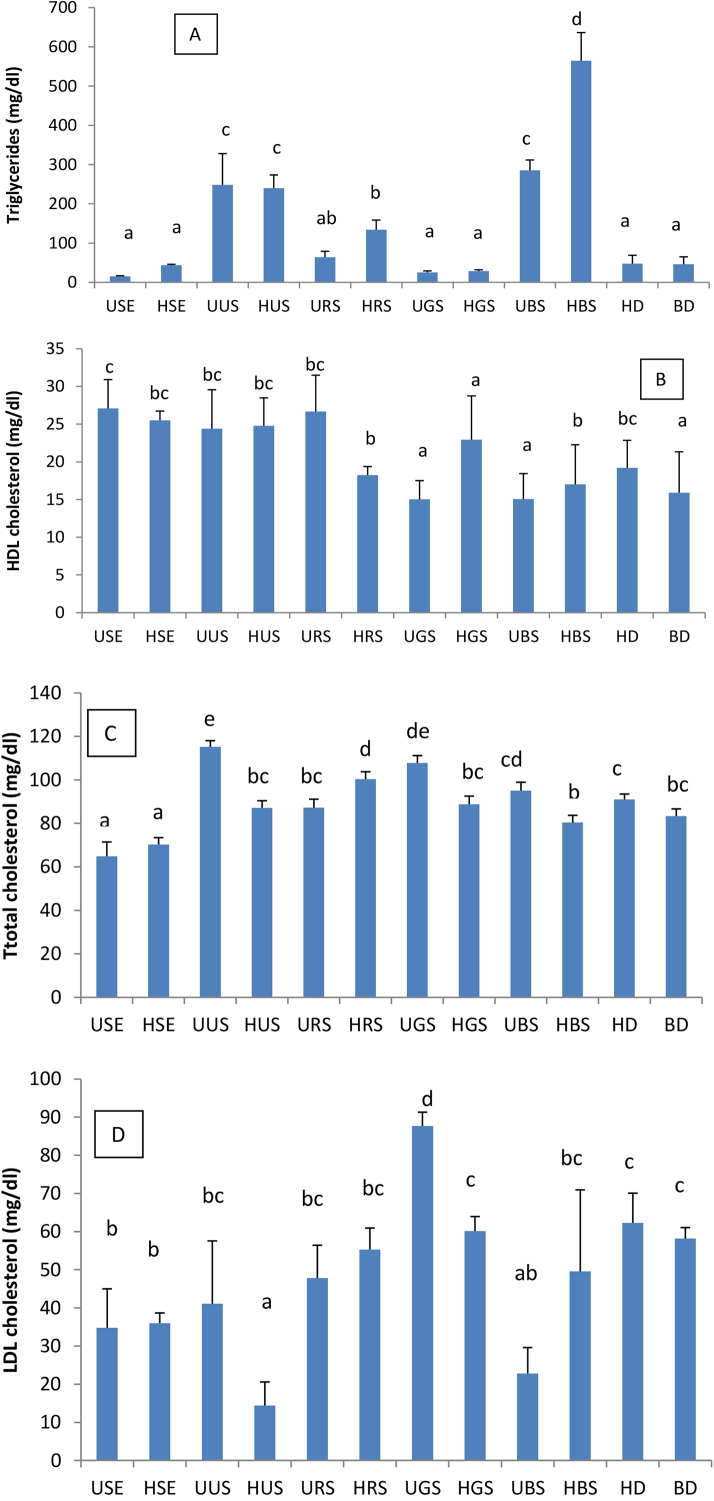
The following Fig. 6 depicts the serum concentration of triglyceride, total, HDL and LDL cholesterol in animals after 28 days of treatment. Lack of a clear mechanism by which soybean exerts its physiological effect limits its clinical utility and wide acceptability (Sharma and Baluja, 2015). Many hypotheses that are still needed to be verified and confirmed are nowadays otherwise accepted.
Fig. 6.
A, B, C and D: Triglyceride, Total, HDL and LDL cholesterol. Parameters were measured on the blood collected at the end of the treatment (day 28). USE: Unhulled Soybean aqueous Extract, HSE: Hulled Soybean aquoeus Extract, UUS: Unhulled Untreated Soybean, HUS: Hulled Untreated Soybean, URS: Unhulled Roasted Soybean, HRS: Hulled Roasted Soybean, UGS: Unhulled Germinated Soybean, HGS: Hulled Germinated Soybean, UBS: Unhulled Boiled Soybean, HBS: Hulled Boiled Soybean, HD: Hypercaloric Diet, BD: Basal Diet. Values with different letters are significantly different at P < 0.05.
Fig. 6A, B, C and D show the serum triglycerides concentrations in animals treated for 28 days with soybean seeds. USE induced the highest decrease in serum triglycerides. HGS is the culinary form that better decreased the serum triglyceride concentrations in comparison with other culinary treatments. These results corroborate data reported by Wanpen et al. (2013). These authors reported that germinated soybean seeds did normalize the serum triglycerides levels in rats, and that activity was correlated to their protein content. The above mentioned results are also in accordance with what obtained by Mona et al. (2014) who reported that isoflavone extracted from soybean seeds significantly lowered the serum triglyceride levels in rats.
Apart from USE which exhibited significant lowering effect on total cholesterol, no significant difference was obtained in serum total cholesterol of animals treated with raw or suspensions of soybean seeds culinary forms. Reduction in total serum cholesterol induced by USE can be related to its high phenolic content (11.15 mGAE/mg), flavonoid content (0.0761 mCE/mg) and also its fibre content since these phytochemicals have already been demonstrated to possess cholesterol lowering abilities (Klosterbuer and Slavin, 2010). Our results corroborate those of Anosike et al. (2008) who studied the benefits of supplementing diets with soybean seeds on serum enzymes markers and blood lipids. Also, UGS is the culinary form that induces the smallest reduction of serum total cholesterol as was also reported. This result corroborate what was reported by Wanpen et al. (2013), who observed that a 10% germinated soybean seeds protein supplemented diet did not significantly affected the serum total, HDL and LDL cholesterol as well as triglyceride when compared to unhulled soybean seeds. The hypocholesteromic potential of soybean seeds could be linked to high content of amino acids, which can, by modifying the insulin/glucagon ratio or the concentration of plasmatic thyroid hormones (increase in thyroxin levels) contribute to a reduction of serum cholesterol. Blood lipid regulatory aptitudes of soybean seeds could equally be the consequence of their fibre content, especially the soluble one, since they can adsorb bile acids, thus allowing their elimination in feces (Anderson and Major, 2002) and contributing to a reduction in serum LDL cholesterol level. Another hypothesis explaining the hypocholesteromic potential of soybean seeds is the reduction of bile acids synthesis by the short chain fatty acids obtained from the fermentation of soluble fibres present in soybean seeds by the symbiotic flora (Anderson and Hanna, 2008).
Fig. 6B shows that USE, followed by URS induced the highest increase in the animals’ HDL cholesterol while UGS and UBS induced the smallest increase in serum HDL cholesterol. These observations could be the result of the differences in the concentration of bioactive principles in soybean seeds forms such as phenols, flavonoid and crude fibres. The observations made in this work are in accordance with data previously reported by Anosike et al. (2008); Mona et al. (2014). Indeed, Mona et al. (2014) observed that isoflavones extracted from soybean seeds significantly normalized the lipid profile of diabetic rats.
Serum LDL cholesterol levels in different groups, estimated by Friedewald equation are shown in Fig. 6D. UBS exhibited the highest potential to lower serum LDL cholesterol while UGS possessed the lowest potential to do so. Significant reduction in LDL cholesterol levels is observed after USE is consistent with what was already reported by Mona et al. (2014) who obtained a significant decrease in serum LDL cholesterol of diabetic rats treated with isoflavone extracted from soybean seeds. UGS, on the other, possessed the smallest potential observed and is contrary to what was obtained by Wanpen et al. (2013) since they reported that soybean seeds did normalize the LDL cholesterol levels in rats. Soybean can stimulate the uptake of LDL cholesterol by liver cells. Indeed soybean seeds’ bioactive principles could stimulate the expression of LDL cholesterol receptors at the level of the liver thus increasing its liver uptake and a consequent decrease in serum. Decrease in serum LDL cholesterol could also be related to the presence of dietary fibres, since they have been demonstrated to adsorb glucose, cholesterol and bile acids, thus limiting their absorption in the gastro-intestinal tract (Zhang et al., 2011).
4. Conclusion
We observed in this study that variable effects were noted according to the treatment method and the concerned phytochemicals. With the exception of antioxidant activity, phenolic contents, we found that domestic cooking methods alter phytochemical contents of soybean seeds, thus lowering their hypoglycemic, slimming and serum lipid regulatory abilities compared to the soybean extracts. Boiling came out as the cooking method that best preserved the bioactive phytochemicals and anti-obesity potential of soybean seeds.
Declarations
Author contribution statement
Cerile Y. Woumbo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Dieudonné Kuate: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hilaire M. Womeni: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Anderson J.W., Hanna T.J. Impact of non-digestible carbohydrates on serum lipoproteins and risk for cardiovascular disease. J. Nutr. 2008;129:1457–1466. doi: 10.1093/jn/129.7.1457S. [DOI] [PubMed] [Google Scholar]
- Anderson J.W., Major A.W. Pulses and lipaemia: short and long-term effect: potential in the prevention of cardiovascular disease. Braz. J. Nutr. 2002;88:263–271. doi: 10.1079/BJN2002716. [DOI] [PubMed] [Google Scholar]
- Anosike C., Obidoa O., Ezeanyika L. Bioactive effects of soybean diet on serum marker enzymes, lipid profile and relative organ weights of Wistar rats. Pakistan J. Nutr. 2008;7(6):817–822. [Google Scholar]
- A.O.A.C . 15th Edn. AOAC international; Washington, D.C: 1990. Official Methods of Analysis of the AOAC. [Google Scholar]
- Barakat H., Rohn S. Effect of different cooking methods on bioactive compounds in vegetarian: broccoli-based bars. J. Funct. foods. 2014;11:407–416. [Google Scholar]
- Bau H., Villaume C., Nicolas J., Mejean L. Effect of germination on chemical composition: biochemical constituents and antinutritional factors of Soya bean (Glycine max) seeds. J. Sci. Food Agr. 1997;73:1–7. [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Carette C., Muzard L., Radu A., Barsamian C., Bretault M., Czernichow S. 2012. Traitement pharmacologique de l’obésité. Médecine Clinique endocrinologie & diabète n° 61. [Google Scholar]
- Chutipanyaporn P., Kruawan K., Chupeerach C., Santivarangkna C., Suttisansanee U. The effect of cooking process on antioxidant activities and total phenolic compounds of five colored beans. Food and Applied Bioscience Journal. 2014;2(3):183–191. [Google Scholar]
- Correia J., Pataky Z., Golay A. Comprendre l’obésité en Afrique: poids du développement et des représentations. Revue Médicale Suisse. 2014;10:712–726. [PubMed] [Google Scholar]
- Dixit A.K., Antony J.I.J., Sharma N.K., Tiwari R.K. Soybean constituents and theirs functional benefits. Opportunity Challenge and Scope of Natural Products in Medicinal Chemistry. 2011:367–383. [Google Scholar]
- Drinkwater M.J., Tsao R., Liu R., Defelice C., Wolyn D.J. Effects of cooking on rutin and glutathione concentrations and antioxidant activity of green asparagus (Asparagus officinalis) spears. J. Func. Foods. 2015;12:342–353. [Google Scholar]
- Finkelstein E.A., Khavjou O.A., Thompson H., Trogdon J.G., Liping P., Bettylou S., Dietz W. Obesity and Severe Obesity Forecasts Through 2030. Am. J. Prev. Med. 2012;42(6):563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Friedewald W., Levy R., Frederickson D. Estimation of the concentration of low- density lipoprotein cholesterol in plasma: without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Ganesan K., Xu B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients. 2017;9(5):455. doi: 10.3390/nu9050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Ohlander M., Jeppsson N., Björk L., Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophaerhamnoides L) during maturation. J. Agric. Food Chem. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- Jinhu T., Jianle C., Feiyan L., Shiguo C., Jianchu C., Donghong L., Xingqian Y. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016;197:1264–1270. doi: 10.1016/j.foodchem.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Klosterbuer A., Slavin J. Functionality of different fibres and their effects on human health. Can. J. Diet. Pract. Res. 2010;71:2. [Google Scholar]
- Kshirsagar R.P., Kothamasu M.V., Patil M.A., Reddy G.B., Kumar B.D., Diwan P.V. Geranium oil ameliorates endothelial dysfunction in high fat high sucrose diet induced metabolic complications in rats. J. Funct. Foods. 2015;15:284–293. [Google Scholar]
- Lemos M.R.B., Siqueira E.M., Arruda S.F., Zambiazi R.C. The effect of roasting on the phenolic contents and antioxidant potential of baru nuts [DipteryxalataVog.] Food Res. Int. 2012;48:592–597. [Google Scholar]
- Martirosyan D., Singh J. A new definition of functional food by FFC: what makes a new definition unique? Functional foods in health and disease. 2015;5(6):209–223. [Google Scholar]
- McCue P., Kwon Y., Shetty K. Anti-diabetic and anti-hypertensive potential of sprouted and solid-state bioprocessed soybean. Asia Pac. J. Clin. Nutr. 2005;14(2):145–152. [PubMed] [Google Scholar]
- Mengmei M., Taihua M. Anti-diabetic effects of soluble and insoluble dietary fibre from deoiled cumin in low-dose streptozotocin and high glucose-fat diet-induced type 2 diabetic rats. J. Funct. Foods. 2016;25:186–196. [Google Scholar]
- Miglio C., Chiavaro E., Visconti A., Fogliano V., Pellegrini A. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008;56:139–147. doi: 10.1021/jf072304b. [DOI] [PubMed] [Google Scholar]
- Mona A.A., Naglaa H.M., Nahed L.Z., Mohamed S.A., Hassan M.S. Effects of Soybean isoflavone on lipid profiles and antioxidant enzyme activity in Streptozotocin induced diabetic rats. Global J. Pharmacol. 2014;8(3):378–384. [Google Scholar]
- Milind P., Bansal N., Kaura S. Take soybean to remain evergreen. International Research Journal of Pharmacy. 2014;5(1):1–6. [Google Scholar]
- Novelli E.L.B., Diniz Y.S., Galhardi C.M., Ebaid G.M.X., Rodrigues H.G., Mani F., Fernandes A.A.H., Cicogna A.C., Novelli F.J.L. Anthropometric parameters and markers of obesity in rats. Lab. Anim. 2007;41:111–119. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- OCDE . 2008. Lignes directrices de l’OCDE pour les essais de produits chimiques: Étude de toxicité orale à dose répétée pendant 28 jours sur les rongeurs [guidelines of OECD for chemicals trials: repeated dose oral toxicity studies for 28 days in rodents] (in French) [Google Scholar]
- Otles S., Ozgoz S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014;13(2):191–202. [PubMed] [Google Scholar]
- Padmaja M., Sravanthi M., Hemalatha K.P.J. Evaluation of antioxidant activity of two Indian medicinal plants. J. Phytol. 2011;3(3):86–91. [Google Scholar]
- Písaříková B., Zralý Z. Dietary fibre content in lupine (Lupinusalbus L.) and soya (Glycine max L.) Seeds. Acta Veterinaria Brunensis. 2010;79:211–216. [Google Scholar]
- Prangthip P., Surasiang R., Charoensiri R., Leardkamolkarn V., Komindr S., Yamborisut U., Vanavichit A., Kongkachuichai R. Amelioration of hyperglycemia, hyperlipidemia, oxidative stress and inflammation in steptozotocin-induced diabetic rats fed a high fat diet by rice berry supplement. J. Funct. Foods. 2013;5(1):195–203. [Google Scholar]
- Shah H.U., Khan U., Alam S., Shad A., Iqbal Z., Parveen S. Effect of home cooking on the retention of various nutrients in commonly consumed pulses in Pakistan. Sarhad J. Agric. 2011;27:279–284. [Google Scholar]
- Sharma A., Baluja Z. Therapeutic Effects of Glycine max (Soybean): A Summary. Int. J. Res. Pharm. Biosci. 2015;2(1):22–27. [Google Scholar]
- Sharma S., Goyal R., Barwal S. Domestic processing effects on physicochemical, nutritional and anti-nutritional attributes in soybean (Glycine max L. Merill) Int. Food Res. J. 2013;20(6):3203–3209. [Google Scholar]
- Trinder P. Determination of glucose in blood using glucose oxidase with alternative oxygen acceptor. Ann. Clin. Biochem. 1969;6:24–27. [Google Scholar]
- Uhegbu F.O., Ugbogu A.E., Nwoku K.C., Ude V.C. Effect of Soybean Oil Supplemented Diet on Fatty Acid Level and Lipid Profile of Albino Rats. Br. J. Pharmacol. Toxicol. 2013;4(4):158–162. [Google Scholar]
- Wanpen M., Yaovadee C., Duangchan H., Saruda L., Sompoch Y. Effects of germinated soybean on serum lipids in rats. Pak. J. Nutr. 2013;12(9):833–836. [Google Scholar]
- Weickert M., Pfeiffer A. Metabolic effects of dietary fibre consumption and prevention of diabetes. J. Nutr. 2008;138:439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- Xu B., Chang S.K.C. Total phenolics, phenolic acids, isoflavones, and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agr. Food Chem. 2008;56:7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]
- Zhang N., Huan C., Ou S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J. Hazard. Mater. 2011;186:236–239. doi: 10.1016/j.jhazmat.2010.10.120. [DOI] [PubMed] [Google Scholar]